Abstract
Aging research has undergone unprecedented advances at an accelerating rate in recent years, leading to excitement in the field as well as opportunities for imagination and innovation. Novel insights indicate that, rather than resulting from a preprogrammed series of events, the aging process is predominantly driven by fundamental non-adaptive mechanisms that are interconnected, linked, and overlap. To varying degrees, these mechanisms also manifest with aging in bone where they cause skeletal fragility. Because these mechanisms of aging can be manipulated, it might be possible to slow, delay, or alleviate multiple age-related diseases and their complications by targeting conserved genetic signaling pathways, controlled functional networks, and basic biochemical processes. Indeed, findings in various mammalian species suggest that targeting fundamental aging mechanisms (eg, via either loss-of-function or gain-of-function mutations or administration of pharmacological therapies) can extend healthspan; ie, the healthy period of life free of chronic diseases. In this review, we summarize the evidence supporting the role of the spectrum of fundamental basic science discoveries contributing to organismal aging, with emphasis on mammalian studies and in particular aging mechanisms in bone that drive skeletal fragility. These mechanisms or aging hallmarks include: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Because these mechanisms are linked, interventions that ameliorate one hallmark can in theory ameliorate others. In the field of bone and mineral research, current challenges include defining the relative contributions of each aging hallmark to the natural skeletal aging process, better understanding the complex interconnections among the hallmarks, and identifying the most effective therapeutic strategies to safely target multiple hallmarks. Based on their interconnections, it may be feasible to simultaneously interfere with several fundamental aging mechanisms to alleviate a wide spectrum of age-related chronic diseases, including osteoporosis.
Keywords: AGING, BONE, OSTEOPOROSIS, DISEASE PREVENTION
Introduction
Aging, which has been defined as the time-dependent accumulation of cellular damage,(1) is associated with progressive decline of physiological functions, resulting in increased susceptibility to complications, pathologies, chronic diseases, and death.(1–3) Indeed, age is by far the greatest risk factor for chronic diseases including cancer, cardiovascular disease, frailty, metabolic dysfunction, macular degeneration, neurodegeneration, osteoarthritis, and osteoporosis, as well as a host of other chronic conditions.(1–3) Because elderly people represent the most rapidly growing segment of the population in most developed countries, there is an increasing need to identify ways to forestall age-related chronic diseases to thereby reduce the enormous burden of healthcare expenditures these diseases create.
The premise that aging results from a preprogrammed series of events is contrary to the now overwhelming and generally accepted experimental evidence establishing that fundamental non-adaptive processes predominantly drive the aging process.(4) Logical evolutionary reasoning implies that adaptive change, which optimizes for fitness and reproduction, does not program aging or longevity given the declining force of natural selection with age.(5) Accordingly, genes with adverse effects later in life can be conserved due to antagonistic pleiotropy, whereby a gene variant controls a phenotypic trait that has beneficial effects in youth but harmful effects in old age.(5) Several examples of antagonistic pleiotropy will be discussed in the context of the spectrum of fundamental basic science mechanisms contributing to organismal aging, and given the complexity the focus herein will be placed on mammalian aging.
Although aging is associated with the decline in a multitude of different biological processes, it is now irrefutable that aging can be manipulated by, eg, only a single genetic mutation.(4) Although at first this is perhaps surprising there are, in fact, hundreds of examples of genes identified in studies ranging across multiple invertebrate species (eg, yeast, worms, and flies), demonstrating that ablated or diminished expression of an individual gene can lead to lifespan extension.(6,7) This is further supported by mounting evidence in mammals establishing that it is possible to slow, delay, or alleviate age-related diseases and their complications by targeting conserved genetic signaling pathways, controlled functional networks, and basic biochemical processes.(1–3) Indeed, findings from studies in various mammalian species suggest that targeting fundamental aging mechanisms (eg, via either loss-of-function or gain-of-function mutations or administration of pharmacological therapies) can extend healthspan; ie, the healthy period of life free of chronic diseases. Collectively, this has led to development of the “geroscience hypothesis,” which posits that manipulation of aging will delay (in parallel) the appearance or severity of multiple chronic diseases because these diseases share the same underlying major risk factor—age itself.(8)
This review summarizes the evidence supporting the role of the spectrum of fundamental basic science discoveries contributing to organismal aging, with emphasis on mammalian studies and particular focus on aging mechanisms in bone that drive skeletal fragility. These mechanisms, in a broader context, have been reviewed in a landmark publication(1) that proposed nine hallmarks of aging. This collaborative effort has helped guide the field to better conceptualize and understand the mechanisms that drive the natural aging process. The nine hallmarks are as follows: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication.(1) To be defined as a hallmark of aging,(1) each fundamental aging mechanism should meet the following criteria: (i) the hallmark will manifest during natural chronological aging; (ii) experimental manipulation of the hallmark will accelerate aging; and (iii) experimental amelioration of the hallmark will forestall the natural aging process and hence extend healthspan. In bone, each hallmark fulfills these criteria to varying degrees and for some only suggestive evidence in this sense currently exists. This will be discussed along with how the aging hallmarks are interconnected, linked, and overlap; thus, interventions that ameliorate one hallmark can in theory ameliorate others. Herein, we focus on evidence implicating each of these fundamental mechanisms in the pathogenesis of osteoporosis as it manifests in the setting of aging.
Genomic Instability
There is extensive evidence that genomic damage accompanies aging and that its artificial induction can provoke aspects of accelerated aging.(1) Of all DNA lesions, DNA double-strand breaks are arguably the most harmful. DNA double-strand breaks can be induced by radiation, radiomimetic chemicals, or ROS, but also during DNA replication when a polymerase encounters a single-strand lesion at a replication fork.(9) DNA double-strand breaks pose problems for cells because their immediate and efficient repair by ligation is often constrained by their physical separation and/or the need to process damaged DNA termini.(10) In the absence of repair, damaged cells can be eliminated by apoptosis. Alternatively, mitotically active cells can respond to DNA double-strand breaks by becoming senescent (see Cellular Senescence).
DNA damage triggers a repair response known as the DNA damage response (DDR). This response is characterized by the activation of Ataxia Telangiectasia Mutated (ATM) and the recruitment of RAD-3–related kinase (ATR)(11) to the site of damage, leading to phosphorylation of Ser-139 of histone H2AX molecules (γ-H2AX) adjacent to the site of DNA damage. The phosphorylation of histone H2AX activates the transducer kinases Chk1 and Chk2, which converge on p53/p21 and p16.(12) Additionally, the DDR stimulates the senescence-associated secretory phenotype (SASP) by upregulating GATA4 and NF-kB. Markers of DNA damage and the DDR are increased in osteoprogenitors and osteocytes from old mice and are associated with increased cell senescence and apoptosis.
Cells contain several DNA repair mechanisms such as the global genome nucleotide excision repair (GG-NER) pathway, which removes helix-distorting damage anywhere in the genome. Transcription-coupled repair (TCR) eliminates lesions in the transcribed strand of active genes when transcription is arrested by DNA damage. This allows for rapid continuation of the transcription process and prevents cell death and senescence.(13,14) In humans, deficiencies in DNA repair mechanisms cause progeroid syndromes such as Werner syndrome, Bloom syndrome, xeroderma pigmentosum, trichothiodystrophy (TTD), Cockayne syndrome, or Seckel syndrome,(15,16) suggesting that DNA damage is causally linked to aging. In several of these syndromes, skeletal abnormalities have been described.(17,18) Similarly, DNA repair-deficient mouse models have reduced lifespan and display multiple symptoms of premature aging including low bone mass.(19–22) However, because several of these mouse models die before 3 months of age, the relevance of these progeroid syndromes to natural skeletal aging is minimal. By contrast, progeroid excision repair cross-complementary group 1 (Ercc1−/Δ) mutant mice have a longer lifespan of about 7 months(20,23) and exhibit low bone mass, associated with low bone formation and increased bone resorption,(21) similar to chronological aging. Furthermore, studies using a mouse model of TTD(24–26) have shown that early bone development is not affected but TTD causes accelerated bone aging from 9 months onward characterized by a decrease in trabecular and cortical bone as well as a loss of periosteal apposition and a reduction in bone strength with age. These effects are associated a reduction in the number of osteogenic and osteoprogenitor cells, as determined in cultures of bone marrow cells. Nevertheless, because of a significant downregulation of the IGF axis and lack of adipose tissue in the TTD mice it remains unknown whether the skeletal defects resulted from bone cell intrinsic effects or were a consequence of the metabolic defects.
In humans, signs of premature aging have been observed in survivors of childhood cancer who received therapies known to induce DNA double-strand breaks, such as chemotherapeutic drugs and radiation.(27,28) In mice, DNA damage due to focal irradiation in long bones causes senescence in osteoblast lineage cells, decreases bone formation, and leads to bone loss.(29) Irradiation also causes changes in osteoprogenitors that are similar to those seen with aging. Together, these studies support the idea that DNA damage interferes with normal skeletal maintenance, and that accumulation of damage with aging contributes to loss of bone mass.
Telomere Attrition
Another hallmark of aging in both animals and humans is telomere attrition. Telomeres are the protective ends of chromosomes that shorten with advancing organismal age. Attrition of telomeres is accelerated in several human diseases driven by pathological telomere dysfunction.(30) Indeed, telomere attrition and mutations in telomere maintenance genes represent a primary hallmark of aging that cause cellular damage, which subsequently activates compensatory or antagonistic aging hallmarks that alleviate the damage in the short-term, but ultimately fail to do so over time eventually leading to tissue dysfunction. For example, telomere attrition induces chromosomal instability that can contribute to tumorigenesis and trigger activation of the cell senescence program (see Cellular Senescence), which in turn is followed by deleterious changes in communication between cells (see Altered Intercellular Communication) as well as a reduction in the proliferation of stem cells and their ability to regenerate tissues (see Stem Cell Exhaustion). In terms of causality, several studies using animals with genetic mutations modeling human telomere syndromes have established the causal role of telomere loss in organismal aging,(30) and have demonstrated that genetic restoration of telomerase (a specialized telomere synthesizing enzyme) can prevent premature aging in telomerase-deficient mice.(31) Taken together, these discoveries have stimulated the development of preclinical telomere-targeted therapeutic approaches to delay aging.(32)
Telomere attrition also represents an intrinsic dysfunctional aging mechanism in cells of the osteoblast lineage as it impairs osteogenic differentiation and has negative effects on skeletal remodeling.(33,34) For example, in osteoprogenitors aging causes dissociation of telomere protective complexes composed of shelterin and associated proteins that together, along with telomerase, normally serve to maintain telomere length, resulting in incomplete replication of chromosomes. This age-related dissociation exposes the ends of chromosomes, leaving them highly vulnerable to persistent insults from DNA damage (see Genomic Instability) that over time leads to activation of the cell senescence program(35) (see Cellular Senescence), subsequent upregulation of a pro-inflammatory secretome (see Altered Intercellular Communication), and diminished self-renewal capacity of osteoprogenitor stem cell pools(33,34) (see Stem Cell Exhaustion). This is an example of how the aging hallmarks in the pathogenesis of age-related bone loss are interconnected, linked, and overlap. Ultimately, these events lead to an age-related decline in osteoprogenitors, deficient bone formation, and the inability to adequately repair and replace damaged or lost bone. Although direct evidence for telomere attrition in osteoblast lineage cells with natural chronological aging is lacking at this time, mice with invalidated telomerase reverse transcriptase (Terc) have been shown to display a low bone mass phenotype, resulting from defective osteoblasts,(36) a pro-inflammatory bone microenvironment that fuels osteoclastogenesis and increased bone resorption,(37) as well as several indicators of increased cell senescence, such as upregulated p53 and p21 levels.(38) Thus, it remains to be seen whether telomere-targeted therapeutic approaches can be applied in the setting of skeletal aging to limit cell damage, prevent the antagonistic or compensatory responses to cell damage, and restore proper intercellular communication as well as adequate numbers of osteoprogenitors. Notwithstanding, caution in doing so may be warranted given that long-term telomerization using gene modifications in stem cells can cause spontaneous genetic changes (see Genomic Instability) and cancer.(39)
Epigenetic Alterations
Chromatin remodeling, DNA methylation, and histone modifications are each specific examples of ways in which the epigenome is regulated by complex enzymatic systems in the setting of both normal and pathological conditions.(1) Indeed, epigenetic posttranscriptional modifications of gene expression are common events that can occur in all cells throughout life either as part of normal physiological programs or can be altered by various age-related changes in external or environmental stressors.(1) For example, aging is associated with chromatin redistribution and distention as well as epigenetic perturbations that can cause shortening of telomeres(30) (see Telomere Attrition) and higher-order unfolding of heterochromatin,(40) which are both hallmark features of senescent cells (see Cellular Senescence). Experimental evidence from gain-of-function and loss-of-function studies in invertebrates has revealed the significance of multiple enzymatic systems designed to regulate epigenetic changes including chromatin remodeling of protein complexes, DNA methyltransferases, methylases/demethylases, and histone acetylases/deacetylases.(1) For example, gene expression can be repressed with aging by DNA methylation, catalyzed by DNA methyltransferases, which prevent regulatory transcription factors from binding to their respective promoter regions. In addition, aging can contribute toward the excessive removal of acetyl groups by histone deacetylases (Hdacs) as well as histone methylation by histone methyltransferases that close the conformation of chromatin and subsequently represses transcription. Noteworthy, however, is that this effect can be reversed by acetylation of lysine residues on histone tails via histone acetyltransferases or Hdac inhibitors. Both of these manipulations can promote transcriptional activation. In addition, aging alters the transcriptional signature of small noncoding RNA molecules, referred to as microRNAs (ie, miRNAs) that also have important roles in posttranscriptional epigenetic regulation of gene expression by blocking mRNA translation or by inducing mRNA degradation, thereby impacting healthspan and longevity. However, at the present time, our understanding of how these complex networks are altered by aging is still in its infancy. Nevertheless, epigenetic alterations not only fulfill all of the criteria of an aging hallmark, but also can in theory be restored and therefore represent realistic therapeutic targets for novel interventions to extend healthspan and prevent age-related bone loss.
Although DNA methylation is one of most extensively studied epigenetic mechanisms, age-related changes in DNA methylation patterns in bone, as well as in other tissues, are complex and still not well understood. Aging was thought for many years to predominantly result in global hypomethylation(41); however, more recent evidence has refuted this apparently oversimplified conclusion.(1) Notwithstanding, there are data suggesting that DNA methyltransferases regulate bone metabolism in vivo, at least in the setting of accelerated aging models. For example, genetic deletion of the histone methyltransferase, Suv39h1, in Zmpste24−/− mice (a model of Hutchinson-Gilford progeria syndrome) increased bone mass and extended the lifespan of these animals.(42) Furthermore, DNA methylation has been shown to influence gene transcription of several crucial factors involved in regulating osteoblast and osteoclast development as well as their activity. These factors include the receptor activator of NF-kB ligand (RANKL)–osteoprotegerin (OPG) system(43) and sclerostin.(44) Thus, based on collective evidence to date, there is at least proof-of-concept precedent for manipulating DNA methylation to delay or alleviate skeletal aging, although there is still a need to rigorously test this hypothesis in the setting of natural chronological aging.
There is also evidence for the role of both histone methylation and demethylation in the regulation of osteoblast development and activity as well as in the control of lineage commitment of mesenchymal stem cells (MSCs) in bone marrow toward osteogenic versus adipogenic cell fates. For example, histone methylation regulates the promoter regions of PPARγ target genes.(45) In addition, osteogenesis is controlled by histone demethylases(46,47) that regulate transcription of several classic osteoblast genes during differentiation, including Runx2 and Osterix.(48,49) However, if and how the epigenetic mechanisms of histone methylation and demethylation are altered with aging in bone, and whether these processes represent viable targets for intervention remains to be seen.
Perhaps even more convincing evidence implicating age-related epigenetic alterations in skeletal aging comes from studies of histone acetylases and deacetylases (eg, Hdacs). Indeed, the family of 18 Hdacs, including the subfamily of seven sirtuins (Sirt1–Sirt7), not only have crucial roles in bone development through control of both intramembranous and endochondral ossification,(50) but have also been implicated in the regulation of several other aging hallmarks such as genomic instability, mitochondrial dysfunction, deregulated nutrient sensing, and altered intercellular communication,(51) again underscoring how the aging hallmarks are interconnected, linked, and overlap. Of particular interest with regard to potential therapeutic approaches to delay or alleviate skeletal aging, inhibition of Hdac or Sirt activity though either genetic manipulations or pharmacological interventions can result in accelerated skeletal aging.(51) By contrast, activation of Sirt1, eg, in mice is associated with a delay in the onset of many agingrelated diseases, including osteoporosis (see Deregulated Nutrient Sensing). Therefore, strategies to activate sirtuins in older individuals could delay or perhaps alleviate several aspects of skeletal aging.
Loss of Proteostasis
Our inevitable functional decline with aging is associated with accumulation of damaged proteins and organelles inside cells, which results in toxic stress that drives age-related tissue dysfunction. This can be the result of endogenous and exogenous stressors that causes proteins within the cell to aggregate, unfold, or even become misfolded.(1) The fate of these misfit proteins is twofold: they can either be reconfigured into their original state or cleared from the cell via proteolysis.(52) With aging, these processes decline as intracellular accumulation of damaged proteins results from failure to restore the structure of misfolded polypeptides, refold unfolded proteins, or from defective proteasomal degradation and impaired autophagy.(1) Despite the remarkable adaptability of these networks to rapidly correct perturbations in proteasomal balance, with aging there is a chronically diminished capacity of these precisely coordinated quality control mechanisms to maintain intracellular protein homeostasis (ie, proteostasis), particularly under stressful conditions leading to proteotoxicity.(52) Therefore, a major goal of current experimental efforts to extend healthspan is to develop interventions that effectively restore proteostasis. Thus far, several interventions that prevent the loss of proteostasis with aging have been shown in both invertebrates and mammals to improve healthspan and some even extend lifespan.(53,54)
One of the essential proteolytic “cleanup” mechanisms that cells can use to maintain proper intracellular proteostasis and prevent proteotoxic stress is autophagy, whereby misfolded, unfolded, aggregated, toxic, or damage proteins are selectively targeted (via chaperone-mediated autophagy or macroautophagy) for destruction.(52) Although we know the efficiency of proteolysis declines with advancing age, our understanding of autophagy in the pathogenesis of age-associated diseases is still evolving. Excessive intracellular accumulation of damaged proteins due to failed autophagy has, however, been established as a common feature observed in neurodegenerative disorders linked with aging such as Alzheimer’s disease and Parkinson’s disease.(52,53) By contrast, situations that stimulate autophagy, such as caloric restriction and physical activity, delay aging-related cellular and tissue degeneration.(55) Furthermore, genetic manipulation that prevents the age-related decline in autophagy in hepatocytes, eg, via upregulation of LAMP-2—a protein involved in lysosomal degradation of damaged proteins, helps delay liver functional decline in old mice.(56) These findings provide compelling evidence reinforcing the premise that proper autophagy counteracts the aging process.
Whether stimulating autophagy to adequately remove damaged proteins in old age causes functional improvements in a multitude of tissues throughout the organism is still unclear. An essential role of autophagy has been hypothesized in bone,(57) for example in osteocytes which, analogous to neurons and hepatocytes, are long-lived postmitotic cells that require defense mechanisms against various forms of endogenous and exogenous types of stress. Work from the Charles O’Brien laboratory has shown that deletion of Atg7, a ubiquitin-activating (E1)-like enzyme that activates LC3—a central protein that stimulates the autophagy pathway—in dentin matrix protein 1 (DMP1)-expressing cells using DMP-cre transgenic mice mimics several aspects of skeletal aging including low bone mass and turnover.(58) In subsequent studies by the same laboratory, deletion of Atg7 using Osterix 1 (Osx1)-Cre transgenic mice, in which deletion occurs at the earliest stages of osteoblast lineage commitment,(59) caused an even more pronounced low bone mass and turnover phenotype as compared to that observed in mice lacking Atg7 in osteocytes.(60) Moreover, defective autophagy in osteoblast progenitors (including their descendants) was associated with diminished maturation of osteocyte morphology as well as retention of endoplasmic reticulum and mitochondria in osteocytes.(60) Taken together, these findings suggest that defective autophagy in osteoblast lineage cells prevents the proper transition of osteoblasts into osteocytes and causes degeneration of the osteocyte canalicular network.(60) Interestingly, the latter phenomenon has recently been associated with natural chronological aging in mice.(61) Given that impaired autophagy in osteoblast lineage cells and osteocytes appears to be a consequence of overwhelming stress that becomes intensified in bone over time, defective autophagy may have a causal role in skeletal aging. However, direct evidence is lacking at this time. Therefore, it will be interesting in future work to examine the potential effects of impaired autophagy in mediating bone loss in the setting of natural chronological aging. Perhaps even more important, will be determining whether interventions that restore autophagy, specifically in osteocytes and/or other cell types within the bone microenvironment such as osteoprogenitors, can delay skeletal aging.
Deregulated Nutrient Sensing
The insulin and IGF-1 signaling pathway is a critical aging mechanism and the most conserved pathway in evolution, affecting worms, flies, and mammals. Current evidence indicates that anabolic signaling accelerates aging, and decreased nutrient signaling, achieved with caloric restricted diets or by stimulation of sirtuins, promotes healthspan and longevity.(62) The FOXO family of transcription factors and the mammalian target of rapamycin (mTOR) complexes are the most relevant downstream effectors of the insulin and IGF-1 signaling pathway for longevity in worms and flies.(63,64)
IGFs
The growth hormone (GH)/IGF axis, acting in an endocrine and autocrine/paracrine fashion, is the critical stimulator of skeletal growth. Studies in rodents have elucidated that IGFs promote both bone length and radial bone growth. IGFs also promote trabecular bone accrual via effects on osteoblasts, osteocytes, and osteoclasts.(65) In the growth plate IGFs stimulate clonal expansion of chondrocytes at the proliferation zone, and maturation of prehypertrophic chondrocytes. IGFs also regulate the mesenchymal and hematopoietic progenitor pools, enhance osteoblasto-genesis, and promote matrix deposition and mineralization. Studies using human bone specimens have elucidated that skeletal content of IGF1 and IGF binding protein 5 (IGFBP-5), which maintains the IGF pool in the bone matrix, severely declines after the second decade of life.(66–68) Clinical studies have attempted to correlate the reductions in serum IGF1 or IGF2 during aging with decreases in bone density, but this has led to conflicting results. Trials with recombinant human growth hormone (rhGH) or rhIGF-I in elderly patients either with primary osteoporosis or frailty have also yielded conflicting outcomes.(69–73)
Studies aimed at elucidating the role of serum IGF1 in the aging skeleton have shown that reductions in serum IGF1 after 5 months of age in mice, using an inducible liver-specific IGF1 deletion model, modestly altered cancellous bone mass and had no effects on the loss of cortical thickness with age.(74–76) Together, the existing evidence indicates that although serum IGF-1 is essential for bone accrual during the postnatal growth phase, its effects after peak bone acquisition (16 weeks) are limited to trabecular bone. Serum IGF-1 has no impact on the age-related loss of cortical bone mass.
FoxOs
The FoxO family of transcription factors exerts seminal influences on survival, proliferation, stress resistance, and longevity in model organisms.(63,77,78) In many mammalian tissues, including bone, FoxO1, FoxO3, and FoxO4 have broad and overlapping patterns of expression.(79,80) FoxOs are primarily regulated by insulin and IGF-1 signaling via PI3K/ Akt-mediated phosphorylates which prevents FoxO activity. Studies using single or combined deletion of FoxO1, FoxO3, and FoxO4 or overexpression of FoxO3 in multiple cell populations within the osteoblast and osteoclast lineage have elucidated the role of these transcription factors on the skeleton.(81) FoxOs decrease osteoblast number by sequestering β-catenin away from T-cell factor (TCF)-mediated transcription in osteoblast progenitors.(82) On the other hand, in mature osteoblasts and osteocytes FoxOs exert pro-survival effects and also inhibit osteoclastogenesis via stimulation of OPG.(80,83,84) Downregulation of OPG, due to inhibition of FoxO activity, mediates the pro-resorptive actions of insulin on bone. In contrast, FoxOs in osteoblast progenitors are dispensable for the pro-osteogenic actions of insulin.(85) These studies indicate that the role of FoxOs in mediating the actions of insulin is cell-type– dependent. It remains unclear whether FoxO levels or activity are altered with aging in bone cells.(80,83) Nevertheless, targeted FoxO1, FoxO3, and FoxO4 deletion in the osteoblast lineage of adult mice increases bone mass; and at 22 month of age bone mass remains higher in these mice.(82) These findings suggest that FoxOs contribute to the decreased bone formation that occurs with aging.
mTOR
The mTOR kinase is part of two distinct multiprotein complexes, mTORC1 and mTORC2, that mediate all aspects of anabolic metabolism by coordinating the nutritional and energetic status with a multitude of biosynthetic activities in the cell.(86) Genetic downregulation of mTORC1 activity in lower organisms extends longevity. In mice, administration of the mTOR inhibitor rapamycin increases healthspan and is the most robust intervention to increase lifespan.(87) mTORC1 and mTORC2 perform important functions in bone development.(88) Genetic disruption of mTORC1 in the osteoblast lineage inhibits the transition of preosteoblasts to mature osteoblasts, and prevents the accrual of normal bone mass or the response to Wnt-induced bone anabolism in mice.(89,90) Accordingly, constitutive activation of mTORC1 increases osteoblast number and bone formation.(91) Disruption of mTORC2 also impairs osteoblast activity causing a severe reduction in cortical bone growth.(92)
Several studies have attempted to elucidate the role mTOR in age-related bone loss. Rictor, a specific component of mTORC2, is downregulated with aging in osteoblasts,(93,94) An increase in miR-188 and miR-218 with aging in either osteoprogenitors or osteoblasts may be responsible for the age-dependent decrease in Rictor expression. These findings implicate a decline in mTORC2 activity in age-related osteoporosis. On the other hand, positive effects of rapamycin have been found in cancellous bone of old rats and mice.(95,96) Rapamycin also attenuates alveolar bone loss in old female C57BL6/JNia mice.(97) Administration of everolimus, another mTOR inhibitor, reduces the loss of bone mass in the ovariectomy model due to an inhibitory effect on bone resorption.(98,99) Genetic evidence for a role of mTOR in aging is provided by the findings that mice deficient in S6K1 (a main mTORC1 substrate) are long-lived and exhibit improved healthspan including higher bone mass, when compared to control mice, at 21 months of age.(100) These results point to mTORC1/S6K1 as a mediator of the effects of aging on the skeleton, but are at odds with the stimulatory actions of mTOR on bone formation during growth. However, due to the systemic nature of the interventions with mTOR inhibitors and S6K1 deletion, it remains unknown whether the skeletal effects resulted from diminished mTOR activity in bone cells. It is also possible that the outcome of mTOR activity in bone cells is different during skeletal growth versus aging. Undoubtedly, further studies are needed to clarify the role of mTOR in agerelated bone loss.
Sirtuins
In addition to the insulin and IGF signaling pathway that participates in glucose-sensing, the sirtuins sense low energy states by detecting high NAD+ levels.(101) The SIR2 family of NAD+ dependent enzymes, known as Sirtuins, are evolutionarily conserved from yeast to humans.(102) These enzymes catalyze the deacetylation of histone and nonhistone proteins such as FoxOs. In mammals there are seven Sirtuins, of which Sirt1 is the most studied.(103) Activation of Sirt1 in mice is associated with a delay in the onset of many aging-related diseases, including osteoporosis.(81,104,105) Sirt1 actions in osteoblast progenitors stimulate osteoblast formation and increase bone mass.(106,107) Deacetylation of FoxOs by Sirt1 prevents FoxO association with β-catenin and potentiates Wnt signaling, leading to increased osteoblast proliferation.(107) The stimulatory actions of Sirt1 on osteoblastogenesis might also be mediated by direct effects on β-catenin and Runx2.(106,108) On the other hand, Sirt1 in osteoclasts inhibits bone resorption.(109,110)
Dietary restriction upregulates Sirt1 and increases lifespan or healthspan in all investigated species, including lower organisms, mice, and nonhuman primates,(62,111,112) underlying the relevance of deregulated nutrient-sensing as a hallmark of aging. Caloric restriction from adulthood to old age in rodents attenuates skeletal aging.(113,114) In addition, a recent study in which calorie restriction was initiated during adulthood (at 4 months of age) in C57BL/6J male mice, and maintained for 4 or 8 months, show a marked increase in bone mass.(110) The beneficial effects of caloric restriction on bone mass are associated with a twofold induction of Sirt1 expression in bone, as seen in other tissues.(115,116) Importantly, long-term administration of Sirt1 activators or overexpression of Sirt1 attenuates the loss of bone mass with aging.(110,117–119) Sirt1 stimulators also cause significant increases in bone mass in the ovariectomy or hind limb unloading models of osteoporosis.(110,119–122) More telling, treatment with resveratrol, a natural Sirt1 activator, caused a significant increase in bone mass in elderly obese men.(123) Thus, Sirt1 may serve as a therapeutic target for combating age-related bone loss.
Mitochondrial Dysfunction
Cellular mitochondrial function declines with aging due to diminished mitochondriogenesis, mutations or deletions in mitochondrial DNA, progressive inefficiency of electron transport chain complexes resulting from destabilization, oxidative stress owing to excessive production of ROS, and defective mitophagy that normally eliminates dysfunctional or damaged mitochondria.(1) Possible links between mitochondrial dysfunction and aging have been proposed long ago (eg, the mitochondrial free radial theory dates back over half a century(124)); however, recent evidence has provoked reevaluation of some of these early theories. Indeed, it is now generally accepted that a gradual increase in cellular levels of ROS, a byproduct of cellular respiration, is well handled under normal conditions by several antioxidant detoxification systems, which are beneficial in responding to various physiological signals and modulating stress response pathways.(125) For example, in bone ROS accumulation promotes the formation and activation of osteoclasts needed to remodel the skeleton.(126) However, as ROS-independent cellular damage elevates with aging, ROS production eventually becomes intensified to a point beyond the level of control by the normal protective defense mechanisms, ultimately leading to ROS toxicity and progressive free-radical damage of proteins, lipids, as well as DNA.(125) These events accelerate age-related degenerative processes in multiple tissues and disease states.
Old bone is no exception to the oxidative stress–induced damage that occurs in response to excessive ROS resulting from age-related accumulation of dysfunctional mitochondria and progressive inefficiency of mitochondrial antioxidant defense mechanisms. As an example, sex steroid deficiency contributes toward increased ROS and oxidative stress levels in the bone microenvironment.(127–130) As in other tissues, bone has several defense mechanisms to protect against ROS toxicity and oxidative stress (reviewed in Manolagas(131)), which include multiple enzymatic reactions (eg, catalase, superoxide dismutases, and thiol-containing oligopeptides) as well as changes in transcription factors (eg, p53 and FoxOs) resulting in gene transcription alterations.
One of the most important lines of defense against ROS toxicity in bone and the age-associated increase in oxidative stress is the FoxO family of transcription factors. Indeed, a growing body of evidence implicates ROS toxicity with aging as an antagonist to Wnt signaling by diverting the limited pool of β-catenin from TCF/lymphoid-enhancer binding factor family of transcription factors to FoxO-mediated transcription in, eg, osteoblasts thereby reducing bone formation in old age.(82,132) Thus, similar to other defense mechanisms against aging, FoxO transcriptional activation protects bone against oxidative stress, but as an unintended consequence suppresses Wnt signaling, thus contributing to defective bone formation in old age.(133)
Additional evidence implicating the deleterious effects of excessive oxidative stress on the skeleton comes from studies showing that pharmacological pro-oxidant administration to mice causes bone loss,(129,130) whereas antioxidant treatments at least partially rescues this effect.(128,134) Even further support comes from genetic mouse models of premature aging(135,136) or global deletion of antioxidant genes (eg, Sod1(137) or Sod2(138)), which are characterized by elevated oxidative stress levels yet low bone mass. Importantly, antioxidant administration to Sod1−/− mice prevents bone loss in these animals,(137) thus supporting the premise that restraining excessive ROS in mitochondria could have beneficial therapeutic effects in old bone. This hypothesis is supported by studies demonstrating that reducing H2O2 (the most abundant species of ROS) production by overexpression of catalase targeted to mitochondria (mitoCAT) extends murine lifespan.(139) Based on this observation, Ucer and colleagues(140) examined the effects of attenuation of H2O2 specifically in cells of the mesenchymal versus myeloid lineages in response to gonadectomy or natural chronological aging. Whereas restraining H2O2 in myeloid lineage cells prevented cortical, but not trabecular, bone loss caused by sex steroid deficiency, it did not prevent age-related bone loss from either skeletal compartment.(140) By contrast, attenuation of H2O2 via mitoCAT in mesenchymal lineage cells partially prevented cortical bone loss with aging as compared to age-matched littermates, although the skeletal phenotype was not completely restored to that observed in young animals suggesting that additional aging mechanisms were still of consequence.(140) Thus, the results from this study suggest that multiple fundamental aging mechanisms, including mitochondrial dysfunction, contribute toward defective bone formation and increased bone resorption, independent of sex steroid deficiency.(140)
Another line of cellular defense against oxidative stress and electrophilic insults that occur with aging is the Kelch-like ECH-associated protein 1-NF-E2-related factor 2 (KEAP1-NRF2) system, which serves as a rapidly inducible mechanism to help maintain redox homeostasis.(141) Under normal circumstances KEAP1 (a cysteine thiol-rich detector of redox status and insults) functions as a repressor of NRF2 to prevent its transcriptional regulation of cytoprotective genes, whereas in response to excessive oxidative stress a derepression mechanism allows NRF2 to be rapidly unbound from KEAP1-mediated repression (thus causing activation of NRF2) to maintain redox homeostasis.(141) The actions of NRF2 on the skeleton have been examined by several laboratories using mice with global deletion of NRF2 or NRF2 hyper-activation due to deletion of KEAP1. These studies indicate that NRF2 is detrimental to the accrual of bone mass and contributes to bone loss in old male mice.(142–144) In contrast, NRF2 is required for the accrual of normal bone mass but has no influence on the loss of bone mass in female mice.(145,146) Interestingly, the expression of phase II detoxifying enzymes is strictly dependent on NRF2 in females but not in males suggesting sex-specific mechanisms for controlling the defense against ROS in bone.(143) Further studies with targeted deletion of NRF2 in bone cell are needed to clarify the cellular and molecular mechanisms of action.
Mounting evidence in nonskeletal tissues points to additional effects of damaged or dysfunctional mitochondria on age-related tissue degeneration, independently of ROS and oxidative stress, through a number of mechanisms, including diminished mitochondriogenesis, mutations or deletions in mitochondrial DNA, destabilization of electron transport chain complexes leading to progressive inefficiency, and defective mitophagy.(1) However, untangling the relative contributions of each of these effects in mediating age-related bone loss remains a challenge to the field and therefore a better understanding of how these processes contribute to skeletal aging will be an important direction of future work.
Cellular Senescence
Cellular senescence is a dynamic process whereby a cell loses its proliferative potential, undergoing an essentially irreversible growth arrest that occurs in response to various stressors, triggering activation of the p16Ink4a/RB and p53/p21 tumor suppressor pathways as well as other pathways that can potentially induce senescence.(147–150) Despite cell cycle arrest, senescent cells remain viable, with high metabolic activity and resistance to apoptosis.(147,148) The process of senescence involves multiple steps that can take days to weeks,(149) although once fully senescent these cells tend to display dramatic phenotypic changes including increased cell size, extensive gene expression changes, telomere shortening, and profound chromatin modifications (ie, decondensation of pericentromeric satellite heterochromatin).(147,148) These common characteristics can be utilized to identify senescent cells, regardless of the initial stressor that induced senescence. A further common feature of senescent cells is the frequent development of a unique secretome, termed the senescence-associated secretory phenotype (SASP), which is comprised of cytokines, chemokines, and extracellular matrix degrading proteins that over prolonged periods have deleterious effects on tissue structure and function (see Altered Intercellular Communication).(151–153) Although the SASP components can vary among distinct senescent cell types, pro-inflammatory cytokines and chemokines appear to be conserved, which implies that an inflammatory response and immune cell attraction are important roles of senescent cells.(149) Along these lines, accumulating evidence suggests that senescent cells have beneficial roles in a number of physiological processes, including protection against unrestricted growth of damaged cells,(154,155) tumor suppression,(155,156) embryonic development,(157,158) wound healing,(159,160) and tissue repair.(161) However, increased senescence in some of these contexts tends to only occur acutely, after which under normal conditions senescent cells are efficiently removed by the immune system.(161,162)
Chronic accumulation of senescent cells occurs in several tissues with aging coinciding with the time and location of agerelated pathology in both rodents and primates.(147–150) This may be due to an increase in the rate of the accumulation of senescent cells(163,164) and/or slowing of the rate of clearance of senescent cells due to the deterioration and inefficiency of the aged immune system.(165,166) Notwithstanding, there is a clear association between increased abundance of senescent cells and tissue dysfunction in the setting of natural chronological aging. Based on this association, the geroscience community hypothesized for many years that senescent cells, through their sustained pro-inflammatory stress, drive multiple age-related pathologies. However, the first evidence demonstrating a causal link between cellular senescence and age-related tissue dysfunction came from the Kirkland and van Deursen laboratories at Mayo Clinic where, at least in a progeroid mouse model of accelerated aging, Baker and colleagues(167) showed that eliminating senescent cells, using a suicide gene–mediated approach to kill p16Ink4a-positive senescent cells (ie, INK-ATTAC– p16Ink4a apoptosis through targeted activation of caspase), can delay multiple aging phenotypes in adipose tissue, skeletal muscle, and the eye. Since this initial discovery, key findings have been reported by several laboratories establishing that reducing the burden of senescent cells in old mice extends healthspan by, for example, improving measures of metabolic dysfunction,(168) hepatic steatosis,(169) vascular dysfunction/ cardiovascular disease,(170,171) and sarcopenia/frailty.(172,173)
To determine which cell types in the bone microenvironment are prone to senescence with natural chronological aging, Farr and colleagues(174) measured senescence and SASP markers in young (6-month-old) and old (24-month-old) mice in vivo in enriched cell populations, all rapidly isolated from bone/marrow without in vitro culture. These studies showed that p16Ink4a levels were significantly higher (5–10 fold) in old relative to young mice in each of the enriched cell populations evaluated (ie, B-cells and T-cells, myeloid cells, osteoprogenitors, osteoblasts, and osteocytes).(174) Similar results were observed in bone biopsies isolated from elderly as compared to young women, indicating parallel findings in humans.(174) Furthermore, the finding of increased osteocyte senescence with aging has also been reported by Piemontese and colleagues(175) who found evidence of elevated DNA damage and senescence biomarkers in osteocyte-enriched bone samples from old relative to young mice. Concordant evidence supporting an accumulation of senescent osteocytes in bone with aging came from the study by Farr and colleagues(174) in which osteocytes in bones of old mice were found to display large-scale unraveling of pericentromeric satellite DNA (ie, senescence-associated distension of satellites [SADS](40,169,174,176)), which is a feature of senescent cells. The finding of increased senescence with aging in a postmitotic, terminally differentiated cell type (ie, the osteocyte) is consistent with recent findings in other tissues indicating that senescence of postmitotic neurons,(177) hepatocytes,(178) and adipocytes(179) may contribute to disease progression through the detrimental effects of the SASP.
Consistent with stem cell exhaustion and dysfunction observed in other tissues with aging, Kim and colleagues(35) demonstrated that in old relative to young mice the number of osteoprogenitors in females and males declined by more than 50% using Osx-Cre;TdRFP mice to fluorescently label and isolate osteoprogenitors from bone marrow.(35) Interestingly, this was due to arrest at the G1 phase of the cell cycle.(35) Moreover, osteoprogenitors from old mice exhibited several characteristics of senescent cells, including elevated levels of γH2AX foci (a marker of DNA damage due to double-strand breaks), phosphorylated p53, elevated p21 expression, and upregulation of several SASP factors such as GATA4 and activation of NF-kB.(35) Complementary to these findings in osteoprogenitors, Farr and colleagues(174) found upregulation of several SASP factors with aging, predominantly in enriched populations of myeloid cells and osteocytes. In addition, expression of the SASP factors, Sdf1 and MMP-13, were also found to be elevated with aging in osteocytes in the study by Piemontese and colleagues.(175) Collectively, these findings suggest that, within the aging bone microenvironment, a subset of cells of various lineages become senescent, although senescent osteoprogenitors, senescent osteocytes, and senescent myeloid cells may be the key producers of the SASP that contribute to age-related skeletal fragility.
In order to address whether senescent cells have a causal role in mediating age-related bone loss, Farr and colleagues(176) used three different approaches, including the suicide gene-mediated approach of ablation of p16Ink4a-expressing senescent cells in INK-ATTAC mice,(167) as well as pharmacological approaches to target senescent cells and examine effects on age-related bone loss. The latter approaches included using the combination of “senolytics”—dasatinib (D; an US Food and Drug Administration [FDA]-approved tyrosine kinase inhibitor(180)) and quercetin (Q; a flavanol present in many fruits and vegetables(181))—that specifically kill senescent cells without affecting proliferating or quiescent, differentiated cells,(182–184) as well as a “senomorphic” approach (ie, using the JAK1/2 inhibitor, ruxolitinib) to blunt the SASP. In old mice, each of these approaches improved bone mass, microarchitecture, and strength with the beneficial effects of targeting senescent cells due to suppressed bone resorption, reduced marrow adiposity, and on endocortical surfaces increased bone formation.(176) Thus, from a therapeutic perspective, targeting senescent cells appears to offer advantages over conventional anti-resorptive therapy (reviewed in Khosla and colleagues(185)). A major goal of future work will be to identify and optimize senotherapeutic approaches that target senescent cells to alleviate multiple chronic disease of aging, including age-related bone loss.
Stem Cell Exhaustion
The decline in regenerative capability of different tissues with aging, along with a significant reduction in the number, proliferative capacity, or differentiation potential of distinct stem cells, has led to the idea that aging is due, at least in part, to the loss of functional adult stem cells needed for tissue repair.(186,187) For example, studies in mice have revealed that the hematopoietic system exhibits myeloid skewing, and diminished production of adaptive immune cells, associated with an increased incidence of anemia and myeloid malignancies.(188,189) Stem cell dysfunction has also been described in the brain,(190,191) reproductive organs,(192,193) and muscle.(194,195) Importantly, administration of muscle-derived stem/progenitor cells isolated from young mice to a mouse model of a human progeria caused a significant healthspan and lifespan extension.(196) These findings suggest that adult stem/progenitor cell dysfunction contributes to aging-related degeneration.
The identity of the MSCs that give rise to the osteoblast lineage has remained elusive due to lack of specific cellular markers.(197) MSCs are commonly defined by their in vitro ability to self-replicate or to differentiate into osteoblasts, chondrocytes, and adipocytes.(198–200) Cultures of bone marrow stromal cells from humans and rodents, used as surrogates for progenitor cells, have been used in an attempt to elucidate changes in MSCs with aging.(201–204) Nevertheless, these studies have provided conflicting results, most likely because of the heterogeneous nature of the cultured cells and different culture conditions. Recent lineage tracing studies in mice have identified subsets of mesenchymal cells in the bone marrow that express smooth muscle α-actin (αSMA), myxovirus resistance-1 (Mx1), leptin receptor, and/or Collagen 2, which can give rise to osteoblasts present on the bone surfaces.(205–208) Of note, marrow cells expressing leptin receptor give origin to the majority of osteoblast and osteocytes present in adult mice. However, whether aging affects the number and osteoblastic differentiation capacity of these or other adult MSC populations in bone remains to be established.
All osteoblasts in bone are derived from progenitor cells expressing Osx1. Studies with bone marrow cells labeled using an Osx1-cre transgene have elucidated that the number of these cells greatly decreases between adulthood and old age in mice. This decline is associated with decreased proliferation and several other markers of cell senescence (see Cellular Senescence).(35) The age-related loss of Osx1-expressing cells has also been attributed to a decrease in type H capillaries at the distal end of the arterial network which are critical for the maintenance of perivascular osteoprogenitors.(209) Aging also leads to an increase in the expression of PPARγ, the master transcription factor for adipogenesis, in Osx1-expressing progenitor cells.(35) Indeed, one well established consequence of aging in MSCs is the increased propensity for adipogenesis leading to the elevated number of adipocytes present in the marrow of old humans and mice.(210) It remains unclear, however, whether any of changes noted in osteoblast progenitors contribute to the decrease in bone formation with aging and are functionally related to skeletal involution.
Altered Intercellular Communication
Mounting evidence indicates that, in addition to changes in cellautonomous processes resulting from aging, age-driven dysfunction in one cell or tissue can cause age-related deterioration systemically in other cells located in various tissues throughout an organism.(1) There are several types of intercellular communication that aging can alter, including elevated inflammation, dysregulated endocrine and neuronal signaling factors, and changes in the crosstalk between microorganisms and their host.(1)
One of the most constant alterations with aging that interferes with intercellular communication is chronic low-grade “sterile” (absence of known pathogens) inflammation, which can stem from several sources and tends to be caused by multiple age-driven processes such as stochastic tissue damage, elevated stress response signals, accumulation of senescent cells (see Cellular Senescence), inability of the debilitated immune system to successfully eliminate pathogens and defective cells, defective autophagy (see Loss of Proteostasis), and transcriptional overactivation of inflammatory pathways.(1) These processes are driven in several tissues, including bone, and are interconnected; that is, activation of one can stimulate the others resulting in persistent inflammation with aging. For example, one of the key signatures of age-related inflammation is stimulation of the NF-kB family of transcription factors, which has a role in driving the aging process in multiple tissues.(211) Indeed, NF-kB transcriptional activity is overstimulated in several tissues with both natural chronological aging, and in, for example, progeroid mouse models of accelerated aging such as Ercc1−/Δ mice (a model of human XPF-ERCC1 (XFE) progeria).(18) These mice develop a well-characterized accelerated aging phenotype (by 6 months of age), caused by defective repair of DNA damage, making them a suitable model for studying age-related diseases. Moreover, compared to their WT littermates, progeroid Ercc1–/Δ mice display overt, progressive osteoporosis by 22 weeks of age, resulting from loss of osteoprogenitors, defective bone formation, and increased osteoclastogenesis in the setting of increased senescence and a pro-inflammatory skeletal phenotype, driven at least in part by upregulated NF-kB signaling.(21) These results suggest that inflammation has a major role in driving the skeletal aging process.
Inflammation is also elevated in response to estrogen deficiency,(212) which is another example of altered intercellular communication with aging that causes bone loss through complex and multifaceted mechanisms (reviewed in Kholsa and Monroe(213) and Almeida and colleagues(214)). In addition to alterations in sex steroid communication, there are several examples of other hormonal changes that are modified with aging in a variety of tissues, including bone. For example, evidence points to the bone-derived hormone, osteocalcin, as having important roles in multiple physiological processes such as energy metabolism,(215) male fertility,(216) and more recently as an anti-aging hormone involved in cognitive function.(217) Indeed, the age-associated reduction in osteocalcin results in elevated anxiety-like behavior in mice as well as deficiencies in memory and learning.(218) Bone-derived osteocalcin also has a role in skeletal muscle where it increases IL-6 production as well as glucose uptake and catabolism for energy production to promote adaptation to exercise.(219) Because the functions of osteocalcin decline in mice with aging, this hormone represents another example of age-related changes in one tissue (ie, bone) that can lead to altered intercellular communication and deterioration in multiple other tissues. In addition to age-associated changes in bone-derived factors that influence skeletal muscle, there are also several examples of altered intercellular communication with aging in the opposite direction; ie, secreted factors from skeletal muscle (so-called myokines(220)) that signal to bone. How muscle-bone crosstalk and vice-versa is altered with aging is an important question at the nexus of identifying novel therapies for treating age-related musculoskeletal diseases, such as sarcopenia and osteoporosis, which often coexist in the elderly population.
Age-associated changes in intercellular communication can also lead to the accumulation of bone marrow fat, which is metabolically distinct from peripheral fat depots and commonly associated with skeletal fragility and increased fracture risk.(221,222) Indeed, mounting data in both animals and humans suggest that bone is progressively replaced with adipose tissue with advancing chronological age, resulting in a reciprocal relationship between marrow adiposity and skeletal parameters such as bone density and strength.(223–225) This is also a consistent observation inmurine modelsof accelerated aging, suchastheP6 strain of senescence-accelerated mice (SAMP6)(226) and Hdac null mice.(227) Although the underlying mechanisms explaining this phenomenon are not completely understood, it has been hypothesized that age-related related changes in transcription factors result in altered levels of several adipokines in the marrow milieu that cause a shift in lineage commitment of bone marrow multipotent MSCs from the osteoblast to the adipogenic lineage.(210) For example, Nishikawa and colleagues(228) demonstrated that expression of Maf (also known as c-Maf), a transcription factor that regulates the cell fate determination of MSCs into osteoblast and adipocyte cell lineages, declines in MSCs with advancing age as a result of prolonged age-related oxidative stress insults (see Mitochondrial Dysfunction), and that partial deletion of Maf in old mice causes accelerated bone marrow adiposity and low bone mass. In addition, aging stimulates adipogenic and suppresses osteogenic programs in MSCs by upregulating the transcription factor PPAR-γ and subsequent activation of the TGF-β/BMP signaling pathways.(229) Therefore, targeting age-related alterations in intercellular communication in the bone microenvironment may be crucial to reversing the age-related switch in the MSC cell fate commitment to ultimately mitigate skeletal fragility.
Additional evidence from multiple laboratories also points to alterations in neuronal regulation of bone metabolism with aging. For example, elevated sympathetic outflow, which occurs in humans with estrogen deficiency and aging,(230) contributes to activation of b-adrenergic receptors on osteoblasts resulting in a relative impairment in bone formation(231) and greater osteoclastic bone resorption by increasing RANKL production.(232) Finally, aging may cause changes in blood-borne circulating factors(233,234) as well as altered interactions between the gut microbiome and host immune system,(235,236) although how these forms of intercellular communication impact the aged skeleton remains to be defined. Collectively, these agerelated changes in intercellular communication represent exciting potential therapeutic targets not only for the prevention and treatment of osteoporosis, but also chronic diseases of aging as a group.
Summary and Perspectives
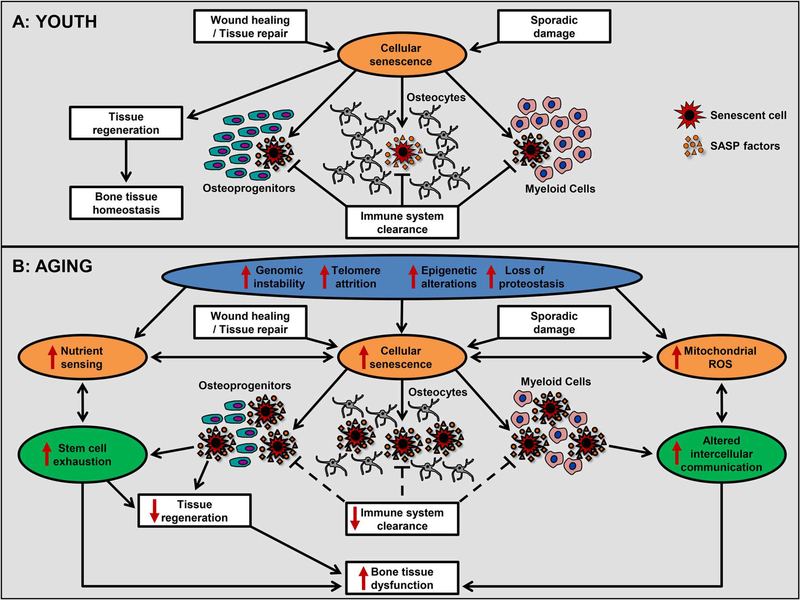
As previously proposed,(1) the nine hallmarks of aging can be collated into three subcategories (Fig. 1A, B): primary hallmarks, antagonistic hallmarks, and integrative hallmarks. The primary hallmarks (depicted in blue) represent the initiating triggers or stressors with clear detrimental effects that over time drive organismal aging, whereas to an extent the antagonistic hallmarks (depicted in orange) represent protective compensatory mechanisms; however, beyond a certain threshold, as discussed earlier, they become harmful with negative consequences that over prolonged periods of time progressively accumulate.(1) Eventually, when compensation can no longer limit the damage caused by the primary and antagonistic hallmarks, a final consequence is the integrative hallmarks (depicted in green), which result in tissue dysfunction and age-related chronic diseases.(1)
Fig. 1.
The hallmarks of skeletal aging. (A) Cellular senescence is shown as an example of an antagonistic hallmark of aging or fundamental aging mechanism that, in youth, acts as a defense response to damage in order to maintain skeletal tissue homeostasis and protect against cancer. (B) Schematic representation of the nine hallmarks of aging: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. The hallmarks are grouped into three categories: the primary hallmarks (depicted in blue), antagonistic hallmarks (depicted in orange), and the integrative hallmarks (depicted in green). Depicted is an overview of how with old age, the aging skeletal hallmarks are potentially interconnected, linked, and overlap. Each hallmark may manifest to varying degrees with chronological aging in bone where it can not only trigger activation other hallmarks, based on their hierarchical relations, but also contribute to the natural skeletal aging process. Cellular senescence is shown as an example of an antagonistic hallmark (depicted in orange) that with aging can be perturbed by the primary hallmarks (depicted in blue) as well as other antagonistic hallmarks, and beyond a certain threshold becomes progressively detrimental rather than compensatory. Ultimately, these events result in activation of the integrative hallmarks (depicted in green) leading to skeletal dysfunction. Because the hallmarks are interconnected, interventions that ameliorate one hallmark can in theory ameliorate others. SASP = senescence-associated secretory phenotype; ROS = reactive oxygen species.
In bone, cellular senescence is an example of an antagonistic hallmark or fundamental aging mechanism that has been conserved due to antagonistic pleiotropy whereby in youth, senescence can be a favorable cell fate serving as a compensatory mechanism to acutely aid in wound healing and tissue repair to promote bone regeneration and in response to sporadic damage from various exogenous and environmental stressors can help prevent the propagation of damaged and oncogenic cells. Under these circumstances, the immune system can efficiently clear senescent cells to prevent their excess accumulation. Subsequently, these cells need to be replaced by recruiting new cells from stem cell pools. Therefore, in youth (as summarized in Fig. 1A) senescence acts as a defense response to damage in order to maintain skeletal tissue homeostasis and protect against cancer. In old age, however (as summarized in Fig. 1B), this turnover system fails to keep up as the primary aging hallmarks that initiate cell damage (ie, genomic instability, telomere attrition, epigenetic alterations, and loss of proteostasis) intensify, thus accelerating the rate of accumulation of senescent cells. At the same time, the primary hallmarks can also perturb the other antagonistic hallmarks causing deregulated nutrient sensing and mitochondrial dysfunction that not only become progressively detrimental (rather than antagonistic or compensatory), but may even contribute to the further accumulation of senescent cells. Regardless of the initial senescence inducer, the senescent cells then need to be replaced at a faster rate contributing to stem cell exhaustion and loss of tissue regenerative capacity. Moreover, the inability of the aged immune system to efficiently eliminate or clear senescent cells can contribute to their even greater burden. Meanwhile, the SASP (an example of altered intercellular communication) continues to amplify, and beyond a certain threshold, becomes a culprit causing dysfunction of neighboring cells and spreading the senescent phenotype. Collectively, this feedback loop creates a vicious cycle that contributes to age-related dysfunction in bone. As noted earlier (see Cellular Senescence), because osteocytes comprise the majority of cells in bone and are long-lived cells they, along with osteoprogenitors and osteoclast precursors (ie, myeloid lineage cells), could be among the most vulnerable cell populations to endure chronic stress from the primary hallmarks of aging. Thus, in response to these continued insults and the resulting accumulation of damage, these cells may be particularly prone in old age to engaging the senescence program. Noteworthy, however, based on the overlap and hierarchical order among the hallmarks, it may be feasible to interfere with a single aging hallmark (eg, cellular senescence or others) to simultaneously impact multiple hallmarks in order to ultimately alleviate a wide spectrum of age-related chronic diseases, including osteoporosis.
Aging research has undergone unprecedented advances at an accelerating rate in recent years, leading to excitement in the field as well as opportunities for imagination and innovation. Current challenges in the field of bone and mineral research include defining the relative contributions of each aging hallmark to the natural skeletal aging process, better understanding the complex interconnections among the hallmarks, and identifying the most effective strategies to target multiple hallmarks. As summarized in Table 1, at this time each hallmark fulfills these criteria to varying degrees and for some only suggestive evidence currently exists. A major goal is to discover, develop, and optimize interventions based on a firm foundation of multidisciplinary discovery science that identifies novel approaches to target multiple fundamental aging mechanisms, which may be possible because of their interconnections. Preferably such targeted approaches will be accomplished with a drug or limited combination thereof to minimize side effects and avoid polypharmacy. Therefore, rather than the current model of treating each age-associated chronic disease individually, which is inherently prone to adverse drug interactions, the hope is that this strategy of targeting fundamental aging mechanisms will forestall age-related chronic diseases as group, ultimately leading to improved healthspan in our elderly population. This concept of intervening in the aging process to extend healthspan is, of course, quite attractive in the aging research field and to most people. Although aging in humans is inevitable and the challenges to delay this fate through extending the healthy period of life free of chronic disease are seemingly insurmountable, a growing body of experimental evidence in model organisms indicates that there is hope in one day achieving this goal.
Table 1.
The Hallmarks of Aging in Bone
| Hallmark of aging | Manifests in bone during natural chronological aging | Experimental manipulation accelerates skeletal aging | Experimental amelioration forestalls skeletal aging |
|---|---|---|---|
| Genomic instability | Markers of DNA damage and the DDR are increased in murine osteoprogenitors and osteocytes(35,175) | DNA repair-deficient mouse models exhibit accelerated skeletal aging(24–26) | Unknown |
| Telomere attrition | Unknown | Mice lacking telomerase reverse transcriptase (Terc) exhibit low bone mass(36–38) | Unknown |
| Epigenetic alterations | Unknown | Genetic or pharmacological inhibition of Hdac or Sirt1 activity cause low bone mass(50,51) | Deletion of the histone methyltransferase Suv39h1 in a model of Hutchinson-Gilford progeria syndrome increases bone mass(42) |
| Loss of proteostasis | Unknown | Mice with deficient autophagy in the osteoblast lineage have low bone mass turnover(58,60) | Unknown |
| Deregulated nutrient sensing | Skeletal content of IGF1 and IGFBP-5 declines after the second decade of life(66–68) | Reductions in serum IGF1 after 5 months of age in mice decrease cancellous bone mass(74–76) | Pharmacological or genetic inhibition of mTORC1 attenuates the loss of bone with age(95,96,100) |
| Rictor, a specific component of mTORC2, decreases with aging in murine osteoblasts(93,94) | Mice lacking Sirt1 in different bone cell populations exhibit low bone mass(81) | Caloric restriction or Sirt1 stimulation attenuates skeletal aging(110,113,114,117–119) Deletion of FoxO1, FoxO3, and FoxO4 in the osteoblast lineage increases bone mass throughout life(82) |
|
| Mitochondrial dysfunction | ROS increases in murine bone(127,128) | Sod1−/− mice have low bone mass(137) Deletion of Sod2 in osteoblasts/osteocytes decreases bone mass(138) | Inhibition of mitochondrial ROS production in cells of the mesenchymal lineage attenuates skeletal aging(140) |
| Cellular senescence | Cellular senescence increases in myeloid cells, osteoprogenitors, and osteocytes(35,174,175) | Unknown | Genetic ablation of p16Ink4a-expressing cells or pharmacological approaches using senolytics or senomorphics in old mice improves bone mass(176) |
| Stem cell exhaustion | Unknown | Unknown | Unknown |
| Altered intercellular communication | Chronic low-grade "sterile"(absence of known pathogens) inflammation(211) | Overstimulation of NF-kB transcriptional activity in, for example, progeroid Ercc1−/Δ mice drives inflammation and accelerated skeletal aging(21) | Unknown |
| Elevated sympathetic outflow(230) | Activation of β-adrenergic receptors on osteoblasts causes an impaired bone formation and greater bone resorption(231,232) | ||
Acknowledgments
This work was supported by grants from the NIH: (National Institute of Arthritis and Musculoskeletal and Skin Diseases [NIAMS] K01 AR070241 [to JNF] and R01 AR056679 [to MA]; National Institute on Aging [NIA] P01 AG013918 [to MA]; and National Institute of General Medical Sciences [NIGMS] P20 GM125503 [to MA]). We thank our colleagues for helpful discussions and comments on the manuscript and apologize to investigators whose relevant work was omitted due to space limitations.
Disclosures
JNF and MA have no conflicts to disclose. JNF was supported by NIH grant K01 AR070241 and Career Development Awards from the Mayo Clinic Robert and Arlene Kogod Center on Aging, as well as the Richard F. Emslander Career Development Award in Endocrinology. MA was supported by NIH grants: R01 AR056679, P01 AG013918, and P20 GM125503.
References
- 1.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging tochronic disease. Cell. 2014;159(4):709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian X, Seluanov A, Gorbunova V. Molecular mechanisms determining lifespan in short- and long-lived species. Trends Endocrinol Metab. 2017;28(10):722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vijg J, Kennedy BK. The essence of aging. Gerontology. 2016;62(4): 381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams GC. Pleitropy, natural selection, and the evolution of senescence. Evolution. 1957;11(4):398–411. [Google Scholar]
- 6.Kennedy BK. The genetics of ageing: insight from genome-wide approaches in invertebrate model organisms. J Intern Med. 2008;263(2):142–52. [DOI] [PubMed] [Google Scholar]
- 7.Liao CY, Kennedy BK. Mouse models and aging: longevity and progeria. Curr Top Dev Biol. 2014;109:249–85. [DOI] [PubMed] [Google Scholar]
- 8.Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. Geroscience. 2017;39(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson SP. Sensing and repairing DNA double-strand breaks.Carcinogenesis. 2002;23(5):687–96. [DOI] [PubMed] [Google Scholar]
- 10.Mine-Hattab J, Rothstein R. Increased chromosome mobility facilitates homology search during recombination. Nat Cell Biol. 2012;14(5):510–7. [DOI] [PubMed] [Google Scholar]
- 11.Rouse J, Jackson SP. Interfaces between the detection, signaling, and repair of DNA damage. Science. 2002;297(5581):547–51. [DOI] [PubMed] [Google Scholar]
- 12.Smogorzewska A, de Lange T. Different telomere damage signaling pathways in human and mouse cells. EMBO J. 2002; 21(16):4338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanawalt PC. Subpathways of nucleotide excision repair and their regulation. Oncogene. 2002;21(58):8949–56. [DOI] [PubMed] [Google Scholar]
- 14.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med.2009;361(15):1475–85. [DOI] [PubMed] [Google Scholar]
- 15.Carrero D, Soria-Valles C, Lopez-Otin C. Hallmarks of progeroid syndromes: lessons from mice and reprogrammed cells. Dis Model Mech. 2016;9(7):719–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burtner CR, Kennedy BK. Progeria syndromes and ageing: what is the connection? Nat Rev Mol Cell Biol. 2010;11(8):567–78. [DOI] [PubMed] [Google Scholar]
- 17.Itin PH, Sarasin A, Pittelkow MR. Trichothiodystrophy: update on the sulfur-deficient brittle hair syndromes. J Am Acad Dermatol. 2001;44(6):891–920; quiz 921–4. [DOI] [PubMed] [Google Scholar]
- 18.Niedernhofer LJ, Garinis GA, Raams A, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somato-troph axis. Nature. 2006;444(7122):1038–43. [DOI] [PubMed] [Google Scholar]
- 19.Vogel H, Lim DS, Karsenty G, Finegold M, Hasty P. Deletion of Ku86 causes early onset of senescence in mice. Proc Natl Acad Sci U S A. 1999;96(19):10770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dollé ME, Kuiper RV, Roodbergen M, et al. Broad segmental progeroid changes in short-lived Ercc1−/Δ7 mice. Pathobiol Aging Age Relat Dis. 2011;1:Article 7219. DOI: 10.3402/pba.v1i0.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q, Liu K, Robinson AR, et al. DNA damage drives accelerated bone aging via an NF-kB-dependent mechanism. J Bone Miner Res. 2013;28(5):1214–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McWhir J, Selfridge J, Harrison DJ, Squires S, Melton DW. Mice with DNA repair gene (ERCC-1) deficiency have elevated levels of p53, liver nuclear abnormalities and die before weaning. Nat Genet. 1993;5(3):217–24. [DOI] [PubMed] [Google Scholar]
- 23.Weeda G, Donker I, de Wit J, et al. Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr Biol. 1997;7(6):427–39. [DOI] [PubMed] [Google Scholar]
- 24.Wijnhoven SW, Beems RB, Roodbergen M, et al. Accelerated aging pathology in ad libitum fed Xpd(TTD) mice is accompanied by features suggestive of caloric restriction. DNA Repair (Amst). 2005;4(11):1314–24. [DOI] [PubMed] [Google Scholar]
- 25.Nicolaije C, Diderich KE, Botter SM, et al. Age-related skeletal dynamics and decrease in bone strength in DNA repair deficient male trichothiodystrophy mice. PLoS One. 2012;7(4):e35246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diderich KE, Nicolaije C, Priemel M, et al. Bone fragility and decline in stem cells in prematurely aging DNA repair deficient trichothiodystrophy mice. Age (Dordr). 2012;34(4):845–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006; 355(15):1572–82. [DOI] [PubMed] [Google Scholar]
- 28.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign ofaccelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013; 31(36):4496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandra A, Lin T, Young T, et al. Suppression of sclerostin alleviates radiation-induced bone loss by protecting bone-forming cells and their progenitors through distinct mechanisms. J Bone Miner Res. 2017;32(2):360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol.2007;3(10):640–9. [DOI] [PubMed] [Google Scholar]
- 31.Jaskelioff M, Muller FL, Paik JH, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469(7328):102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez P, Blasco MA. Telomere-driven diseases and telomere-targeting therapies. J Cell Biol. 2017;216(4):875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kassem M, Marie PJ. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell. 2011;10(2):191–7. [DOI] [PubMed] [Google Scholar]
- 34.Marie PJ. Bone cell senescence: mechanisms and perspectives.J Bone Miner Res. 2014;29(6):1311–21. [DOI] [PubMed] [Google Scholar]
- 35.Kim HN, Chang J, Shao L, et al. DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell. 2017;16(4):693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pignolo RJ, Suda RK, McMillan EA, et al. Defects in telomere maintenance molecules impair osteoblast differentiation and promote osteoporosis. Aging Cell. 2008;7(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saeed H, Abdallah BM, Ditzel N, et al. Telomerase-deficient mice exhibit bone loss owing to defects in osteoblasts and increased osteoclastogenesis by inflammatory microenvironment. J Bone Miner Res. 2011;26(7):1494–505. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Chen Q, Lee SH, et al. Impairment of osteoblast differentiation due to proliferation-independent telomere dysfunction in mouse models of accelerated aging. Aging Cell. 2012; 11(4):704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burns JS, Abdallah BM, Guldberg P, et al. Tumorigenic heterogeneity in cancer stem cells evolved from long-term cultures of telomerase-immortalized human mesenchymal stem cells. Cancer Res. 2005;65(8):3126–35. [DOI] [PubMed] [Google Scholar]
- 40.Swanson EC, Manning B, Zhang H, Lawrence JB. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J Cell Biol. 2013;203(6):929–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Letarouilly JG, Broux O, Clabaut A. New insights into the epigenetics of osteoporosis. Genomics. Forthcoming. Epub 2018 May 4. DOI: 10.1016/j.ygeno.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Liu B, Wang Z, Zhang L, et al. Depleting the methyltransferaseSuv39h1 improves DNA repair and extends lifespan in a progeria mouse model. Nat Commun. 2013;4:1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delgado-Calle J, Sanudo C, Fernandez AF, et al. Role of DNA methylation in the regulation of the RANKL-OPG system in human bone. Epigenetics. 2012;7(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delgado-Calle J, Sanudo C, Bolado A, et al. DNA methylation contributes to the regulation of sclerostin expression in human osteocytes. J Bone Miner Res. 2012;27(4):926–37. [DOI] [PubMed] [Google Scholar]
- 45.Takada I, Mihara M, Suzawa M, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-g. Nat Cell Biol. 2007;9:1273–85. [DOI] [PubMed] [Google Scholar]
- 46.Wei Y, Chen YH, Li LY, et al. CDK1-dependent phosphorylation ofEZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat Cell Biol. 2011;13(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye L, Fan Z, Yu B, et al. Histone demethylases KDM4B and KDM6Bpromotes osteogenic differentiation of human MSCs. Cell Stem Cell. 2012;11(1):50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang D, Okamura H, Nakashima Y, Haneji T. Histone demethylaseJmjd3 regulates osteoblast differentiation via transcription factors Runx2 and osterix. J Biol Chem. 2013;288(47):33530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinha KM, Yasuda H, Zhou X, deCrombrugghe B. Osterix and NO66 histone demethylase control the chromatin of Osterix target genes during osteoblast differentiation. J Bone Miner Res. 2014;29(4): 855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradley EW, McGee-Lawrence ME, Westendorf JJ. Hdac-mediated control of endochondral and intramembranous ossification. Crit Rev Eukaryot Gene Expr. 2011;21(2):101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradley EW, Carpio LR, van Wijnen AJ, McGee-Lawrence ME, Westendorf JJ. Histone deacetylases in bone development and skeletal disorders. Physiol Rev. 2015;95(4):1359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med. 2015;21-(12): 1406–15. [DOI] [PubMed] [Google Scholar]
- 53.Vilchez D, Saez I, Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun.2014;5:5659. [DOI] [PubMed] [Google Scholar]
- 54.Morimoto RI, Cuervo AM. Proteostasis and the aging proteome inhealth and disease. J Gerontol A Biol Sci Med Sci. 2014;69 Suppl 1: S33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joysand frustrations of autophagy. Annu Rev Physiol. 2010;72: 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14(9):959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alm JJ, Koivu HMA, Heino TJ, et al. Circulating plastic adherent mesenchymal stem cells in aged hip fracture patients. J Orthop Res. 2010;28(12):1634–42. [DOI] [PubMed] [Google Scholar]
- 58.Onal M, Piemontese M, Xiong J, et al. Suppression of autophagy in osteocytes mimics skeletal aging. J Biol Chem. 2013;288(24): 17432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodda SJ, McMahon AP. Distinct roles for hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133(16):3231–44. [DOI] [PubMed] [Google Scholar]
- 60.Piemontese M, Onal M, Xiong J, et al. Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage. Sci Rep. 2016;6:24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tiede-Lewis LM, Xie Y, Hulbert MA, et al. Degeneration of the osteocyte network in the C57BL/6 mouse model of aging. Aging (Albany NY). 2017;9(10):2190–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fontana L, Partridge L, Longo VD. Extending healthy life span— from yeast to humans. Science. 2010;328(5976):321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993; 366(6454):461–4. [DOI] [PubMed] [Google Scholar]
- 64.Slack C, Giannakou ME, Foley A, Goss M, Partridge L. dFOXO-independent effects of reduced insulin-like signaling in Drosophila. Aging Cell. 2011;10(5):735–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yakar S, Werner H, Rosen CJ. Insulin-like growth factors: actions on the skeleton. J Mol Endocrinol. 2018;61(1):T115–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicolas V, Prewett A, Bettica P, et al. Age-related decreases in insulin-like growth factor-I and transforming growth factor-b in femoral cortical bone from both men and women: implications for bone loss with aging. J Clin Endocrinol Metab. 1994;78(5): 1011–6. [DOI] [PubMed] [Google Scholar]
- 67.Nicolas V, Mohan S, Honda Y, et al. An age-related decrease in the concentration of insulin-like growth factor binding protein-5 in human cortical bone. Calcif Tissue Int. 1995;57(3):206–12. [DOI] [PubMed] [Google Scholar]
- 68.Mohan S, Baylink DJ. Serum insulin-like growth factor binding protein (IGFBP)-4 and IGFBP-5 levels in aging and age-associated diseases. Endocrine. 1997;7(1):87–91. [DOI] [PubMed] [Google Scholar]
- 69.Marcus R, Butterfield G, Holloway L, et al. Effects of short term administration of recombinant human growth hormone to elderly people. J Clin Endocrinol Metab. 1990;70:519–27. [DOI] [PubMed] [Google Scholar]
- 70.Rudman D, Feller AG, Nagraj HS, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323(1):1–6. [DOI] [PubMed] [Google Scholar]
- 71.Ghiron LJ, Thompson JL, Holloway L, et al. Effects of recombinant insulin-like growth factor-I and growth hormone on bone turnover in elderly women. J Bone Miner Res. 1995;10:1844–52. [DOI] [PubMed] [Google Scholar]
- 72.Thompson JL, Butterfield GE, Marcus R, et al. The effects of recombinant human insulin-like growth factor-I and growth hormone on body composition in elderly women. J Clin Endocrinol Metab. 1995;80(6):1845–52. [DOI] [PubMed] [Google Scholar]
- 73.Holloway L, Kohlmeier L, Kent K, Marcus R. Skeletal effects of cyclic recombinant human growth hormone and salmon calcitonin in osteopenic postmenopausal women. J Clin Endocrinol Metab. 1997;82:1111–7. [DOI] [PubMed] [Google Scholar]
- 74.Courtland HW, Elis S, Wu Y, et al. Serum IGF-1 affects skeletal acquisition in a temporal and compartment-specific manner. PLoS One. 2011;6(3):e14762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ashpole NM, Herron JC, Estep PN, et al. Differential effects of IGF-1 deficiency during the life span on structural and biomechanical properties in the tibia of aged mice. Age (Dordr). 2016;38(2):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ashpole NM, Herron JC, Mitschelen MC, et al. IGF-1 regulates vertebral bone aging through sex-specific and time-dependent mechanisms. J Bone Miner Res. 2016;31(2):443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278(5341):1319–22. [DOI] [PubMed] [Google Scholar]
- 78.Ogg S, Paradis S, Gottlieb S, et al. The Fork head transcription factorDAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389(6654):994–9. [DOI] [PubMed] [Google Scholar]
- 79.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005; 24(50):7410–25. [DOI] [PubMed] [Google Scholar]
- 80.Ambrogini E, Almeida M, Martin-Millan M, et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010;11(2):136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim HN, Iyer S, Ring R, Almeida M. The role of FoxOs in bone health and disease. Curr Top Dev Biol. 2018;127:149–63. [DOI] [PubMed] [Google Scholar]
- 82.Iyer S, Ambrogini E, Bartell SM, et al. FOXOs attenuate bone formation by suppressing Wnt signaling. J Clin Invest. 2013; 123(8):3409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rached MT, Kode A, Xu L, et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11(2):147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142(2):296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iyer S, Han L, Ambrogini E, et al. Deletion of FoxO1, 3, and 4 in osteoblast progenitors attenuates the loss of cancellous bone mass in a mouse model of type 1 diabetes. J Bone Miner Res. 2017;32(1):60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen J, Long F. mTOR signaling in skeletal development and disease. Bone Res. 2018;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen J, Tu X, Esen E, et al. WNT7B promotes bone formation in part through mTORC1. PLoS Genet. 2014;10(1):e1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J, Long F. mTORC1 signaling promotes osteoblast differentiation from preosteoblasts. PLoS One. 2015;10(6):e0130627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riddle RC, Frey JL, Tomlinson RE, et al. Tsc2 is a molecular checkpoint controlling osteoblast development and glucose homeostasis. Mol Cell Biol. 2014;34(10):1850–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen J, Holguin N, Shi Y, Silva MJ, Long F. mTORC2 signaling promotes skeletal growth and bone formation in mice. J Bone Miner Res. 2015;30(2):369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li CJ, Cheng P, Liang MK, et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest. 2015;125(4):1509–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lai P, Song Q, Yang C, et al. Loss of Rictor with aging in osteoblasts promotes age-related bone loss. Cell Death Dis. 2016;7(10):e2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luo D, Ren H, Li T, Lian K, Lin D. Rapamycin reduces severity of senile osteoporosis by activating osteocyte autophagy. Osteoporos Int. 2016;27(3):1093–101. [DOI] [PubMed] [Google Scholar]
- 96.Ma Y, Qi M, An Y, et al. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell. 2018;17: e12709 DOI: 10.1111/acel.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.An JY, Darveau R, Kaeberlein M. Oral health in geroscience: animal models and the aging oral cavity. Geroscience. 2018;40(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kneissel M, Luong-Nguyen NH, Baptist M, et al. Everolimus suppresses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts. Bone. 2004;35(5):1144–56. [DOI] [PubMed] [Google Scholar]
- 99.Browne AJ, Kubasch ML, Gobel A, et al. Concurrent antitumor and bone-protective effects of everolimus in osteotropic breast cancer. Breast Cancer Res. 2017;19(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Selman C, Tullet JM, Wieser D, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949): 140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31(2):194–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guarente L Sirtuins in aging and disease. Cold Spring Harb SympQuant Biol. 2007;72:483–8. [DOI] [PubMed] [Google Scholar]
- 103.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17(11):679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simic P, Zainabadi K, Bell E, et al. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating β-catenin. EMBO Mol Med. 2013;5(3):430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iyer S, Han L, Bartell SM, et al. Sirtuin1 (Sirt1) promotes cortical bone formation by preventing β-catenin sequestration by FoxO transcription factors in osteoblast progenitors. J Biol Chem. 2014;289(35):24069–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zainabadi K, Liu CJ, Guarente L. Sirt1 is a positive regulator of the master osteoblast transcription factor, RUNX2. PLoS One. 2017; 12(5):e0178520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Edwards JR, Perrien DS, Fleming N, et al. Silent information regulator (Sir)T1 inhibits NF-kB signaling to maintain normal skeletal remodeling. J Bone Miner Res. 2013;28(4):960–9. [DOI] [PubMed] [Google Scholar]
- 110.Zainabadi K, Liu CJ, Caldwell ALM, Guarente L. Sirt1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis. PLoS One. 2017;12(9):e0185236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kalu DN, Hardin RR, Cockerham R, et al. Lifelong food restriction prevents senile osteopenia and hyperparathyroidism in F344 rats. Mech Ageing Dev. 1984;26(1):103–12. [DOI] [PubMed] [Google Scholar]
- 114.Tatsumi S, Ito M, Asaba Y, Tsutsumi K, Ikeda K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology. 2008;149(2):634–41. [DOI] [PubMed] [Google Scholar]
- 115.Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the Sirt1 deacetylase. Science. 2004;305(5682):390–2. [DOI] [PubMed] [Google Scholar]
- 116.Nisoli E, Tonello C, Cardile A, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310(5746):314–7. [DOI] [PubMed] [Google Scholar]
- 117.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8(2):157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Herranz D, Munoz-Martin M, Canamero M, et al. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mercken EM, Mitchell SJ, Martin-Montalvo A, et al. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell. 2014;13(5):787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Momken I, Stevens L, Bergouignan A, et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011;25(10):3646–60. [DOI] [PubMed] [Google Scholar]
- 121.Su JL, Yang CY, Zhao M, Kuo ML, Yen ML. Forkhead proteins are critical for bone morphogenetic protein-2 regulation and antitumor activity of resveratrol. J Biol Chem. 2007;282(27):19385–98. [DOI] [PubMed] [Google Scholar]
- 122.Artsi H, Cohen-Kfir E, Gurt I, et al. The Sirtuin1 activator SRT3025 down-regulates sclerostin and rescues ovariectomy-induced bone loss and biomechanical deterioration in female mice. Endocrinology. 2014;155(9):3508–15. [DOI] [PubMed] [Google Scholar]
- 123.Ornstrup MJ, Harslof T, Kjaer TN, Langdahl BL, Pedersen SB. Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2014;99(12):4720–9. [DOI] [PubMed] [Google Scholar]
- 124.Harman D Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. [DOI] [PubMed] [Google Scholar]
- 125.Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011;21(10):569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garrett IR, Boyce BF, Oreffo ROC, et al. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jilka RL, Almeida M, Ambrogini E, et al. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell. 2010;9(5):851–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Almeida M, Han L, Martin-Millan M, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282(37):27285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lean JM, Chow JW, Chambers TJ. The rate of cancellous bone formation falls immediately after ovariectomy in the rat. J Endocrinol. 1994;142(1):119–25. [DOI] [PubMed] [Google Scholar]
- 130.Jagger CJ, Lean JM, Davies JT, Chambers TJ. Tumor necrosis factor-α mediates osteopenia caused by depletion of antioxidants. Endocrinology. 2005;146(1):113–8. [DOI] [PubMed] [Google Scholar]
- 131.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31(3):266–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Manolagas SC, Almeida M. Gone with the Wnts: β-catenin, t-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol. 2007;21:2605–14. [DOI] [PubMed] [Google Scholar]
- 133.Manolagas SC. The quest for osteoporosis mechanisms and rational therapies: how far we’ve come, how much further we need to go. J Bone Miner Res. 2018;33(3):371–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lean JM, Davies JT, Fuller K, et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112(6):915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tyner SD, Venkatachalam S, Choi J, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002; 415(6867):45–53. [DOI] [PubMed] [Google Scholar]
- 136.de Boer J, Andressoo JO, de Wit J, et al. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296(5571): 1276–9. [DOI] [PubMed] [Google Scholar]
- 137.Nojiri H, Saita Y, Morikawa D, et al. Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J Bone Miner Res. 2011;26(11):2682–94. [DOI] [PubMed] [Google Scholar]
- 138.Kobayashi K, Nojiri H, Saita Y, et al. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Sci Rep. 2015;5:9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schriner SE, Linford NJ, Martin GM, et al. Extension of murine lifespan by overexpression of catalase targeted to mitochondria. Science. 2005;308(5730):1909–11. [DOI] [PubMed] [Google Scholar]
- 140.Ucer S, Iyer S, Kim HN, et al. The effects of aging and sex steroid deficiency on the murine skeleton are independent and mechanistically distinct. J Bone Miner Res. 2017;32(3):560–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: athiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98(3):1169–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Park CK, Lee Y, Kim KH, et al. Nrf2 is a novel regulator of bone acquisition. Bone. 2014;63:36–46. [DOI] [PubMed] [Google Scholar]
- 143.Pellegrini GG, Cregor M, McAndrews K, et al. Nrf2 regulates mass accrual and the antioxidant endogenous response in bone differently depending on the sex and age. PLoS One. 2017;12(2): e0171161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yoshida E, Suzuki T, Morita M, et al. Hyperactivation of Nrf2 leads tohypoplasia of bone in vivo. Genes Cells. 2018;23(5):386–92. [DOI] [PubMed] [Google Scholar]
- 145.Kim JH, Singhal V, Biswal S, Thimmulappa RK, DiGirolamo DJ. Nrf2 is required for normal postnatal bone acquisition in mice. Bone Res. 2014;2:14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ibanez L, Ferrandiz ML, Brines R, et al. Effects of Nrf2 deficiency on bone microarchitecture in an experimental model of osteoporosis. Oxid Med Cell Longev. 2014;2014:726590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40. [DOI] [PubMed] [Google Scholar]
- 148.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123(3):966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.van Deursen JM. The role of senescent cells in ageing. Nature.2014;509(7501):439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescencein aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21(12):1424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Coppe JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Acosta JC, Banito A, Wuestefeld T, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15(8):978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. [DOI] [PubMed] [Google Scholar]
- 155.Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279(5349):349–52. [DOI] [PubMed] [Google Scholar]
- 156.Campisi J Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–22. [DOI] [PubMed] [Google Scholar]
- 157.Munoz-Espin D, Canamero M, Maraver A, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155(5):1104–18. [DOI] [PubMed] [Google Scholar]
- 158.Storer M, Mas A, Robert-Moreno A, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155(5):1119–30. [DOI] [PubMed] [Google Scholar]
- 159.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12(7):676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Demaria M, Ohtani N, Youssef SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445(7128):656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. [DOI] [PubMed] [Google Scholar]
- 165.Ageing Nikolich-Zugich J. and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8(7):512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang J, Geiger H, Rudolph KL. Immunoaging induced by hematopoietic stem cell aging. Curr Opin Immunol. 2011;23(4): 532–6. [DOI] [PubMed] [Google Scholar]
- 167.Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4apositive senescent cells delay aging-associated disorders. Nature. 2011;479(7372):232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu M, Palmer AK, Ding H, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4: e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ogrodnik M, Miwa S, Tchkonia T, et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Roos CM, Zhang B, Palmer AK, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Childs BG, Baker DJ, Wijshake T, et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354(6311):472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu M, Tchkonia T, Ding H, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S.A. 2015;112(46):E6301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. Forthcoming. Epub 2018 Jul 9. DOI: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Farr JN, Fraser DG, Wang H, et al. Identification of senescent cells in the bone microenvironment. J Bone Miner Res. 2016;31(11): 1920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Piemontese M, Almeida M, Robling AG, et al. Old age causes de novo intracortical bone remodeling and porosity in mice. JCI Insight. 2017;2(17):e93771 DOI: 10.1172/jci.insight.93771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Farr JN, Xu M, Weivoda MM, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(9): 1072–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jurk D, Wang C, Miwa S, et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012;11(6):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jurk D, Wilson C, Passos JF, et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun. 2014;2:4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Minamino T, Orimo M, Shimizu I, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15(9):1082–7. [DOI] [PubMed] [Google Scholar]
- 180.Yi JS, Huang Y, Kwaczala AT, et al. Low-dose dasatinib rescues cardiac function in Noonan syndrome. JCI Insight. 2016;1(20): e90220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.D’Andrea G Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–71. [DOI] [PubMed] [Google Scholar]
- 182.Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kirkland JL, Tchkonia T. Clinical strategies and animal models for developing senolytic agents. Exp Gerontol. 2015;68:19–25. DOI: 10.1016/j.exger.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The clinical potential of senolytic drugs. J Am Geriatr Soc. 2017;65(10): 2297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Khosla S, Farr JN, Kirkland JL. Inhibiting cellular senescence: a new therapeutic paradigm for age-related osteoporosis. J Clin Endocrinol Metab. 2018;103(4):1282–90. DOI: 10.1210/jc.2017-02694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–13. [DOI] [PubMed] [Google Scholar]
- 187.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132(4):681–96. [DOI] [PubMed] [Google Scholar]
- 188.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol. 2010;22(4):507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Signer RA, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12(2):152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Molofsky AV, Slutsky SG, Joseph NM, et al. Increasing p16INK4aexpression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443(7110):448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang X, Ebata KT, Robaire B, Nagano MC. Aging of male germ line stem cells in mice. Biol Reprod. 2006;74(1):119–24. [DOI] [PubMed] [Google Scholar]
- 193.Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24(6):1505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302(5650):1575–7. [DOI] [PubMed] [Google Scholar]
- 195.Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z.Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294(1):50–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lavasani M, Robinson AR, Lu A, et al. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun. 2012;3:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ono N, Kronenberg HM. Mesenchymal progenitor cells for the osteogenic lineage. Curr Mol Biol Rep. 2015;1(3):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–40. [DOI] [PubMed] [Google Scholar]
- 199.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. [DOI] [PubMed] [Google Scholar]
- 200.Bianco P, Robey PG. Skeletal stem cells. Development. 2015;142(6): 1023–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–26. [DOI] [PubMed] [Google Scholar]
- 202.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells.Ageing Res Rev. 2006;5(1):91–116. [DOI] [PubMed] [Google Scholar]
- 203.Kasper G, Mao L, Geissler S, et al. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells. 2009;27(6):1288–97. [DOI] [PubMed] [Google Scholar]
- 204.Coipeau P, Rosset P, Langonne A, et al. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy. 2009;11(5):584–94. [DOI] [PubMed] [Google Scholar]
- 205.Park D, Spencer JA, Koh BI, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10(3):259–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Grcevic D, Pejda S, Matthews BG, et al. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells. 2012;30(2):187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ono N, Ono W, Nagasawa T, Kronenberg HM. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol. 2014;16(12):1157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Horowitz MC, Berry R, Holtrup B, et al. Bone marrow adipocytes.Adipocyte. 2017;6(3):193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-kB in aging and disease. Aging Dis. 2011;2(6):449–65. [PMC free article] [PubMed] [Google Scholar]
- 212.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116:1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Khosla S, Monroe DG. Regulation of bone metabolism by sex steroids. Cold Spring Harb Perspect Med. 2018;8(1):a031211 DOI: 10.1101/cshperspect.a031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Almeida M, Laurent MR, Dubois V, et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017;97(1):135–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wei J, Ferron M, Clarke CJ, et al. Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest. 2014;124(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Oury F, Ferron M, Huizhen W, et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest. 2013;123(6):2421–33. DOI: 10.1172/JCI65952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Oury F, Ferron M, Huizhen W, et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest. 2015;125(5):2180 Erratum. DOI: 10.1172/JCI81812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Khrimian L, Obri A, Karsenty G. Modulation of cognition and anxiety-like behavior by bone remodeling. Mol Metab. 2017;6(12): 1610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mera P, Laue K, Ferron M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2017;25(1):218. [DOI] [PubMed] [Google Scholar]
- 220.Pedersen BK. Muscle as a secretory organ. Compr Physiol. 2013;3(3):1337–62. [DOI] [PubMed] [Google Scholar]
- 221.Griffith JF, Yeung DK, Ma HT, et al. Bone marrow fat content in the elderly: a reversal of sex difference seen in younger subjects. J Magn Reson Imaging. 2012;36(1):225–30. [DOI] [PubMed] [Google Scholar]
- 222.Pansini V, Monnet A, Salleron J, et al. 3 Tesla (1) H MR spectroscopy of hip bone marrow in a healthy population, assessment of normal fat content values and influence of age and sex. J Magn Reson Imaging. 2014;39(2):369–76. [DOI] [PubMed] [Google Scholar]
- 223.Gasparrini M, Rivas D, Elbaz A, Duque G. Differential expression of cytokines in subcutaneous and marrow fat of aging C57BL/6J mice. Exp Gerontol. 2009;44(9):613–8. [DOI] [PubMed] [Google Scholar]
- 224.Schellinger D, Lin CS, Fertikh D, et al. Normal lumbar vertebrae: anatomic, age, and sex variance in subjects at proton MR spectroscopy—initial experience. Radiology. 2000;215(3):910–6. [DOI] [PubMed] [Google Scholar]
- 225.Schwartz AV, Sigurdsson S, Hue TF, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98(6): 2294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kodama Y, Takeuchi Y, Suzawa M, et al. Reduced expression ofinterleukin-11 in bone marrow stromal cells of senescence-accelerated mice (SAMP6): relationship to osteopenia with enhanced adipogenesis. J Bone Miner Res. 1998;13(9):1370–7. [DOI] [PubMed] [Google Scholar]
- 227.McGee-Lawrence ME, Carpio LR, Schulze RJ, et al. Hdac3 deficiency increases marrow adiposity and induces lipid storage and glucocorticoid metabolism in osteochondroprogenitor cells. J Bone Miner Res. 2016;31(1):116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nishikawa K, Nakashima T, Takeda S, et al. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J Clin Invest. 2010;120(10): 3455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell. 2004;3(6):379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Farr JN, Charkoudian N, Barnes JN, et al. Relationship of sympathetic activity to bone microstructure, turnover, and plasma osteopontin levels in women. J Clin Endocrinol Metab. 2012; 97(11):4219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111: 305–17. [DOI] [PubMed] [Google Scholar]
- 232.Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–20. [DOI] [PubMed] [Google Scholar]
- 233.Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–4. [DOI] [PubMed] [Google Scholar]
- 234.Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153(4):828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li JY, Chassaing B, Tyagi AM, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6):2049–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jones RM, Mulle JG, Pacifici R. Osteomicrobiology: the influence of gut microbiota on bone in health and disease. Bone. Forthcoming. Epub 2017 Apr 27. DOI: 10.1016/j.bone.2017.04.009. [DOI] [PubMed] [Google Scholar]