Abstract
Thirteen novel benzothiazole derivatives incorporating glycine, methionine, alanine, and phenylalanine were synthesised by facile acylation reactions through benzotriazole or DCC mediated reactions and their structures were identified by 1H-NMR, 13C-NMR, and FT-IR spectroscopic techniques and elemental analysis. The carbonic anhydrase (CA, EC 4.2.1.1) inhibitory activity of the new compounds was assessed against four human (h) isoforms, hCA I, hCA II, hCA V, and hCA XIII. Some of the synthesised compounds showed good in vitro carbonic anhydrase inhibitory properties, with inhibition constants in the micromolar level. The new amino acid benzothiazole conjugates found to be more effective against hCA V and hCA II inhibition. In vitro antioxidant activities of the novel compounds were determined by DPPH method. Most of the synthesised compounds showed moderate to low antioxidant activities compared to the control antioxidant compounds (BHA and α-tocopherol).
Keywords: Benzothiazole; amino acids; carbonic anhydrase; antioxidant; benzotriazole methodology
1. Introduction
Benzothiazole derivatives have been extensively studied in drug chemistry1,2 and they exhibit diverse activities such as antitubercular3, antimicrobial4–6, antimalarial7, anticonvulsant8, antioxidant9, antidiabetic10, antitumor11, carbonic anhydrase (CA)12, and tryptase inhibitors13. Moreover, benzothiazole moieties are also found in fluorescent pH indicators14, iminocoumarin based zinc sensors15, bioluminogenic agent16,17, vulcanisation process of rubber18 and ligands for transition metal catalysts19.
On the other hand, CAs are a class of well-studied metalloenzymes that are widely distributed in all living organisms20. These enzymes (hCAs) are zinc-containing enzymes that catalyse the reversible hydration of carbon dioxide to bicarbonate and a proton (CO2 + H2O⇆HCO3− + H+). Fifteen isoforms of human CA (hCA I–XV) have been isolated, their presence being fundamental for the regulation of many physiological processes21,22.
The interest in finding an effective carbonic anhydrase enzyme inhibitor12,23–26 has been increasing in recent years, especially with the exploring of possible relationships between carbonic anhydrase and cancer22,2,7–29. A similar interest is also observed in synthesising effective antioxidants for food products and drugs.
With the hope to obtain an effective carbonic anhydrase enzyme inhibitor having good antioxidant properties, we planned to synthesise new amino acid-benzothiazole conjugates using the benzotriazole methodology and to explore their carbonic anhydrase enzyme inhibition and antioxidant properties.
2. Material and methods
2.1. Chemistry
The starting materials and reagents used in the reactions were supplied commercially by Acros (Newark, NJ), Aldrich (St. Louis, MO), Fluka (Munich, Germany), or Merck (Kenilworth, NJ). The solvents were dried by standard methods and freshly distilled prior to use. All microwave assisted reactions were carried in a microwave oven system manufactured by Milestone (Milestone Start S Microwave Labstation for Synthesis, Valbrembo, Italy). 1H NMR (300.13 MHz) and 13C NMR (75.47 MHz) spectra were recorded using a Bruker Avance 300 MHz Ultrashield high performance digital FT NMR spectrometer (Bruker, Billerica, MA). Infrared spectra were recorded as KBr pellets in the range 4000–400 cm−1 on a Perkin Elmer FT-IR spectrophotometer (PerkinElmer, Waltham, MA). Mass spectra were obtained using an Agilent 6460 Series Triple LC/MS instrument (Santa Clara, CA, USA). Elemental analyses were performed with a LECO CHNS-932 elemental analyser (LECO, ST. Joseph, MI). Melting points were recorded using an electrothermal-9200 melting point apparatus (Electrothermal Engineering, Essex, UK) and are uncorrected.
2.2. Synthesis of amino acid-benzothiazole derivatives
Benzyl (2-(1H-benzo[d][1,2,3]triazol-1-yl)-2-oxoethyl)carbamate (I), tert-butyl (2-(1H-benzo[d][1,2,3]triazol-1-yl)-2-oxoethyl)carbamate (II), tert-butyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxopropan-2-yl)carbamate (III), tert-butyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxo-3-phenylpropan-2-yl)carbamate (IV), (S)-benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxo-3-phenylpropan-2-yl)carbamate (V), (9H-fluoren-9-yl)methyl (2-(1H-benzo[d][1,2,3]triazol-1-yl)-2-oxoethyl)carbamate (VI) and benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-4-(methylthio)-1-oxobutan-2-yl)carbamate (VII) were prepared according to the literature procedures30–33.
2.2.1. General procedure for the synthesis of benzothiazole conjugates 1–13
A mixture of equivalent amounts of the appropriate N-protected aminoacylbenzotriazole and appropriate benzothiazole derivative was subjected to microwave irradiation (100 W, 70 °C) in anhydrous dichloromethane for 30 min. After the completion of the reaction, all volatiles were removed by rotary evaporator and the obtained crude product was crystallised from ethanol.
2.2.2. Benzyl (3–(6-methylbenzo[d]thiazol-2-yl)-2-oxopropyl)carbamate (1)
White crystals (69,88%), mp 213–214 °C. ν(CO)amide: 1710 cm−1, ν(CO)carbamate: 1670 cm−1. 1H-NMR (DMSO-d6) δ: 12.38 (s, 1H, NHCO), 7.77 (s, 1H, Ar-H), 7.69 (t, 1H, Ar-H, J = 8.0 Hz), 7.64 (d, 1H, Ar-H, J = 8 0.0 Hz), 7.39–7.21 (m, 6H, Ar-H + NH), 5.07 (s, 2H, CH2Ph), 4.99 (d, 2H, NHCH2CO, J = 8.0 Hz). 13C-NMR (DMSO-d6) δ: 169.1 (NHCO), 156.4(CH2OCO), 146.7 (SCHN), 136,9, 133.0, 131.6, 128.3, 127.8, 127.7, 127.4, 127.2, 121.3, 120.2 (Ar-C), 65.6 (CH2Ph), 43.5 (NHCH2CO), 20.9 (CH3). Elemental analysis: C18H17N3O3S required C, 60.83; H, 4.82; N, 11.82; S, 9.02, found C, 61.04; H, 4.99; N, 11.72; S, 8.89. MS m/z for C18H17N3O3S [M] + calcd. 355.1 found 355.9.
2.2.3. Tert-butyl (3–(6-methylbenzo[d]thiazol-2-yl)-2-oxopropyl)carbamate (2)
White crystals (72.6%), mp 212–213 °C. ν(CO)amide: 1706 cm−1, ν(CO)carbamate: 1666 cm−1. 1H-NMR (DMSO-d6) δ: 12.32 (s, 1H, NHCO), 7.76 (s, 1H, Ar-H), 7.62 (d, 1H, Ar-H, J = 8.2 Hz), 7.26–7.17 (m, 2H, Ar-H + NH), 3.89 (d, 2H, NHCH2CO, J = 6.0 Hz), 1.40 (s, 9H, C(CH3)3 . 13C-NMR (DMSO-d6) δ: 169.4 (NHCO), 156.8(CH2OCO), 155.9 (SCHN), 146,5, 132.9, 131.5, 127.4, 121.3, 120.1 (Ar-C), 78.2 (C(CH3)3), 43.2 (NHCH2CO), 28.1 (C(CH3)3, 20.9 (CH3). Elemental analysis: C15H19N3O3S required C, 56.06; H, 5.96; N, 13.07; S, 9.98, found. C, 55.95; H, 5.80; N, 12.95; S, 9.80, MS m/z for C15H19N3O3S [M] + calcd. 321.1 found 321.9.
2.2.4. Tert-butyl (4–(6-methylbenzo[d]thiazol-2-yl)-3-oxobutan-2-yl)carbamate (3)
White crystals (73.0%), mp 207–208 °C. ν(CO)amide: 1711 cm−1, ν(CO)carbamate: 1671 cm−1. 1H-NMR (DMSO-d6) δ: 12.35 (s, 1H, NHCO), 7.75 (s, 1H, Ar-H), 7.63 (d, 1H, Ar-H, J = 8.2 Hz), 7.30 (d, 1H, Ar-H, J = 8.2 Hz), 7.24 (d, 1H, NH, J = 8.2 Hz), 4.29 (q, 1H, CHCH3, J = 8.0 Hz), 2.41 (s, CH3), 1,51 (s, 9H, C(CH3)3), 1.27 (d, 3H, CHCH3, J = 8.0 Hz). 13C-NMR (DMSO-d6) δ: 172.9 (NHCO), 157.0(CH2OCO), 155.2 (SCHN), 146,5, 132.9, 131.6, 127.4, 121.3, 120.1 (Ar-C), 78.2 (C(CH3)3), 49.8 (NHCHCO), 20.9 (C(CH3)3, 17.4 (CH3). Elemental analysis: C16H21N3O3S required C, 57.29; H, 6.31; N, 12.53; O, 14.31; S, 9.56, found. C, 57.73; H, 6.08; N, 12.53; S, 9.30, MS m/z for C16H21N3O3S [M] + calcd. 335.1 found 321.9.
2.2.5. Tert-butyl (4–(6-methylbenzo[d]thiazol-2-yl)-3-oxo-1-phenylbutan-2-yl)carbamate (4)
White crystals (80.4%), mp 167–168 °C. ν(CO)amide: 1713 cm−1, ν(CO)carbamate: 1672 cm−1. 1H-NMR (DMSO-d6) δ: 12.55 (s, 1H, NHCO), 7.77 (s, 1H, Ar-H), 7.64 (d, 1H, Ar-H, J = 8.2 Hz), 7.54–6.88 (m, 7H + NH), 4.29 (m, 1H, NHCHCO), 3.08–3.02 and 2.88–2.80 (m, 2H, CH2Ph), 2.42 (s, CH3), 1,32 (s, 9H, C(CH3)3). 13C-NMR (DMSO-d6) δ: 171.9 (NHCO), 157.0(CH2OCO), 155.4 (SCHN), 146,5, 133.0, 131.6, 129.3, 128.0, 127.4, 126.4, 121.3, 120.1 (Ar-C), 78.3 (C(CH3)3), 56.1 (NHCHCO), 36.1 (CH2Ph), 28.1 (CH3), 21.0 (C(CH3)3. Elemental analysis: C22H25N3O3S required C, 63.98; H, 6.13; N, 10.28; S, 7.77, found. C, 64.21; H, 6.12; N, 10.21; S, 7.79, MS m/z for C22H25N3O3S [M] + calcd. 411.2 found 411.9.
2.2.6. Benzyl (3–(6-ethoxybenzo[d]thiazol-2-yl)-2-oxopropyl)carbamate (5)
White crystals (67.27%), mp 214–215 °C. ν(CO)amide: 1712 cm−1, ν(CO)carbamate: 1673 cm−1. 1H-NMR (DMSO-d6) δ: 7.84–7.10 (m, 8H, Ar-H + NH), 6.92 (dd, 1H, Ar-H, J = 2.4 Hz, 1H), 5.06 (s, 2H, CH2Ph), 4.04 (q, 2H, CH2CH3, J = 6.9 Hz), 3.88 (d, 2H, CH2CO, J = 5.8 Hz), 1.34 (t, 3H, CH2CH3, J = 6.9 Hz). 13C-NMR (DMSO-d6) δ: 170.6 (NHCO), 156.4(CH2OCO), 155.7, 154.5 (SCHN), 143.3, 137,1, 133.1, 128.3, 127.8, 127.7, 120.2, 114.3, 105.3 (Ar-C), 65.4 (CH2Ph), 63.5 (OCH2CH3) , 44.5 (NHCH2CO), 14.7 (CH2CH3). Elemental analysis: C19H19N3O3S required C, 59.21; H, 4.97; N, 10.90; S, 8.32, found C, 59.14; H, 4.95; N, 10.41; S, 7.92. MS m/z for C19H19N3O3S [M] + calcd. 385.1 found 385.9.
2.2.7. Benzyl (4–(6-ethoxybenzo[d]thiazol-2-yl)-3-oxo-1-phenylbutan-2-yl)carbamate (6)
White crystals (71%), mp 160–161 °C. ν(CO)amide: 1713 cm−1, ν(CO)carbamate: 1675 cm−1. 1H-NMR (DMSO-d6) δ: 12.57 (s, 1H, NH), 7.86 (d, 1H, NH, J = 8.1 Hz), 7.74–6.66 (m, 13H, Ar-H), 4.97 (s, 2H, CH2Ph), 4.74–4.40 (m, 1H, CH), 4.75 (q, 2H, CH2CH3, J = 8.0 Hz), 3.13–3.07 and 2.90–2.82 (m, 2H, CHCH2), 1,36 (t, 3H, CH2CH3, J = 8.0 Hz). 13C-NMR (DMSO-d6) δ: 171.5 (NHCO), 156.0 (CH2OCO), 155.7 (SCHN), 155.4, 142.5, 137,4, 136.8, 132.8, 129.3, 128.3, 128.1, 127.8, 127.6, 121.1, 115.2, 105.4 (Ar-C), 65.4 (CH2Ph), 63.6 (NHCHPh), 56.4 (OCH2CH3) , 36.9 (CHCH2Ph), 14.7 (CH2CH3). Elemental analysis: C26H25N3O4S required C, 65.67; H, 5.30; N, 8.84; S, 6.74, found C, 65.62; H, 5.25; N, 8.74; S, 6.72. MS m/z for C26H25N3O4S [M] + calcd. 475.2 found 476.0.
2.2.8. Tert-butyl (3–(6-ethoxybenzo[d]thiazol-2-yl)-2-oxopropyl)carbamate (7)
White crystals (86%), mp 171–172 °C. ν(CO)amide: 1710 cm−1, ν(CO)carbamate: 1670 cm−1. 1H-NMR (DMSO-d6) δ: 12.25 (s, 1H, NH), 7.63 (d, 1H, Ar-H, J = 12.0 Hz), 7.55 (d, 1H, Ar-H, J = 4.0 Hz), 7.19 (t, 1H, NH, J = 8.0 Hz) 7.01 (dd, 1H, Ar-H, J = 8.8 and 2.6 Hz), 4.06 (q, 2H, CH2CO, J = 6.9 Hz), 3.88 (d, 2H, CH2CO, J = 8.0 Hz), 1,41 (s, 9H, C(CH3)3), 1.35 (t, 3H,CH2CH3, J = 6.9 Hz). 13C-NMR (DMSO-d6) δ: 169.2 (NHCO), 155.9(CH2OCO), 155.6 (SCHN), 155.3, 142.5, 132,7, 121.1, 115.2, 105.4 (Ar-C), 78.2 (CH2Ph), 63.6 (OCH2CH3) , 43.1 (NHCH2CO), 28.1 (C(CH3)3), 14.6 (CH2CH3). Elemental analysis: C16H21N3O4S required C, 54.68; H, 6.02; N, 11.96; S, 9.12, found C, 54.77; H, 5.98; N, 11.88; S, 9.02. MS m/z for C19H19N3O3S [M] + calcd. 351.1 found 352.0.
2.2.9. Benzyl (3–(6-(methylsulfonyl)benzo[d]thiazol-2-yl)-2-oxopropyl)carbamate (8)
White crystals (63%), mp 178–179 °C. ν(CO)amide: 1714 cm−1, ν(CO)carbamate: 1677 cm−1. 1H-NMR (DMSO-d6) δ: 13.03 (s, 1H, NH), 8.67 (s, 1H, Ar-H), 7.96 (d, 3H, NH, J = 4.0 Hz + Ar-H), 7.40–7.27 (m, 10H, Ar-H), 4.98 (s, 1H, CH2Ph), 4.74–4.40 (m, 1H, CH), 3.27 (s, 3H, CH3), 3.16–3.10 and 2.99–2.62 (m, 2H, CHCH2). 13C-NMR (DMSO-d6) δ: 172.4 (NHCO), 161.8 (C-SO2Me), 156.4 (CH2OCO), 152.0 (SCHN), 137.2, 136.7, 135,5, 132.0, 129.3, 128.3, 128.1, 127.9, 127.6, 126.6, 124.9, 122.1, 120.9 (Ar-C), 65.3 (CH2Ph), 56.6 (CHCH2Ph), 44.0 (CH3), 36.7 (CHCH2Ph). Elemental analysis: C25H23N3O5S2 required C, 58.92; H, 4.55; N, 8.25; S, 12.58, found C, 58.95; H, 4.56; N, 8.26; S, 12.61. MS m/z for C25H23N3O5S2 [M]+ calcd. 509.1 found 510.1.
2.2.10. (9H-Fluoren-9-yl)methyl (2-((6-methylbenzo[d]thiazol-2-yl)amino)-2-oxoethyl)carbamate (9)
White crystals (81%), mp 220–221 °C. ν(CO)amide: 1709 cm−1, ν(CO)carbamate: 1671 cm−1. 1H-NMR (DMSO-d6) δ: 12.42 (s, 1H, NHCO), 7.91 (d, 2H, Ar-H), 7.82–7.70 (m, 4H, Ar-H + NH), 7.44 (t, 2H, Ar-H, J = 7.6 Hz), 7.36 (t, 2H, Ar-H, J = 7.6 Hz), 7.30–7.14 (m, 2H, Ar-H) , 4.50–4.15 (m, 3H, CH + CH2O), 4.99 (d, 2H, NHCH2CO, J = 6.0 Hz), 2.58 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ: 169.7 (NHCO), 157.4(CH2OCO), 157.1 (SCHN), 148,1, 144.3, 141.2, 130.3, 128.1, 127.6, 127.1, 125.7, 124.0, 120.6, 119.6, 110.2 (Ar-C), 66.3 (CH2OCO), 47.1 (CH), 43.9 (NHCH2CO), 18.4 (CH3). Elemental analysis: C25H21N3O3S required C, 67.70; H, 4.77; N, 9.47; S, 7.33, found C, 67.54; H, 4.65; N, 9.32; S, 7.19. MS m/z for C25H21N3O3S [M] + calcd. 443.1 found 443.7.
2.2.11. (9H-Fluoren-9-yl)methyl(2-((6-(methylsulfonyl)benzo[d]thiazol-2-yl)amino)-2-oxoethyl)carbamate (10)
White crystals (70%), mp 230–211 °C. ν(CO)amide: 1711 cm−1, ν(CO)carbamate: 1674 cm−1. 1H-NMR (DMSO-d6) δ: 12.77 (bs, 1H, NHCO), 8.66 (s, 1H, Ar-H), 7.99–7.87 (m, 4H, Ar-H) , 7.81 (t, 1H, N-H, J = 6 Hz), 7.74 (d, 2H, Ar-H, J = 7.6 Hz), 7.74 (t, 2H, Ar-H, J = 7.2 Hz), 7.36 (t, 2H, Ar-H, J = 7.2 Hz), 4.41–4.16 (m, 3H, CH + CH2O), 4.07 (d, 2H, NHCH2CO, J = 6.0 Hz), 3.26 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ: 170.5 (NHCO), 162.3 (C-SO2Me), 157.1 (CH2OCO), 152,6 (SCHN), 144.3, 141.2, 135.9, 132.5, 128.1, 127.6, 125.7, 125.3, 122.6, 121.3, 120.6 (Ar-C), 66.3 (CH2OCO), 47.1 (CH), 44.5 (NHCH2CO), 44.1 (CH3). Elemental analysis: C25H21N3O5S2 required C, 59.16; H, 4.17; N, 8.28; S, 12.63, found C, 58.90; H, 4.34; N, 7.97; S, 12.10. MS m/z for C25H21N3O5S2 [M] + calcd. 507.1 found 507.9.
2.2.12. (9H-Fluoren-9-yl)methyl (2-((4-methylbenzo[d]thiazol-2-yl)amino)-2-oxoethyl)carbamate (11)
White crystals (76%), mp 218–219 °C. ν(CO)amide: 1713 cm−1, ν(CO)carbamate: 1672 cm−1. 1H-NMR (DMSO-d6) δ: 12.35 (s, 1H, NHCO), 7.91 (d, 2H, Ar-H, J = 7.5 Hz), 7.83–7.70 (m, 4H, Ar-H + NH), 7.63 (d, 1H, Ar-H, J = 8.4 Hz), 7.25 (d, 2H, Ar-H, J = 7.6 Hz), 7.43 (t, 2H, Ar-H, J = 7.4 Hz), 7.34 (t, 2H, Ar-H, J = 7.4 Hz), 7.25 (d, 1H, Ar-H, J = 8.4 Hz), 4.39–4.18 (m, 3H, CH + CH2O), 3.99 (d, 2H, NHCH2CO, J = 6.0 Hz), 2.41 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ: 169.6 (NHCO), 157.4 (SCHN), 157.1 (CH2OCO), 144,3, 141.2, 133.5, 128.1, 127.6, 125.7, 121.9, 121.8, 120.7, 120.6, 120.5, 110.2 (Ar-C), 66.3 (CH2OCO), 47.1 (CH), 44.0 (NHCH2CO), 21.4 (CH3). Elemental analysis: C25H21N3O3S required C, 67.70; H, 4.77; N, 9.47; S, 7.23, found C, 67.54; H, 4.65; N, 9.32; S, 7.19. MS m/z for C25H21N3O3S [M] + calcd. 443.1 found 443.9.
2.2.13. Benzyl (1-((4–(6-methylbenzo[d]thiazol-2-yl)phenyl)amino)-1-oxo-3-phenylpropan-2-yl)carbamate (12)
White crystals (70,62%), mp 227–228 °C. ν(CO)amide: 1711 cm−1, ν(CO)carbamate: 1673 cm−1. 1H-NMR (DMSO-d6) δ: 10.47 (s, 1H, NH), 8.25–7.52 (m, 7H, Ar-H + NH), 7.45–6.80 (m, 11H, Ar-H), 4.99 (s, 2H, CH2Ph), 4.56–4.35 (m, 1H, CH), 3.15–2.99 (m, 1H, CHHPh), 2.98– 2.80 (m, 1H, CHHPh), 2.45 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ: 171.0 (NHCO), 165.8 (SCHN), 156.0 (CH2OCO), 151.8, 141.44, 137.70, 136.88, 135.00, 134.41, 129.22, 128.28, 128.08, 128.00, 127.83, 127.72, 127.56, 126.39, 122.13, 121.75, 119.50 (Ar-C), 65.4 (CH2Ph), 57.1 (CHCH2Ph), 37.3 (CHCH2Ph) , 21.1 (CH3). Elemental analysis: C31H27N3O3S required C, 71.38; H, 5.22; N, 8.06; S, 6.15, found C, 71.22; H, 5.21; N, 8.08; S, 6.17. MS m/z for C31H27N3O3S [M] + calcd. 521.2 found 521.9.
2.2.14. Benzyl (1-((4–(6-methylbenzo[d]thiazol-2-yl)phenyl)amino)-4-(methylthio)-1-oxobutan-2-yl)carbamate (13)
Beige crystals (70,85%), mp 200–201 °C. ν(CO)amide: 1713 cm−1, ν(CO)carbamate: 1676 cm−1. 1H-NMR (DMSO-d6) δ: 10.42 (s, 1H, NH), 8.04 (d, 2H, Ar-H), 7.98–7.86 (m, 2H, Ar-H +NH), 7.82 (d, 2H, Ar-H, J = 8.7 Hz), 7.75 (d, 1H, Ar-H, J = 7.7 Hz), 7.49–6.91 (m, 6H, Ar-H), 5.06 (s, 2H, CH2Ph), 4.37–4.20 (m, 1H, CH), 2.64–2.48 (m, 5H, CH2 + CH3), 2.45 (s, 3H, CH3), 2.00–1.65 (m, 1H, CH2S). 13C-NMR (DMSO-d6) δ: 171.1 (NHCO), 165.8 (SCHN), 156.1 (CH2OCO), 151.8, 141.5, 136.9, 135.0, 134.4, 128.3, 127.8, 122.1, 121.7, 119.5(Ar-C), 65.5 (CH2Ph), 54.8 (CHCH2CH2S), 31.3 (CH2CH2S) , 229.7 (CH3), 21.01 (SCH3), 4.6 (CH2SCH3). Elemental analysis: C27H27N3O3S2 required C, 64.13; H, 5.38; N, 8.31; S, 12.68, found C, 64.17; H, 5.28; N, 8.43; S, 12.71. MS m/z for C27H27N3O3S2 [M] + calcd. 505.2 found 505.9.
2.3. CA inhibition
An applied photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity by using method of Khalifah34. Phenol red (at a concentration of 0.2 mM) has been used as an indicator, working at the absorbance maximum of 557 nm, with 20 mM HEPES (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled–deionised water and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow the formation of the E–I complex. The inhibition constants were obtained by non-linear least-square methods using PRISM (www.graphpad.com), and non-linear least squares methods, values representing the mean of at least three different determinations, as described earlier by us35–39.
2.4. Antioxidant testing
2.4.1. DPPH radical scavenging activity
Antioxidant activity was determined based on the ability of the antioxidants to act as radical scavengers towards the stable free radical, 1,1-diphenyl-2-picrylhydrazyl (DPPH). As detailed by Yang et al.40, 1 ml of antioxidant solution (solubilised in ethanol) was added to 3 ml of a 0.1 mM ethanolic solution of DPPH. After 30 min at ambient temperature in darkness, absorbance readings were taken at 517 nm. Inhibition (%) was calculated using the equation
where As is the absorbance reading for samples containing antioxidant, Ao is the absorbance of the antioxidant in pure methanol and Ab corresponded to the absorbance of the DPPH solution.
3. Results and discussion
Benzothiazole is an important scaffold for drug development, because it exhibits a broad spectrum of pharmacological activities1,2. In this study, 13 new amino acid–benzothiazole derivatives were successfully synthesised and their human carbonic anhydrase enzyme inhibition and antioxidant capacities were determined by using the stopped flow methodology and DPPH method.
Among the carbonic anhydrase (CA, EC 4.2.1.1) enzymes (four human (h) isoforms, hCA I, hCA II, hCA V, and hCA XIII) the new amino acid benzothiazole conjugates showed more effective inhibitory activity against hCA V and hCA II than hCA I and hCA XII. In vitro antioxidant activities of the novel compounds were determined by DPPH method. Most of the synthesised compounds showed moderate to low antioxidant activities compared to the control antioxidant compounds (BHA and α-tocopherol).
3.1. Synthesis and characterisation of the new amino acid–benzothiazole derivatives
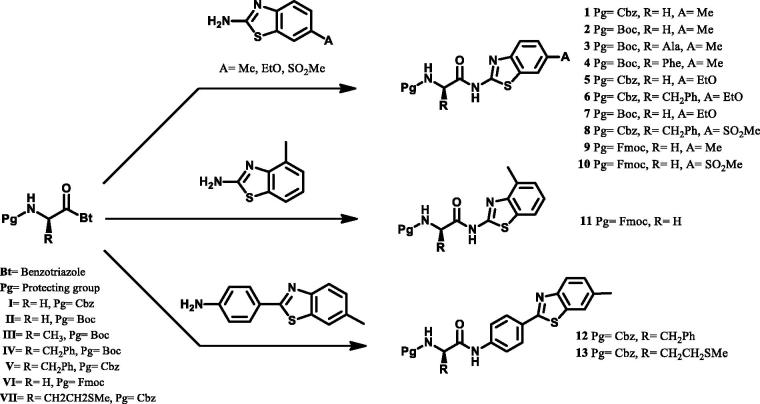
The syntheses of novel amino acid–benzothiazole derivatives reported in this study are depicted in Scheme 1. Because benzotriazole acts as easy leaving group, we chose benzotriazole-mediated methodology to synthesise the desired amino acid benzothiazole conjugates. Compounds 1–13 were prepared through a facile benzotriazole mediated acylation reaction in one step (Scheme 1) at 70 °C under microwave irradiation for 30 min in dry dichloromethane with good or high yields. To learn the effect of the protection group, we used several protection groups such as Cbz, Boc, or Fmoc. All the compounds were fully characterised by 1H, 13C NMR, MS, and FTIR (ATR) spectroscopy and elemental analyses. All spectral data were in agreement with the proposed structures. The characteristic NH resonances of the benzothiazole part of the amino acid–benzothiazole conjugates 1–13 were observed at 10.42–13.03 ppm region as singlet peak in the 1H NMR spectrum. The carbamate NH resonances of compounds 3, 6, 7, 8, and 10 were observed at 7.24, 7.86, 7.19, 7.96, and 7.8 ppm as triplet, respectively, whereas for compounds, 1, 2, 4, 5, 9, 11, 12, and 13 were observed in the aromatic region together with aromatic protons. Both NH protons were confirmed by D2O exchange. The singlet that peaks around 5.00 ppm for compounds 1, 5, 6, 8, 12, and 13 was assigned to the CH2 protons for benzyloxycarbonyl protected group whereas the upfield singlet that signals around 1.40 ppm was assigned to the tert-butyl protons of Boc-protected group for compounds, 2, 3, 4, and 7. Carbonyl resonances of the amide carbonyls and carbamate carbonyl were observed around 171 and 156 ppm, respectively. All other aliphatic and aromatic protons and carbons were observed in the expected regions. The molecular ion peaks were observed for all proposed structures of novel compounds in the mass spectra. The IR spectra of amino acid–benzothiazole conjugates, 1–13, showed characteristic amide carbonyl peaks around between 1706 and 1719 cm−1, whereas the carbamate carbonyl peaks around between 1666 and 1677 cm−1. All other spectral data were in accordance with the assumed structures.
Scheme 1.
Synthetic pathways of amino acid-benzothiazole derivatives (1–13).
3.2. Carbonic anhydrase inhibition
All the synthetised amino acid-benzothiazole conjugates have been evaluated by means of a stopped flow CO2 hydrase assay34 to test their inhibitory potency against four human (h) CA isoforms (hCA I, hCA II, hCA V, and hCA XIII). Inhibition data are reported in Table 1, along with those referred to acetazolamide (AAZ), used as standard sulphonamide inhibitor. In order to evaluate the effect of substitution at both amino and benzothiazole parts of the amino acid–benzothiazole conjugates several new compounds have been prepared from the reaction of the corresponding N-protected amino acid and appropriate benzothiazole moieties. The following structure–activity relationship (SAR) has been delineated:
Table 1.
Inhibition data of hCA I, hCA II, hCA V, and hCA XIII with compounds 1–13 and the standard sulfonamide inhibitor acetazolamide (AAZ) by a stopped flow CO2 hydrase assay.
| KI (µM)a | ||||
|---|---|---|---|---|
| Comp | hCA I | hCAII | hCA V | hCA XIII |
| 1 | 4.3 | 32.1 | 4.3 | 94.6 |
| 2 | >100 | 74.8 | 65.5 | >100 |
| 3 | 90.5 | 50.4 | 86.9 | >100 |
| 4 | >100 | >100 | >100 | >100 |
| 5 | >100 | 82.3 | >100 | >100 |
| 6 | >100 | >100 | >100 | >100 |
| 7 | >100 | >100 | 60.0 | >100 |
| 8 | 89.9 | >100 | 9.0 | >100 |
| 9 | 71.9 | >100 | 7.3 | >100 |
| 10 | 7.1 | >100 | 2.9 | >100 |
| 11 | >100 | 88.1 | 74.5 | >100 |
| 12 | >100 | 37.0 | >100 | >100 |
| 13 | >100 | 39.1 | 41.2 | 84.9 |
| AAZ | 0.25 | 0.012 | 0.063 | 0.017 |
aMean from three different assays, by a stopped flow technique (errors were in the range of ±5–10% of the reported values).
Compounds 1, 3, 8–10 showed Ki values in the low micromolar levels ranging from 4.3 to 90.5 µM (Table 1). Among these compounds, Me and SO2Me substituents at 6 position of the benzothiazole incorporating glycine, alanine and phenylalanine made positive contribution to the inhibition activity.
Compounds 1–3, 5, and 11–13 showed considerable inhibition against CA II with Ki values in the low micromolar levels, ranging from 32.1 to 88.1 µM (Table 1).
As seen in Table 1, nearly all compounds displayed potent inhibition against CA V with Ki values in the low micromolar levels, ranging from 2.9 to 86.9 µM, except compounds 4–6 and 12.
Among the compounds, only compounds 1 and 13 showed some inhibition against the tumour associated isoform CA XII with Ki values with 94.6 and 84.9 µM, respectively.
3.3. Antioxidant testing
3.3.1. DPPH radical scavenging activity
The antioxidant activity of the compounds was determined based on the ability of the antioxidants to act as radical scavengers towards the stable free radical, 1,1-diphenyl-2-picrylhydrazyl (DPPH)40.
The antioxidant results of the new compounds were given in Table 2. Among the tested compounds seen in Scheme 1, the compounds bearing ethoxy group at 6 position of the benzothiazole ring was found to be the most effective antioxidant at 62.5 and 125 μg/ml concentrations with 32 and 38%. The compounds bearing methylsulfonyl at 6 position of the benzothiazole ring was found to be next effective antioxidant at 62.5 and 125 μg/ml concentrations with 22 and 23%. With respect to the amino acid part of the compounds, glycine and phenylalanine derivatives seemed to be more active for the antioxidant activities.
Table 2.
Antioxidant activities of the synthesised amino acid-benzothiazole derivatives.
| Antioxidant activity, % |
|||||
|---|---|---|---|---|---|
| Comp. no | 12.5 μg/ml | 25 μg/ml | 37.5 μg/ml | 62.5 μg/ml | 125 μg/ml |
| 1 | 0 | 0 | 0 | 11.4 | 12.5 |
| 2 | 0 | 0 | 3.9 | 4.6 | 6.1 |
| 3 | 3.3 | 3.4 | 5.3 | 7.5 | 7.7 |
| 4 | 0 | 5.7 | 5.8 | 9.6 | 10,4 |
| 5 | 12.0 | 15.0 | 25.0 | 32.0 | 38.0 |
| 6 | 11.0 | 14.0 | 23.0 | 32.0 | 37.0 |
| 7 | 13.0 | 15.0 | 22.5 | 24.8 | 28.3 |
| 8 | 0 | 2.1 | 6.6 | 19.2 | 20.7 |
| 9 | 4 | 4.6 | 5.3 | 5.8 | 6.4 |
| 10 | 0 | 0 | 22.5 | 23.1 | 24.5 |
| 11 | 5 | 6.7 | 6.9 | 7.1 | 8.2 |
| 12 | 0 | 3 | 3.4 | 5.8 | 6.1 |
| 13 | 5 | 6.7 | 6.1 | 7.1 | 8.2 |
| α-Toc. | 62.9 | 63.4 | 68.4 | 72.8 | 74.0 |
| BHA | 61.1 | 63.0 | 67.5 | 71.0 | 72.4 |
4. Conclusions
In this study, 13 new amino acid-sulfathiazole conjugates were synthesised, and their carbonic anhydrase inhibitory properties were determined against human carbonic anhydrase hCA I, hCA II, hCA V, and hCA XII. The new amino acid-benzothiazole conjugates showed considerable inhibition against, hCA V and hCA II with Ki values in the micromolar levels, ranging from 2.9 to 88.1 µM. Among the compounds, compound 1 showed potent enzyme inhibition against all carbonic anhydrase enzymes studied in this work with micromolar levels. In vitro antioxidant activities of the novel compounds were determined by DPPH method. On the other hand, most of the synthesised compounds showed moderate to low antioxidant activities compared to the control antioxidant compounds (BHA and α-tocopherol).
Funding Statement
We thank Inönü University, Turkey (BAPB – Grand No-2016/94) and Universita` degli Studi di Firenze, Italy.
Disclosure statement
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Gill RK, Rawal RK, Bariwal J. Recent advances in the chemistry and biology of benzothiazoles. Arch Pharm (Weinheim) 2015;348:155–78. doi: 10.1002/ardp.201400340 [DOI] [PubMed] [Google Scholar]
- 2.Sharma PC, Sinhmar A, Sharma A, et al. Medicinal significance of benzothiazole scaffold: an insight view. J Enzyme Inhib Med Chem 2013;28:240–66. [DOI] [PubMed] [Google Scholar]
- 3.Telvekar VN, Bairwa VK, Satardekar K, Bellubi A. Novel 2-(2-(4-aryloxybenzylidene) hydrazinyl)benzothiazole derivatives as anti-tubercular agents. Bioorganic Med Chem Lett 2012;22:649–52. [DOI] [PubMed] [Google Scholar]
- 4.Küçükbay H, Çetinkaya E, Durmaz R. Synthesis and antimicrobial activity ofsubstituted benzimidazole, benzothiazole and imidazole derivatives. Arzneim Forsch/Drug Des 1995;45:1331–4. [Google Scholar]
- 5.Küçükbay H, Durmaz B. Antifungal activity of organic and organometallic derivatives of benzimidazole and benzothiazole. Arzneim Forsch/Drug Des 1997;47:667–70. [PubMed] [Google Scholar]
- 6.Stella A, Segers K, De Jonghe S, et al. Synthesis and antibacterial evaluation of a novel series of 2-(1,2-dihydro-3-oxo-3H-pyrazol-2-yl)benzothiazoles. Chem Biodivers 2011;8:253–65. [DOI] [PubMed] [Google Scholar]
- 7.Venugopala KN, Krishnappa M, Nayak SK, et al. Synthesis and antimosquito properties of 2,6-substituted benzo[d]thiazole and 2,4-substituted benzo[d]thiazole analogues against Anopheles arabiensis. Eur J Med Chem 2013;65:295–303. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui N, Alam MS, Sahu M, et al. Design, synthesis, anticonvulsant evaluation and docking study of 2-[(6-substituted benzo[d]thiazol-2-ylcarbamoyl)methyl]-1-(4-substituted phenyl)isothioureas. Bioorg Chem 2017;71:230–43. [DOI] [PubMed] [Google Scholar]
- 9.Thaslim Basha S, Sudhamani H, Rasheed S, et al. Microwave-assisted neat synthesis of α-aminophosphonate/phosphinate derivatives of 2-(2-aminophenyl)benzothiazole as potent antimicrobial and antioxidant agents. Phosphorus Sulfur Silicon Relat Elem 2016;191:1339–43. [Google Scholar]
- 10.Meltzer-Mats E, Babai-Shani G, Pasternak L, et al. Synthesis and mechanism of hypoglycemic activity of benzothiazole derivatives. J Med Chem 2013;56:5335–50. [DOI] [PubMed] [Google Scholar]
- 11.Sović I, Jambon S, Kraljević Pavelić S, et al. Synthesis, antitumor activity and DNA binding features of benzothiazolyl and benzimidazolyl substituted isoindolines. Bioorganic Med Chem 2018;26:1950–60. [DOI] [PubMed] [Google Scholar]
- 12.Küçükbay FZ, Buğday N, Küçükbay H, et al. Synthesis, characterization and carbonic anhydrase inhibitory activity of novel benzothiazole derivatives. J Enzyme Inhib Med Chem 2016;31:1221–5. [DOI] [PubMed] [Google Scholar]
- 13.Costanzo MJ, Yabut SC, Almond HR, et al. Potent, small-molecule inhibitors of human mast cell tryptase. Antiasthmatic action of a dipeptide-based transition-state analogue containing a benzothiazole ketone. J Med Chem 2003;46:3865–76. [DOI] [PubMed] [Google Scholar]
- 14.Yao S, Schafer-Hales KJ, Belfield KD. A new water-soluble near-neutral ratiometric fluorescent pH indicator. Org Lett 2007;9:5645–8. [DOI] [PubMed] [Google Scholar]
- 15.Komatsu K, Urano Y, Kojima H, Nagano T. Development of an iminocoumarin-based zinc sensor suitable for ratiometric fluorescence imaging of neuronal zinc. J Am Chem Soc 2007;129:13447–54. [DOI] [PubMed] [Google Scholar]
- 16.Ioka S, Saitoh T, Iwano S, et al. Synthesis of firefly luciferin analogues and evaluation of the luminescent properties. Chem A Eur J 2016;22:9330–7. [DOI] [PubMed] [Google Scholar]
- 17.Gabr MT, Pigge FC. Rhenium complexes of bis(benzothiazole)-based tetraarylethylenes as selective luminescent probes for amyloid fibrils. Chem A Eur J 2018;24:11729–37. doi: 10.1002/chem.201801801 [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Cheng X, Wang L, et al. Hydrogenation of tar residue derived from the synthesis of rubber vulcanization accelerator 2-mercaptobenzothiazole. Asia Pacific J Chem Eng 2017;12:400–5. [Google Scholar]
- 19.Lalinde E, Lara R, López IP, et al. Benzothiazole-based cycloplatinated chromophores: Synthetic, optical, and biological studies. Chem A Eur J 2018;24:2440–56. [DOI] [PubMed] [Google Scholar]
- 20.Meleddu R, Distinto S, Cottiglia F, et al. Tuning the dual inhibition of carbonic anhydrase and cyclooxygenase by dihydrothiazole benzensulfonamides. ACS Med Chem Lett 2018;9:1045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiaramonte N, Romanelli MN, Teodori E, Supuran CT. Amino acids as building blocks for carbonic anhydrase inhibitors. Metabolites 2018;8:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Supuran CT, Alterio V, Di Fiore A, et al. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: Three for the price of one. Med Res Rev 2018;38:1799–836. [DOI] [PubMed] [Google Scholar]
- 23.Küçükbay FZ, Küçükbay H, Tanc M, Supuran CT. Synthesis and carbonic anhydrase inhibitory properties of amino acid – coumarin/quinolinone conjugates incorporating glycine, alanine and phenylalanine moieties. J Enzyme Inhib Med Chem 2016;31:1198–202. [DOI] [PubMed] [Google Scholar]
- 24.Küçükbay FZ, Küçükbay H, Tanc M, Supuran CT. Synthesis and carbonic anhydrase I, II, IV and XII inhibitory properties of N-protected amino acid – sulfonamide conjugates. J Enzyme Inhib Med Chem 2016;31:1476–83. [DOI] [PubMed] [Google Scholar]
- 25.Buğday N, Küçükbay FZ, Küçükbay H, et al. Synthesis of novel dipeptide sulfonamide conjugates with effective carbonic anhydrase I, II, IX, and XII inhibitory properties. Bioorg Chem 2018; 81:311–8. [DOI] [PubMed] [Google Scholar]
- 26.Küçükbay H, Buğday N, Küçükbay FZ, et al. Bioorganic chemistry synthesis and carbonic anhydrase inhibitory properties of novel 4- (2-aminoethyl) benzenesulfonamide-dipeptide conjugates. Bioorg Chem 2019;83:414–23. [DOI] [PubMed] [Google Scholar]
- 27.von Neubeck B, Gondi G, Riganti C, et al. An inhibitory antibody targeting carbonic anhydrase XII abrogates chemoresistance and significantly reduces lung metastases in an orthotopic breast cancer model in vivo. Int J Cancer 2018;143:2065–75. [DOI] [PubMed] [Google Scholar]
- 28.Angeli A, Trallori E, Carta F, et al. Heterocoumarins are selective carbonic anhydrase IX and XII inhibitors with cytotoxic effects against cancer cells lines. ACS Med Chem Lett 2018;9:947–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vullo D, Innocenti A, Nishimori I, et al. Carbonic anhydrase inhibitors. Inhibition of the transmembrane isozyme XII with sulfonamides – a new target for the design of antitumor and antiglaucoma drugs? Bioorganic Med Chem Lett 2005;15:963–9. [DOI] [PubMed] [Google Scholar]
- 30.El Khatib M, Jauregui L, Tala SR, et al. Solution-phase synthesis of chiral O-acyl isodipeptides. Medchemcomm 2011;2:1087–92. [Google Scholar]
- 31.Panda SS, Ibrahim MA, Küçükbay H, et al. Synthesis and antimalarial bioassay of quinine - peptide conjugates. Chem Biol Drug Des 2013;82:361–6. [DOI] [PubMed] [Google Scholar]
- 32.Katritzky AR, Singh A, Haase DN, Yoshioka M. N-(Fmoc-α-aminoacyl)benzotriazoles: Versatile synthetic reagents from proteinogenic amino acids. Arkivoc 2009;2009:47–56. [Google Scholar]
- 33.Ibrahim MA, Panda SS, Oliferenko AA, et al. Macrocyclic peptidomimetics with antimicrobial activity: Synthesis, bioassay, and molecular modeling studies. Org Biomol Chem 2015;13:9492–503. [DOI] [PubMed] [Google Scholar]
- 34.Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73. [PubMed] [Google Scholar]
- 35.Mincione F, Starnotti M, Menabuoni L, et al. Carbonic anhydrase inhibitors: 4-sulfamoyl-benzenecarboxamides and 4-chloro-3-sulfamoyl-benzenecarboxamides with strong topical antiglaucoma properties. Bioorganic Med Chem Lett 2001;11:1787–91. [DOI] [PubMed] [Google Scholar]
- 36.Del Prete S, Vullo D, De Luca V, et al. Biochemical characterization of recombinant β-carbonic anhydrase (PgiCAb) identified in the genome of the oral pathogenic bacterium Porphyromonas gingivalis. J Enzyme Inhib Med Chem 2015;30:366–70. [DOI] [PubMed] [Google Scholar]
- 37.Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–50. [DOI] [PubMed] [Google Scholar]
- 38.Maresca A, Vullo D, Scozzafava A, et al. Inhibition of the β-class carbonic anhydrases from mycobacterium tuberculosis with carboxylic acids. J Enzyme Inhib Med Chem 2013;28:392–6. [DOI] [PubMed] [Google Scholar]
- 39.Scozzafava A, Passaponti M, Supuran CT, Gülçin I. Carbonic anhydrase inhibitors: Guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX and XII). J Enzyme Inhib Med Chem 2015;30:586–91. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Guo J, Yuan J. In vitro antioxidant properties of rutin. LWT Food Sci Technol 2008;41:1060–6. [Google Scholar]