Abstract
Activation of the endocannabinoid system modulate dopaminergic pathways that are involved in the effects of psychostimulants including amphetamine, cocaine, nicotine and other drugs of abuse. Genetic deletion or pharmacological activation of CB2 cannabinoid receptor is involved in the modulation of the effects of psychostimulants and their rewarding properties. Here we report on the behavioral effects of psychostimulants in DAT-Cnr2 conditional knockout (cKO) mice with selective deletion of type 2 cannabinoid receptors in dopamine neurons. There was enhanced psychostimulant induced hyperactivity in DAT-Cnr2 cKO mice, but the psychostimulant-induced sensitization was absent in DAT-Cnr2 cKO compared to the WT mice. Intriguingly lower doses of amphetamine reduced locomotor activity of the DAT-Cnr2 cKO mice. While cocaine, amphetamine and methamphetamine produced robust conditioned place preference (CPP) in both DAT-Cnr2 cKO and WT mice, nicotine at the dose used induced CPP only in the WT but not in the DAT-Cn2 cKO mice. However pre-treatment with the CB2R selective agonist JWH133, blocked cocaine and nicotine induced CPP in the WT mice. The deletion of CB2Rs in dopamine neurons modified the levels of tyrosine hydroxylase, and reduced the expression of dopamine transporter gene expression in DAT-Cnr2 cKO midbrain region. Taken together, our data suggest that CB2Rs play a role in the modulation of dopamine-related effects of psychostimulants and could be exploited as therapeutic target in psychostimulant addiction and other psychiatric disorders associated with dopamine dysregulation.
Keywords: CB2 cannabinoid receptors, mice, amphetamine, cocaine, nicotine, conditioned place preference
1. Introduction
There is increasing and compelling evidence that the endocannabinoid system (ECS) plays an important role in the reinforcing properties of drugs of abuse and in the control of rewarding behaviors, predominantly through neuromodulatory function in the central nervous system (CNS) [1–5]. The activation of the ECS mediates retrograde signaling in neuronal tissues that are involved with the regulation of synaptic transmission to suppress neurotransmitter release by presynaptic cannabinoid receptors (CBRs). This modulatory action on synaptic transmission has functional implications and interactions with the effects of abused substances. Furthermore, activation of the ECS modulates dopaminergic pathways that are involved in the effects of psychostimulants including amphetamine, cocaine, nicotine and other drugs of abuse. Therefore, the ECS is an important component of the reward mechanisms in the brain [6]. The ECS consists of two receptor subtypes, CB1 and CB2 cannabinoid receptors (CB1Rs, CB2Rs), as well as the endogenous ligands, the endocannabinoids, and the enzymes responsible for their biosynthesis and degradation [7]. Dopamine is a monoamine neurotransmitter and it is involved in several physiological and behavioral processes including cognition, locomotion, mood, motivation, and reward. Dopamine is produced mainly in the dopaminergic neurons in the ventral tegmental area (VTA) and the substantia nigra of the midbrain and in the arcuate nucleus of the hypothalamus. Dopaminergic neurons projecting from the VTA to the nucleus accumbens constitute the main components of the mesolimbic system, and are involved in neurobehavioral effects of addictive drugs [8–10]. Psychostimulants produce their effects mainly by enhancing dopamine transmission in the brain, especially in limbic areas such as the nucleus accumbens in humans and in rodents [11–13]. Thus, dopamine plays a key role in the behavioral and reinforcing effects of psychostimulants. Both CB1Rs and CB2Rs are coupled to Gi/o protein, which negatively modulates adenylyl cyclase. The CB2R has 44% amino acid identity with the CB1R [14]. CBRs are heterogeneously distributed in motor, limbic and cognitive regions of the brain [15, 16]. CB1Rs, the most abundant G-protein coupled receptors in mammalian CNS, are abundantly expressed mainly at pre- and some postsynaptic sites [17], and have been implicated in various aspects of the rewarding properties of drugs of abuse [4]. Although initial studies were not able to detect CB2R expression in the brain [14, 18], compelling evidence demonstrates CB2Rs expression in the CNS with their presence detected on microglia and neurons [15, 19–24]. CB2Rs expression has been found in several brain areas such as: the hippocampus, the striatum and the brain stem [19, 22]. They are expressed in postsynaptic somatodendritic area of the neuron and on glial cells of the brain at much lower levels than the CB1Rs [21]. The role of CB1Rs on the effects of psychostimulants has been fairly well studied and addressed, but the role of CB2Rs have received little attention. However, we and others have demonstrated the neuronal expression and reported that CB2Rs are involved in the effects of drug abuse and synaptic plasticity [23–25]. Furthermore, CB2Rs have been linked to cocaine self-administration and changes in extracellular dopamine (DA) levels in the nucleus accumbens in mice [25]. Research indicates that the ECS seems to be involved in some properties of psychostimulants including cocaine, amphetamine, and their derivatives methamphetamine and 3, 4-methylenedioxymethamphetamine (MDMA). Similarly, CB2R is involved in the rewarding properties of other psychostimulants. For example, a study demonstrated that systemic administration of the CB2R agonist O-1966 inhibited nicotine-induced conditioned place preference (CPP) in WT mice whereas CB2R knockout mice did not display nicotine CPP [26].
The objective of this work was to characterize the role of dopaminergic CB2Rs in the modulation of psychomotor behaviors and in the rewarding properties of psychostimulants. To achieve this, we evaluated the acute locomotor responses and the sensitization to motor responses induced by cocaine, nicotine, methamphetamine, and d-amphetamine in DAT-Cnr2 conditional knock-out (cKO) mice. The underlining molecular impact of the endocannabinoid/CB2R system following deletion of CB2Rs in dopamine neurons was determined by tyrosine hydroxylase (TH) immunoblotting, dopamine transporter (DAT) gene expression, and the anatomical and structural integrity of dopaminergic neurons in the midbrain of DAT-Cnr2 cKO, and compared to the WT type mice.
2. Materials and methods
2.1. Animals
The experiments were performed using DAT-Cnr2 cKO mice and C57BL/J6 mice as the wild type (WT) controls. We used the Cre-lox technology for the generation of DAT-Cnr2 cKO mice. Briefly, the strategy crossed the Cnr2-floxed mice with the DAT-Cre mice. The DAT-Cre mice expressed the Cre- recombinase enzyme under DAT promoter and it is capable of ablate Cnr2 gene from the midbrain DA neurons. Genotyping and RNAscope in situ hybridization confirmed the cell selective deletion of CB2Rs in dopamine cells in the homozygous, but not in the heterozygous and wild type mice [24]. The C57BL/J6 mice is the genetic background of the DAT-Cnr2 cKO mice and we demonstrate that the performances of the genotypes: CB2R-floxed, DAT-Cre and C57BL/6J were not significantly different in motor function and in other tests. This is the reason for using the C57BL/6J as the WT control in our studies [24]. The experiments were performed in adult male mice (20–30 g body weight). The animals were bred in the mouse laboratory at William Paterson University of New Jersey. The animals were maintained under controlled room temperature (25±2°C) and light-dark (12:12 hour) conditions with free access to food and water. The experimental procedures followed the Guide for the Care and Use of Laboratory Animals and were approved by William Paterson University animal care and use committee (IACUC).
2.2. Drugs
(−) cocaine hydrochloride (cocaine), (+)-amphetamine sulphate (amphetamine), methamphetamine and (−) nicotine hydrogen tartrate salt (nicotine) were dissolved in 0.9% saline (0.9% NaCl). The CB2R agonist JHW133 was dissolved in a vehicle composed of a mixture of Tween, DMSO, and saline solution (1:2:7). All drugs were purchased from Sigma-Aldrich Chem. Co. (St. Louis, Mo, USA). Drugs were administered into the peritoneum (i.p.) at a volume of 0.01 ml/g body weight. The vehicle was given to the control animals in the same volume.
2.3. Behavioral experiments
The behavioral tests were conducted in a room under dim light during the dark phase of the cycle. Mice were taken into the behavioral room for approximately 45 minutes for them to habituate before the start of the experiments. Mice were gently handled for a week before the experiments to reduce anxiety. The apparatus were cleaned with 70% alcohol solution between subjects.
2.3.1. Apparatus
The effects of psychostimulants on spontaneous locomotor activity was evaluated by placing the mouse into an infrared photobeam-controlled open-field test chamber (ENV −510: MED Associates Inc., St. Albans, VT, USA). The role of CB2Rs in the rewarding properties of the psychostimulants was investigated using the CPP of the DAT-Cnr2 cKO and wild type mice in a two chamber paradigm. The CPP apparatus consisted of a two-compartment (13 cm width x 24 cm length x15 cm depth) Plexiglas chamber insert (ENV-512). The floor for compartment-1 had parallel rods (3-mm radius, 8 mm center to center spacing) with black cardboard paper covering the outside. Compartment-2 had a stainless steel wire mesh (6X6) floor with white cardboard paper covering the outside of the walls. A removable Plexiglas wall divided both sides for pre- and post-conditioning test sessions, and a 5-cm opening in the center wall that allowed access to both compartments. During the conditioning sessions, the opening was closed to restrict animals to a single compartment. Time spent in each compartment was recorded by using the computer controlled animal activity monitoring system.
2.3.2. Conditioned Place Preference (CPP) Procedure
The CPP protocol involved three phases over a period of 8 days (pre-conditioning, conditioning and post-conditioning). Each phase was separated by one day. During the pre-conditioning session mice were placed individually in the CPP box for 15 minutes. The time spent in each of the compartments was recorded using the activity monitoring system. Mice were then removed and placed in their home cages. During the conditioning phase, mice were randomly assigned to one of two groups: vehicle or experimental group (n=10/group). Mice were injected with saline by their weight and confined to compartment 2 of the CPP apparatus for 15 minutes. Four hours after the first session, mice designated as control received saline injections, and were placed in compartment 1 for 15 minutes. The other half of the animals received the drug injection and were also confined to compartment 1 for 15 minutes. This was repeated for 4 consecutive days. During the postconditioning session, mice were placed in the CPP box for 15 minutes. The activity monitoring system recorded time spent in each of the compartments. The CPP score was determined by the time spent in the drug-paired compartment minus the time spent in the saline-paired compartment during the CPP test.
2.3.3. Acute Spontaneous locomotor activity
Acute spontaneous locomotor activities were evaluated in the open-field test boxes. Mice were individually placed in the center of the box (43.2×43.2×30.5 cm) and allowed to freely explore the chamber for 20 minutes, except for the amphetamine dose-response curve in which the time was 30 minutes for each dose. The spontaneous locomotor activity was monitored using 16 evenly spaced infrared transmitters and receivers positioned around the periphery of the four sides of the chambers (Med Associates Inc, USA). The test boxes were connected to a computer, and total distances were obtained before and after drug or saline administration.
2.3.4. Sensitization to motor response
Mice from both genotypes were selected and divided in groups receiving saline or drug i.p injection once daily for 6 consecutive days at 10 am. The evaluation of motor sensitization was carried out by measuring the distance traveled by the animals in the open-field test for 20 minutes, under baseline conditions, and 15 minutes after drug/saline administration on day 1. After the last drug/saline administration on day 6, mice remained abstinent for one day (day 7). On day 8, mice were challenged with the drug or saline and 15 minutes after injection and the locomotor activity was measured.
2.3.5. Temperature measurement
Core body temperature was recorded using a thermometer equipped with a mouse rectal probe. The animals were gently restrained and habituated to the procedure before the experiment.
2.4. Biochemical and molecular experiments
2.4.1. Tissue sample preparation
Mice were decapitated, and their brains rapidly removed from the skull. Their brains were quickly frozen in liquid nitrogen to facilitate dissection. The midbrain region was quickly dissected and lysed in 300 μg of RIPA buffer, homogenized with ultrasonic homogenizer, and incubated for 20 minutes at 4°C. The homogenates were centrifuged at 12000 RPM for 15 minutes at room temperature. Aliquots of the resulting supernatants were taken, and after the protein concentration was determined, they were frozen and stored at −80°C until use for immunoblotting experiments.
2.4.2. Immunoblotting
Western blotting and immunodetection protocol was used to determine tyrosine hydroxylase (TH) levels. Briefly, 20 μg of protein was mixed with RIPA buffer and Laemli solution. The samples were boiled at 100 °C for 5 minutes, and were loaded onto a Mini-protean® TGX Stain free™ Precast Gel (BIO-RAD) for 40 minutes at 200 mV. Proteins were then transferred to membrane Mini format, 0.2 um PVDF Trans-Blot® Turbo™ (BIO-RAD). Membranes were blocked for 90 minutes with blocking buffer. After blocking, the membranes were incubated overnight at room temperature with a primary mouse anti-TH monoclonal antibody (1: 1000; Abcam). After washing, membranes were incubated with anti-rabbit IgM secondary antibody AP (1: 10000) for 2 h. Mouse monoclonal anti β-actin antibody (1: 1000: Abcam) served as a control for loading uniformity for each lane and was used to normalize differences in TH expression and protein content. Immunoreactive protein was visualized using a detection kit (NBT/BCIP in carbonate buffer). After exposure, the membranes were scanned.
2.4.3. Quantitative reverse-transcription (qRT) PCR for mRNA quantification
qRT-PCR was used to estimate dopamine transporter (DAT) mRNA in mouse midbrain. Two pairs of intron-spanning PCR primers with a Tm of 56–60°C were designed and one of them was selected for qRT-PCR based on a single peak in melting curve, an amplification coefficient (AC) of “2” in a series of dilutions assay and/or a lower Ct value.
2.4.4. Sampling of ventral tegmental area (VTA) tissue from mice midbrain
Adult mice were killed by rapid decapitation for brain collection. Midbrains were promptly dissected in metal mouse matrix (ZIVIC, PA, USA) from the brains, and VTA was sliced out of the dissected coronal sections and transferred to pre-chilled tubes for RNA extraction.
2.4.5. RNA extraction
Total RNA of midbrain tissue (N = 5 per group), was isolated by using 200 μl/5.0 mg tissue of TRIzol reagents (Ambion, MA, USA), following the manufacturer’s protocol, and reconstituted in 20 μl RNase-free water. RNA concentration was estimated with NanoDrop Lit (Thermo Fisher Scientific). Approximately 10 μg RNA was extracted from every 5.0 mg VTA tissue. RNA samples were stored at −80°C till cDNA synthesis.
2.4.6. cDNA synthesis
One hundred ng RNA was reverse-transcribed into cDNA by using Verso cDNA synthesis kit (ThermoFisher Scientific) with oligo dT primers following the manufacturer’s protocol. cDNA was diluted by 5 folds with DNase-free water prior to quantification by qRT-PCR or before being stored at −20°C.
2.4.7. qRT-PCR analysis of relative mRNA levels
cDNA samples were amplified in triplicates or quadruplicates by incubation in the Bio-Rad CFX Connect real-time system (Bio-rad, CA, USA). The amplification condition was 95°C for 5 minutes, then for 49 cycles of 95°C for 15 sec, 55°C for 20 sec and 72°C for 30 sec using SsoAdvanced Universal SYBR green supermix (Bio-rad, CA, USA) in a final volume of 12.5 μl, containing 1 μl of cDNA and a final concentration of 0.5 μM for forward and reverse primers. Amplification coefficient (AC) was calculated from the Ct slope of the standard curve using the following formula: AC=10−1/slope. 1:2 serial dilutions of the starting template were prepared to generate eight points, and the Ct vs log cDNA concentration plot was constructed to calculate the Ct slope. This AC value was used in data analysis for relative mRNA levels, which were normalized with reference gene.
2.4.8. Immunohistochemical staining for TH- positive neurons
DAT-Cnr2 cKO and WT mice (N=5 mice per group) were intracardially perfused with 0.9% saline followed by 4% paraformaldehyde (PFA). The brains were extracted and put into a container filled with PFA overnight. The next day the brains were changed into a 30% sucrose solution and stored at −80 °C until use. Coronal sections (μm) containing the midbrain were obtained. The slices were incubated overnight at 4°C with antibody against TH polyclonal antibody (Abcam, Cambridge, MA, USA). After washing, sections were incubated with fluorescence secondary antibody. The sections were mounted on slides and examined using confocal microscopy.
2.5. Statistical analysis
Data are presented as mean ± SEM. Sigma Plot 12.0 statistical program was used. We verified the normality test (Shapiro-Wilk) before completing all of the tests. The statistical analysis were performed by the one-way and two-way analysis of variance (ANOVA). Post hoc comparisons of means was carried out with Dunnett’s or Tukey’s test for multiple comparisons when appropriate. The confidence limit of p < 0.05 was considered statistically significant. One of the factors of the ANOVA was the genotype (DAT-Cnr2 or WT mice) and the other factor was treatment groups (saline or drug). We used One-way ANOVA together with post hoc Tukey test for the time spent in the drug-paired compartment in the CPP model. For the psychostimulant-induced locomotor sensitization experiments, in each of the three phases of the test [i.e., baseline, acute response to drug on day 1, and the response after repeated drug administration on day 8] were analyzed using ANOVA with repeated measures followed by Dunnett’s post hoc comparisons. The effect of the pre-treatment with the CB2R agonist (JWH133) was evaluated in the cocaine- and nicotine-induce CPP only in the WT C57BL/6J control mice. The Pearson correlation coefficient was used for DAT mRNA level linear correlation. A p<0.05 was considered statistically significant.
3. Results
3.1. Deletion of CB2R in DAT-Cnr2 cKO mice enhances amphetamine- and cocaine-induced hyperlocomotor activity
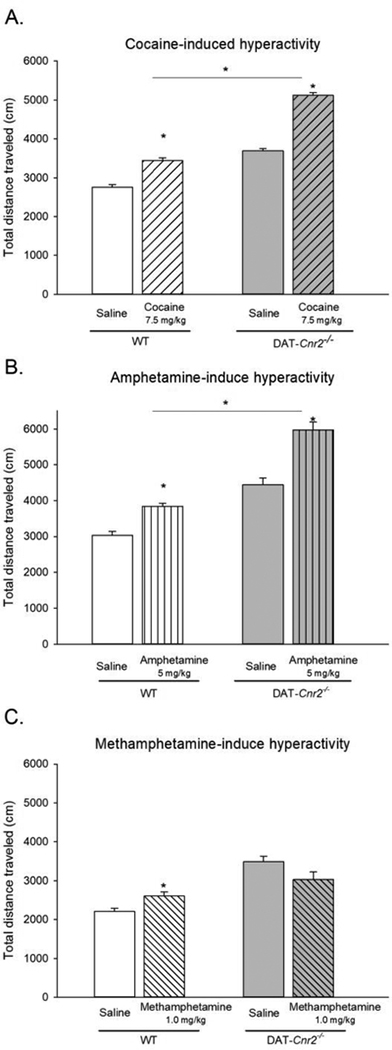
The effects of selective deletion of CB2Rs in DAT-Cnr2 cKO mice on the locomotor effects of selected psychostimulants were evaluated. DAT-Cnr2 cKO and WT mice were treated acutely with the selected doses of cocaine (7.5 mg/kg), amphetamine (5.0 mg/kg) or methamphetamine (1.0 mg/kg) or vehicle, and the distance traveled in 20 minutes in the open field test were recorded and analyzed 15 minutes after i.p. injection (Fig. 1). In general, DAT-Cnr2 cKO mice with selective deletion of CB2Rs exhibit a hyperactive phenotype characterized by hyper-locomotor activity compared with the WT mice as we previously reported [24]. Cocaine (7.5 mg/kg) increased the distance traveled in both WT and DAT-Cnr2 cKO mice (Fig. 1A). Two-way ANOVA showed genotypic effect [F(1,36) = 318.09, p<0.001], treatment effect [F(1,36) = 219.31, p<0.001], and interaction effect was also significant [F(1,36) = 27.72, p<0.001]. Post-hoc test showed that 7.5 mg/kg cocaine increased the distance traveled relative to vehicle in both and between genotypes (p<0.05). Amphetamine (5.0 mg/kg) significantly increased the distance traveled in both WT and DAT-Cnr2 cKO mice (Fig. 1B). Two-way ANOVA showed genotypic effect [F(1,36) = 209.73, p< <0.001], treatment effect [F(1,36) = 89.76, p<0.001], and interaction effect was significant [F(1,36) = 19.920, p<0.001]. Post hoc test showed that amphetamine (5.0 mg/kg) increased the distance traveled relative to vehicle in both, and between genotypes (p<0.05). Methamphetamine (1.0 mg/kg) increased the distance traveled in WT, but not in the DAT-Cnr2 cKO mice (Fig. 1C). Two-way ANOVA showed genotypic effect [F(1,36) = 35.02, p< <0.001] but not significant for treatment effect [F(1,36) = 0.008, p = 0.926]. However, the interaction effect was significant [F(1,36) = 7.87, p=0.008]. Post hoc test showed that 1.0 mg/kg methamphetamine increased the distance traveled relative to vehicle in WT mice (p<0.05). The distance traveled between vehicle treated mice was different in both genotypes (p<0.05). In WT mice, all treatments - cocaine, amphetamine and methamphetamine - produced significant increases in the distances traveled. However, in the DAT-Cnr2 cKO mice, the increase in the distance traveled induced by cocaine and amphetamine (but not methamphetamine) was higher than in the WT mice. This effect was observed in addition to the hyper-locomotor characteristic of the DAT-Cnr2 cKO mice as we previously reported [24].
Fig. 1.
Acute effects of selected doses of psychostimulants, cocaine, 7.5 mg/kg (A), amphetamine 5.0 mg/kg (B) and methamphetamine 1.0 mg/kg (C) on locomotor activity in DAT-Cnr2 cKO and WT mice. These acute studies were performed 15 minutes after drug/saline i.p. injection as measured by the total distances traveled in 20 minutes in the open-field test. The white bars represent the WT and the grey bars represent DAT-Cnr2 cKO mice. There was significant increase in locomotor activities with the same doses of cocaine and amphetamine but not with methamphetamine in DAT-Cnr2 cKO compared to WT mice. Data are expressed as mean ± SEM (n = 10 mice per group). Two-way ANOVA followed by Dunnett’s post hoc test *p < 0.05.
3.2. Biphasic effects induced by amphetamine on locomotion in DAT-Cnr2 cKO mice.
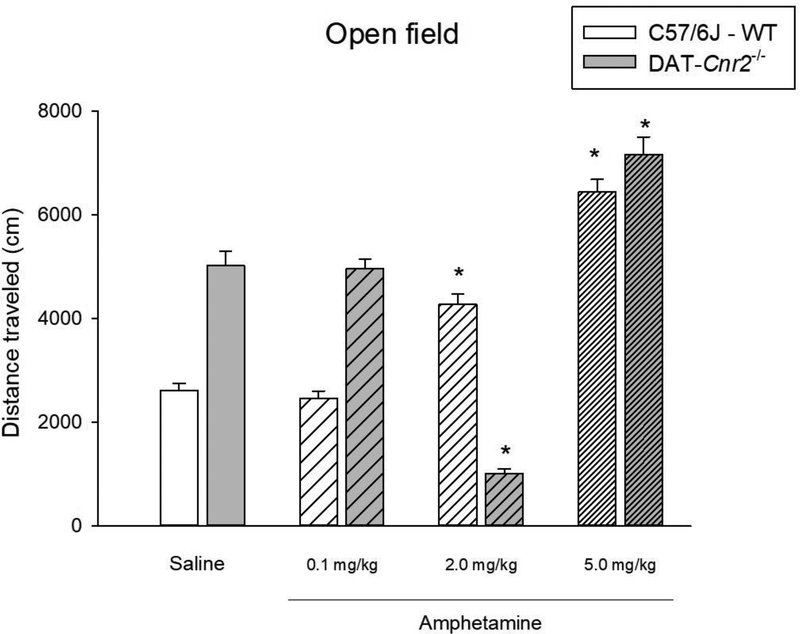
The effects of deletion of CB2Rs in DAT-Cnr2 cKO mice on the locomotor activity of three selected doses of amphetamine were evaluated to test the hypothesis that the DAT-Cnr2 cKO mice can be used as a mouse model of ADHD. The distance traveled during a 30 minute period, 15 minutes after the injection of the three selected doses of amphetamine (0.1, 2.0 and 5.0 mg/kg) in DAT-Cnr2 cKO and WT mice is shown in Fig. 2. Two-way ANOVA showed genotypic effect [F(1,72) = 14.76, p< <0.001], treatment effect [F(3,72) = 136.31, p<0.001] and interaction effect was also significant [F(3,72) = 77.95, p<0.001]. Dunnett’s post hoc test showed that the low dose of 0.1 mg/kg had no significant effect on the distance traveled in both genotypes. The 2.0 mg/kg dose of amphetamine significantly increased the distance traveled in WT animals (p<0.05), but not in DAT-Cnr2 cKO mice that was significantly reduced (p<0.05). However, the 5.0 mg/kg dose of amphetamine significantly increased the distance traveled in both genotypes (p<0.05).
Fig. 2.
Biphasic effects of amphetamine on locomotor activity in DAT-Cnr2 cKO mice. Distance traveled in 30 min in the open field after administration of amphetamine (0.1 – 5.0 mg/kg) or saline to DAT-Cnr2 cKO and WT mice. The white bars represent the WT and the grey bars represent DAT-Cnr2 cKO mice. Data are expressed as mean ± SEM (n = 10 mice per group). Two way ANOVA followed by Dunnett’s post hoc test *p < 0.05.
3.3. Effects of a low and high dose of cocaine on locomotor sensitization in DAT-Cnr2 cKO mice
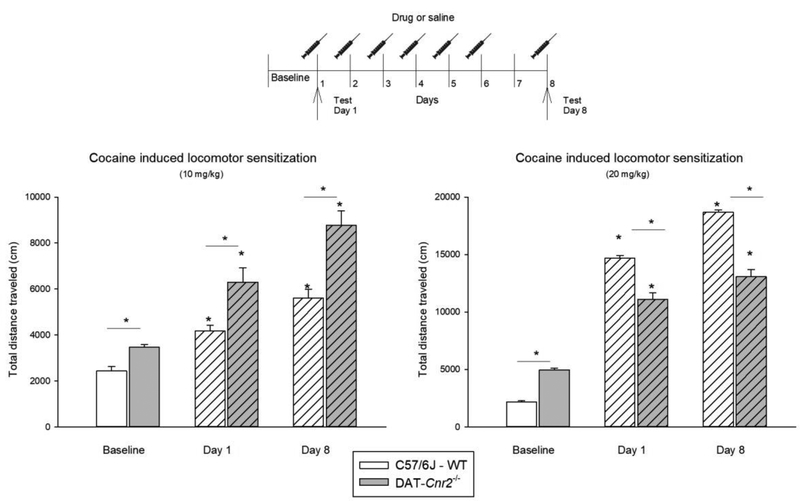
A previous report demonstrated that there was decreased cocaine motor sensitization in mice overexpressing CB2Rs [27]. The sensitization of the locomotor effects of cocaine were investigated in DAT-Cnr2 cKO mice. (Fig. 3). DAT-Cnr2 cKO and WT mice were subjected to a locomotor cocaine sensitization protocol with two doses of cocaine (10 mg/kg and 20 mg/kg). There was a significant genotype × time interaction for total distance traveled after 10 mg/kg cocaine injection on day 1, and after mice had received repeated cocaine treatment on day 8 [F(2,18) = 4.19, P<0.032]. Post hoc analysis showed that the distance traveled by DAT-Cnr2 cKO and WT mice following the baseline, acute administration on day 1, and after repeated administration on day 8 were significantly different. There was also a significant genotype × time interaction for total distance traveled after 20 mg/kg cocaine injection on day 1, and after mice had received repeated cocaine treatment on day 8 [F(2,18) = 51.52, P<0.001]. Post hoc analysis showed that the distance travel by DAT-Cnr2 cKO and WT mice following the baseline, acute administration on day 1, and after repeated administration on day 8 were significantly different. The sensitization protocol produced a dose-related enhancement in cocaine-induced motor activity in WT, but not in the DAT-Cnr2 cKO mice. DAT-Cnr2 cKO mice showed significantly increased sensitization to cocaine motor effects after the 10 mg/kg dose when compared to WT mice. However, after the 20 mg/kg dose, DAT-Cnr2 cKO mice showed less sensitization than the WT mice treated with the same dose of cocaine (Fig. 3). The results with cocaine indicate that the sensitivity of the DAT-Cnr2 cKO is a useful model in dissecting and identifying distinct modes of sensitization of psychostimulant drug action.
Fig. 3.
Locomotor-induced sensitization by cocaine (10 and 20 mg/kg) in DAT-Cnr2 cKO and WT mice. Mice received one cocaine injection (10 or 20 mg/kg) daily for 6 consecutive days and the locomotor activity was measured for 20 minutes before injection as the baseline, 15 minutes after administration on day 1 and on day 8 (no injection on day 7). The white bars represent the WT and the grey bars represent DAT-Cnr2 cKO mice. Data are expressed as mean ± SEM (n = 10 mice per group). Two way ANOVA followed by Dunnett’s post hoc test *p < 0.05.
3.4. Locomotor sensitization induced by amphetamine is absent in DAT-Cnr2 cKO mice
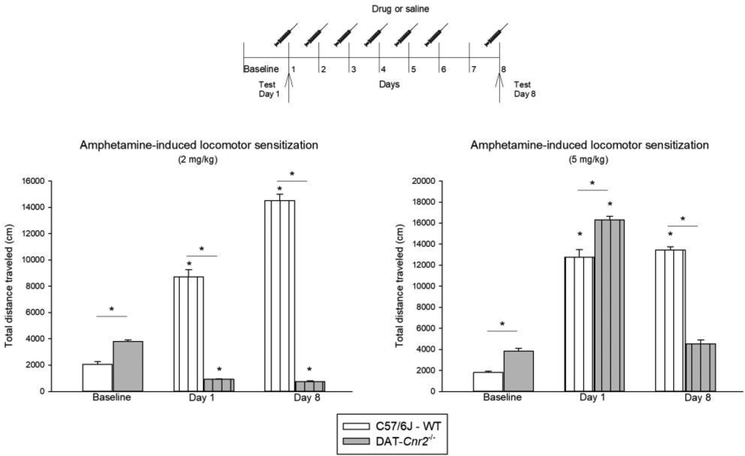
Locomotor sensitization induced by two doses of amphetamine (2.0 and 5.0 mg/kg) were evaluated in DAT-Cnr2 cKO and WT mice (Fig. 4). Two-way ANOVA showed significant interaction between genotype and time for the distance traveled after 2 mg/kg of amphetamine [F(2,18) = 456.26, P<0.001]. Post hoc analysis revealed that the baseline locomotor activity, after acute administration on day 1, and after repeated administration on day 8 of amphetamine were significantly different between DAT-Cnr2 cKO and WT mice. There was significant genotype × time interaction for total distance traveled after 5.0 mg/kg amphetamine injection on day 1, and after mice had received repeated amphetamine treatment on day 8 [F(2,18) = 39.53, P<0.001]. Post hoc analysis showed that the distance traveled by WT mice was different on day 1 and day 8 in comparison with the baseline (p<0.05). Whereas in the DAT-Cnr2 cKO mice, only the acute administration produced an increase in locomotor activity in comparison with the baseline. The difference is not significant on day 8 versus baseline. In the locomotor sensitization paradigm, amphetamine doses of 2.0 and 5.0 mg/kg induced dose-dependent sensitization in WT, but not in DAT-Cnr2 cKO mice.
Fig. 4.
Locomotor-induced sensitization by amphetamine (2 and 5 mg/kg) in DAT-Cnr2 cKO and WT mice. Mice received one amphetamine injection (2 or 5 mg/kg) daily for 6 consecutive days and the locomotor activity was measured for 20 minutes before injection as the baseline, 15 minutes after administration on day 1 and on day 8 (no injection on day 7). The white bars represent the WT and the grey bars represent DAT-Cnr2 cKO mice. Data are expressed as mean ± SEM (n = 10 mice per group). Two way ANOVA followed by Dunnett’s post hoc test *p < 0.05.
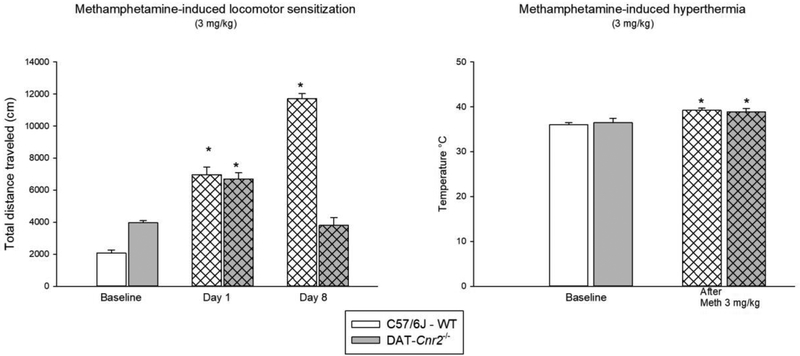
3.5. Methamphetamine-induced motor sensitization is absent in DAT-Cnr2 cKO mice, but methamphetamine-induced hyperthermia is present
We determined and compared methamphetamine induced behavioral sensitization and hyperthermia in DAT-Cnr2 cKO and WT mice (Fig. 5). There was significant interaction between genotype and interval for total distance traveled after 3.0 mg/kg methamphetamine on day 1 and on day 8, in comparison with the baseline of WT and DAT-Cnr2 cKO mice [F(2,18) =142.594, P<0.001]. Post hoc analysis showed that the distance traveled on day 1 and on day 8 significantly increased in comparison with the baseline in WT mice (p<0.05). For DAT-Cnr2 cKO, the increase was only present on day 1 (p<0.05). Methamphetamine (3.0 mg/kg) induced locomotor sensitization in WT but not in DAT-Cnr2 cKO mice (Fig. 5). Curiously methamphetamine-induced hyperthermia was present in both genotypes (Fig. 5).
Fig. 5.
Locomotor sensitization induce by methamphetamine (3 mg/kg) and methamphetamine-induced hyperthermia in DAT-Cnr2 cKO and WT mice. Mice received one methamphetamine injection (3 mg/kg) daily for 6 consecutive days and the locomotor activity was measured for 20 minutes before injection as the baseline, 15 minutes after administration on day 1 and on day 8 (no injection on day 7). Rectal temperature was measured in centigrade before and after methamphetamine administration. The white bars represent the WT and the grey bars represent DAT-Cnr2 cKO mice. Data are expressed as mean ± SEM (n = 10 mice per group). Two way ANOVA followed by Dunnett’s as post hoc test *p < 0.05. Paired t-test * p < 0.05 baseline vs methamphetamine. Student t-test n.s. WT vs DAT-Cnr2 cKO mice.
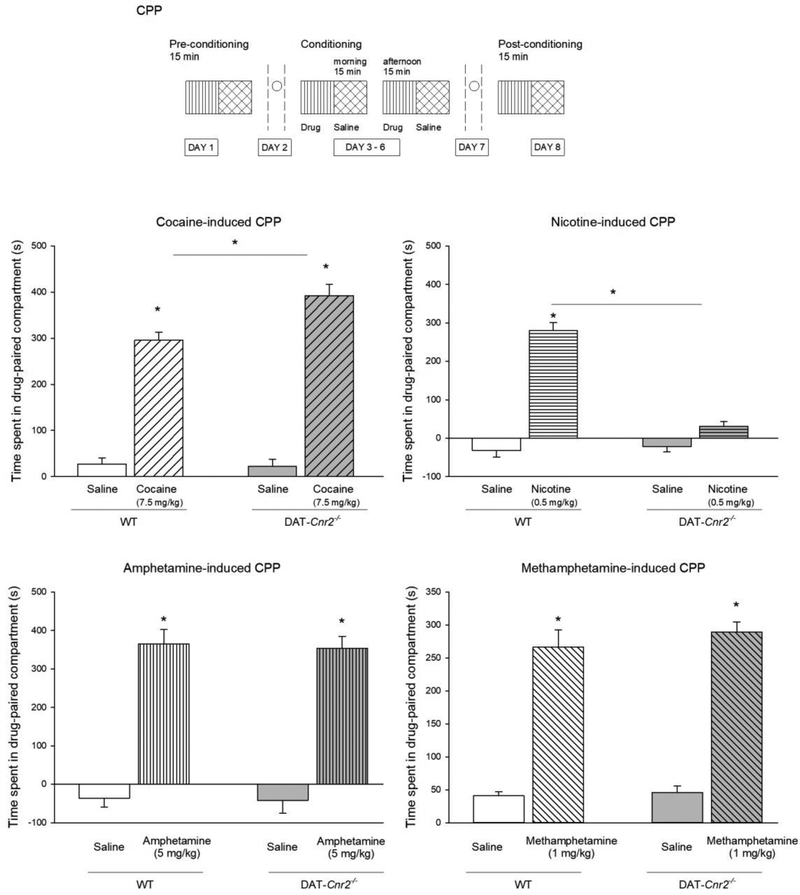
3.6. DAT-Cnr2 cKO mice displayed increased cocaine, amphetamine and methamphetamine-induced CPP, but not nicotine-induced CPP
In the next set of studies, we investigated the role of CB2Rs in the rewarding properties of the selected psychostimulants following deletion of CB2Rs in dopamine neurons using the CPP paradigm. We have reported that the CB2Rs in dopamine neurons modify alcohol and cocaine CPP [24]. Here, we extended the study to include the determination of the role of CB2Rs in dopamine neurons on nicotine, methamphetamine and cocaine CPP. Cocaine (7.5 mg/kg) significantly increased the preference score for the cocaine associated compartment in WT and DAT-Cnr2 cKO mice. The preference score was significantly higher in DAT-Cnr2 cKO mice compared to WT animals. Cocaine (7.5 mg/kg) increased the time spent in the drug-pair compartment in both WT and DAT-Cnr2 cKO mice (Fig. 6). Two-way ANOVA showed genotypic effect [F(1,36) = 4.06, p = 0.051] and treatment effect [F(1,36) = 116.54, p<0.001]. The interaction effect was also significant [F(1,36) = 7.458, p = 0.01]. Post hoc analysis showed that 7.5 mg/kg cocaine increased amount of the time spent in the drug-paired compartment relative to vehicle in both genotypes (p<0.05). The time spent in the drug-paired compartment with cocaine was different for WT and DAT-Cnr2 cKO mice (p<0.05). Amphetamine (5.0 mg/kg) increased the time spent in the drug-paired compartment in both WT and DAT-Cnr2 cKO mice (Fig. 6). Two-way ANOVA showed treatment effect [F(1,36) = 66.97, p< <0.001]. Post hoc analysis showed that in both genotypes, amphetamine (5.0 mg/kg) increased the amount of time spent in the drug-paired compartment (p<0.05). Methamphetamine (1.0 mg/kg) increased the amount of time spent in the drug-paired compartment in both WT and DAT-Cnr2 cKO mice (Fig. 6). Two-way ANOVA showed treatment effect [F(1,36) = 205.02, p< <0.001]. Post hoc analysis showed that in both genotypes, methamphetamine (1.0 mg/kg) increased the amount of time spent in the drug-paired compartment (p<0.05). Nicotine at 0.5 mg/kg (a dose used in most studies to establish nicotine CPP) [26, 30–33] induced robust CPP in the WT but not in DAT-Cnr2 cKO mice. Thus, nicotine (0.5 mg/kg) increased the amount of time spent in the drug-paired compartment in WT but not in DAT-Cnr2 cKO mice (Fig. 6). Two-way ANOVA showed genotypic effect [F(1,36) = 9.94, p=0.003] and treatment effect [F(1,36) = 5.250, p<0.028]. Post hoc analysis showed that 0.5 mg/kg nicotine increased the time spent in the drug-paired compartment relative to vehicle in WT (p<0.05). The time spent in the drug-paired compartment with nicotine was different for WT and DAT-Cnr2 cKO mice (p<0.05). Nicotine did not produce CPP in the DAT-Cnr2-cKO mice, unlike cocaine, amphetamine and methamphetamine, which produced robust CPP in DAT-Cnr2 cKO mice. Interestingly, modification of components of ECS is associated with reduction or enhancement of nicotine CPP [26, 30–33]. Thus, our results are in agreement with previous data suggesting that CB2Rs play opposing roles in nicotine- and cocaine induced CPP [26, 31].
Fig. 6.
Conditioned place preference (CPP) induced by psychostimulants. CPP-induced by cocaine (7.5 mg/kg), nicotine (0.5 mg/kg), amphetamine (5.0 mg/kg), and methamphetamine (1.0 mg/kg). Time spent in the drug-paired compartment during post-conditioning minus the time spent in the drug-paired compartment in the pre-conditioning session. Data shows the mean ± SEM. (n = 10 mice per group). Two way ANOVA followed by Dunnett’s post hoc test *p < 0.05.
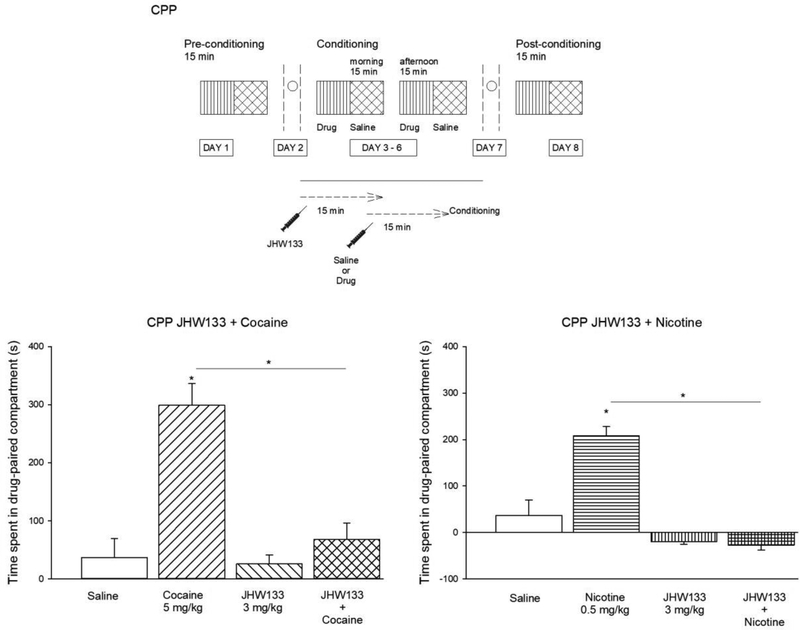
3.7. Effects of JWH133 on cocaine and nicotine-induced CPP in WT mice
Our previous data [24] indicated that alcohol induced CPP in the WT mice was significantly inhibited by the selective CB2R agonist JHW 133 [24]. Here we examined the effect of JWH133 on cocaine and nicotine – induced CPP only in the WT mice (Fig. 7). We found that pre-treatment with JWH133 (3.0 mg/kg) blocked the development of cocaine– induced CPP in the WT mice. There was significant difference between groups determined by one-way ANOVA [F(3,32) = 12.06, p<0.001]. A Tukey post hoc analysis revealed that the time spent in the drug-paired compartment was significantly lower in the group of mice treated with JWH133 prior cocaine administration compared with the group treated with cocaine alone (p<0.05). The pre-treatment with the CB2R agonist, JWH133 (3.0 mg/kg), blocked the development of cocaine– induced CPP in the WT mice. One-way ANOVA [F(3,36) = 5.32, p=0.004]. Post hoc Tukey analysis showed that the time spent in the drug-paired compartment in the group pre-treated with JWH133 prior the nicotine administration was significantly lower than in the WT mice treated with nicotine alone (p<0.05). Collectively with our previous data [24], these results indicate the involvement of CB2Rs in nicotine-induced CPP in the mouse model.
Fig. 7.
Effects of pre-treatment with the CB2R antagonist, JWH133, on the development of cocaine or nicotine – induced CPP. Time spent in the drug-paired compartment in the group treated with vehicle, cocaine (5.0 mg/kg), JWH133 (3.0 mg/kg) or the combination of JHW133 + Cocaine. Time spent in the drug-paired compartment in the group treated with vehicle, nicotine (0.5 mg/kg), JWH133 (3.0 mg/kg) or the combination of JHW133 + Cocaine. Cocaine or nicotine induced CPP in the WT mice was significantly inhibited by the selective CB2R agonist JWH133. Data shows the mean ± SEM. (n = 10 mice per group). One way ANOVA followed by Tukey’s post hoc test *p < 0.05.
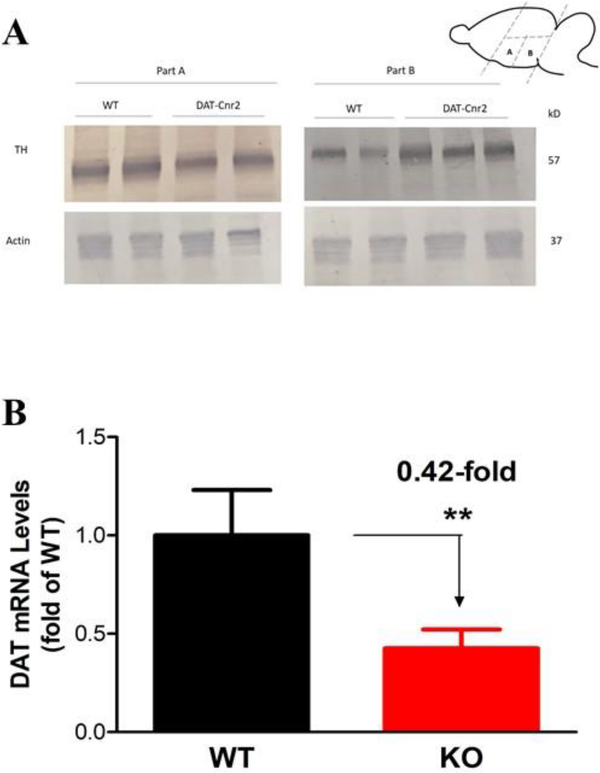
3.8. Levels of TH in DAT-Cnr2 cKO and WT mice
Western blot analysis in WT and DAT-Cnr2 cKO midbrain regions, showed that the antibody used against the expression of the dopaminergic neuronal marker tyrosine hydroxylase (TH) revealed bands with the expected molecular weight in the two midbrain regions labeled A and B in (Fig. 8A). The expression of TH was lower in DAT-Cnr2 cKO in brain area A than in area B where the expression of TH was higher in the DAT-Cnr2 cKO mice in comparison with that of the WT. The differential TH protein expression in midbrain regions of DAT-Cnr2 cKO suggests the involvement of CB2Rs in dopaminergic neuronal function as we previously reported [24]. This was supported by the determination and analysis of dopamine transporter (DAT) - the principal regulator of dopamine transmission
Fig. 8A and 8B.
Levels of tyrosine hydroxylase (TH) protein. Representative amounts of TH protein compared with actin, in midbrain areas, part A and part B, following the deletion of CB2Rs in DA neurons in DAT-Cnr2 cKO and the WT mice is shown in Fig. 8A. The quantification of dopamine transporter (DAT) mRNA levels and normalized by GAPDH mRNA in mouse midbrain after the deletion of CB2Rs in DA neurons is shown in Fig. 8B. **p < 0.05 for DAT-Cnr2 cKO compared with the WT mice.
3.9. Analysis of DAT and EIF3F gene expression in DAT-Cnr2 cKO and WT mice
To investigate the effects of deletion of CB2Rs from DA neurons, qRT-PCR was used to estimate DAT mRNA expression in mouse midbrain region. DAT is involved in the re-uptake of DA in the synapse and it has been suggested that EIF3F function as a transcription that activates DAT (Communication with Dr. Lin). The expression of mDAT mRNA was significantly reduced in DAT-Cnr2 cKO but unaffected in the WT mice (Fig. 8B), whereas the expression of mEIF3F (data not shown) was slightly elevated in the DAT-Cnr2 cKO but unchanged in the WT mice. This further supports the neuronal expression of CB2Rs and its involvement in midbrain dopamine function.
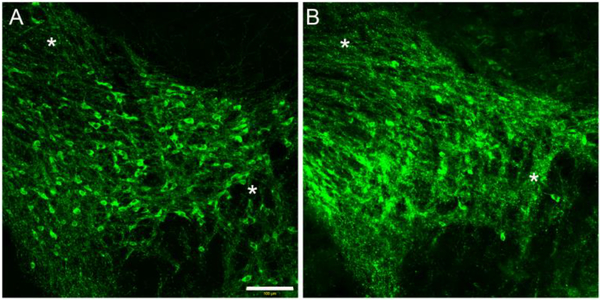
3.10. Immunohistochemical staining for TH- positive neurons in DAT-Cnr2 cKO and WT mice
In order to determine the anatomical and structural integrity of dopaminergic neurons, we immunostained for tyrosine hydroxylase (TH) in the midbrain of the DAT-Cnr2 cKO and WT mice. As shown in Fig. 9, there was an increase in TH immunofluorescence at the level of neurites in the neuropil of DAT-Cn2 cKO (Fig. 9A), in comparison with the WT (Fig. 9B) mice. Taken together the decreased DAT mRNA gene expression and the modification of the levels of TH protein provides additional evidence for the existence of neuronal CB2Rs following its deletion from dopamine neurons.
Fig. 9.
Tyrosine hydroxylase (TH) immunofluorescence staining of the midbrain of DAT-Cnr2 cKO (A) and WT mice (B). Microphotographs showing increased qualitative TH immunofluorescence at the levels of neurites in the neuropil of DAT-Cnr2 cKO in comparison with the WT mice.
4. Discussion
There is a lingering debate [34], about the functional neuronal expression of CB2Rs in the brain. This is because CB2Rs were previously thought to be restricted to peripheral tissues and predominantly in immune cells. However, accumulating evidence, along with genome-wide atlas of gene expression in the adult mouse brain [35], indicate neuronal expression of CB2Rs. In the current study, we investigated and characterized the molecular basis of the behavioral effects of selected psychostimulants in mutant mice with cell-type specific deletion of CB2Rs in dopamine neurons. The principal findings arising from the present study are: (1) The increase in locomotor activity induced by cocaine and amphetamine was enhanced in the DAT-Cnr2 cKO mice. The psychostimulant-induced sensitization was absent in DAT-Cnr2 cKO but present in the WT mice. (2) DAT-Cnr2 cKO mice displayed cocaine, amphetamine and methamphetamine-induced- but not to nicotine induced CPP. Pre-treatment with the CB2R agonist JWH133 blocked cocaine and nicotine CPP in WT mice. (3) The deletion of CB2Rs in DAT-Cnr2 cKO mice modified the expression of TH, DAT mRNA expression and the anatomical and structural integrity of dopaminergic neurons.
Psychomotor activity has been widely used to study neural mechanisms underlying addictive drug action [36]. The role of CB2Rs on cocaine induced hyperlocomotion and rewarding properties has been investigated. For example, there were reduced locomotor responses in mice overexpressing CB2Rs (CB2xP) treated with acute cocaine (10–20 mg/kg), compared with WT mice [27]. In the present study, we found that deletion of CB2Rs in DAT-Cnr2 cKO mice provoked hyperactivity and treatment with acute cocaine further exaggerated this hyperactivity. It is remarkable that the effect of the high dose of cocaine tested did not present the same pattern of hyperactivity. Cocaine was found to induce hyperlocomotion in both genotypes which was dose dependent in the WT but not in the in the DAT-Cnr2 cKO mice. However, in the DAT-Cnr2 cKO mice the increase in locomotor activity induced by the highest dose of cocaine was not as significant as expected by the dosage. In fact, it was slightly lower than the increase shown by WT mice. This result is similar to the one observed with the CB1R deficient mice. The animals responded significantly less to high doses of cocaine [37]. Furthermore, cocaine induced conditioned place aversion (CPA) in the CB2xP mice [27], whereas in DAT-Cnr2 cKO mice cocaine-induced CPP. In WT mice, the pretreatment with a selective CB2R agonist JHW133 prior to cocaine administration reduced cocaine CPP induction. A similar finding was reported in rats [38], with another CB2R agonist, O-1966, that decreased cocaine-induced CPP [26]. JWH133 was also shown to inhibit intravenous cocaine self-administration [25], and cocaine self-administration was reduced in CB2xP [27]. Therefore, our results contribute to the existing evidence regarding the role of the CB2Rs in the reinforcing effects of cocaine. Taken together, these data support the notion that CB1Rs and CB2Rs have different and perhaps opposing roles in modulating cocaine’s rewarding and psychomotor stimulant effects.
In dopaminergic neurons, CB2Rs are localized postsynaptically [19], and their activation leads to a decrease in VTA’s neuronal firing [39] as well as basal and cocaine-induced dopamine release in the nucleus accumbens [25]. Cocaine self-administration is impaired in CB1R knockout mice [40] as well as cocaine induced-CPP [41]. CB1Rs and CB2Rs also play an important role in the regulation of rewarding behaviors through the modulation of medium spiny neuron (MSN) activity [1, 2]. Within the VTA, CB1Rs have been identified on terminals of inhibitory GABAergic and excitatory glutamatergic neurons [42]. Therefore, regulation of dopamine neuron function could be under the indirect stimulatory effect of CB1Rs that are located on VTA afferents and under the direct inhibitory effect of CB2Rs that are located on dopaminergic neurons. In support of this notion, under basal conditions, we reported that deletion of CB2Rs from dopamine neurons enhances motor activities of the DAT-Cnr2 cKO mice in comparison to WT mice [24]. It has been reported that CB1R KO mice displayed decreased basal locomotor activity compared with their wild-type [43,44], suggesting that under physiological conditions, the activation of CB1Rs tends to activate locomotion and may therefore contribute to the maintenance of normal locomotor activity in wild-type mice. The finding of the basal hyperactive phenotype of DAT-Cnr2 cKO mice is the first evidence of the role of CB2Rs in the control of psychomotor behavior. These results supports our report that CB2Rs puts a “brake” on locomotor activation by dopamine neurons, and its deletion in the DAT-Cnr2 cKO mice enhances psychomotor behavior [24]. Furthermore, we found that the acute administration of low doses of d-amphetamine induced a decrease in locomotor activity in DAT-Cnr2 cKO mice, and this issue should be addressed in further studies, since this paradoxical effect of amphetamine is observed in attention deficit hyperactivity disorder (ADHD) models in mice. The involvement of CB1R and CB2R activity in the development of behavioral sensitization to methamphetamine was studied in naïve mice and it was suggested that the activity of the ECS is involved in the neuronal circuitry underlying the development of sensitization to methamphetamine [29].
The repeated administration of psychostimulants in rodents results in a progressive and enduring augmentation of locomotor and stereotyped behaviors. This response termed behavioral sensitization [45, 46], is supported by the demonstration that CB1R mutant mice display impaired locomotor sensitization to cocaine or d-amphetamine [37, 47]. Results from the present study show that the DAT-Cnr2 cKO mice were less sensitive to psychostimulants-induced locomotor sensitization. Therefore, this data demonstrate that CB2Rs are implicated in the development of behavioral sensitization to psychostimulants. Another prominent characteristic change produced by amphetamine is an increase in extracellular dopamine levels. Amphetamine not only inhibit the reuptake of released dopamine in the synaptic cleft, but also trigger dopamine release from the cytosol to the extracellular space by means of reverse transport through the plasma membrane dopamine transporter [48–50]. Psychostimulants such as amphetamine, methamphetamine and cocaine affect mesolimbic dopaminergic terminals, raising dopamine levels in the NAcc by the direct action on dopaminergic axon terminals. Cocaine inhibits the reuptake of dopamine, serotonin and norepinephrine by blocking their respective transporters, DAT, SERT and NET. Therefore, the study of the role of CB2Rs in the behavioral effects of these compounds is important in unraveling the differential effects of psychostimulants. Interestingly, nicotine, which is a potent ganglionic and CNS stimulant, exerts its effects through binding to nicotinic cholinergic receptors (nAChR) that are located in the brain, autonomic ganglia, and adrenal glands, and at the neuromuscular junction [51]. By a different mechanism, nicotine also activates DA neurons via nAChR, which are essential for nicotine-induced reinforcement and are associated with dopamine signaling [52, 53]. Thus, nicotine acts indirectly on the dopaminergic system. For that reason, studying the role of CB2Rs on the rewarding properties is vital, as it has been suggested that CB2Rs plays a role in the development of nicotine CPP [26]. Our data support this finding and indicates the involvement of CB2Rs in nicotine-induced CPP. Our results indicate that deletion of CB2Rs in dopamine neurons induces hyperlocomotion, whereas the depletion of dopamine increases endocannabinoid levels with reduction of locomotor activity [54].
Our current data implicate an interaction between endocannabinoid/CB2Rs and dopaminergic pathways indicating that CB2Rs plays an active role in modulating the rewarding properties of psychostimulants in the mouse model. Furthermore, in this study we report that the selective deletion of CB2Rs dopamine neurons induced strong CPP to the psychostimulants, cocaine, amphetamine and methamphetamine in the DAT-Cnr2 cKO mice. However, nicotine, another psychostimulants, showed the opposite effect as the DAT-Cnr2 cKO were resistant to the rewarding properties of nicotine, measured by the CPP paradigm. In the case of nicotine, the CNS stimulant effect is associated with the cholinergic system and contributes to the reinforcing properties of nicotine. Interestingly modification of components of the ECS was associated with reduction or enhancement of nicotine place conditioning [26, 30–33]. Thus our results are in agreement with previous data suggesting that CB2Rs play opposing roles in nicotine- and cocaine induced CPP [26, 31].
We found differential expression of TH in DAT-Cnr2 cKO mice in contrast to WT animals in the two brain areas analyzed. TH is the rate-limiting enzyme in the biosynthesis of dopamine [55]. Under basal conditions and in brain areas containing VTA and SN, the expression of TH was higher in the DAT-Cnr2 cKO than in the WT mice. There was also an increase in TH immunofluorescence at the level of neurites in the neuropil of DAT-Cnr2 cKO compared to WT mice. The expression of mDAT mRNA was significantly reduced in DAT-Cnr2 cKO, but unchanged in the WT mice. This may be due to an adaptive system response caused by the hyperdopaminergic tone and supports the neuronal expression of CB2Rs and its involvement in midbrain dopamine function. The results suggest an enhanced dopamine turnover, and consequently, an enhanced expression of the enzyme required for its biosynthesis. Circuits important for psychostimulant reward and locomotion are not likely to be limited to those using monoaminergic neurotransmitters alone. Nevertheless, the mesolimbic dopaminergic pathway that is engaged by drugs of abuse is among the brain circuits modulated by endocannabinoids [56]. As the pharmacological manipulation of CB1R ligands have provided disappointing results in clinical trials and clinical outcomes, attention has increasingly focused on CB2Rs, and the emerging link of CB2Rs in immune and inflammatory signaling with psychiatric and neurological disorders. Studies have shown that apart from forming dimers with other GPCRs, CB1Rs and CB2Rs can form homo-and heterodimers in neuronal cells and in the brain [57]. Such oligomerization may affect receptor signaling, trafficking and ligand binding. This has been demonstrated for CB1R-CB2R heteromers with the ability of CB1R antagonists to block the effect of CB2R agonists and, conversely, the ability of CB2R antagonists to block the effect of CB1R agonists, showing a bidirectional cross antagonism phenomenon. The cell type selective deletion of CB1Rs or CB2Rs may shed light on the mechanism by which CB2Rs can negatively modulate CB1R function [57].
5. Conclusions
A number of conclusions can be drawn from these studies that characterized the behavioral effects of psychostimulants in mutant mice with cell-type specific deletion of CB2Rs in dopamine neurons. We have shown that CB2Rs expressed in dopaminergic neurons participate in the locomotor and differential rewarding properties of the selected psychostimulants used in this study. There was enhanced psychostimulant induced hyperactivity in the DAT-Cnr2 cKO mice and surprisingly the psychostimulant-induced sensitization was absent in DAT-Cnr2 cKO compared to the WT mice. In addition, DAT-Cnr2 cKO mice displayed cocaine, amphetamine and methamphetamine induced-CPP but not to nicotine induced CPP, and pre-treatment with the CB2R agonist JWH133 blocked cocaine and nicotine CPP in WT mice. The deletion of CB2Rs in DAT-Cnr2 cKO mice modified the expression of TH, DAT mRNA expression, and the anatomical and structural integrity of dopaminergic neurons. Furthermore, our data of reduction in DAT gene expression and enhanced TH activity in the midbrain of DAT-Cnr2 cKO supports the results demonstrating reduction of methamphetamine effects by Δ9-THC [58]. Our study contributes to the growing importance of the functional neuronal expression of CB2Rs as revealed by the DAT-Cnr2 cKO mice. The results indicate that the DAT-Cnr2 cKO is a useful preclinical model in discriminating the reward-like properties as well as identifying distinct mode of sensitization of psychostimulants. It was therefore concluded that further studies can exploit the therapeutic potential of targeting the endocannabinoid/CB2R system in psychostimulant addiction and other psychiatric disorders associated with dopamine dysregulation.
Acknowledgement
This work was partly supported by William Paterson University. Ana Canseco-Alba was supported by the Mexican National Council of Science and technology (CONACYT # CVU332502/232728). Zhicheng Lin is supported by NIH grants DA021409 and DA031573. Qing-Rong Liu is supported by the Intramural Research Program of NIA-NIH and Emmanuel S. Onaivi was supported by NIH grant DA032890. We thank Patricia Tagliaferro for the IHC image preparation. This work will not be possible without the student worker support for maintenance of mice in the animal laboratory by the Dean of the College of Science and Health at William Paterson University, Dr. Venkat Sharma. We dedicate this work in memory of the sudden and tragic loss of our co-author and Senior Lab. technician, Norman Schanz.
Footnotes
Conflict of interest
The authors declare that they do not have conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].L Gardner E, Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacology biochemistry, and behavior 81 (2005) 263–284. [DOI] [PubMed] [Google Scholar]
- [2].Lupica CR, Riegel AC, Hoffman AF, Marijuana and cannabinoid regulation of brain reward circuits. British journal of pharmacology 143 (2004) 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maldonado R, Valverde O, Berrendero F, Involvement of the endocannabinoid system in drug addiction. Trends in neurosciences 29 (2006) 225–232. [DOI] [PubMed] [Google Scholar]
- [4].Parolaro D, Vigano D, Realini N, Rubino T, Role of endocannabinoids in regulating drug dependence. Neuropsychiatric disease and treatment 3 (2007) 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Solinas M, Goldberg SR, Piomelli D, The endocannabinoid system in brain reward processes. British journal of pharmacology 154 (2008) 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, et al. , Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: From mice to human subjects. PLoS One 3 (2008) e1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Di Marzo V, Melck D, Bisogno T, De Petrocellis L, Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends in neurosciences 21 (1998) 521–528. [DOI] [PubMed] [Google Scholar]
- [8].Di Chiara G, Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural brain research 137 (2002) 75–114. [DOI] [PubMed] [Google Scholar]
- [9].Pierce RC, Kumaresan V, The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neuroscience and biobehavioral reviews 30 (2006) 215–238. [DOI] [PubMed] [Google Scholar]
- [10].A Sutton M, Beninger RJ, Psychopharmacology of conditioned reward: evidence for a rewarding signal at D1-like dopamine receptors. Psychopharmacology 144 (1999) 95–110. [DOI] [PubMed] [Google Scholar]
- [11].Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR, The dopamine theory of addiction: 40 years of highs and lows. Nat. Rev Neurosci 16 (2015) 305–312. [DOI] [PubMed] [Google Scholar]
- [12].Wise RA, Dopamine, learning and motivation. Nat. Neurosci 5 (2004) 483–494. [DOI] [PubMed] [Google Scholar]
- [13].Ikemoto S, Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 56 (2007) 27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Munro S, Thomas KL, Abu-Shaar M, Molecular characterization of a peripheral receptor for cannabinoids. Nature 365 (1993) 61–65. [DOI] [PubMed] [Google Scholar]
- [15].P Gong J, S Onaivi E, Ishiguro H, Liu QR, Tagliaferro P, Brusco A, et al. , Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain research 1071 (2006) 10–23. [DOI] [PubMed] [Google Scholar]
- [16].Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM, Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83 (1998) 393–411. [DOI] [PubMed] [Google Scholar]
- [17].Ong WY, Mackie K, A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience 92 (1999) 1177–1191. [DOI] [PubMed] [Google Scholar]
- [18].Griffin G, Wray EJ, Tao Q, McAllister SD, Rorrer WK, Aung MM, et al. , Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: further evidence for CB2 receptor in the rat central nervous system. Eur J Pharmacol. 377 (1999) 117–125. [DOI] [PubMed] [Google Scholar]
- [19].Brusco A, Tagliaferro P, Saez T, Onaivi ES, Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse 62 (2008) 944–949. [DOI] [PubMed] [Google Scholar]
- [20].A Cabral G, Marciano-Cabral F, Cannabinoid receptors in microglia of the central nervous system: immune functional relevance. Journal of leukocyte biology 78 (2005) 1192–1197. [DOI] [PubMed] [Google Scholar]
- [21].Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, et al. , Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Annals of the New York Academy of Sciences 1074 (2006) 514–536. [DOI] [PubMed] [Google Scholar]
- [22].Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. , Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310 (2005) 329–332. [DOI] [PubMed] [Google Scholar]
- [23].Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK, et al. , Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron 90 (2016) 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu Q-R, Canseco-Alba A, Zhang HY, Tagliaferro P, Chung M, Dennis E, et al. , Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Scientific Reports 7 (2017) 17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xi ZX, Q Peng X, Li X, Song R, Zhang HY, Liu QR, et al. , Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nature neuroscience 14 (2011) 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].M Ignatowska-Jankowska B, Muldoon PP, Lichtman AH, I Damaj M, The cannabinoid CB2 receptor is necessary for nicotine-conditioned place preference, but not other behavioral effects of nicotine in mice. Psychopharmacology 229 (2013) 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Aracil-Fernandez A, Trigo JM, Garcia-Gutierrez MS, Ortega-Alvaro A, Ternianov A, Navarro D, et al. , Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB(2) receptors. Neuropsychopharmacol. 37 (2012) 1749–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Renard J, Loureiro M, Rosen LG, Zunder J, Oliveira CD, Schmid S, et al. , Cannabidiol counteracts amphetamine-induced neuronal and behavioral sensitization of the mesolimbic dopamine pathway through a novel mTOR/p70S6 kinase signaling pathway. J Neurosci. 36 (2016) 5160–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Landa L, Sulcova A, Slais K, Involvement of cannabinoid CB1 and CB2 receptor activity in the development of behavioral sensitization to methamphetamine effects in mice. Neuro. Endocrinol Lett 27 (2006) 63–69. [PubMed] [Google Scholar]
- [30].Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI. The endogenous cannabinoid system modulates nicotine reward and dependence. J. Pharmacol Exp Ther 326 (2008) 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Navarrete F, Rodriguez-Arias M, Martin-Garcia E, Navarro D, Garcia-Gutierrez MS, Aguilar MA, et al. , Role of CB2 cannabinoid receptors in the rewarding, reinforcing, and physical effects of nicotine. Neuropsychopharmacology 38 (2013) 2515–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kutlu MG, Ortega LA, Gould TJ, Strain-dependent performance in nicotine-induced conditioned place preference. Behav Neurosci. 129 (2015) 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bowers MS, Jackson A, Maldoon PP, Damaj MI, N-acetylcysteine decreased nicotine reward-like properties and withdrawal in mice. Psychopharmacology 233 (2016) 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lopez A, Aparicio N, Pazos MR, Grande MT, Barreda-Manso MA, Benito-Cuesta I, et al. , Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. Journal Neuroinflammation 15 (2018) 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. , Genome-wide atlas of gene expression in the adult mouse brain. Nature 445 (2007) 168–176. [DOI] [PubMed] [Google Scholar]
- [36].Cornish JL, Kalivas PW , Cocaine sensitization and craving: differing roles for dopamine and glutamate in the nucleus accumbens. J Addict Dis 20 (2001) 43–54. [DOI] [PubMed] [Google Scholar]
- [37].Corbille AG, Valjent E, Marsicano G, Ledent C, Lutz B, Herve D et al. , Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci 27 (2007) 6937–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Delis F, Polissidis A, Poulia N, Justinova Z, Nomikos GG, Goldberg SR, et al. , Attenuation of cocaine-induced conditioned place preference and motor activity via cannabinoid CB2 receptor agonism and CB1 receptor antagonism in rats. Int. J. Neuropsychopharmacol 20 (2017) 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, et al. , Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci. 111 (2014) E5007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Soria G, Mendizábal V, Touriño C, Robledo P, Ledent C, Parmentier M, Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology 30 (2005)1670–1680. [DOI] [PubMed] [Google Scholar]
- [41].Miller LL, Ward SJ, Dykstra LA, Chronic unpredictable stress enhances cocaine-conditioned place preference in type 1 cannabinoid receptor knockout mice. Behav Pharmacol 19 (2008) 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Matyas F, Urban GM, Watanabe M, Mackie K, Zimmer A, Freund TF, Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology, 54 (2008) 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI, Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci. 96 (1999) 5780–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li X, Hoffman AF, Peng X-Q, Lupica CR Gardner EL, Xi ZX. Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice. Psychopharmacology 204 (2009) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Segal DS, Behavioral and neurochemical correlates of repeated d-amphetamine administration. Adv Biochem Psychopharmacol 13 (1975) 247–62. [PubMed] [Google Scholar]
- [46].Robinson TE, Berridge KC, The neural basis of drug craving: an incentive sensitization theory of addiction. Brain Res Rev 18 (1993) 247–291. [DOI] [PubMed] [Google Scholar]
- [47].Thiemann G, Di Marzo V, Molleman A, Hasenohrl RU, The CB1 cannabinoid receptor antagonist AM251 attenuate amphetamine-induced behavioral sensitization while causing monamine changes in nucleus accumbens and hippocampus. Pharmacol Biochem Behav. 89 (2008) 384–391. [DOI] [PubMed] [Google Scholar]
- [48].Fleckenstein AE, Gibb JW, Hanson GR, Differential effects of stimulants on monoaminergic transporters: pharmacological consequences and implications for neurotoxicity. Eur J Pharmacol. 406 (2000) 1–13. [DOI] [PubMed] [Google Scholar]
- [49].Kilty JE, Lorang D, Amara SG, Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science 254 (1991) 578–579. [DOI] [PubMed] [Google Scholar]
- [50].Hyman SE, Malenka RC, Nestler EJ, Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 29 (2006) 565–598. [DOI] [PubMed] [Google Scholar]
- [51].Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu. Rev. Pharmacol. Toxicol, 36 (1996) 597–613. [DOI] [PubMed] [Google Scholar]
- [52].Drenan RM, Grady SR, Steele AD, McKinney S, Patzlaff NE, McIntosh JM, Cholinergic modulation of locomotion and strialta dopamine release is mediated by alpha6alpha4* nicotinic acetylcholine receptors. J Neurosci. 30 (2010) 9877–9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM et al. , Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391 (1998) 173–77. [DOI] [PubMed] [Google Scholar]
- [54].Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, et al. , Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutaminergic transmission. J. Neurosci. 22 (2002) 6900–6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kumer SC, E K, Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 67 (1996) 443–462. [DOI] [PubMed] [Google Scholar]
- [56].Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, et al. , Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J. Neurosci 27 (2007) 791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Callen L, Moreno E, Barroso-Chinea P, Moreno-Delgado D, Cortes A, Mallol J, et al. , Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J. Biol. Chem 287 (2012) 20851–20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Castelli MP, Madeddu C, Casti A, Casu A, Casti P, Scherma M, et al. , Δ9-Tetrahydrocannabinol prevents methamphetamine-induced neurotoxicity. PLoS ONE 9(5) (2014) e98079. [DOI] [PMC free article] [PubMed] [Google Scholar]