Abstract
Protection from yearly recurring, highly acute infections with a pathogen that rapidly and continuously evades previously induced protective neutralizing antibodies, as seen during seasonal influenza virus infections, can be expected to require a B cell response that too is highly variable, able to adapt rapidly and to reduce morbidity and death when sterile immunity cannot be garnered quickly enough. As we outline in this brief review, the influenza-specific B cell response is exactly that: It is multifaceted, involves both innate-like and conventional B cells, provides early and later immune protection, employs B cells with distinct BCR repertoires and distinct modes of activation, and continuously adapts to the ever-changing virus while enhancing overall protection. A formidable response to a formidable pathogen.
INTRODUCTION
B cell-derived antibodies generated in response to influenza virus are critical for preventing death from acute respiratory tract infections, promoting a more rapid and full recovery, and for providing continued protection from future infection-induced illness and/or death. This is despite the fact that the yearly re-emerging seasonal strains of influenza virus undergo rapid point mutations, “antigenic drift”, which reduces the effectiveness of strain-specific antibodies generated from one year to the next (1). Nonetheless, pre-existing, non-neutralizing humoral immunity, even if unable to prevent re-infection, can prevent serious disease and/or reduce mortality. This is well illustrated by the fact that individuals most at risk from dying following influenza infection are the very young and old, or those with immuno-deficiencies- Thus patients in whom an effective immune system has either not developed or is compromised. In addition to the mostly strain-specific antibodies generated following an infection, influenza-specific B cell responses can include rare responses against highly conserved antigenic epitopes. Responses to such epitopes can provide cross-protection against multiple influenza strains, i.e. induce “heterosubtypic” influenza-specific immunity. The specificity of these cross-protective antibodies and their protective capacities have been a recent focus of anti-influenza vaccine development efforts, and are reviewed in detail elsewhere (2).
Advances in our understanding of B cell responses to infections have revealed their complexity: In addition to the generation of antibodies, B cells also generate cytokines with which they can regulate the immune response, and they can act as antigen-presenting cells to CD4 and CD8 T cells (3). Their elaboration of cytokines and interaction with T cells is likely to induce responses that are distinct from those provided by other APCs, shaping T cell-immunity in yet to be determined ways. Moreover, the presence of virus-induced innate responses can also affect B cell functions, and this may differ depending on the tissue in which B cells receive such signals, thus indicating further complexities based on B cell tissue location and interaction with innate immune cells. Further complexities are the extensive heterogeneity among B cells with regard to their developmental origins, initial B cell receptor (BCR) affinity for influenza antigens, and their differentiation state, all of which shape B cell response outcomes.
Here we review the multiple aspects of B cell immunity to influenza virus infection, focusing on experimental mouse models and citing human studies when possible. We discuss the role of B cell responses to innate signals and B cell-derived cytokines involved in anti-viral responses, as well as describe how B cells transform these signals into functional changes. We review innate-like B cell responses, early extrafollicular plasmablast and later germinal center responses, and argue that the latter two are of equal importance in the development of strong and durable humoral immunity to influenza virus infection. Understanding the complexities involving B cell responses to influenza infections may help to overcome the challenges of inducing long-term immunity through vaccination.
B cells shape the early immune response to influenza infection
Mice never exposed to influenza virus, and even those kept under germ-free conditions, nonetheless have circulating “natural” IgM antibodies, a fraction of which can bind to a variety of different influenza virus strains (4–6). This IgM antibody is generated in the bone marrow and spleen of mice by differentiated, neonatally-derived subsets of innate-like B cells called B-1 cells (6, 7) and can reduce viral loads and mortality rates from infection (8, 9). In addition to this steady-state function, innate-like B-1 cells also respond to influenza virus infection with rapid migration from the pleural cavity to the draining mediastinal lymph nodes (medLN) (5, 10). Accumulation in the medLN, which occurs as early as two-days after influenza infection, depends on Type I IFN production in the respiratory tract, which was shown to cause integrin CD11b activation on the surface of pleural cavity B-1 cells, facilitating their retention in the medLN (10). Once there, some B-1 cells begin to produce IgM, including IgM that can bind multiple strains of influenza virus (4, 5). The mechanisms leading to induction of antibody secretion by these cells remains unknown.
Secreted (s)IgM is a potent facilitator of complement activation (11) and can bind the Complement receptors (CR1/2) as well as the Fc receptor for IgM (FcμR) expressed on dendritic cells (DCs), macrophages, as well as B cells (12, 13). Lack of IgM in mice led to delayed influenza clearance (8, 9, 14, 15). IgM-mediated protection from influenza infection is mediated at least in part through activation of complement (15). In addition, lack of sIgM and/or lack of FcμR expression on B cells caused decreases in influenza-specific IgG, likely further contributing to reduced viral clearance (9). Thus, early production of IgM in response to influenza optimizes the overall antibody response and is a potential catalyst in priming both innate and adaptive immunity.
Immediate early responses by bone marrow-derived conventional follicular B cells (also termed B-2 cells), which make up the vast majority of B cells in lymphoid and non-lymphoid tissues, are less well characterized. Some potential functions may be inferred from other infection models. In addition to populating secondary lymphoid tissues, naïve B cells are dispersed throughout the conductive airways and lung parenchyma at homeostasis (16), and are thus able to respond rapidly to respiratory infections. In support of this, lung-associated B cells were observed transporting antigen to the spleen, but not the lymph nodes, within 2 hours post intranasal immunization of mice with virus-like particles (17). Following intratracheal administration of anthrax spores, B cells were found to deliver antigen within just 6 hours to the lung-draining lymph nodes in a BCR-independent manner (18). These observations indicate early functional roles of B cells in antigen delivery from the periphery to lymphoid tissues to secondary lymphoid tissues. The nature of the antigen and/or the inflammatory responses induced to distinct pathogens may affect subsequent localization of these B cells and therefore the initiation of the adaptive response.
B cell responses during influenza infection are present in both medLN and spleen. However, splenic responses appear to be minimal and of shorter duration compared to those in the medLN, indicating that the medLN are the main sites of B cell response activation after intranasal influenza infection, at least in experimental settings. In humans, where severe infections usually proceed from the upper to the lower respiratory tract, the involvement of upper respiratory tract draining cervical lymph nodes likely play a greater role compared to experimental infections of mice, in which the virus is usually applied deep into the respiratory tract.
The medLN significantly increase in size and cellularity within 1–2 days after infection (19). Influenza antigen-carrying CD11b+ DCs migrate into these lymph nodes, thereby promoting the activation of CD4 and CD8 T cells. Interestingly, lymph node expansion following LCMV infection was shown to depend on the provision of lymphotoxin α1β2 by B cells (20). Furthermore, two days after immunization with the antigen keyhole limpet hemocyanin (KLH) in CFA, B cells facilitated a six-fold increase in lymph node cellularity by secreting vascular endothelial growth factor A (VEGF-A) (21), which promotes both blood and lymph vasculature remodeling and growth (22). Without B cells or B cell-derived VEGF-A, dendritic cell trafficking and lymph node cellularity were reduced to homeostatic levels (21). Thus, B cells actively regulate immune responses by shaping secondary lymphoid tissue remodeling after infection.
Within the lymph nodes, B cells become subjugated to cues for activation provided by innate signals. Global gene expression analysis on medLN B cells of day 2 influenza-infected mice revealed a Type I IFN signature (23). Type I IFN was shown to cause rapid global B cell activation, likely prior to first antigen-encounter, including immediate upregulation of TLR3 and 7 and increases in expression of CD69 and CD86 (23). This renders B cells more responsive to innate signals and increases their ability to interact with T cells by trapping them in the lymph node, as well as increasing expression of co-stimulatory molecules. Indeed, these innate-signaling derived activation signals were shown to be important for B cell response induction, as B cell-specific deletion of the Type I IFN receptor diminished the influenza-specific antibody response as early as 3 days post-infection (23). Thus, adaptive B cell response activation is integrated in, and dependent on, the network of infection-induced innate signals. Further exploration of how innate activation of B cells affects the quality and quantity of anti-influenza responses will be important for the development of adjuvants that support stronger and longer-lasting humoral immunity.
B cell activation leads to rapid induction of extrafollicular and slower development of germinal center responses in draining lymph nodes after influenza infection
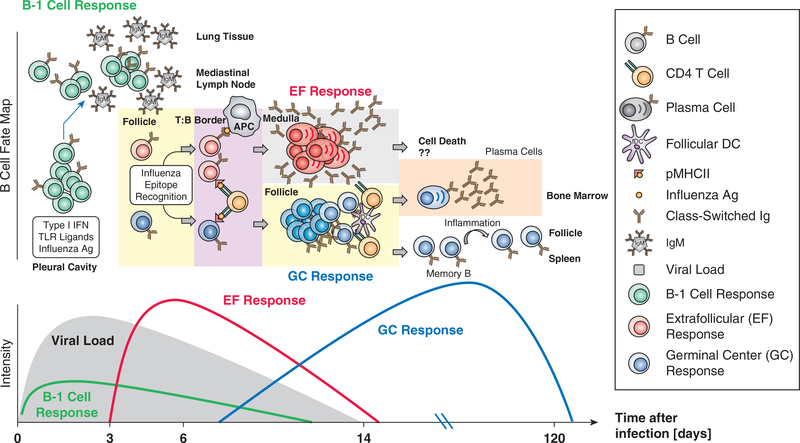
As discussed above, respiratory tract-draining lymph nodes are the main site of influenza-induced B cell activation. A critical outcome of B cell activation is the induction of antibody-secreting cells (ASCs) producing class-switched, protective antibodies (24, 25). Strong influenza-specific antibody responses are produced by extrafollicular (EF) B cells well before Germinal center (GC) -derived plasma cells are generated (Figure 1). EF responses are situated in the lymph node medulla, where rapid and strong B cell proliferation is followed by differentiation of these cells into short-lived plasmablasts that secrete IgM, IgA and IgG (26).
Figure 1. The heterogenous nature of B cell responses to influenza virus infection.
(Top) B-1 cells, activated by various innate signals, including type I IFN, respond to initial influenza infection with migration to the draining medLN, where these cells differentiate to IgM-producing cells. Conventional B cells, activated by influenza antigen will migrate to the T-B boder where they receive “T cell help”. Thus activated, B cells will differentiate along one of two pathways: Extrafollicular foci (EF), which induce strong and rapid clonal expansion and differentiation to antibody secreting plasmablasts and germinal centers in the B cell follicles (GC). EF-derived plasmablasts are thought to live for only 3–5 days, while the outcome of GC responses is the development of long-lived antibody-secreting plasma cells in the bone marrow and circulating non-secreting memory B cells. Inflammatory signals may activate these memory B cells to rapidly migrate to spleen and lymph nodes and to differentiate to antibody secreting cells, or undergo new diversification in germinal center responses. (Bottom) B-1 and EF responses are the only B cell responses that are sufficiently fast to influence viral clearance after a primary challenge.
As we explore below, the explicit mechanisms that drive B cells into either the EF or the GC fate have not yet been completely resolved. Of importance, the kinetics of the EF response correlate with the peak and resolution of primary influenza infection (27), indicating that this rapid, strong, and early humoral response contributes to virus clearance. In contrast, GC responses do not usually form until the infection has nearly resolved, indicating GCs are geared towards generating and maintaining immunological memory by producing long-lived antibody-secreting plasma cells and memory B cells. The latter importantly enhances the precursor frequency of influenza-specific B cells, some of which can respond to the next influenza virus challenge either by initiating a rapid EF response, or by forming new GCs (Figure 1).
Apart from the above-discussed migration of antigen-carrying B cells into secondary lymphoid tissues, most B cells likely encounter antigen in the lymph node follicles through stochastic movement that bring them near the follicle’s capsular plane, where they encounter antigen tethered on sub-capsular macrophages (28). B cells can also encounter antigen elsewhere in the lymph node (reviewed in (29)), though whether B cell response quality differs depending on the type and site of antigen encounter is unknown. The antigen-specific activation of B cells and CD4 T cells induce genetic reprogramming that strongly promotes their interaction, specifically their chemokine-mediated migration towards each other. This is facilitated initially by the upregulation of CCR7 and downregulation of CXCR5 in B cells, and the inverse in T cells, which leads to their congregation at the para-cortical ridge (30), otherwise known as the T:B border. Movement of B cells is also affected by the orphan receptor Ebi2, which is expressed differentially at the many stages of B cell activation and differentiation in the lymph node (31). Furthermore, antigen-stimulation induces secretion of CCL3 and CCL4 by B cells, which will further attract activated CCR5-expressing CD4 T cells and thus enhance T-B interaction and humoral immunity (32).
Extrafollicular Responses
Antigen-activated B cells can form EF foci independent of CD4 T cell “help”, and such T-independent B cell responses can confer some protection against influenza virus infection (33, 34). However, most protective B cell responses to influenza, including those generated in EF responses, are T-dependent. The local expansion and rapid differentiation of B cells into plasmablasts, seen in the EF response, requires B cell genetic programs to polarize towards an ASC fate. The ASC state is characterized by expression of transcription factors Blimp1, XBP1, and the high expression of IRF4, which is upregulated upon initial B cell activation together with IRF8. However, IRF4 and IRF8 become mutually antagonistic after reaching certain expression thresholds, influencing polarization towards an effector (IRF4) or a GC fate (IRF8) (35).
The signals that preferentially induce IRF4 over IRF8 and thereby an ASC fate are subject of ongoing investigation. Elegant previous work has begun to shed light on some potential mechanisms. Of particular significance, a strong correlation was observed between BCR affinity for antigen and the ASC fate (36–38). Reducing a high-affinity interaction of B cells to the model antigen hen-egg lysozyme (HEL), by alterations made to the cognate antigen, showed that only high-affinity BCR-antigen interaction could promote EF responses (36). This is supported also by in vivo studies showing that GC-derived, memory B (Bmem) cells will rapidly form EF responses during a recall response preferentially over generating new GCs (39). At the molecular level, and consistent with those observations, IRF4 is known to be upregulated by increasing affinities of the BCR for antigen. Furthermore, IRF4 is induced in a dose-dependent manner by the engagement of B cell-expressed co-receptor CD40 with CD40L on T cells (40). These observations strongly suggest that BCR signal strength, as well as T cell help, contribute towards differentiation of activated B cells into plasmablasts. Whether strong BCR-signaling alone can overcome the need for CD40 engagement in the formation of T-independent EF responses, or whether IRF4 induction might be induced by innate signals in addition to BCR-signaling, remains to be determined.
A model of rapid EF response induction that depends on the presence of high-affinity BCR is consistent with data demonstrating that these responses can provide antibody rapidly and of sufficient quality to contribute to immunity after influenza infection (27). However, it also suggests that rapid EF responses are formed only when B cells of “sufficient” antigen-affinity are present in a given host prior to an infection. The high morbidity and mortality rates observed in newborns and the very young after influenza infection might be in part explained by the fact that these individuals lack such a robust and broad naïve B cell repertoire (41), and that they do not yet carry influenza-specific Bmem cells that could provide clones specific for influenza (see also below).
Once an activated B cells differentiates into a plasmblast, their populations begin to expand rapidly in the lymph node medulla and secrete predominantly class-switched antibody, peaking between 7 to 14 days post-influenza infection (27, 42). In humans, peak influenza-specific plasmablast numbers in the peripheral blood peak around 7 days after symptom onset during infection (43) and after immunization (44). However, after immunization plasmablasts appear at a much reduced frequency compared to infection (45) and have even higher levels of somatic hypermutation (SHM) than circulating Bmem cells (46), indicating they were recruited from memory pools and thus previously generated in GC rather than EF.
Once an EF response is initiated, its maintenance does not appear to require further T cell help (47). Interestingly, it was shown that an increased presence of neutrophils in the draining lymph nodes led to increases in plasmablast numbers after sub-cutaneous CFA immunization (48). The mechanisms of that interaction were inferred to be dependent on the cytokine B cell-activating factor (BAFF) secreted by neutrophils. However, other studies more explicitly showed that the survival factor A PRoliferation-Inducing Ligand (APRIL), also secreted by neutrophils, was vital in maintaining ASC populations within niches of the lower respiratory tract submucosa (49), as well the splenic marginal zone (50). Plasmablasts from the EF response thus seem to be maintained, or at least significantly supported, by antigen-independent means, including through the secretion of survival factors by effectors of the innate compartment.
Germinal Center Responses
GC-derived expansion of B cell clones is minimal during initial influenza infection and virus clearance, i.e. the first 7 to 10 days after virus exposure. Instead, GC-associated B cell numbers peak well into the contraction phase of the immune response (51) (Figure 1). To initiate GC formation, B cells activated by antigen and cognate CD4 T cell help return to the B cell zone (follicle) from the T:B border, along with their cognate CD4 T cells, where follicular DCs display non-processed antigens (52). Antigen-binding B cell clones then undergo massive proliferation as well as SHM, the latter supported by the expression of the enzyme aicda (AID), forming the dark zone of the GC with cells known as centroblasts. This process leads to B cell sub-clones with genetic alterations in the antigen-binding site of their BCR. Centroblasts then migrate to the light zone of the GC for validation of antigen-specificity. There these cells, known as centrocytes, engage with CD4 follicular helper T (Tfh) cells to determine B cell fate (53). Positive selection will result in either a return to the dark zone for further rounds of proliferation and mutations, or in an exit from the GC as either Bmem cells or terminally-differentiated plasma cells. It is not known what signals instruct the differentiation of GC clones to either a Bmem or plasma cell fate, but lower-affinity clones were shown to preferentially accumulate within the Bmem pool (54–56), while high-affinity clones are shunted preferentially towards a plasma cell fate (25–27), in much the same manner as seen during the EF response. Therefore, it appears that both plasmablasts and post-GC plasma cells may use the similar differentiation pathways to reach an ASC state. However, while EF-derived ASCs appear to be short-lived and confined to the site of their induction, the post-GC plasma cells migrate to the bone marrow in a CXCR4-dependent manner. There they can secrete antibody and survive long-term, supported by local, stromal cell-derived survival signals (57).
Extensive immunoglobulin sequencing studies of circulating Bmem cells in humans have shown that strong antibody repertoire diversity is generated in response to repeated infection with, or exposure to, seasonal influenza viruses. The data suggest similar strong, ongoing GC responses to occur in humans following influenza infection, as those studied in experimental mouse models (58). Given that both the influenza virus and the B cells are relying on mutations to outcompete each other, the GC response is likely the main B cell response engaged in the “arms race” between the virus and the humoral response, as somatic hypermutation in EF responses is limited (59, 60). Indeed, sequencing studies have been able to trace the evolution of B cell responses that occur in response to repeated exposures to seasonal influenza virus strains (61–63), clearly revealing a GC-derived Bmem cell repertoire that is shaped by repeated exposures to mutated viruses.
Humoral immunity to influenza integrates the B-1 response and B-2-derived EF and GC responses
Thus, humoral immunity to influenza can be categorized into immediate early, early and late responses, each facilitated by a distinct set of B cells. Control of an acute influenza infection by B cells involves both innate-like B-1 cells as well as rapidly responding conventional B cell-derived EF responses both having the capacity to control early tissue virus load. This control is critical, as a rapid containment of this rapidly-dividing virus can reduce the risk of overshooting cytokine and CD8 T cell responses, which can cause extensive and excessive accumulation of leukocytes in the lung parenchyma, lung pathology, and eventual organ failure.
GC B cell responses on the other hand must fulfill distinct roles in combating influenza infection. The kinetics of these responses precludes important roles during primary infection. Yet, the induction and then maintenance of GC responses for months after the clearance of the virus suggests that their main function is the strengthening of the initial line (i.e. B-1 and EF) of humoral defense during recall responses, either through generation of antibody-producing plasma cells or through provision of a large repertoire of Bmem cells. The production of affinity-matured, neutralizing antibodies by plasma cells per se seems of limited value against an infection that can rapidly mutate targeted epitopes. Therefore, the generation of a broad repertoire of Bmem cells seems very important, as these B cells are generated to a variety of influenza antigens and can respond, depending on their initial affinity to antigen, by either rapidly generating antibodies or by inducing strong GC responses. It may also explain why such Bmem cells are not of as high affinity for the inducing antigen as the effector plasma cells: The next round of infections might bring mutated antigens that bind differently to these BCR. Recent studies with human Bmem cells support the notion that this compartment has a broad repertoire and includes cross-reactive BCR-bearing cells that are continuously shaped by repeat exposure to seasonal influenza virus strains (58, 64). The broadening of this repertoire would ensure that there are B cell pools available to bind viral antigens, even if altered by mutations, as seen during the seasonal encounters with influenza by humans.
Although EF and GC responses to influenza are generated by conventional B cells, early sequencing studies showed a remarkable lack of overlap in the V-gene usage of HA-specific B cells early and later after immunization with influenza virus (27, 65). Recent elegant studies by the Yewdell group confirmed these early studies using influenza virus infection of mice (51). The data are consistent with the above discussed findings that only high-affinity antigen-BCR interactions drive EF responses (36), while lower-affinity interactions initiate GC responses. Given the strength of the EF response after infection, this suggests that humoral responses to influenza virus infection, and likely other infections, do NOT begin with the elaboration of low-affinity antibodies that over time affinity-mature into higher affinity antibody responses. Rather high-affinity antibody responses produced by the EF response are replaced later by high-affinity antibody responses generated from the GC response.
If our analysis is correct, this would be a significant departure from the text-book idea of antibody response that “mature” over time, but very consistent with conclusions drawn from previous studies with VSV infection of mice that failed to demonstrate overall changes in serum antibody affinity over the course of that virus infection (66). For an acute infection, like infections with influenza virus, the immediate early activation of B cells into ASC from pre-existing high-affinity clones may make the difference between life and death, while during more protracted infections the influence of the EF response may be more modest.
Respiratory tract B cell activation during influenza infection
In addition, and similar to other mucosal sites, strong and sustained respiratory tract tissue injury, including injury induced by influenza infection, can result in the formation of broncho-associated lymphoid tissue (BALT) in the submucosa of the upper respiratory tract and within the parenchyma of the lower respiratory tract airways (67–69). Although the importance of BALT formation in immune defense is still not resolved, such inducible BALT was shown to contain GCs and to generate influenza-specific antibody responses after influenza infection in mice that lacked all secondary lymphoid organs, including all draining lymph nodes (67), indicating their potential to catalyze adaptive T and B cell responses. Interestingly, following local ablation of BALT with diphtheria toxin, significant reductions in influenza-specific serum antibody titers were noted after intranasal Influenza inoculation (70). However, another study showed that after influenza infection BALT formed only if mice had received repeated intranasal administration of LPS as neonates (71). Differences in virus strain and mouse models may be the cause of these discrepancies. Together, the data indicate that tertiary lymphoid tissues in the respiratory tract may contribute to immunity during strong and/or chronic immune activation.
The GC generates ASCs and Bmem cells capable of recall responses and long-term influenza-specific antibody production
Following the resolution of influenza infection, ASC populations are found long-term in bone marrow as well as locally in the respiratory tract (72). The differentiation pathways of the local ASC have not been resolved, while those residing in the bone marrow likely arise from GC responses. Both influenza infection-induced bone marrow and lung ASCs were shown to require TACI (a receptor for APRIL) for long-term survival, while lymph node and spleen ASCs were unaffected by the loss of that receptor (73). Given that lymph node ASCs are derived mainly from EF responses, as they disappear with the resolution of the EF response and before robust GC responses develop (42), this could suggest that cell-fate decisions between plasmablasts and plasma cells seem to be the result of broadly overlapping genetic programs. Differences might still exist between individual ASC populations based on tissue location and/or whether their differentiation pathways involved EF or GC responses.
GC-derived plasma cell development leads to sustained influenza-specific serum antibody titers. Antigen-specific antibody has been detected decades after infection and sometimes after immunization in humans (74). Carbon dating on plasma cells in the gastrointestinal tract confirmed this subset’s aging capacity (75). Similarly, survivors of the 1918 Influenza pandemic showed sero-reactivity to the 1918 virion 90 years later (74). This indicates that influenza infection can generate essentially life-long humoral protection against the same (homosubtypic) virus strain. Whether boosting by subsequent seasonal influenza exposures contributes towards maintenance of such antibody responses is not known and should be considered.
Few data are available revealing the lifespan and aging dynamics of Bmems cells. It has been well demonstrated, however, that these cells respond acutely to secondary challenges, partaking in both EF and GC responses in local lymph nodes (39, 76), as well as migrating directly to sites of insult (77, 78). More than 5 months after influenza challenge, class-switched, CD38+ Bmem cells were found in spleen, medLN, and lungs of BALB/c mice (77). Furthermore, reactivation of lung Bmem populations was shown to contribute to enhanced immunity during virus challenge (77, 79) and the loss of Bmem cells, as seen in HIV-infected patients, reduced the patient’s ability to respond to influenza vaccinations (80). As outlined above, repeated exposure to influenza virus continuously alters the virus-specific class-switched Bmem compartment of humans, indicating that Bmem cells respond to and help control influenza infections, likely including many that cause subclinical infections.
B cells as regulators of T-dependent antiviral immunity
Several influenza virus infection studies in mice demonstrated B cell synergy with either CD4 or CD8 T cells in optimizing protection against a primary viral challenge. While lack of CD8 T cells delayed clearance of influenza A/PR8 (H1N1) significantly in mice (81), so did a lack of B cells, albeit not as strongly (14). The targeting and elimination of DCs by cytotoxic CD8 T cells may also affect B cells directly by reducing priming of robust CD4 T cell responses that support development of virus-specific antibodies. Consistent with that, the lack of CD8 T cells actually led to increased antibody responses (82). Absence of both CD8 T cells and B cells led to uncontrolled viral dissemination and death (34), indicating CD4 T cells alone cannot protect against this infection. The same held true for CD8 T cells, as lack of both CD4 T cells and B cells resulted in significant mortality after low-dose H1N1 infection (83).
This raises the question of how B cells cooperate with T cells to prevent influenza virus-induced immunopathology and death. A lack of antigen-specific antibody in a Listeria-LCMV challenge mouse model led to uncontrolled CD4 T cell activity, cytokine storm, and death, which was abrogated upon transfer of antigen-specific B cells (84). In that case, antigen-specific antibody was shown to reduce antigen load and thereby prevent continued overly strong activation of effector CD4 T cells. Consistent with that data, influenza-infected mice lacking B cells and chronically depleted of CD8 T cells, but with an intact CD4 T cell compartment, rapidly succumbed to infection, while replenishment with virus-specific B cells rescued these mice (34). The data indicate that generation of protective antibodies provides a bridge between CD4-driven, innate and antigen-directed responses.
Inclusive with this dynamic of suppressive feedback, B cells also promotes CD4 T cell activity. Absence of B cells in influenza-infected mice was associated with reduced systemic CD4 T cell proliferation and reduced polarization of Th1 cells in the lungs, which resulted in decreased viral clearance (14). This might be due at least in part through the ability of B cells to produce numerous cytokines, as B cell-derived IFNγ, IL-6, and TNFα were shown to support Th1 responses by CD4 T cells (85–87). In addition, production of IL-6 by B cells was shown to promote the development and/or maintenance of TFH cells and thereby the regulation of antiviral antibody responses (88).
Triggering of cytokine production by B cells might be induced through their expression of a wide array of pattern-recognition receptors (PRRs), including toll-like receptors (TLRs), retinoic acid inducible gene I (RIG-I) -like receptors (RLRs), and NOD-like receptors (NLRs) (89–91). Specifically, the activation of TLR7 on murine B cells has been shown to induce IFN-α production during Influenza, although not to EBV infection (92, 93). B cells also express inflammasome complexes (94), and thus can promote Caspase-1-mediated processing of the inflammatory cytokines IL-1, IL-18, and IL-33 into their active forms (95). IL-1β is particularly interesting, as IL-1 KO mice showed increased morbidity and mortality following Influenza infection, which correlated with decreases in amounts of serum and airway influenza-binding IgM (96).
Cytokine production by B cells may also modulate potential overshooting cytokine responses, the so called “cytokine storm”, observed following infection with particularly pathogenic influenza viruses. IL-10 can suppress such overshooting responses (97) and IL-10 production by regulatory B cells (“Bregs”) and plasma cells has been shown to be effective in modulating a number of inflammatory conditions (reviewed in: (98)). Given the close interaction of B cells with recently activated CD4 T cells in the bridging channels of activated lymph nodes, further analysis of immunomodulation of antiviral T cell responses by cytokine-producing B cells may provide important mechanistic insight into the regulation of effective antiviral immunity to influenza.
Effective heterosubtypic immunity to influenza
Effective immunity to influenza virus requires the presence of T and B cells that respond to more than one sub-strain of influenza, so called “heterosubtypic” immunity. Studies conducted following the 2009 pandemic showed that humans harbored influenza-specific memory CD4 T cells that were cross-reactive between seasonal and 2009 pandemic H1N1 strains (99), as well as cross-reactive Bmem cells. CD8 T cell immunity to influenza has long been known to target highly conserved internal proteins of influenza, particularly the nuclear protein (NP), which can provide some heterosubtypic immune recognition (100).
The contribution of each adaptive immune compartment to heterosubtypic immunity was evaluated in mice, demonstrating a most potent role for T-dependent antibody responses. Genetic ablation of CD4 T cells in mice following a sublethal dose of a H3N2 virus infection resulted in significantly enhanced mortality to subsequent challenge with a lethal dose of H1N1 (33). The same studies with B-cell deficient (μMT) mice resulted in even greater mortality, while depletion of CD8 T cells had no effect (33). This was despite existing cross-reactivity of H3N2-primed CD8 T cells. As expected, CD8 T cell-deficient mice generated protective levels of neutralizing serum antibody against the heterosubtypic virus, while influenza-specific antibody titers in CD4 T cell deficient mice barely rose above that of their naïve controls (33).
Recent studies have identified the highly-conserved “stalk” domain of the trimeric HA molecule as a target of broadly cross-reactive and indeed cross-protective antibodies (reviewed elsewhere (2)). A current challenge for exploiting this information for the better design of vaccines is the finding that these epitopes are neither immunodominant, nor do they induce neutralizing antibodies, i.e. antibodies that directly prevent the binding of the virus to target cells. Their effectiveness instead relies on Fc-mediated effector functions by innate immune cells, including the enhancement of antibody-directed cell cytotoxicity (ADCC) facilitated by binding of specific Ig isotypes to activating Fc-receptors (101, 102). Indeed, broadly-neutralizing, monoclonal antibodies (mAbs) with disrupted Fc-receptor binding did not protect against lethal influenza infection in mice, whereas identical clones with full Fc-receptor binding potential completely prevented infection-induced mortality (103, 104). Unfortunately, immunization of mice with antigens holding ADCC-associated epitopes led to increased alveolar damage and mortality after boost with intact virus (105), demonstrating the double-edged sword of Fc-mediated ADCC during influenza infection, especially as a potential vaccine target.
Given the limitations of these naturally induced broadly-protective antibodies, it appears likely that the observed naturally-developing T-dependent, B cell-mediated heterosubtypic immunity is provided not only by such cross-reactive protective antibodies, but also by the ongoing reshaping of the overall influenza-specific Bmem compartment by seasonal influenza virus exposures. Such exposures constantly boost and shape an increasingly broad repertoire of Bmem cells, generating a range of antibody specificities that are both neutralizing and non-neutralizing. Together they provide strong protection, if not always from virus-induced disease, but protection from serious illness and death. When failure of protection is observed, i.e. in the very young as well as the elderly, the lack of a functional broad and robust repertoire of responding B cells, either because they are under-developed or no longer functional, is likely the main driver of this failure of immune protection.
It seems counterintuitive then that the presence of pre-existing, humoral memory to influenza virus has been shown to prevent the induction of strong neutralizing responses to a related but distinct influenza strain under certain conditions (106, 107), a phenomenon known as “original antigenic sin”. In those situations, the presence of a broad repertoire of pre-existing, influenza-specific Bmem cells might lead to the activation of non-protective B cells over naïve, but potentially neutralizing B cells. Alternatively, the rapid capture of antigen may prevent strong immune response activation. While animal models show clear evidence for such a phenomenon, the extent to which such scenario may contribute to the lack of protection in humans remains unclear.
CONCLUSIONS
B cells are the drivers of immunity to influenza virus infection during both primary and repeat infections. Their responses differ with the stages of the infection and provide a) pre-existing natural and antigen-induced antibodies to blunt the initial infection; b) rapid antibody responses generated in EF responses from pre-existing high-affinity B cell clones that contribute to the early control of virus infection; and c) GC B cell responses that generate a broad repertoire of antibodies and Bmem cells in preparation of the next exposure to this virus. The repeated exposures to influenza virus continuously shape the repertoire of influenza-specific B cells that can continue this cycle of responses. Naturally-induced B cell responses, while not always successful in preventing influenza infection per se, are generally extremely robust, allowing humans to draw, if not win, the battle against this rapidly mutating and highly successful pathogen– by surviving it.
Acknowledgments
JHL is supported by NIH-T32 training grant HL007013. Recent work from this laboratory described in the review was supported by NIH/NIAID U19AI109962 and R01AI117890
List of Abbreviations
- APRIL
A proliferation-inducing ligand
- ASCs
Antibody-secreting cells
- BAFF
B cell activating factor
- DCs
Dendritic Cells
- EF
Extrafollicular
- GC
Germinal Center
- HEL
Hen-egg lysozyme
- KLH
Keyhole limpet hemocyanin
- medLN
Mediastinal Lymph Nodes
- sIgM
Secreted IgM
- SHM
Somatic hypermutation
- Tfh
CD4 T follicular helper cells
- VEGF-A
Vascular endothelial growth factor A
REFERENCES
- 1.Altman MO 2018. Antibody Immunodominance:The key to understanding influenza virus antigenic drift. Viral Immunol 2: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coughlan L, and Palese P 2018. Overcoming barriers in the path to a universal influenza virus vaccine. Cell Host Microbe 24: 18–24. [DOI] [PubMed] [Google Scholar]
- 3.Shen P, and Fillatreau S 2015. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol 15: 441–451. [DOI] [PubMed] [Google Scholar]
- 4.Baumgarth N, Herman O, Jager G, Brown L, Herzenberg L, and Herzenberg L 1999. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. J Exp Med 96: 2250–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi YS, and Baumgarth N 2008. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med 205: 3053–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savage HP, Yenson VM, Sawhney SS, Mousseau BJ, Lund FE, and Baumgarth N 2017. Blimp-1-dependent and -independent natural antibody production by B-1 and B-1-derived plasma cells. J Exp Med 214: 2777–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi YS, Dieter JA, Rothaeusler K, Luo Z, and Baumgarth N 2012. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol 42: 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, and Chen J 2000. B-1 and B-2 Cell–Derived Immunoglobulin M Antibodies Are Nonredundant Components of the Protective Response to Influenza Virus Infection. J Exp Med 192: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen TT, Graf BA, Randall TD, and Baumgarth N 2017. sIgM-FcmuR Interactions Regulate Early B Cell Activation and Plasma Cell Development after Influenza Virus Infection. J Immunol 199: 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waffarn EE, Hastey CJ, Dixit N, Soo Choi Y, Cherry S, Kalinke U, Simon SI, and Baumgarth N 2015. Infection-induced type I interferons activate CD11b on B-1 cells for subsequent lymph node accumulation. Nat Commun 6: 8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shulman M, Collins C, Pennell N, and Hozumi N 1987. Complement activation by IgM: evidence for the importance of the third constant domain of the mu heavy chain. Eur J Immunol 17: 549–554. [DOI] [PubMed] [Google Scholar]
- 12.Sorman A, Zhang L, Ding Z, and Heyman B 2014. How antibodies use complement to regulate antibody responses. Mol Immunol 61: 79–88. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Coligan JE, and Morse HC 3rd. 2016. Emerging Functions of Natural IgM and Its Fc Receptor FCMR in Immune Homeostasis. Front Immunol 7: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopf M, Brombacher F, and MF B 2002. Role of IgM antibodies versus B cells in influenza virus-specific immunity. Eur J Immunol 32: 2229–2236. [DOI] [PubMed] [Google Scholar]
- 15.Jayasekera JP, Moseman EA, and Carroll MC 2007. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol 81: 3487–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Garnier C, Filgueira L, Wikstrom M, Smith M, Thomas JA, Strickland DH, Holt PG, and Stumbles PA 2005. Anatomical Location Determines the Distribution and Function of Dendritic Cells and Other APCs in the Respiratory Tract. J Immunol 175: 1609–1618. [DOI] [PubMed] [Google Scholar]
- 17.Bessa J, Zabel F, Link A, Jegerlehner A, Hinton HJ, Schmitz N, Bauer M, Kundig TM, Saudan P, and Bachmann MF 2012. Low-affinity B cells transport viral particles from the lung to the spleen to initiate antibody responses. Proc Natl Acad Sci U S A 109: 20566–20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rayamajhi M, Delgado C, Condon TV, Riches DW, and Lenz LL 2012. Lung B cells promote early pathogen dissemination and hasten death from inhalation anthrax. Mucosal Immunol 5: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingulli E, Funatake C, Jacovetty EL, and Zanetti M 2008. Cutting Edge: Antigen Presentation to CD8 T Cells after Influenza A Virus Infection. J Immunol 182: 29–33. [DOI] [PubMed] [Google Scholar]
- 20.Kumar V, Scandella E, Danuser R, Onder L, Nitschke M, Fukui Y, Halin C, Ludewig B, and Stein JV 2010. Global lymphoid tissue remodeling during a viral infection is orchestrated by a B cell-lymphotoxin-dependent pathway. Blood 115: 4725–4733. [DOI] [PubMed] [Google Scholar]
- 21.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, and Randolph GJ 2006. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 24: 203–215. [DOI] [PubMed] [Google Scholar]
- 22.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, and Streilein JW 2004. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 113: 1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coro ES, Chang WLW, and Baumgarth N 2006. Type I IFN Receptor Signals Directly Stimulate Local B Cells Early following Influenza Virus Infection. J Immunol 176: 4343–4351. [DOI] [PubMed] [Google Scholar]
- 24.Sealy R, Surman S, Hurwitz J, and Coleclough C 2002. Antibody response to influenza infection of mice: different patterns for glycoprotein and nucleocapsid antigens. Immunology 108: 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sangster MY, Riberdy JM, Gonzalez M, Topham DJ, Baumgarth N, and Doherty PC 2003. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. J Exp Med 198: 1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLennan I, Toellner K, Cunningham A, Serre K, Sze D, Zúñiga E, Cook M, and Vinuesa C 2003. Extrafollicular antibody responses. Immunol Rev 194: 8–18. [DOI] [PubMed] [Google Scholar]
- 27.Kavaler J, Caton A, Staudt L, and Gerhard W 1991. A B cell population that dominates the primary response to influenza virus hemagglutinin does not participate in the memory response. Eur J Immunol 21: 2687–2695. [DOI] [PubMed] [Google Scholar]
- 28.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, and von Andrian UH 2007. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450: 110–114. [DOI] [PubMed] [Google Scholar]
- 29.Batista FD, and Harwood NE 2009. The who, how and where of antigen presentation to B cells. Nat Rev Immunol 9: 15–27. [DOI] [PubMed] [Google Scholar]
- 30.Reif K, Ekland E, Ohl L, Nakano H, Lipp M, Förster R, and Cyster J 2002. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature 416: 94–99. [DOI] [PubMed] [Google Scholar]
- 31.Pereira JP, Kelly LM, Xu Y, and Cyster JG 2009. EBI2 mediates B cell segregation between the outer and centre follicle. Nature 460: 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damdinsuren B, Zhang Y, Khalil A, Wood WH 3rd, Becker KG, Shlomchik MJ, and Sen R 2010. Single round of antigen receptor signaling programs naive B cells to receive T cell help. Immunity 32: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen H, van Ginkel F, Vu H, McGhee J, and Mestecky J 2001. Heterosubtypic immunity to influenza A virus infection requires B cells but not CD8+ cytotoxic T lymphocytes. J Infect Dis. 183: 368–376. [DOI] [PubMed] [Google Scholar]
- 34.Mozdzanowska K, Furchner M, Zharikova D, Feng J, and Gerhard W 2005. Roles of CD4+ T-cell-independent and -dependent antibody responses in the control of influenza virus infection: evidence for noncognate CD4+ T-cell activities that enhance the therapeutic activity of antiviral antibodies. J Virol 79: 5943–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H, Chaudhri VK, Wu Z, Biliouris K, Dienger-Stambaugh K, Rochman Y, and Singh H 2015. Regulation of bifurcating B cell trajectories by mutual antagonism between transcription factors IRF4 and IRF8. Nat Immunol 16: 1274–1281. [DOI] [PubMed] [Google Scholar]
- 36.Paus D, Phan TG, Chan TD, Gardam S, Basten A, and Brink R 2006. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med 203: 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, and Brink R 2006. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med 203: 2419–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krautler NJ, Suan D, Butt D, Bourne K, Hermes JR, Chan TD, Sundling C, Kaplan W, Schofield P, Jackson J, Basten A, Christ D, and Brink R 2017. Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J Exp Med 214: 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sze D, Toellner K, García de Vinuesa C, Taylor D, and MacLennan I 2000. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med 192: 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ochiai K, Maienschein-Cline M, Simonetti G, Chen J, Rosenthal R, Brink R, Chong AS, Klein U, Dinner AR, Singh H, and Sciammas R 2013. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity 38: 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cancro M, Wylie D, Gerhard W, and Klinman N 1979. Patterned acquisition of the antibody repertoire: diversity of the hemagglutinin-specific B-cell repertoire in neonatal BALB/c mice. Proc Natl Acad Sci U S A 76: 6577–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothaeusler K, and Baumgarth N 2010. B-cell fate decisions following influenza virus infection. Eur J Immunol 40: 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang KY, Li CK, Clutterbuck E, Chui C, Wilkinson T, Gilbert A, Oxford J, Lambkin-Williams R, Lin TY, McMichael AJ, and Xu XN 2014. Virus-specific antibody secreting cell, memory B-cell, and sero-antibody responses in the human influenza challenge model. J Infect Dis 209: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 44.Halliley JL, Kyu S, Kobie JJ, Walsh EE, Falsey AR, Randall TD, Treanor J, Feng C, Sanz I, and Lee FE 2010. Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine 28: 3582–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee FE, Halliley JL, Walsh EE, Moscatiello AP, Kmush BL, Falsey AR, Randall TD, Kaminiski DA, Miller RK, and Sanz I 2011. Circulating human antibody-secreting cells during vaccinations and respiratory viral infections are characterized by high specificity and lack of bystander effect. J Immunol 186: 5514–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, and Wilson PC 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453: 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toellner K, Gulbranson-Judge A, Taylor D, Sze D, and MacLennan I 1996. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J Exp Med 183: 2303–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parsa R, Lund H, Georgoudaki AM, Zhang XM, Ortlieb Guerreiro-Cacais A, Grommisch D, Warnecke A, Croxford AL, Jagodic M, Becher B, Karlsson MC, and Harris RA 2016. BAFF-secreting neutrophils drive plasma cell responses during emergency granulopoiesis. J Exp Med 213: 1537–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, Donze O, Frossard C, Chizzolini C, Favre C, Zubler R, Guyot JP, Schneider P, and Roosnek E 2008. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest 118: 2887–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, and Cerutti A 2011. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 13: 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Angeletti D, Gibbs JS, Angel M, Kosik I, Hickman HD, Frank GM, Das SR, Wheatley AK, Prabhakaran M, Leggat DJ, McDermott AB, and Yewdell JW 2017. Defining B cell immunodominance to viruses. Nat Immunol 18: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heesters BA, Chatterjee P, Kim YA, Gonzalez SF, Kuligowski MP, Kirchhausen T, and Carroll MC 2013. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity 38: 1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, and Förster R 2000. Follicular B Helper T Cells Express Cxc Chemokine Receptor 5, Localize to B Cell Follicles, and Support Immunoglobulin Production. J Exp Med 192: 1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith K, Light A, Nossal G, and Tarlinton D 1997. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO 16: 2996–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, and Nussenzweig MC 2010. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 143: 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shinnakasu R, Inoue T, Kometani K, Moriyama S, Adachi Y, Nakayama M, Takahashi Y, Fukuyama H, Okada T, and Kurosaki T 2016. Regulated selection of germinal-center cells into the memory B cell compartment. Nat Immunol 17: 861–869. [DOI] [PubMed] [Google Scholar]
- 57.Wols HAM, Underhill GH, Kansas GS, and Witte PL 2002. The Role of Bone Marrow-Derived Stromal Cells in the Maintenance of Plasma Cell Longevity. J Immunol 169: 4213–4221. [DOI] [PubMed] [Google Scholar]
- 58.McCarthy KR, Watanabe A, Kuraoka M, Do KT, McGee CE, Sempowski GD, Kepler TB, Schmidt AG, Kelsoe G, and Harrison SC 2018. Memory B Cells that Cross-React with Group 1 and Group 2 Influenza A Viruses Are Abundant in Adult Human Repertoires. Immunity 48: 174–184 e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacob J 1992. In situ studies of the primary immune response to (4-hydroxy-3- nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med 176: 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McHeyzer-Williams MG 1993. Antigen-driven B cell differentiation in vivo. J Exp Med 178: 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fonville JM, Wilks SH, James SL, Fox A, Ventresca M, Aban M, Xue L, Jones TC, Le NMH, Pham QT, Tran ND, Wong Y, Mosterin A, Katzelnick LC, Labonte D, Le TT, van der Net G, Skepner E, Russell CA, Kaplan TD, Rimmelzwaan GF, Masurel N, de Jong JC, Palache A, Beyer WEP, Le QM, Nguyen TH, Wertheim HFL, Hurt AC, Osterhaus A, Barr IG, Fouchier RAM, Horby PW, and Smith DJ 2014. Antibody landscapes after influenza virus infection or vaccination. Science 346: 996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrews SF, Huang Y, Kaur K, Popova LI, Ho IY, Pauli NT, Henry Dunand CJ, Taylor WM, Lim S, Huang M, Qu X, Lee JH, Salgado-Ferrer M, Krammer F, Palese P, Wrammert J, Ahmed R, and Wilson PC 2015. Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med 7: 316ra192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andrews SF, Kaur K, Pauli NT, Huang M, Huang Y, and Wilson PC 2015. High preexisting serological antibody levels correlate with diversification of the influenza vaccine response. J Virol 89: 3308–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu Y, Zhang Z, Sheehan J, Avnir Y, Ridenour C, Sachnik T, Sun J, Hossain MJ, Chen LM, Zhu Q, Donis RO, and Marasco WA 2016. A broadly neutralizing anti-influenza antibody reveals ongoing capacity of haemagglutinin-specific memory B cells to evolve. Nat Commun 7: 12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kavaler J, Caton A, Staudt L, Schwartz D, and Gerhard W 1990. A set of closely related antibodies dominates the primary antibody response to the antigenic site CB of the A/PR/8/34 influenza virus hemagglutinin. J Immunol 145: 2312–2321. [PubMed] [Google Scholar]
- 66.Roost H, Bachmann M, Haag A, Kalinke U, Pliska V, Hengartner H, and Zinkernagel R 1995. Early high-affinity neutralizing anti-viral IgG responses without further overall improvements of affinity. Proc Natl Acad Sci U S A 92: 1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, and Randall TD 2004. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 10: 927–934. [DOI] [PubMed] [Google Scholar]
- 68.Chvatchko Y 1996. Germinal Center Formation and Local Immunoglobulin E (IgE) Production in the Lung after an Airway Antigenic Challenge. J Exp Med 184: 2353–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tschernig T, and Pabst R 2000. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology 68: 1–8. [DOI] [PubMed] [Google Scholar]
- 70.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, and Lambrecht BN 2009. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med 206: 2339–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, and Randall TD 2011. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol 12: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones P, and Ada G 1986. Influenza virus-specific antibody-secreting cells in the murine lung during primary influenza virus infection. J Virol 60: 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolf AI, Mozdzanowska K, Quinn WJ 3rd, Metzgar M, Williams KL, Caton AJ, Meffre E, Bram RJ, Erickson LD, Allman D, Cancro MP, and Erikson J 2011. Protective antiviral antibody responses in a mouse model of influenza virus infection require TACI. J Clin Invest 121: 3954–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu X, Tsibane T, McGraw PA, House FS, Keefer CJ, Hicar MD, Tumpey TM, Pappas C, Perrone LA, Martinez O, Stevens J, Wilson IA, Aguilar PV, Altschuler EL, Basler CF, and Crowe JE Jr. 2008. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature 455: 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Landsverk OJ, Snir O, Casado RB, Richter L, Mold JE, Reu P, Horneland R, Paulsen V, Yaqub S, Aandahl EM, Oyen OM, Thorarensen HS, Salehpour M, Possnert G, Frisen J, Sollid LM, Baekkevold ES, and Jahnsen FL 2017. Antibody-secreting plasma cells persist for decades in human intestine. J Exp Med 214: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McHeyzer-Williams LJ, Milpied PJ, Okitsu SL, and McHeyzer-Williams MG 2015. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat Immunol 16: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Onodera T, Takahashi Y, Yokoi Y, Ato M, Kodama Y, Hachimura S, Kurosaki T, and Kobayashi K 2012. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc Natl Acad Sci U S A 109: 2485–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shenoy GN, Chatterjee P, Kaw S, Mukherjee S, Rathore DK, Bal V, Rath S, and George A 2012. Recruitment of memory B cells to lymph nodes remote from the site of immunization requires an inflammatory stimulus. J Immunol 189: 521–528. [DOI] [PubMed] [Google Scholar]
- 79.Koutsakos M, Wheatley A, Loh L, Clemens E, Sant S, Nüssing S, Fox A, Chung A, Laurie K, Hurt A, Rockman S, Lappas M, Loudovaris T, Mannering S, Westall G, Elliot M, Tangye S, Wakim L, Kent S, Nguyen T, and Kedzierska K 2018. Circulating T(FH) cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci Transl Med 14: 428. [DOI] [PubMed] [Google Scholar]
- 80.Wheatley AK, Kristensen AB, Lay WN, and Kent SJ 2016. HIV-dependent depletion of influenza-specific memory B cells impacts B cell responsiveness to seasonal influenza immunisation. Sci Rep 6: 26478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eichelberger M 1991. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J Exp Med 174: 875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Webby RJ, Andreansky S, Stambas J, Rehg JE, Webster RG, Doherty PC, and Turner SJ 2003. Protection and compensation in the influenza virus-specific CD8+ T cell response. Proc Natl Acad Sci U S A 100: 7235–7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mozdzanowska K, Maiese K, and Gerhard W 2000. Th Cell-Deficient Mice Control Influenza Virus Infection More Effectively Than Th- and B Cell-Deficient Mice: Evidence for a Th-Independent Contribution by B Cells to Virus Clearance. J Immunol 164: 2635–2643. [DOI] [PubMed] [Google Scholar]
- 84.Penaloza-MacMaster P, Barber D, Wherry E, Provine N, Teigler J, Parenteau L, Blackmore S, Borducchi E, Larocca R, Yates K, Shen H, Haining W, Sommerstein R, Pinschewer D, Ahmed R, and Barouch D 2015. Vaccine-elicited CD4 T cells induce immunopathology after chronic LCMV infection. Science. Science 16: 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barr TA, Brown S, Mastroeni P, and Gray D 2010. TLR and B cell receptor signals to B cells differentially program primary and memory Th1 responses to Salmonella enterica. J Immunol 185: 2783–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O’Connor RA, Anderton SM, Bar-Or A, Fillatreau S, and Gray D 2012. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209: 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Menard LC, Minns LA, Darche S, Mielcarz DW, Foureau DM, Roos D, Dzierszinski F, Kasper LH, and Buzoni-Gatel D 2007. B Cells Amplify IFN- Production By T Cells via a TNF- -Mediated Mechanism. J Immunol 179: 4857–4866. [DOI] [PubMed] [Google Scholar]
- 88.Karnowski A, Chevrier S, Belz GT, Mount A, Emslie D, D’Costa K, Tarlinton DM, Kallies A, and Corcoran LM 2012. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J Exp Med 209: 2049–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, and Hartmann G 2002. Quantitative Expression of Toll-Like Receptor 1–10 mRNA in Cellular Subsets of Human Peripheral Blood Mononuclear Cells and Sensitivity to CpG Oligodeoxynucleotides. J Immunol 168: 4531–4537. [DOI] [PubMed] [Google Scholar]
- 90.Masumi A, Ito M, Mochida K, Hamaguchi I, Mizukami T, Momose H, Kuramitsu M, Tsuruhara M, Takizawa K, Kato A, and Yamaguchi K 2010. Enhanced RIG-I expression is mediated by interferon regulatory factor-2 in peripheral blood B cells from hepatitis C virus-infected patients. Biochem Biophys Res Commun 391: 1623–1628. [DOI] [PubMed] [Google Scholar]
- 91.Petterson T, Jendholm J, Mansson A, Bjartell A, Riesbeck K, and Cardell LO 2011. Effects of NOD-like receptors in human B lymphocytes and crosstalk between NOD1/NOD2 and Toll-like receptors. J Leukoc Biol 89: 177–187. [DOI] [PubMed] [Google Scholar]
- 92.Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, and Marsland BJ 2007. TLR Signaling Fine-Tunes Anti-Influenza B Cell Responses without Regulating Effector T Cell Responses. J Immunol 178: 2182–2191. [DOI] [PubMed] [Google Scholar]
- 93.Gram AM, Sun C, Landman SL, Oosenbrug T, Koppejan HJ, Kwakkenbos MJ, Hoeben RC, Paludan SR, and Ressing ME 2017. Human B cells fail to secrete type I interferons upon cytoplasmic DNA exposure. Mol Immunol 91: 225–237. [DOI] [PubMed] [Google Scholar]
- 94.Ali MF, Dasari H, Van Keulen VP, and Carmona EM 2017. Canonical Stimulation of the NLRP3 Inflammasome by Fungal Antigens Links Innate and Adaptive B-Lymphocyte Responses by Modulating IL-1beta and IgM Production. Front Immunol 8: 1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ogura Y, Sutterwala FS, and Flavell RA 2006. The inflammasome: first line of the immune response to cell stress. Cell 126: 659–662. [DOI] [PubMed] [Google Scholar]
- 96.Schmitz N, Kurrer M, Bachmann MF, and Kopf M 2005. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol 79: 6441–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arimori Y, Nakamura R, Yamada H, Shibata K, Maeda N, Kase T, and Yoshikai Y 2013. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antiviral Res 99: 230–237. [DOI] [PubMed] [Google Scholar]
- 98.Rosser EC, and Mauri C 2015. Regulatory B cells: origin, phenotype, and function. Immunity 42: 607–612. [DOI] [PubMed] [Google Scholar]
- 99.Richards KA, Topham D, Chaves FA, and Sant AJ 2010. Cutting edge: CD4 T cells generated from encounter with seasonal influenza viruses and vaccines have broad protein specificity and can directly recognize naturally generated epitopes derived from the live pandemic H1N1 virus. J Immunol 185: 4998–5002. [DOI] [PubMed] [Google Scholar]
- 100.Yewdell JW, Bennink JR, Smith GL, and Moss B 1985. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A 82: 1785–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leon PE, He W, Mullarkey CE, Bailey MJ, Miller MS, Krammer F, Palese P, and Tan GS 2016. Optimal activation of Fc-mediated effector functions by influenza virus hemagglutinin antibodies requires two points of contact. Proc Natl Acad Sci U S A 113: E5944–E5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vanderven HA, Jegaskanda S, Wheatley AK, and Kent SJ 2017. Antibody-dependent cellular cytotoxicity and influenza virus. Curr Opin Virol 22: 89–96. [DOI] [PubMed] [Google Scholar]
- 103.DiLillo DJ, Palese P, Wilson PC, and Ravetch JV 2016. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest 126: 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.DiLillo DJ, Tan GS, Palese P, and Ravetch JV 2014. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med 20: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ye ZW, Yuan S, Poon KM, Wen L, Yang D, Sun Z, Li C, Hu M, Shuai H, Zhou J, Zhang MY, Zheng BJ, Chu H, and Yuen KY 2017. Antibody-Dependent Cell-Mediated Cytotoxicity Epitopes on the Hemagglutinin Head Region of Pandemic H1N1 Influenza Virus Play Detrimental Roles in H1N1-Infected Mice. Front Immunol 8: 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Justewicz D, Morin M, Robinson H, and Webster R 1995. Antibody-forming cell response to virus challenge in mice immunized with DNA encoding the influenza virus hemagglutinin. J Virol 69: 7712–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim JH, Skountzou I, Compans R, and Jacob J 2009. Original antigenic sin responses to influenza viruses. J Immunol 183: 3294–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]