Highlights
-
•
Proteobacteria, Bacteroidetes, Firmicutes, Actinobacteria and Fusobacteria. dominated the bacteria of Labroides dimidiatus.
-
•
A total of 36 classes and 132 families were identified of the 1,426,740 amplicon sequence reads obtained.
-
•
The bacterial identified is composed of both pathogenic zoonotic and non-harmful groups.
Keywords: Bluestreak cleaner wrasse, Skin mucus, Bacterial community, Microbiota
Abstract
This study was designed to evaluate the bacterial composition of the Labroides dimidiatus and its surrounding water. Fish and carriage water samples were obtained from corals of the Karah Island in Terengganu Malaysia. DNA was extracted and the bacteria communities on the skin mucus and stomach as well as water sample were classified (to family level) using the 16S rRNA-based metagenomics analysis. 1,426,740 amplicon sequence reads corresponding to 508 total operational taxonomic units were obtained from the three metagenomics libraries in this study. The Proteobacteria, Bacteroidetes, Firmicutes, Actinobacteria and Fusobacteria were the most dominant bacterial phyla in all samples. A total of 36 different classes and 132 families were identified, many of which had shared presence in all samples while others were exclusive to different sample. Thirty-three of these were identified as pathogenic zoonotic bacterial. The results obtained indicate a strong influence of host environment on the composition of its microbiota. Knowing the composition of the microbiota is the first step toward exploring proper management of this ornamental fish in captivity.
1. Introduction
Interest in ornamental fish keeping is rapidly growing globally. It is currently considered the second largest global hobby next to photography [1]. However, majority of the marine ornamental fishes are caught from the wild [2]. Hence, understanding the microbial community associated with wild ornamental fishes could provide useful information about the husbandry requirement, health management, as well as help dictate effective biosecurity measure for these fishes to be raised in captivity. Gerzova et al., [3] had earlier suggested the fact that ornamental fishes are grossly understudied with respect to microbial communities, and could be potential sources of pathogenic infections. This is a public health concern in the pet industry due to the possibility of cross infections (between fishes) and zoonotic infections (between fish and man). The knowledge of the susceptibility of these fish to different microbes in the wild could help dictate measures that would prevent transfer of potentially pathogenic bacteria from infected fish to others fish group reared together in captivity and vice versa.
The Labroides dimidiatus is a very popular marine ornamental fish accounting for about 36% of the total ornamental imports to the United States of America [4]. This species is part of a larger group of fishes commonly known as the “Bluestreak cleaner wrasse”. The group name reflects their “cleaning behavior” in the ocean; hence, they play an important ecological role in the coral reefs [[5], [6], [7]]. Generally, they specialize in removing ecto-parasites, diseased and injured tissue, as well as unwanted food particles from the fishes that visit them [8]. This “cleaning” attribute has also been exploited in the Aquaculture industries in some part of the world. For instance in Norway, different wrasse species are commonly used for the control of sea-lice in the Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) industries [9]. Hence, the environmental condition as well as the life cycles of these fishes could predispose them to a wide range of microbial infections.
However, there is paucity of information on the bacteria composition associated with many Bluestreak cleaner wrasse species. One of the first steps to ensuring a heathy husbandry condition for aquarium fishes is to have a good understanding of the microbiome balance [10]. More so, knowledge of the bacterial communities (pathogenic or not) would help understand the complex relationship between the bacteria and its host (fish). In this study therefore, the bacterial composition on the skin and in the stomach of L. dimidiatus as well as that around in the surrounding water were investigated. We employed the metagenomics technique combined with 16S rRNA gene and the Next Generation Sequencing (NGS) analyses to identify and compare the diversities of bacteria observed.
2. Materials and methods
2.1. Sample collection and preparation
Samples of L. dimidiatus (between 0.5–2.8 g) were obtained from different location at the coral area of the Karah Island, Terengganu Malaysia. The fish caught were immediately transported to the Anatomy and Physiology Laboratory in the School of Fisheries and Aquaculture Sciences, Universiti Malaysia Terengganu for analysis. Ten healthy fish were tranquilized with 150 mg/1 solutions of tricaine methane sulphonate (MS222) [11] and killed by pitching. They were gently washed thrice with sterile seawater to remove the dirt on the fish without compromising the microbial colonies on the skin. The skin mucus was collected by dorsolateral scraping of the surface of the dead L. dimidiatus specimens with the aid of a sterile scalpel [12]. The collected mucus (i.e. ten samples from the ten fish) was placed in 1.5 ml microcentrifuge tubes, centrifuged for 10 min (12, 000 × g for at 4 °C) and stored at −80 °C until DNA analysis was done. The technique for sample collection from the fish stomach used in this study was a modification of earlier technique reported by Balcázar et al., [12]. The stomach of the ten fish samples were carefully removed and flashes frozen in 50 ml sterile falcon tubes, and then stored at −80 °C until DNA extraction was done. Water samples for analysis (ten in number) were earlier collected simultaneously during fish sampling using the method adopted by Smith et al., [13]. In brief, water samples were collected in sterilized blue cap bottles, store in cold storage boxes and transported to the laboratory for analysis.
2.2. DNA extraction
DNA was extracted using the NucleoSpin® Tissue Kit (Machery-Nagel, Germany) following the manufacturer’s protocol. Samples of the skin mucus were first centrifuged for 5 min (13000rcf) before DNA was extracted. 25 mg of the collected fish stomach was homogenised in 50–75 μL phosphate buffer saline (PBS). The water sample on the other hand was conditioned following the method specified by Wolf et al., [14] before DNA extraction was done. After DNA extraction from the different samples, the quality (102.69 to 381.87 ng/ul) and concentration (1.8–2.08) was determined (using biodrop machine) and found to within recommended range before storage at −20 °C.
2.3. 16S rRNA bacterial Amplification
The 16S rRNA gene in this study was amplified using the universal bacterial primer set 63 F (5‟-CAGGCCTAACACATGCAAGTC-3‟) and 1389R (5‟-ACGGGCGGTGTGTACAAG-3‟) [15]. A reaction volume of 50 μL was used for the PCR containing DNA template, 1× Reaction Buffer, 2.5 mM MgCl2, 200 μM of each dATP, dCTP, dGTP and dTTP (Vivantis Technologies, Malaysia), 0.5 μM of each primer and 2 U of Taq polymerase (Vivantis Technologies, Malaysia). The PCR condition were programmed for a 2 min initial denaturation at 95 °C, followed by 24 cycles of denaturation (at 95 °C for 30 s), annealing (at 53 °C for 1 min), and extension (at 72 °C for 2 min) and a final extension at 72 °C for 10 min. The reaction was performed on a PTC-0200 G thermo cycler (Bio-Rad Laboratories, Inc., USA). Beside PCR clean up done to purify the PCR product in this study, it is important to state that a negative control was included in the PCR amplification to ensure there was no contamination of reagents.
2.4. Illumina library generation: amplicon PCR
In a second PCR, 1 μL of amplicon was used. By following the method described by Bartram et al., [16], the V3 hypervariable region of the 16S rRNA genes was selected for this method because of its taxonomic resolution [17], conserved flanking regions [18], and length [19] (≥170 to 190 nucleotides), which is compatible with paired-end 125-base read assembly. Hence, the V3 region of the 16S rRNA gene was amplified using modified 341 F and 518R primers [18]. In addition, the V3 specific priming regions primers were complementary to the standard Illumina forward and reverse primers (Table 1). Hence, the primers contained a 6-bp indexing sequence to allow for multiplexing. Amplification primers were designed with Illumina adapters. PCR amplifications were carried out for each sample, using a reaction volume of 25 μL containing 12.5 μL of 2X KAPA HiFi HotStart Ready Mix, 0.75 μL of each 10μM Forward and Reverse primers, 1 μL of 12.5 ng/μL DNA templates and PCR-grade water up to 25 μL. The PCR conditions for amplification were as follows: 95 °C initial denaturation for 3 min, followed by 15 cycles of denaturation (at 98 °C for 20 s), annealing (at 67 °C for 15 s) and extension (at 72 °C for 15 s), and a final extension at 72 °C for 1 min. The PCR was performed in a DNA Engine thermocycler (Bio- Rad, Mississauga, Ontario, Canada).
Table 1.
Primers profiles used for the Illumina library construction.
| Sample | Primer name | Oligonucleotide sequence (5’–3’) | Reference |
|---|---|---|---|
| All samples | V3_F | aatcatacggcgaccaccgagatctacactctttccctacacgacgctcttccgatctCCTACGGGAGGCAGCAG | Bartram et al., (2011) |
| Skin mucus | V3_7R | caagcagaagacggcatacgagatGATCTGgtgactggagttcagacgtgtgctcttcccgatctATTACCGCGGCTGCTGG | Bartram et al., (2011) |
| Stomach content | V3_5R | caagcagaagacggcatacgagatCACTGTgtgactggagttcagacgtgtgctcttcccgatctATTACCGCGGCTGCTGG | Bartram et al., (2011) |
| Carriage Water | V3_13R | caagcagaagacggcatacgagatCGTACTgtgactggagttcagacgtgtgctcttcccgatctATTACCGCGGCTGCTGG | Bartram et al., (2011) |
| All samples | 341 F | CCTACGGGAGGCAGCAG | Muyzer et al., 1993 |
| All samples | 518R | ATTACCGCGGCTGCTGG | Muyzer et al., 1993 |
Lowercase letters denote adapter sequences necessary for binding to the flow cell, underlined lowercase are binding sites for the Illumina sequencing primers, bold uppercase highlight the index sequences (the first 12 indexes were obtained from Illumina) and regular uppercase are the V3 region primers (341 F on for the forward primers and 518R for the reverse primers) (Bartram et al., 2011).
Using a gel electrophoresis (2% agarose), the PCR products of the correct size were separated from the primers/primer dimers and recovered using a Gel Extraction and PCR Purification Combo Kit, Spin-column (BioTeke Corporation, Beijing, China). The purified PCR products were then sequenced using Illumina MiSeq Desktop Sequencer (Illumina, Inc.) (Service provided by the Science Vision Sdn. Bhd., Malaysia).
2.5. 16S rRNA-Based taxonomic analysis
The generated multi-million reads were trimmed and assembled using Mothur software [20]. Overlapping regions within Illumina paired-end reads were aligned to generate “contigs.” If a mismatch was discovered, the paired end sequences involved in the assembly were discarded. All sequences with ambiguous base calls were also discarded. Sequences were then assigned taxonomic affiliations based on naïve Bayesian classification (RDP classifier) [21].
After trimming, screening and alignment of the sequences; they were assigned to operational taxonomic units of six samples of 16S rRNA gene fragments. The sequenced data was connected to server and the fastq file was downloaded. A tab-delimited “oligos” file containing the primer and barcode information was created. Then, the Greengenes reference files from the Mothur website (http://www.mothur.org/wiki/Taxonomy_outline) was downloaded, and the data analysis was performed. The operational taxonomic units (OTU) of the bacterial colonies were defined by a pairwise similarity cutoff of 97% using the Ribosomal Database Project [22] pyrosequencing pipeline.
3. Results
The total DNA successfully purified from skin mucus, stomach and water samples was appropriate for the subsequent PCR amplification (DNA yield ranged between 102.69–381.87 ng/μL; DNA purity ranged from 1.8 to 2.08). A near-full length of ˜1400bp was successfully amplified in all samples for the 16S rRNA gene. Similarly, a ˜330bp fragment was amplified for the nested PCR (with V3 regions) in all samples examined. The secondary amplification created a single amplicon of conserved region for compatibility with Illumina index and sequencing adapters.
3.1. Bacteria composition in Labroides dimidiatus from Karah Island, Terengganu Malaysia
Total number of amplicon sequences reads obtained from the skin mucus and stomach of L. dimidiatus as well as the carriage water was 1,426,740 reads (Table 2). The highest reads of 731,548 was obtained from the stomach samples, followed by the skin mucus (455,709 reads), while the carriage water had lower reads of the study (239,483 reads). In contrast, the operational taxonomy units (OTU) in the carriage water was more diverse (227) than those of the skin mucus (164) or stomach samples (117).
Table 2.
Characteristics of 16S rRNA metagenomic libraries of Labroides dimidiatus from Karah Island, Terengganu Malaysia.
| Skin mucus | Stomach content | Carriage water | |
|---|---|---|---|
| Amplicon sequences | 455,709 | 731,548 | 239,483 |
| Total of single-reads OTUs | 164 | 117 | 227 |
| Percentage of total single reads OTUs (%) | 37.104 | 26.471 | 51.357 |
3.2. Phyla of associated Bacteria isolated in Labroides dimidiatus from Karah Island, Terengganu Malaysia
Taxonomic analysis of the V3 16S rRNA gene amplicon reads yielded a total of twelve classifiable phyla (Table 3). Five of which were dominant in the entire sample namely; Proteobacteria (57.7–65.9%), Bacteroidetes (7.7–13.4%), Firmicutes (10.4–15.4%), Actinobacteria (7.3–12.0%) and Fusobacteria (1.3–1.8%). The skin mucus harbored the higher percentage of Proteobacteria, Bacteroidetes and Fusobacteria. Similarly, the highest percentage of Actinobacteria was observed in the stomach. While, the highest percentage of Firmicutes observed were identified both in the stomach and the carriage water. However, the carriage water samples contained the highest number of phyla [11] when compared to those identified in the stomach (8 phyla) and skin mucus (7 phyla). In addition to the five abundant and the unclassified phyla identified across all samples, Acidobacteria, planctomycetes, Nitrospira, TM7 and Tenericutes were isolated in the carriage water; however, at very low quantities (0.4%). Also, the Spirochaetes phylum was a unique phyla detected only in stomach of L. dimidiatus.
Table 3.
Percentage of bacterial phyla associated with Labroides dimidiatus from Karah Island, Terengganu Malaysia.
| Phyla2 | Skin mucus | Stomach content | Carriage water |
|---|---|---|---|
| Proteobacteria | 65.9 | 60.7 | 57.7 |
| Bacteroidetes | 13.4 | 7.7 | 12.8 |
| Firmicutes | 10.4 | 15.4 | 15.4 |
| Actinobacteria | 7.3 | 12.0 | 8.4 |
| Fusobacteria | 1.8 | 1.7 | 1.3 |
| Acidobacteria | 0.0 | 0.9 | 2.2 |
| Spirochaetes | 0.0 | 0.9 | 0.0 |
| Unclassified | 0.6 | 0.9 | 0.4 |
| Nitrospira | 0.0 | 0.0 | 0.4 |
| Planctomycetes | 0.6 | 0.0 | 0.4 |
| TM7 | 0.0 | 0.0 | 0.4 |
| Tenericutes | 0.0 | 0.0 | 0.4 |
Phylum taxonomy and abundance were classified based on a confidence using the RDP Classifier. Values are given as a percentage.
3.3. Distribution of bacterial community at class level
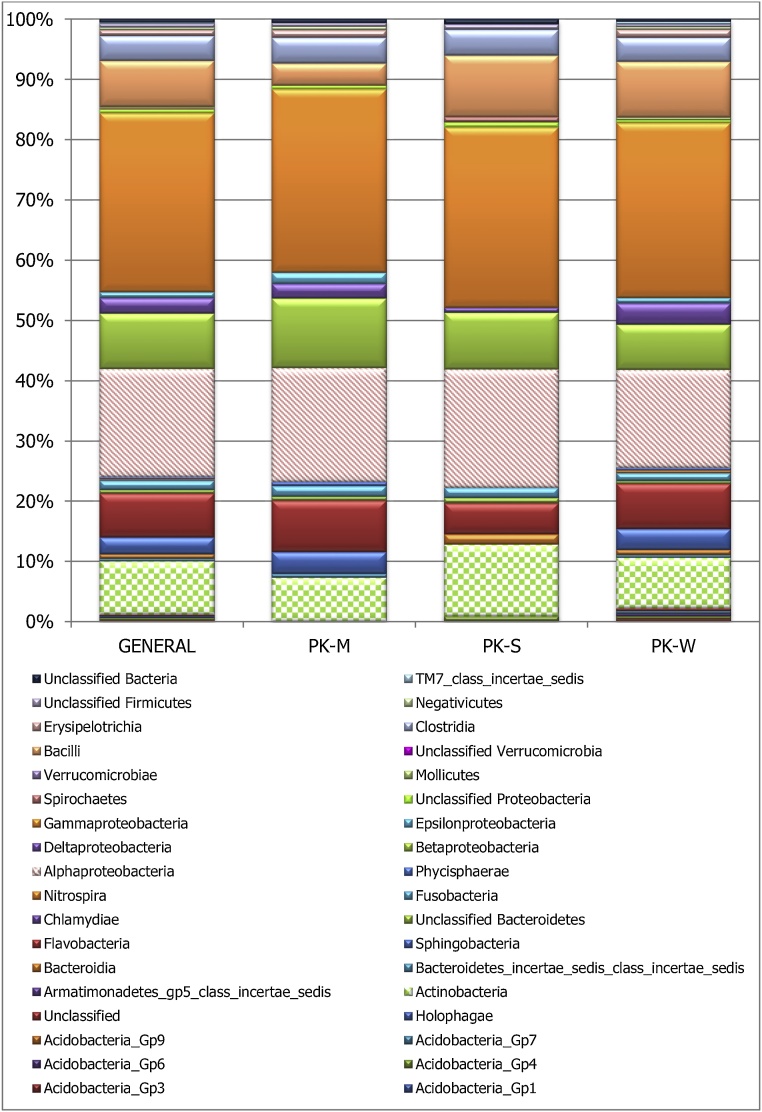
In this study, a total of 36 different classes of bacteria were identified in this study. The associated bacteria classes in the carriage water were more diverse than those in the skin mucus as well as those in the stomach of L. dimidiatus. However, most of these classes were about 1% or less of the total bacterial isolated, hence, limiting their resolution (Fig. 1). In general, the Gammaproteobacteria represented about 29.7%. While the Alphaproteobacteria and Betaproteobacteria were found at 29.7% and 17.91%. Deltaproteobacteria and Epsilonproteobacteria in the phyla Protebacteria were detected at 2.6% and less than 1% respectively. Actinobacteria, Bacilli, Flavobacteria, were also found at more than 5%. However, Clostridia, Sphingobacteria, Deltaproteobacteria, Fusobacteria were found at less than 5%.
Fig. 1.
Taxonomic diversity and relative abundance the different classes of Bacteria associated with Labroides dimiditus from Karah Island, Terengganu Malaysia.
PK = Karah Island, M = skin mucus, S = stomach W = carriage water. General = PK-M + PK-S + PK-W (i.e. the cumulative Bacteria classes associated with the Labroides dimiditus as observed in the mucus, stomach and carriage water).
3.4. Distribution of bacterial community at order level
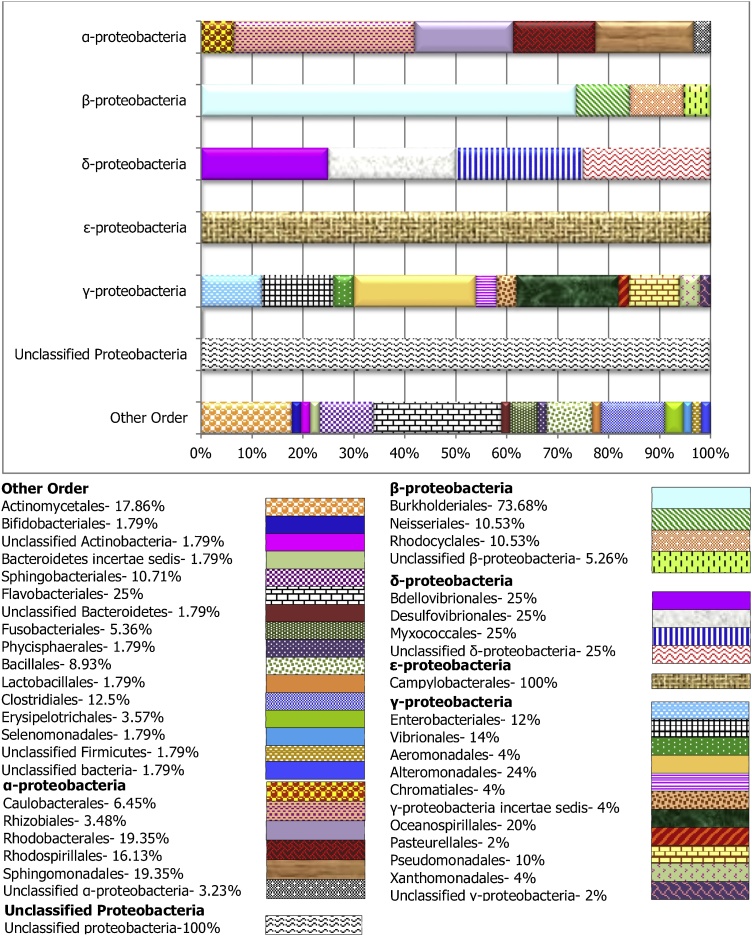
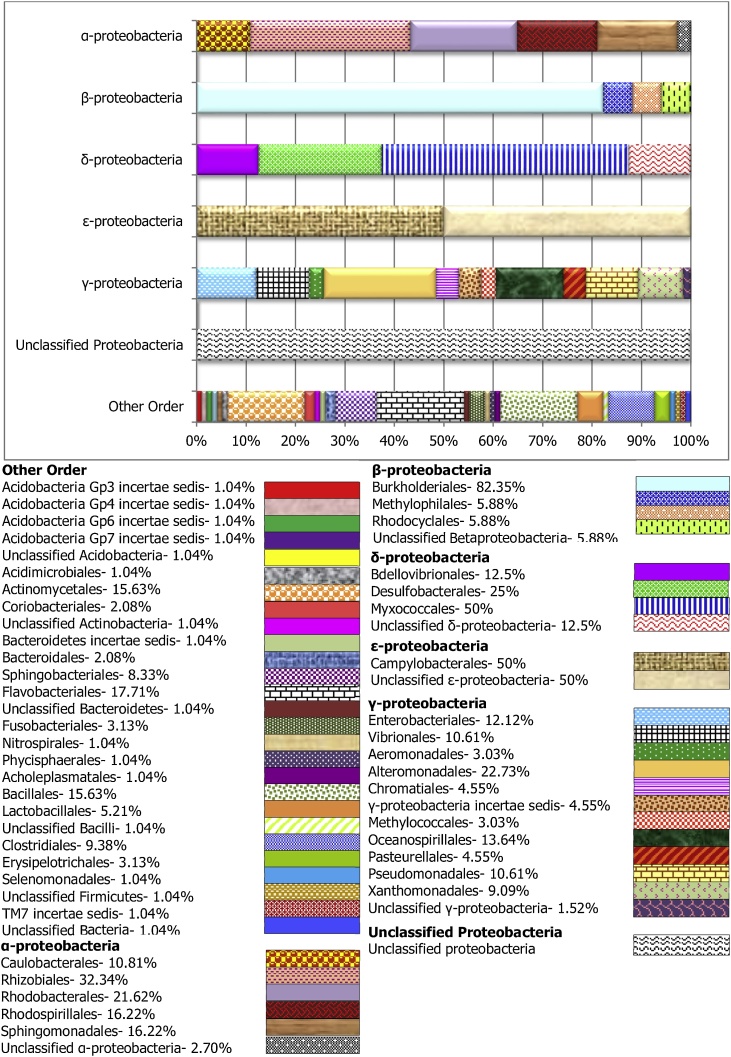
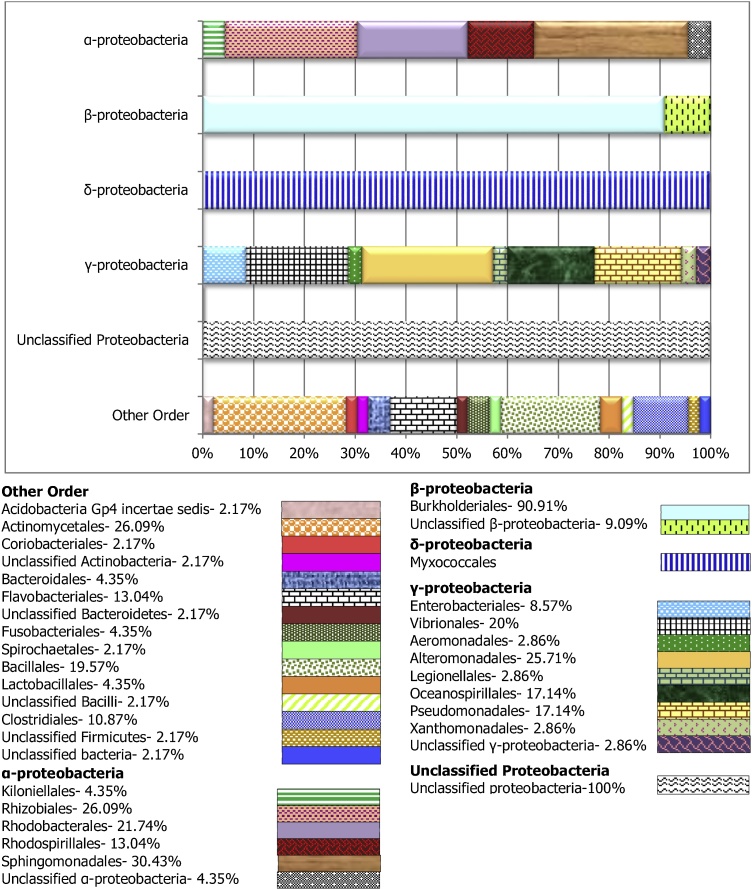
To assess the microbial composition, the most abundant class observed; Proteobacteria was categorized into different order level, while the remaining “orders” were grouped as “other” (Figs. 2, 3 and 4). The Gammaproteobacteria, were the largest chunk of Proteobacteria observed in this study, and with the largest number of orders isolated. The most prominent members include the Alteromonadales and Oceanospirillales. The Betaproteobacteria in all the sample groups were dominated by the Burkholderiales, while, the Alphaproteobacteria were composed primarily of Rhizobiales, Rhodobacterales, Caulobacterales and Sphingomonadales. Similarly, the Bdellovibrionales, Desulfovibrionales, and Myxococcales are commonly orders of the Deltaproteobacteria found in the carriage water (Fig. 4) and the skin (Fig. 2). However, only the Myxococcales was observed in the stomach for this group of bacterial (Fig. 3). Among the dominate orders grouped as “others” in this study are the Bacillales, Flavobacteriales, Actinomycetales, and Sphingobacteriales. Both Flavobacteriales and Sphingobacteriales are members of the phylum Bacteroidetes while Actinomycetales is a member of the Actinobacteria. These two phyla were earlier reported to be among the dominant isolated phylum after Proteobacteria in this study.
Fig. 2.
Taxonomic diversity and relative abundance at the order level of bacteria associated with skin mucus sample of Labroides dimiditus from Karah Island, Terengganu Malaysia. Each bar denotes individually orders in Alpha-, Beta-, Delta-, Epsilon-, and Gamma-proteobacteria respectively, while the last bar (others) shows orders in others phylum.
Fig. 4.
Taxonomic composition and relative abundance at the order level of bacteria associated with carriage water from Karah Island, Terengganu Malaysia. Each bar denotes individually orders in Alpha-, Beta-, Delta-, Epsilon-, and Gamma-proteobacteria respectively, while the last bar (others) shows orders in others phylum.
Fig. 3.
Taxonomic diversity and relative abundance at the order level of bacteria associated with stomach sample of Labroides dimiditus from Karah Island, Terengganu Malaysia. Each bar denotes individually orders in Alpha-, Beta-, Delta-, Epsilon-, and Gamma-proteobacteria respectively, while the last bar (others) shows orders in others phylum.
3.5. Distribution of bacterial community in Labroides dimidatus by family
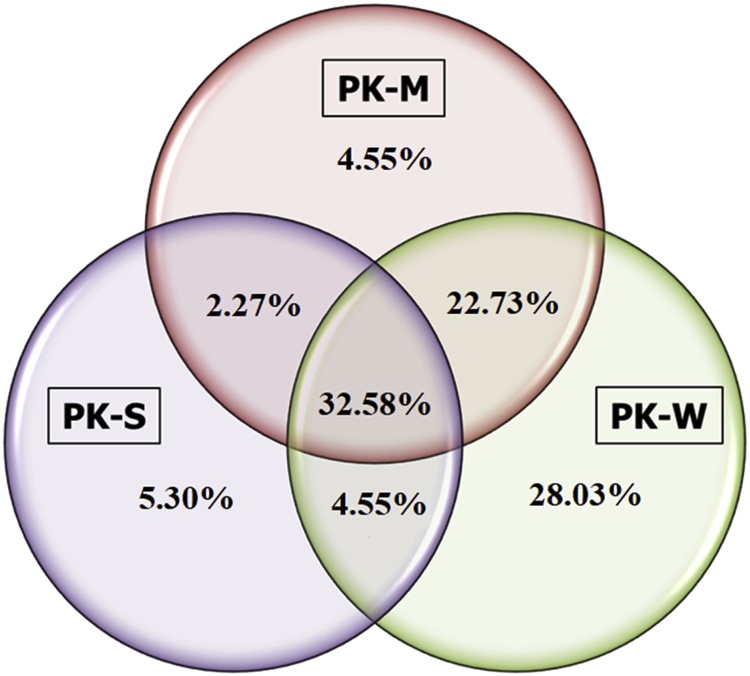
A total of 82 and 59 families of bacteria were identified in the skin mucus and stomach respectively, while 116 bacteria families were identified in the carriage water. Forty-three of these families (32.58% of total identified microbes) were present in all three samples (Table 4, Fig. 5), while three (2.27%) were common only on the skin mucus and in the stomach of L. dimidiatus (Table 5, Fig. 5). Similarly, six other bacterial families (4.55%) found in the stomach were present in the carriage water samples (Table 6, Fig. 5). However, thirty bacteria families (22.73%) were shared between the skin mucus and carriage water (Table 7, Fig. 5). It is worth mentioning that, some thirty-seven families (28.03%) were found exclusively in the carriage water (Table 8 Fig. 5). However, only seven families (5.30%) were exclusive in the stomach (Table 9, Fig. 5) while, another six (4.55%) were only present in the skin mucus of the L. dimidiatus (Table 10, Fig. 5). A total of thirty-three pathogenic zoonotic bacterial were isolated in this study.
Table 4.
Shared bacteria families’ in the skin mucus and stomach of Labroides dimidiatus as well as the water from Karah Island, Terengganu Malaysia.
| Serial Number | Class | Family |
|---|---|---|
| 1 | Alphaproteobacteria | Acetobacteraceae |
| 2 | Clostridia | Lachnospiraceae |
| 3 | Actinobacteria | Micrococcaceae |
| 4 | Alphaproteobacteria | Bradyrhizobiaceae |
| 5 | Betaproteobacteria | Burkholderiaceae |
| 6 | Betaproteobacteria | Burkholderiales_incertae_sedis |
| 7 | Alphaproteobacteria | Bradyrhizobiaceae |
| 8 | Betaproteobacteria | Burkholderiaceae |
| 9 | Betaproteobacteria | Burkholderiales_incertae_sedis |
| 10 | Actinobacteria | Corynebacteriaceae |
| 11 | Actinobacteria | Propionibacteriaceae |
| 12 | Actinobacteria | Unclassified Actinomycetales |
| 13 | Actinobacteria | Unclassified Actinobacteria |
| 14 | Flavobacteria | Flavobacteriaceae |
| 15 | Flavobacteria | Unclassified Flavobacteriales |
| 16 | Unclassified Bacteroidetes | Unclassified Bacteroidetes |
| 17 | Fusobacteria | Fusobacteriaceae |
| 18 | Alphaproteobacteria | Hyphomicrobiaceae |
| 19 | Alphaproteobacteria | Methylobacteriaceae |
| 20 | Alphaproteobacteria | Unclassified Rhizobiales |
| 21 | Alphaproteobacteria | Rhodobacteraceae |
| 22 | Alphaproteobacteria | Unclassified Rhodospirillales |
| 23 | Alphaproteobacteria | Erythrobacteraceae |
| 24 | Alphaproteobacteria | Sphingomonadaceae |
| 25 | Alphaproteobacteria | Unclassified Sphingomonadales |
| 26 | Alphaproteobacteria | Unclassified Alphaproteobacteria |
| 27 | Betaproteobacteria | Comamonadaceae |
| 28 | Betaproteobacteria | Oxalobacteraceae |
| 29 | Betaproteobacteria | Unclassified Burkholderiales |
| 30 | Betaproteobacteria | Unclassified Betaproteobacteria |
| 31 | Gammaproteobacteria | Unclassified Oceanospirillales |
| 32 | Gammaproteobacteria | Moraxellaceae |
| 33 | Gammaproteobacteria | Pseudomonadaceae |
| 34 | Gammaproteobacteria | Xanthomonadaceae |
| 35 | Gammaproteobacteria | Unclassified Gammaproteobacteria |
| 36 | Unclassified Proteobacteria | Unclassified Proteobacteria |
| 37 | Bacilli | Bacillaceae_1 |
| 38 | Bacilli | Staphylococcaceae |
| 39 | Bacilli | Unclassified Bacillales |
| 40 | Clostridia | Peptostreptococcaceae |
| 41 | Clostridia | Unclassified Clostridiales |
| 42 | Clostridia | Unclassified Firmicutes |
| 43 | Unclassified bacteria | Unclassified Bacteria |
Fig. 5.
Venn diagram of the percentages of shared and unique bacteria families identified in three different types of samples collected in this study. PK = Karah Island, M = skin mucus, S = stomach, W = carriage seawater.
Table 5.
Shared bacteria families’ in the skin mucus and stomach of Labroides dimidiatus from Karah Island, Terengganu Malaysia.
| Serial Number | Class | Family |
|---|---|---|
| 1 | Bacilli | Carnobacteriaceae |
| 2 | Actinobacteria | Brevibacteriaceae |
| 3 | Actinobacteria | Dermacoccaceae |
Table 6.
Shared bacteria families’ in the stomach of Labroides dimidiatus and water from Karah Island, Terengganu Malaysia.
| Serial Number | Class | Family |
|---|---|---|
| 1 | Actinobacteria | Coriobacteriaceae |
| 2 | Alphaproteobacteria | Rhizobiaceae |
| 3 | Deltaproteobacteria | Nannocystaceae |
| 4 | Actinobacteria | Microbacteriaceae |
| 5 | Deltaproteobacteria | Unclassified Myxococcales |
| 6 | Bacilli | Paenibacillaceae_1 |
Table 7.
Shared bacteria families’ in the skin mucus of Labroides dimidiatus and water from Karah Island, Terengganu Malaysia.
| Serial Number | Class | Family |
|---|---|---|
| 1 | Betaproteobacteria | Rhodocyclaceae |
| 2 | Deltaproteobacteria | Phaselicystidaceae |
| 3 | Epsilonproteobacteria | Helicobacteraceae |
| 4 | Erysipelotrichia | Erysipelotrichaceae |
| 5 | Negativicutes | Veillonellaceae |
| 6 | Actinobacteria | Actinomycetaceae |
| 7 | Actinobacteria | Nocardiaceae |
| 8 | Alphaproteobacteria | Caulobacteraceae |
| 9 | Gammaproteobacteria | Pasteurellaceae |
| 10 | Clostridia | Clostridiales_Incertae_Sedis_XI |
| 11 | Acidobacteria_Gp4 | Acidobacteria_Gp4_family_incertae_sedis |
| 12 | Actinobacteria | Nocardioidaceae |
| 13 | Bacilli | Lactobacillaceae |
| 14 | Bacteroidetes_incertae_ sedis_class_incertae_sedis | Bacteroidetes_incertae_sedis_family_incertae_sedis |
| 15 | Sphingobacteria | Flammeovirgaceae |
| 16 | Sphingobacteria | Cytophagaceae |
| 17 | Phycisphaerae | Phycisphaeraceae |
| 18 | Alphaproteobacteria | Hyphomonadaceae |
| 19 | Flavobacteria | Cryomorphaceae |
| 20 | Alphaproteobacteria | Rhodospirillaceae |
| 21 | Deltaproteobacteria | Bacteriovoracaceae |
| 22 | Deltaproteobacteria | Unclassified Deltaproteobacteria |
| 23 | Gammaproteobacteria | Colwelliaceae |
| 24 | Gammaproteobacteria | Ferrimonadaceae |
| 25 | Gammaproteobacteria | Ferrimonadaceae |
| 26 | Gammaproteobacteria | Moritellaceae |
| 27 | Gammaproteobacteria | Chromatiaceae |
| 28 | Gammaproteobacteria | Unclassified Chromatiales |
| 29 | Gammaproteobacteria | Alcanivoracaceae |
| 30 | Gammaproteobacteria | Gammaproteobacteria_family_ incertae_sedis |
Table 8.
Bacteria families’ exclusive in water from Karah Island, Terengganu Malaysia.
| Serial Number | Class | Family |
|---|---|---|
| 1 | Gammaproteobacteria | unclassified Xanthomonadales |
| 2 | Bacilli | Paenibacillaceae_2 |
| 3 | Bacilli | Enterococcaceae |
| 4 | Acidobacteria_Gp3 | Acidobacteria_Gp3_family_incertae_sedis |
| 5 | Acidobacteria_Gp6 | Acidobacteria_Gp6_family_incertae_sedis |
| 6 | Acidobacteria_Gp7 | Acidobacteria_Gp7_family_incertae_sedis |
| 7 | unclassified Acidobacteria | unclassified Acidobacteria |
| 8 | Actinobacteria | Acidimicrobiaceae |
| 9 | Bacteroidia | Porphyromonadaceae |
| 10 | Alphaproteobacteria | unclassified Caulobacterales |
| 11 | Deltaproteobacteria | Desulfobacteraceae |
| 12 | Deltaproteobacteria | Desulfobulbaceae |
| 13 | Gammaproteobacteria | Methylococcaceae |
| 14 | Mollicutes | Acholeplasmataceae |
| 15 | Bacilli | Alicyclobacillaceae |
| 16 | Bacilli | Bacillales_Incertae_Sedis_XII |
| 17 | Bacilli | Thermoactinomycetaceae_1 |
| 18 | Bacilli | Leuconostocaceae |
| 19 | Bacilli | unclassified Lactobacillales |
| 20 | Clostridia | Ruminococcaceae |
| 21 | Actinobacteria | Pseudonocardiaceae |
| 22 | Sphingobacteria | Sphingobacteriaceae |
| 23 | Alphaproteobacteria | Bartonellaceae |
| 24 | Betaproteobacteria | Alcaligenaceae |
| 25 | Betaproteobacteria | Methylophilaceae |
| 26 | Gammaproteobacteria | Unclassified Pseudomonadales |
| 27 | Bacilli | Planococcaceae |
| 28 | Bacilli | Streptococcaceae |
| 29 | Gammaproteobacteria | Hahellaceae |
| 30 | Bacilli | Bacillaceae_2 |
| 31 | Clostridia | Clostridiaceae_1 |
| 32 | Bacteroidia | unclassified Bacteroidales |
| 33 | Nitrospira | Nitrospiraceae |
| 34 | TM7_class_incertae_ sedis | TM7_family_incertae_sedis |
| 35 | Sphingobacteria | Chitinophagaceae |
| 36 | Alphaproteobacteria | Phyllobacteriaceae |
| 37 | Gammaproteobacteria | Sinobacteraceae |
Table 9.
Bacteria families’ exclusive to the stomach of Labroides dimidiatus collected from Karah Island, Terengganu Malaysia.
| Serial Number | Class | Family |
|---|---|---|
| 1 | Actinobacteria | Streptomycetaceae |
| 2 | Bacteroidia | Prevotellaceae |
| 3 | Bacilli | Bacillales_Incertae_Sedis_XI |
| 4 | Gammaproteobacteria | Legionellaceae |
| 5 | Epsilonproteobacteria | Unclassified Epsilonproteobacteria |
| 6 | Alphaproteobacteria | Kiloniellaceae |
| 7 | Spirochaetes | Unclassified Spirochaetales |
Table 10.
Bacteria families’ exclusive to the skin mucus of Labroides dimidiatus collected from Karah Island, Terengganu Malaysia.
| Serial Number | Class | Family |
|---|---|---|
| 1 | Actinobacteria | Dermatophilaceae |
| 2 | Actinobacteria | Bifidobacteriaceae |
| 3 | Betaproteobacteria | Neisseriaceae |
| 4 | Epsilonproteobacteria | Unclassified Campylobacterales |
| 5 | Sphingobacteria | Unclassified Sphingobacteriales |
| 6 | Epsilonproteobacteria | Campylobacteraceae |
4. Discussion
Metagenomics method used in this study successful identified a number of bacterial associated with L. dimidiatus. To our knowledge, this is the first of such study aimed at characterising the microbiota of wild L. dimidiatus using the Next Generation Sequencing technology. This method allows a more complex view of the composition of the microbiota of fish and its environment with high precision of taxonomic classification using thousands of reads [23]. The five most common phyla, in this study (Proteobacteria, Bacteroidetes, Firmicutes, Actinobacteria and Fusobacteria) had earlier been reported by Gerzova et al., [3] in carriage water of ornamental fish from three different continents. Similar finding have been reported by Boutin et al., [24]. Larsen et al. [25], had earlier isolated these groups of bacterial in the skin of mullet Mugil cephalus, red snapper Lutjanus campechanus, spotted seatrout Cynoscion nebulosus, sand seatrout Cynoscion arenarius, Atlantic croaker Micropogonias unduluatus, and pinfish Lagodon rhomboides. Similarly, Gerzova et al., [3] had suggested that these are common phyla in the stomach of channel catfish Ictalurus punctatus, largemouth bass Micropterus salmoides, and bluegill Lepomis macrochirus and some ornamental fishes. It is a known fact that microbes form symbiotic relationships with their host organisms and offers several advantages [26]. However, the origins of most fish microbes have not yet been studied; hence, this phenomenon is poorly understood [27].
The findings of this study suggest that the structure of fish microbiomes from the Karah Island, Terengganu, Malaysia were largely influenced by the environments water where they are found. Fish skin is constantly in contact with the aquatic environment, hence, the largely synchronised bacteria composition on the skin mucus of the L. dimidiatus and that of the carriage water in the study was expected. Georgala, [28] and Horsley [29], had earlier suggested that the external bacteria communities of many fishes are strong reflection of the microbial community present in the surrounding water. Beyond the bios of the surrounding waters, distribution of microbial communities in the stomach may also be largely influenced by the type of diet fed by the fish [30]. Schmidt et al. [31], and Gajardo et al., [32] had earlier justified this assumption in their study with Salmo salar. This position cannot be suggested for the current study, as no attempt was made to characterise the microbes of the feed fed by the fish in the wild. However, the mutualism interaction between L. dimidiatus and its client (i.e. the fishes it helps remove parasites and bacteria infection from) could present a new pathogenic infection partway in this fish [33]. Similarly, this could be a peculiar form of parasitic transmission pattern in other cleaner wrasses fishes.
Out of the many genera isolated in this study, Alteromonas and Vibrio were two of the dominant genera of the Gammaproteobacteria as observed in all samples. This has been reported prevalent in many other marine environments [[34], [35], [36]]. Alteromonas has also been reported in some species of fish reared in a re-circulatory aquaculture system [37,38]. The bacteria group Alteromonas have great carbon cycling potential, hence are composite of member of the marine master recyclers [39,40]. Also, the genus Vibrio, have been reported in other members of wrasse [[41], [42], [43], [44]]. Several species of Vibrio are typically associated with marine and freshwater environments [45]. The Vibrio infections can spread rapidly when fish are confined in very high stocking densities a scenario found in commercial aquaculture systems where morbidity may reach 100% in affected facilities [13]. Vibrio sp. are pathogenic to human, and responsible for disease in wild and reared organisms, which includes fish, molluscs, crustaceans, rotifers, and corals, [46]. Another genus of interest in abundance isolated in the skin mucus of L. dimidiatus was the Sandarakinotalea. The presence of this bacteria group may be due to the burrowing activity of the fish in the sand for the purpose of predatory avoidance. This has been earlier demonstrated in the study reported by Slobodkin and Fishelson, [47].
Out of the 132 microbes identified in this study, 32% were shared/found in all site examined (i.e. mucus, stomach and the carriage water). Members of the Gammaproteobacteria were the most commonly shared bacteria among all the samples in this study. So were the members of the Alphaproteobacteria, Betaproteobacteria, Actinobacteria, Flavobacteria, Fusobacteria, Bacilli and Clostridia. Usually, shared bacteria communities within a population, are reflection of symbiotic association possibly because of metabolic benefits between the bacteria and it host [48]. Most of the bacteria identified are involved in nutrient cycling. Rhodobacteraceae for instance is deeply involved in sulfur and carbon biogeochemical cycling and symbiosis with aquatic micro- and macro-organisms [49,50]. Carbon cycling and biodegradative capabilities are widespread characteristic within the members of the Alpha- and Gammaproteobacteria. These include the Alteromonadaceae, Pseudomonadaceae, Sphingomonadaceae, and Vibrionaceae as well as Cytophagaceae, Flavobacteriaceae and Bacteroidetes, which are members of the Cytophaga-Flavobacteria-Bacteroides (CFB) clade [51].
Lactobacillus spp., Lactococcus spp., Enterococcus spp., Shewanella spp., Bacillus spp., Aeromonas spp., Vibrio, Enterobacter spp., Pseudomonas spp., Clostridium spp., and Kocuria spp. identified in this study are common probiotic bacteria used in the aquaculture industries [52,53]. These probiotic bacteria have been isolated in several healthy fish species such as Indian carp, Labeo rohita, Nile tilapia, Oreochromis niloticus, gilt-head bream, Sparus aurata [[54], [55], [56]]. They function to improve growth, immune responses, resistance to pathogenic bacteria, and expression of different genes involved in inflammation, development and digestion processes in the fish host [[54], [55], [56]]. In this study, 28% of the identified microbes were found unique to the carriage water, while about 5% were unique to the mucus and stomach of the fish. The observation of some bacteria exclusive to different samples or environment is not new. Earlier study by Kormas et al., [57], Bakke et al., [58], Estruch et al. [59], and Li et al. [60], have showed that aquaculture microbiota composition in the fish was different from those found in the surrounding environment. Cardinale et al., [61], however, had earlier concluded that the differences in the structure of bacteria communities of the environment and the host organism might simply be opportunistic infection, rather than symbiotic partnership.
Fish can also be a source of human infections caused by pathogens transmitted from fish or the aquatic environment. Depending on season, and the level of exposure pathogenic bacteria could be transmitted to human. Thirty-three of such zoonotic pathogenic bacteria were observed in this study. Notable among them is the Mycobacteria. This is one of the most common fish-borne bacteria pathogens known to man. It can cause granulomatous inflammation of the human skin [62]. Similarly, Erysipelothrix spp. is transmissible from fish to humans, and causes infection of cutaneous wounds. This typically results in localized, painful, self-limiting cellulitis, with purple discoloration and oedema (‘fish rose’) [63,64]. In addition to these two, Streptococcus spp, Photobacterium spp, and Vibrio spp, are other fish-borne zoonosis bacteria associated with L. dimidiatus worth mentioning in this study. Gram positive bacteria such as Clostridium, Lactococcus and Staphylococcus were only found in the stomach and water sample while Streptococcus is found in all samples of L. dimidiatus. Clostridium is commonly commensal in the intestines of marine animals and in the sediments of the environment/decaying organic matter where paralytic neurotoxin is produced [65]. Lactococcus and Streptococcus are also zoonotic fish-borne bacteria found in a wide variety of temperate and warm water fishes [66,67]. Pathogens of gram negative bacteria such as Aeromonas spp, Edwardsiella spp., Enterobacteriaceae, Francisella spp., Pseudomonas, and Vibrio spp. isolated mostly in this study have been implicated for zoonotic infections of fish and humans in previous studies [[68], [69], [70], [71]].
Earlier studies by Rasmussen & Sorensen, [72], Horner-Devine et al. [73], Leflaive et al., [74] Sala et al. [75,76], had concluded that functional bacteria composition in a systems are further driven by changes in the environmental conditions such as nutrient availability and pollutants. Similarly, changes in salinity, season and geographic location could severely influence the composition of free-living and symbiotic bacteria communities in the fish [77,78]. This means that changes in the dynamics of the external environment such as those obtained when wild fishes are raise in captivity could result in substantial differences in the bacteria community than what was reported in this study. In line with this assumption, Smith et al. [13], had suggested the possibility of ornamental fish raised in captivity harbouring novel microbial communities and pathogens that could pose potential risks to the pet industry, fish trade, humans and other fish species. Hence, there is need to intensify research in this regards for biosecurity purposes and to ensure suitable husbandry of aquarium held wild ornamental fishes such as L. dimidiatus.
4.1. Limitations of the study
The collected samples of fish used for this study were gently washed thrice with sterile seawater to remove the dirt on the fish. This was done with the hope that the microbial colonies on the skin were not compromised. However, this is likely not the case. In correction of the limitation in this study, it is recommended that DNA be extracted from the sterilized water used to wash the fish’s skin in future studies.
Acknowledgements
The authors would like to express appreciation to the Institute of Tropical Aquaculture (AKUATROP) and Biosystem Laboratory, School of Fisheries and Aquaculture Sciences (FISHA), University Malaysia Terengganu for providing laboratory facilities for this study. The authors would like to thank Miss Nor Aiffa Wahyu Abu Bakar and staff of Net loft, FISHA for their assistance in sample collection. This research is funded by a grant from the Ministry of Higher Education Malaysia.
References
- 1.Balachandran N.D., Thipramalai T.A.K., Balasubramanian T., Kapila T. A study on the effect of using mangrove leaf extracts as a feed additive in the progress of bacterial infections in marine ornamental fish. J. Coast. Life Med. 2013;1(3):217–224. [Google Scholar]
- 2.Noga E.J. In: Fish Disease : Diagnosis and Treatment. second, ed. Noga E.J., editor. Wiley-Blackwell; US: 2010. pp. 5–7. [Google Scholar]
- 3.Gerzova L., Videnska P., Faldynova M., Sedlar K., Provaznik I., Cizek A., Rychlik I. Characterization of microbiota composition and presence of selected antibiotic resistance genes in carriage water of ornamental fish. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0103865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhyne A.L., Tlusty M.F., Schofield P.J. Revealing the appetite of the marine aquarium fish trade: the volume and biodiversity of fish imported into the United States. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0035808. e35808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNEP-WCMC . Prepared for the European Commission Directorate General E – Environment ENV.E.2. – Development and Environment; 2007. SRG 42/8/a International Trade in Aquatic Ornamental Species. [Google Scholar]
- 6.UNEP-WCMC . European Commission Directorate General E – Environment ENV.E.2. – Development and Environment; 2008. Consultation Process on Monitoring of International Trade in Ornamental Fish. [Google Scholar]
- 7.Wabnitz C., Taylor M., Green E., Razak T. UNEP-WCMC; Cambridge, UK: 2003. The Global Trade in Marine Ornamental Species from Ocean to Aquarium. [Google Scholar]
- 8.Potts G.W. The ethology of Labroides dimidiatus (cuv & val) Labridae, pisces on Aldabra. Anim. Behav. 1973;21:250–291. [Google Scholar]
- 9.Rimstad E., Basic D., Gulla S., Hjeltnes B., Mortensen S. Norwegian Scientific Committee for Food and Environment (VKM); Oslo, Norway: 2017. Risk Assessment of Fish Health Associated With the Use of Cleaner Fish in Aquaculture. Opinion of the Panel on Animal Health and Welfare of the Norwegian Scientific Committee for Food and Environment. VKM Report 2017:32, ISBN: 978-82-8259-289-5, ISSN: 2535-4019. [Google Scholar]
- 10.Gómez G.D., Balcázar J.L. A review on the interactions between gut microbiota and innate immunity of fish. Federation of European Microbiological Societies. Immunol. Med. Microbiol. 2008;52:145–154. doi: 10.1111/j.1574-695X.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 11.Wagner E.J., Jensen T., Arndt R., Routedge M.D., Brddwisch Q. Effects of rearing density upon cut throat trout haematology, hatchery performance, fin erosion and general health and condition. Prog. Fish-Cult. 1997;59:173–187. [Google Scholar]
- 12.Balcázar J.L., Lee N.M., Pintado J., Planas M. Phylogenetic characterization and in situ detection of bacterial communities associated with seahorses (Hippocampus guttulatus) in captured. Syst. Appl. Microbiol. 2010;33:71–77. doi: 10.1016/j.syapm.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Smith K.F., Schmidt V., Rosen G.E., Amaral-Zettler L. Microbial diversity and potential pathogens in ornamental fish aquarium water. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0039971. e39971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf J., Prüss-Ustün A., Cumming O. Review of the evidence relating drinking-water and sanitation to diarrhoea: a meta-regression. Trop. Med. Int. Health. 2014;19(8) doi: 10.1111/tmi.12331. 928942. [DOI] [PubMed] [Google Scholar]
- 15.Hongo Y., Yuzawa H., Ohkuma M., Kudo T. Evaluation of primers and PCR conditions for the analysis of 16S rRNA genes from a natural environment. Federation of European Microbiological Societies. Microbiol. Lett. 2003;221:299–304. doi: 10.1016/S0378-1097(03)00218-0. [DOI] [PubMed] [Google Scholar]
- 16.Bartram A.K., Lynch M.D., Stearns J.C., Moreno-Hagelsieb G., Neufeld J.D. Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end Illumina reads. Appl. Environ. Microbiol. 2011;77(11):3846–3852. doi: 10.1128/AEM.02772-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huse S.M., Dethlefsen L., Huber J.A., Welch D.M., Relman D.A., Sogin M.L. Exploring microbial diversity and taxonomy using SSU rRNA hypervariable tag sequencing. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muyzer G., de Waal E.C., Uitterlinden A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gloor G.B., Hummelen R., Macklaim J.M., Dickson R.J., Fernandes A.D., MacPhee R. Microbiome profiling by Illumina sequencing of combinatorial sequence-tagged PCR products. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., Sahl J.W., Stres B., Thallinger G.G., Van Horn D.J., Weber C.F. Introducing mothur: open-source, platform-independent, community- supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75(23):7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole J.R., Wang Q., Cardenas E., Fish J., Chai B., Farris R.J., Kulam-Syed-Mohideen A.S., McGarrell D.M., Marsh T., Garrity G.M., Tiedje J.M. The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:141–145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiteley A.S., Jenkins S., Waite I., Kresoje N., Payne H., Mullan B. Microbial 16S rRNA ion tag and community metagenome sequencing using the ion torrent (PGM) platform. J. Microbiol. Methods. 2012;91:80–88. doi: 10.1016/j.mimet.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Boutin S., Bernatchez L., Audet C., Derôme N. Network analysis highlights complex interactions between pathogen, host and commensal microbiota. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0084772. e84772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen A.M., Mohammed H.H., Arias C.R. Characterization of the gut microbiota of three commercially valuable warmwater fish species. J. Appl. Microbiol. 2014;116:1396–1404. doi: 10.1111/jam.12475. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy J., Marchesi J.R., Dobson A.D.W. Marine metagenomics: strategies for the discovery of novel enzymes with biotechnological applications from marine environments (Review) Microb. Cell Fact. 2008;7(27):1–8. doi: 10.1186/1475-2859-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen A.M., Tao Z., Bullard S.A., Arias C.R. Diversity of the skin microbiota of fishes: evidence for host species specificity. Federation of European Microbiological Societies. Microbiol. Ecol. 2013;85:483–494. doi: 10.1111/1574-6941.12136. [DOI] [PubMed] [Google Scholar]
- 28.Georgala D.L. The bacterial flora of the skin of North Sea cod. J. Gen. Microbiol. 1958;18:84–91. doi: 10.1099/00221287-18-1-84. [DOI] [PubMed] [Google Scholar]
- 29.Horsley R.W. A review on the bacterial flora of teleosts and elamobrachs, includings methods for its analysis. J. Fish Biol. 1977;10:529–553. [Google Scholar]
- 30.Landeira-Dabarca A., Sieiro C., Álvarez M. Change in food ingestion induces rapid shifts in the diversity of microbiota associated with cutaneous mucus of Atlantic salmon Salmo salar. J. Fish Biol. 2013;82:893–906. doi: 10.1111/jfb.12025. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt V., Amaral-Zettler L., Davidson J., Summerfelt S., Good C. The influence of fishmeal-free diets on microbial communities in Atlantic salmon Salmo salar recirculation aquaculture systems. Appl. Environ. Microbiol. 2016;82:4470–4481. doi: 10.1128/AEM.00902-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gajardo K., Jaramillo-Torres A., Kortner T., Merrifield D., Tinsley J., Bakke A. Alternative protein sources in the diet modulate microbiota and functionality in the distal intestine of Atlantic salmon (Salmo salar) Appl. Environ. Microbiol. 2017;83:e02615–16. doi: 10.1128/AEM.02615-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grutter A.S. Parasite removal rates by the cleaner wrasse Labroides dimidiatus. Inter-Res. Mar. Ecol. Programme Ser. 1996;130:61–70. [Google Scholar]
- 34.Juteau P., Larocque R., Rho D., LeDuy A. Analysis of the relative abundance of different types of bacteria capable of toluene degradation in a compost biofilter. Appl. Microbiol. Biotechnol. 1999;52:863–868. doi: 10.1007/s002530051604. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe K., Teramoto M., Harayama S. An outbreak of nonflocculating catabolic populations caused the breakdown of a phenol-digesting activated-sludge process. Appl. Environ. Microbiol. 1999;65:2813–2819. doi: 10.1128/aem.65.7.2813-2819.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palleroni N.J., Port A.M., Chang H.K., Zylstra G.J. Hydrocarboniphaga effusa gen. nov., sp nov., a novel member of the gamma-Proteobacteria active in alkane and aromatic hydrocarbon degradation. Int. J. Syst. Evol. Microbiol. 2004;54:1203–1207. doi: 10.1099/ijs.0.03016-0. [DOI] [PubMed] [Google Scholar]
- 37.Fjellheim A.J., Playfoot K.J., Skjermo J., Vadstein O. Vibrionaceae dominates the microflora antagonistic towards Listonella anguillarum in the intestine of cultured Atlantic cod (Gadus morhua L.) larvae. Aquaculture. 2007;269:98–106. [Google Scholar]
- 38.van der Meeren T., Brunvold L., Sandaa R.-A., Bergh Ø., Castberg T., Thyrhau R., Mangor-Jensen A. Water quality and microbial community structure in juvenile Atlantic cod (Gadus morhua L.) Aquaculture. 2011;316:111–120. [Google Scholar]
- 39.Nelson C.E., Wear E.K. Microbial diversity and the lability of dis- solved organic carbon. Proc. Natl. Acad. Sci. 2014;111:7166–7167. doi: 10.1073/pnas.1405751111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buchan A., LeCleir G.R., Gulvik C.A., González J.M. Master recy-clers: features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 2014;12:686–698. doi: 10.1038/nrmicro3326. [DOI] [PubMed] [Google Scholar]
- 41.Jensen S., Samuelsen O.B., Andersen K., Torkildsen L., Lambert C., Choquet G., Paillard C., Bergh Ø. Characterisation of strains of Vibrio splendidus and V. Tapetis isolated from corkwing wrasse Symphodus melops suffering vibriosis. Dis. Aquat. Org. 2003;53:25–31. doi: 10.3354/dao053025. [DOI] [PubMed] [Google Scholar]
- 42.Bergh Ø., Samuelsen O.B. Susceptibility of corkwing wrasse Symphodus melops, goldsinny wrasse Ctenolabrus rupestris, and Atlantic salmon Salmo salar smolt, to experimental challenge with Vibrio tapetis and Vibrio splendidusisolated from corkwing wrasse. wrasse. Aquac. Int. 2007;15:11–18. [Google Scholar]
- 43.Harkestad L.S. University of Bergen, Department of Biology; 2011. Experimental Infections of Corkwing, Symphodus melops, with the Vibrio tapetis Strains CECT 4600, LP2 and NRP45. (In Norwegian). Msc Thesis. 136 pages. [Google Scholar]
- 44.Colquhoun D., Duodu S., Nilsen H.K., Sviland C., Vågnes Ø. Helsesituasjonen hos marin fisk 2011 (In Norwegian) In: Olsen A.B., Hellberg H., editors. vol.2012. Veterinærinstiuttet; 2012. pp. 33–38. ((2012) Fiskehelserapporten 2011). [Google Scholar]
- 45.Nilsen A.V., Viljugrein H., Røsaeg M.V., Colquhoun D. The Norwegian Veterinary Institute’s Report Series; 2014. Rensefiskhelse - kartlegging av dødelighet og dødelighetsårsaker (Cleaner fish health - a survey of mortalities and causes of death; in Norwegian) p. 12. [Google Scholar]
- 46.Gomez-Gil B., Thompson C.C., Matsumura Y., Sawabe T., Iida T., Christen R., Thompson F., Sawabe T. The famlily Vibrionaceae. In: Rosenberg E., DeLong E.F., Lory S., Stackebrandt E., Thompson F., editors. The Prokaryotes– Gammaproteobacteria. Springer; Berlin Heidelberg: 2014. pp. 660–713. [Google Scholar]
- 47.Slobodkin L.B., Fishelson L. The effect of the cleaner-fish Labroides dimidiatus on the point diversity of fishes on theReef front at Eilat. Am. Nat. 1974;108(961):369–376. http://www.jstor.org/stable/2459898 May - Jun., 1974. [Google Scholar]
- 48.Grube M., Cardinale M., Vieira de Castro J., Müller H., Berg G. Species-specific structural and functional diversity of bacterial communities in lichen symbioses. ISME J. 2009;3:1105–1115. doi: 10.1038/ismej.2009.63. [DOI] [PubMed] [Google Scholar]
- 49.Brinkhoff T., Giebel H.-A., Simon M. Diversity, ecology, and genomics of the Roseobacter clade: a short overview. Arch. Microbiol. 2008;189:531–539. doi: 10.1007/s00203-008-0353-y. [DOI] [PubMed] [Google Scholar]
- 50.Pujalte M., Lucena T., Ruvira M.A., Arahal D.R., Macián M.C. The family Rhodobacteraceae. In: Rosenberg E., DeLong E.F., Lory S., Stackebrandt E., Thompson F., editors. The Prokaryotes- Alphaproteobacteria and Betaproteobacteria. Springer; Berlin Heidelberg: 2014. pp. 439–512. [Google Scholar]
- 51.Wackett L.P. Bacteria in sand. Environ. Microbiol. 2013;15:2144–2145. doi: 10.1111/1462-2920.12176. [DOI] [PubMed] [Google Scholar]
- 52.Péréz-Sánchez T., Ruiz-Zarzuela I., deBlas I., Balcázar J.L. Probiotics in aquaculture: a current assessment. Rev. Aquac. 2014;6:133–146. [Google Scholar]
- 53.Nayak S.K. Probiotics and immunity: a fish perspective (Review) Fish Shellfish Immunol. 2010;29:2–14. doi: 10.1016/j.fsi.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Giri S.S., Sukumaran V., Oviya M. Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol. 2013;34:660–666. doi: 10.1016/j.fsi.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Del’Duca A., Evangelista C.D., Galuppo D.C., Cesar A.P. Evaluation of the presence and efficiency of potential probiotic bacteria in the gut of tilapia (Oreochromis niloticus) using the fluorescent in situ hybridization technique. Aquaculture. 2013;391:115–121. [Google Scholar]
- 56.Cerezuela R., Meseguer J., Esteban M.A. Effects of dietary inulin, Bacillus subtilis and microalgae on intestinal gene expression in gilthead seabream (Sparus aurata L.) Fish Shellfish Immunol. 2013;34:843–848. doi: 10.1016/j.fsi.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 57.Kormas K.A., Meziti A., Mente E., Frentzos A. Dietary differences are reflected on the gut prokaryotic community structure of wild and commercially reared sea bream (Sparus aurata) Microbiol. Open. 2014;3(5):718–728. doi: 10.1002/mbo3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bakke I., Coward E., Andersen T., Vastein O. Selection in the host structures the microbiota associated with developing cod larvae (Gadus morhua) Environ. Microbiol. 2015;17:3914–3924. doi: 10.1111/1462-2920.12888. [DOI] [PubMed] [Google Scholar]
- 59.Estruch G., Collado M.C., Pe-aranda D.S., Tomás Vidal A., Jover Cerdá M., Pérez, Martínez G. Impact of fishmeal replacement in diets for gilthead sea bream (Sparus aurata) on the gastrointestinal microbiota determined by pyrosequencing the 16S rRNA gene. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li T., Long M., Gatesoupe F.-J., Zhang Q., Li A., Gong X. Comparative analysis of the intestinal bacterial communities in different species of carp by pyrosequencing. Microbiol. Ecol. 2015;69:25–36. doi: 10.1007/s00248-014-0480-8. [DOI] [PubMed] [Google Scholar]
- 61.Cardinale B.J., Srivastava D.S., Duffy J.E., Wright J.P., Downing A.L., Sankaran M., Jouseau C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443:989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- 62.Gauthier D.T. Bacterial zoonoses of fishes: a review and appraisal of evidence for linkages between fish and human infections. Vet. J. 2015;203:27–35. doi: 10.1016/j.tvjl.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 63.Reboli A.C., Farrar W.E. Erysipelothrix rhusiopathiae: an occupational pathogen. Clin. Microbiol. Rev. 1989;2:354–359. doi: 10.1128/cmr.2.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Q., Chang B.J., Riley T.V. Erysipelothrix rhusiopathiae. Vet. Microbiol. 2010;140:405–417. doi: 10.1016/j.vetmic.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Bean N.H., Griffin P.M. Foodborne disease outbreaks in the United States, 1973–1987: pathogens, vehicles, and trends. J. Food Prot. 1990;53:804–817. doi: 10.4315/0362-028X-53.9.804. [DOI] [PubMed] [Google Scholar]
- 66.Chan J.F.W., Woo P.C.Y., Teng J.L.L., Lau S.K.P., Leung S.S.M., Tam F.C.C., Yuen K.Y. Primary infective spondylodiscitis caused by Lactococcus garvieae and a review of human L. Garvieae infections. Infection. 2011;39:259–264. doi: 10.1007/s15010-011-0094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim J.H., Go J., Cho C.R., Kim J.I., Lee M.S., Park S.C. First report of human acute acalculous cholecystitis caused by the fish pathogen Lactococcus garvieae. J. Clin. Microbiol. 2013;51:712–714. doi: 10.1128/JCM.02369-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hejazi A., Falkiner F.R. Serratia marcescens. J. Med. Microbiol. 1997;46:903–912. doi: 10.1099/00222615-46-11-903. [DOI] [PubMed] [Google Scholar]
- 69.McCoy E., Morrison J., Cook V., Johnston J., Eblen D., Guo C. Foodborne agents associated with the consumption of aquaculture catfish. J. Food Prot. 2011;74:500–516. doi: 10.4315/0362-028X.JFP-10-341. [DOI] [PubMed] [Google Scholar]
- 70.Weir M., Rajic A., Dutil L., Cernicchiaro N., Uhland F.C., Mercier B., Tusevljak N. Zoonotic bacteria, antimicrobial use and antimicrobial resistance in ornamental fish: a systematic review of the existing research and survey of aquaculture-allied professionals. Epidemiol. Infect. 2012;140:192–206. doi: 10.1017/S0950268811001798. [DOI] [PubMed] [Google Scholar]
- 71.Camus A., Berliner A., Clauss T., Hatcher N., Marancik D. Serratia marcescens associated ampullary system infection and septicaemia in a bonnethead shark, Sphyrna tiburo (L.) J. Fish Dis. 2013;36:891–895. doi: 10.1111/jfd.12107. [DOI] [PubMed] [Google Scholar]
- 72.Rasmussen L.D., Sorensen S.J. Effects of mercury contamination on the culturable heterotrophic, functional and genetic diversity of the bacterial community in soil. FEMS Microbiol. Ecol. 2001;36:1–9. doi: 10.1111/j.1574-6941.2001.tb00820.x. [DOI] [PubMed] [Google Scholar]
- 73.Horner-Devine M.C., Carney K.M., Bohannan B.J.M. An ecological perspective on bacterial biodiversity. Proc. R. Soc. Lond., B, Biol. Sci. 2004;271:113–122. doi: 10.1098/rspb.2003.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leflaive J., Danger M., Lacroix G., Lyautey E., Oumarou C., Ten-Hage L. Nutrient effects on the genetic and functional diversity of aquatic bacterial communities. FEMS Microbiol. Ecol. 2008;66:379–390. doi: 10.1111/j.1574-6941.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- 75.Sala M.M., Estrada M., Gasol J.M. Seasonal changes in the functional diversity of bacterioplankton in contrasting coastal environments of the NW Mediterranean. Aquat. Microb. Ecol. 2006;44:1–9. [Google Scholar]
- 76.Sala M.M., Terrado R., Lovejoy C., Unrein F., Pedros-Alio C. Metabolic diversity of heterotrophic bacterioplankton over winter and spring in the coastal Arctic Ocean. Environ. Microbiol. 2008;10:942–949. doi: 10.1111/j.1462-2920.2007.01513.x. [DOI] [PubMed] [Google Scholar]
- 77.Sullam K.E., Essinger S.D., Lozupone C.A., O’Connor M.P., Rosen G.L., Knight R., Kilham S.S., Russell J.A. Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis. Mol. Ecol. 2012;21:3363–3378. doi: 10.1111/j.1365-294X.2012.05552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zarkasi K.Z., Abell G.C.J., Taylor R.S., Neuman C., Hatje E., Tamplin M.L., Katouli M., Bowman J.P. Pyrosequencing-based characterization of gastrointestinal bacteria of Atlantic salmon (Salmo salar L.) within a commercial mariculture system. J. Appl. Microbiol. 2014;117:18–27. doi: 10.1111/jam.12514. [DOI] [PubMed] [Google Scholar]