Abstract
Importance
Attention-deficit/hyperactivity disorder (ADHD) is conceptualized as a neurodevelopmental disorder that is strongly heritable. However, to our knowledge, no study to date has examined the genetic and environmental influences explaining interindividual differences in the developmental course of ADHD symptoms from childhood to adolescence (ie, systematic decreases or increases with age). The reason ADHD symptoms persist in some children but decline in others is an important concern, with implications for prognosis and interventions.
Objective
To assess the proportional impact of genes and the environment on interindividual differences in the developmental course of ADHD symptom domains of hyperactivity/impulsivity and inattention between ages 8 and 16 years.
Design, Setting, and Participants
A prospective sample of 8395 twin pairs from the Twins Early Development Study, recruited from population records of births in England andWales between January 1, 1994, and December 31, 1996. Data collection at age 8 years took place between November 2002 and November 2004; data collection at age 16 years took place between February 2011 and January 2013.
Main Outcomes and Measures
Both DSM-IV ADHD symptom subscales were rated 4 times by participants’ mothers.
Results
Estimates from latent growth curve models indicated that the developmental course of hyperactivity/impulsivity symptoms followed a sharp linear decrease (mean score of 6.0 at age 8 years to 2.9 at age 16 years). Interindividual differences in the linear change in hyperactivity/impulsivity were under strong additive genetic influences (81%; 95% CI, 73%-88%). More than half of the genetic variation was specific to the developmental course and not shared with the baseline level of hyperactivity/impulsivity. The linear decrease in inattention symptoms was less pronounced (mean score of 5.8 at age 8 years to 4.9 at age 16 years). Nonadditive genetic influences accounted for a substantial amount of variation in the developmental course of inattention symptoms (54%; 95% CI, 8%-76%), with more than half being specific to the developmental course.
Conclusions and Relevance
The large genetic influences on the developmental course of ADHD symptoms are mostly specific and independent of those that account for variation in the baseline level of symptoms. Different sets of genes may be associated with the developmental course vs the baseline level of ADHD symptoms and explain why some children remit from ADHD, whereas others persist. Recent longitudinal imaging data indicate that the maintenance or increase in symptoms is underpinned by atypical trajectories of cortical development. This may reflect a specific genetic liability, distinct from that which contributes to baseline ADHD symptoms, and warrants closer follow-up.
Attention-deficit/hyperactivity disorder (ADHD) is conceptualized as a neurodevelopmental disorder1–3 under substantial genetic influences.4,5 However, research modeling specifically the developmental course of ADHD symptoms is rare.2 A better understanding of the developmental course of symptoms (ie, systematic decreases or increases with age) may inform clinicians on the clinical course of the condition6,7 and prognosis2,8; it may also provide critical insights regarding the mechanisms underlying ADHD symptoms2 and preventive interventions.9 Although children experiencing a systematic decline or increase in symptoms with age show a differential long-term prognosis,2,10,11 the origins of these interindividual differences in the developmental course of ADHD symptoms are largely unknown.8 This study is the first, to our knowledge, to examine the respective impact of genetic and environmental influences on the developmental course of the ADHD symptom domains of hyperactivity/impulsivity and inattention, using a large population-based sample of twins followed up from childhood to adolescence.
In both clinical and population samples, the 2 symptom domains of ADHD, hyperactivity/impulsivity and inattention, have repeatedly demonstrated concurrent, predictive, and discriminant validity1,6,12–14 and follow a different developmental course.6,7,15 Whereas hyperactive/impulsive symptoms tend to decline steadily after early childhood,6,15–17 inattention symptoms, after an initial increase in early childhood,16 tend to be stable or follow a less pronounced decline.2,6,15,18 Although these trends describe the mean change in symptoms with age, there is also substantial variation between individuals, eg, some children experience a steady decline in inattention symptoms with age, whereas symptoms persist or increase for others.10,19 These interindividual differences reflect more than nonsystematic transient change or random noise. For example, population-based studies have shown that children with increasing levels of inattention were more at risk for long-term poor academic outcomes, even when controlling for baseline or mean levels of inattention.10,11 Interestingly, a longitudinal imaging study in a clinical sample suggested that, independent of baseline symptom severity, interindividual differences in trajectories of cerebral cortical development in childhood and adolescence are associated with a differential clinical course (ie, remission vs persistence).8 Taken together, these findings suggest that, independent of the baseline level of ADHD symptoms, the heterogeneity between individuals in the developmental course of symptoms may be underpinned by differences in cortical trajectories and associated with differential prognosis. However, the origins of the interindividual differences in ADHD symptoms (ie, why they persist in some children but decline in others) are still largely unknown.
Behavioral genetic designs can help in addressing this question. Extant longitudinal twin studies4,5,20 have shown that genetic factors are largely responsible for the stability of ADHD symptoms (ie, genetic stability) but also that new genetic factors emerge at different developmental stages (ie, genetic innovation; for instance, different genetic variants may be associated with ADHD in childhood and adulthood9,21,22). Although the models used in these studies point toward the importance of genetic influences on the developmental course of ADHD, they focus on age-to-age change in ADHD symptoms. One approach to examine systematic long-term change in symptoms is modeling latent growth curve factors (intercept and slope).23,24 The mean of these factors captures the sample average in the baseline level (the intercept) and the average systematic change over time (the slope, eg, an overall linear decrease in hyperactivity symptoms). The variance of these factors captures interindividual differences in baseline level and systematic change (ie, symptoms do not decline at the same pace for all children). With the twin design, the variance of these factors as well as their covariation can be decomposed into genetic and environmental influences. Phenotypic and imaging studies8,10,11 suggest that the differential prognosis associated with interindividual differences in the developmental course of ADHD symptoms cannot be entirely explained by interindividual differences in the baseline level of symptoms. Such interindividual differences in the course of symptoms may reflect specific genetic liability.
Herein, we applied genetically informative growth curve models to a population-based sample of twins to examine the respective impact of environmental and genetic influences on interindividual differences in the baseline level and the developmental course of inattention and hyperactivity/impulsivity.
Methods
Participants
Participants were drawn from the Twins Early Development Study, a longitudinal study of twin pairs recruited from population records of twin births in England and Wales between January 1, 1994, and December 31, 1996.25 The current study sample included a total of 8395 twin pairs for whom both twins had ADHD symptoms data for at least 1 assessment between ages 8 and 16 years. Data collection at age 8 years took place between November 2002 and November 2004; data collection at age 16 years took place between February 2011 and January 2013. The study sample is fairly representative of the UK population as compared with the data obtained by the Office of National Statistics (eAppendix 1 and eTable 1 in the Supplement). Ethical authorization was given by the Institute of Psychiatry Ethics Committee. Parents were given a letter describing the general purpose of the study and written consent was required. It was made clear that participation was voluntary and participants could withdraw from the study whenever they wished.
Measures
The DSM-IV ADHD symptom subscale, taken from the Conners’ Parent Rating Scales–Revised,26 was completed by mothers to assess inattentive and hyperactive/impulsive symptoms at the following mean ages of the participants: 7.9, 11.3, 14.1, and 16.3 years. As in the DSM-IV, the measure comprised 18 items (9 for hyperactivity/impulsivity and 9 for inattention). Each item was rated on a 4-point Likert scale ranging from 0 (not at all true) to 3 (very much true), leading to final scores ranging from 0 to 18. Standardized Cronbach α across the 4 ages ranged between 0.83 and 0.85 for hyperactivity/impulsivity and 0.90 and 0.92 for inattention. These scores measure population symptoms dimensionally and not the clinical disorder.
Statistical Analysis
All analyses were conducted separately for hyperactivity/impulsivity and inattention. All scores were regressed on sex and age prior to analyses.
A latent growth curve model was fitted to examine the developmental course of hyperactivity/impulsivity and inattention between ages 8 and 16 years. First, a phenotypic latent growth curve (ie, without genetic decomposition) was fitted to the data (detailed specifications can be found in Figure 3 of the article by Olsen and Kenny27). This model was used to determine the baseline level (intercept) and the growth factors (eg, linear slope) required to account for the observed hyperactivity/impulsivity and inattention scores across time. Second, the resulting best model was modified to include the genetic and environmental influences on the growth factors. Two sets of genetic models were considered, an ACE model (A indicates additive genetic influence; C, common or shared environment; and E, nonshared environment) and an ADE model (D indicates nonadditive or dominant genetic influence, which reflects effects of interactions between alleles at the same or different loci). These models also enabled the estimation of how much of the genetic and environmental influences on the developmental course (eg, linear slope) were shared with the baseline level (ie, intercept). The residuals (variance at each time not explained by the growth factors) were also decomposed into ACE and ADE factors.24 See eAppendix 2 in the Supplement for details on the estimation and the interpretation of ACE and ADE models.
Model Fit and Estimator
For each model, we report χ2, the Akaike information criterion, and additional approximate fit indexes (eTable 2 in the Supplement).28 Full information maximum likelihood was used to deal with missing data. A maximum likelihood estimator with robust standard errors (MLR) and scaled test statistics were used to account for skewness, while 95% confidence intervals were obtained by bootstrapping (5000 repetitions). The structural equation modeling package lavaan version 0.5-16 was used for phenotypic and biometric models29 and implemented within R software30 version 3.02.
Results
Preliminary Analyses
Consistent with previous studies,20,31 we detected no differences in genetic and environmental etiologies across boys and girls for symptom scores; therefore, sex differences were not considered in subsequent multivariate analyses. Table 1 shows the number of complete monozygotic and dizygotic twin pairs and twin correlations at each age (complete descriptive statistics are shown in eTable 3 and eTable 4 in the Supplement). The monozygotic to dizygotic correlation ratios suggested that a model with additive genetic influence (ACE) was more appropriate for hyperactivity/impulsivity, whereas a model with dominance genetic effects (ADE) was more adequate for inattention (dizygotic correlations less than half of the monozygotic correlations at all ages). We fitted a standard Cholesky decomposition, as is commonly used on longitudinal data.32 For hyperactivity/impulsivity scores, an ACE model indeed fitted the data better (results are presented in Table 2; fit indices are shown in eTable 2 in the Supplement). The influence of additive genetic influences was pervasive at all ages, explaining around 80% of the total variance at each age. From a longitudinal perspective, there was evidence for both (1) genetic continuity, for instance, genetic factors explaining hyperactivity/impulsivity at age 8 years still explained 28% of the total variance at age 16 years (A1 at 16 years,Table 2); and (2) genetic innovation, for instance, 27% of the variance at age 16 years was independent of genetic influences at previous ages (A4 at 16 years, Table 2). No evidence of shared environmental influences on hyperactivity/impulsivity emerged. Nonshared environmental influences were small and largely age specific. Results for inattention (Table 3) were different, as a model with nonadditive genetic effects fitted the data better. Between 37% and 49% of the variance at each age was explained by additive genetic influences, whereas between 28% and 42% was explained by nonadditive genetic effects.
Table 1. MZ and DZ Correlations at Each Agea.
| Twins | Age, y | |||
|---|---|---|---|---|
| 8 | 12 | 14 | 16 | |
| Hyperactivity score, correlation (95% CI) | ||||
| MZ | 0.87 (0.85-0.89) | 0.87 (0.84-0.89) | 0.84 (0.81-0.87) | 0.78 (0.74-0.82) |
| DZ | 0.42 (0.39-0.46) | 0.45 (0.42-0.49) | 0.38 (0.32-0.43) | 0.41 (0.37-0.45) |
| Inattention score, correlation (95% CI) | ||||
| MZ | 0.79 (0.76-0.81) | 0.75 (0.72-0.78) | 0.77 (0.73-0.82) | 0.71 (0.67-0.75) |
| DZ | 0.29 (0.26-0.32) | 0.33 (0.30-0.37) | 0.33 (0.29-0.38) | 0.33 (0.29-0.37) |
| Twin pairs, No. | ||||
| MZ | 2345 | 2099 | 1285 | 1820 |
| DZ | 4314 | 3735 | 2076 | 3264 |
| Total | 6659 | 5834 | 3361 | 5084 |
Abbreviations: DZ, dizygotic; MZ, monozygotic.
The total study sample number is superior to time-specific numbers as all twin pairs with 1 complete pair of data at 1 time or more were included in the latent growth model (eg, a pair of twins with missing value[s] at 8 years but available scores at 12 years was included). Data were complete at all 4 assessments for 2154 pairs, at 3 assessments for 2096 pairs, at 2 assessments for 1889 pairs, and at 1 assessment for 2256 pairs, summing to the total study sample number of 8395 twin pairs.
Table 2. Cholesky Decomposition of Additive Genetic Influences, Shared Environmental Influences, and Nonshared Environmental Influences for Hyperactivity/Impulsivity Score From Ages 8 to 16 Yearsa.
| Age, y | Proportion (95% CI) by Assessment No. | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | |
| Additive genetic influences | A1 | A2 | A3 | A4 | Total a2 |
| 8 | 0.85 (0.80 to 0.89) | 0.85 (0.80 to 0.89) | |||
| 12 | 0.48 (0.43 to 0.53) | 0.35 (0.30 to 0.39) | 0.83 (0.76 to 0.87) | ||
| 14 | 0.36 (0.32 to 0.41) | 0.16 (0.12 to 0.21) | 0.34 (0.29 to 0.39) | 0.86 (0.83 to 0.89) | |
| 16 | 0.28 (0.23 to 0.32) | 0.14 (0.09 to 0.21) | 0.10 (0.05 to 0.15) | 0.27 (0.20 to 0.32) | 0.79 (0.71 to 0.85) |
| Shared environmental influences | C1 | C2 | C3 | C4 | Total c2 |
| 8 | 0.02 (0.00 to 0.07) | 0.02 (0.00 to 0.07) | |||
| 12 | 0.00 (−0.04 to 0.05) | 0.05 (0.01 to 0.10) | 0.05 (0.01 to 0.10) | ||
| 14 | 0.00 (−0.01 to 0.01) | 0.00 (0.00 to 0.01) | 0.00 (−0.03 to 0.00) | 0.00 (0.00 to 0.00) | |
| 16 | 0.00 (−0.02 to 0.06) | 0.00 (−0.03 to 0.05) | 0.02 (0.00 to 0.11) | 0.00 (0.00 to 0.00) | 0.03 (0.00 to 0.09) |
| Nonshared environmental influences | E1 | E2 | E3 | E4 | Total e2 |
| 8 | 0.13 (0.11 to 0.15) | 0.13 (0.11 to 0.15) | |||
| 12 | 0.03 (0.02 to 0.05) | 0.09 (0.08 to 0.11) | 0.13 (0.11 to 0.15) | ||
| 14 | 0.02 (0.01 to 0.04) | 0.02 (0.01 to 0.03) | 0.10 (0.08 to 0.13) | 0.14 (0.11 to 0.17) | |
| 16 | 0.01 (0.01 to 0.02) | 0.01 (0.00 to 0.03) | 0.03 (0.02 to 0.06) | 0.12 (0.10 to 0.15) | 0.18 (0.15 to 0.22) |
The values presented are standardized components of variance. For instance, additive genetic influences explain 83% of the total variance at age 12 years, of which 48% comes from factor A1 that corresponds to age 8 years and 35% comes from factor A2 specific to age 12 years. Finally, a2 + c2 + e2 = 1 at each age (last column). Significant paths are in bold.
Table 3. Cholesky Decomposition of Additive and Dominant Genetic Influences and Nonshared Environmental Influences for Inattention Score From Ages 8 to 16 Yearsa.
| Age, y | Proportion (95% CI) by Assessment No. | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | |
| Additive genetic influences | A1 | A2 | A3 | A4 | Total a2 |
| 8 | 0.37 (0.24-0.50) | 0.37 (0.24-0.50) | |||
| 12 | 0.17 (0.07-0.29) | 0.31 (0.26-0.36) | 0.49 (0.37-0.61) | ||
| 14 | 0.15 (0.04-0.29) | 0.08 (0.02-0.14) | 0.23 (0.15-0.31) | 0.46 (0.30-0.62) | |
| 16 | 0.12 (0.03-0.27) | 0.06 (0.01-0.10) | 0.03 (0.00-0.08) | 0.26 (0.19-0.31) | 0.47 (0.33-0.60) |
| Dominant genetic influences | D1 | D2 | D3 | D4 | Total d2 |
| 8 | 0.42 (0.28-0.56) | 0.42 (0.28-0.56) | |||
| 12 | 0.26 (0.14-0.39) | 0.03 (0.00-0.10) | 0.28 (0.16-0.41) | ||
| 14 | 0.19 (0.07-0.34) | 0.15 (0.04-0.29) | 0.00 (0.00-0.00) | 0.34 (0.17-0.52) | |
| 16 | 0.13 (0.03-0.27) | 0.16 (0.05-0.30) | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | 0.29 (0.14-0.44) |
| Nonshared environmental influences | E1 | E2 | E3 | E4 | Total e2 |
| 8 | 0.21 (0.18-0.24) | 0.21 (0.18-0.24) | |||
| 12 | 0.04 (0.03-0.06) | 0.18 (0.16-0.21) | 0.23 (0.20-0.26) | ||
| 14 | 0.03 (0.02-0.05) | 0.03 (0.02-0.05) | 0.14 (0.12-0.16) | 0.20 (0.16-0.24) | |
| 16 | 0.02 (0.01-0.04) | 0.03 (0.02-0.05) | 0.05 (0.03-0.07) | 0.14 (0.12-0.16) | 0.24 (0.20-0.28) |
The values presented are standardized components of variance. See Table 2 for an explanation of the values. Finally, a2 + d2 + e2 = 1 at each age (last column). Significant paths are in bold.
Latent Growth Curve Models
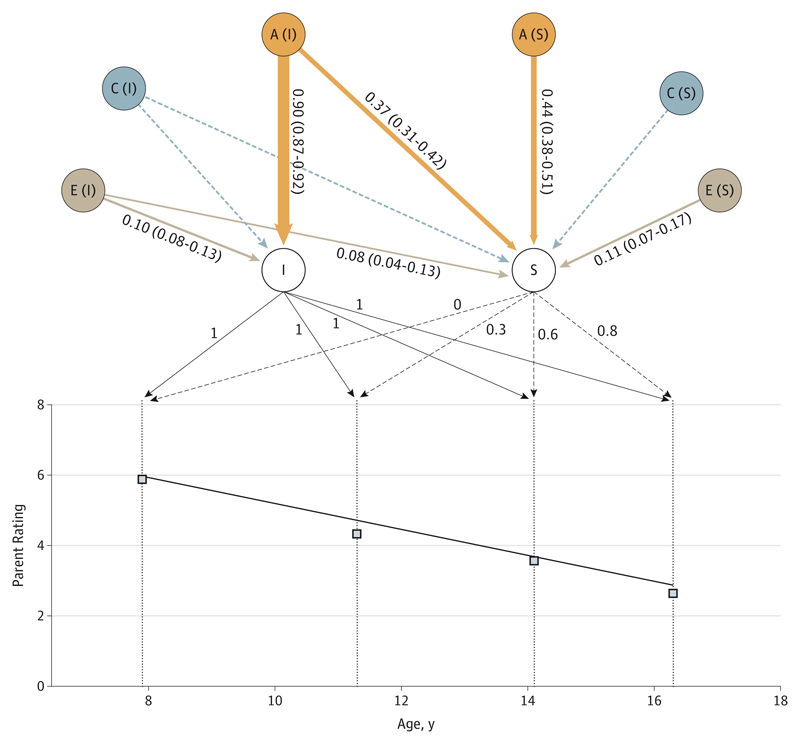
In the phenotypic model, the score of hyperactivity/impulsivity decreased sharply and linearly, with a 3-point decrease from a score of 6.0 at age 8 years to a score of 2.9 at age 16 years (Figure 1). An ACE model (with additive genetic influence) fitted best (eTable 2 in the Supplement). Heritability of the baseline level (intercept) of hyperactivity/impulsivity was high: 90% (95% CI, 87%-92%) of the variance was explained by additive genetic influences. Interindividual differences in the linear systematic change (slope) of hyperactivity/impulsivity was also highly influenced genetically: 81% (95% CI, 73%-88%). Figure 1 shows that more than half of this influence was not shared with the genetic factors influencing the baseline level (ie, 44% specific to the slope and 37% shared with the intercept, summing to 81%). No shared environmental influences and little nonshared environmental influences were detected on either the intercept or the slope.
Figure 1. Genetic and Environmental Influences on the Intercept and Slope of Hyperactivity/Impulsivity.
Observed mean values of hyperactivity/impulsivity (squares) and model-fitted linear decrease (black line) are represented. The intercept (I), slope (S), and their loadings are indicated (slope loadings equal distance in years from the first measurement, divided by 10 to facilitate computations). The heritability (A), shared environment (C), and nonshared environment (E) standardized components of variance and 95% bootstrapped confidence estimates are provided for I and S (except for the nonsignificant dotted arrows). The width of the arrows is proportional to the effect.
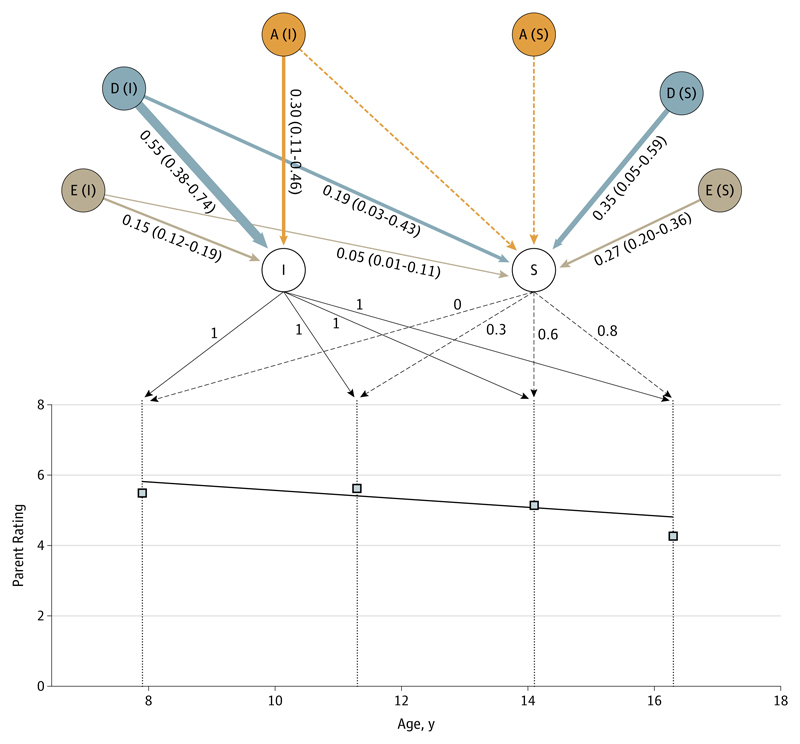
Results for inattention differed in 2 ways. First, in the phenotypic model, the significant linear decline was much less pronounced, with a 1-point decrease from a score of 5.8 at age 8 years to 4.9 at age 16 years (Figure 2 and eTable 4 in the Supplement). The eFigure in the Supplement displays interindividual differences in the developmental course of symptoms: the distributions of the slopes for hyperactivity/impulsivity and inattention show that, although the slope was negative for inattention, a substantial minority of the sample had increasing levels of inattention, in contrast to hyperactivity/impulsivity. eTable 5 in the Supplement provides additional information on children with increasing inattention. A model including nonadditive genetic variance (ADE) fitted the model better (estimates are presented in Figure 2; fit indices are shown in eTable 2 in the Supplement). The nonadditive genetic component (D) explained more than half of the total variance of the baseline level (55%; 95% CI, 38%-74%) and 54% (95% CI, 8%-76%) of the slope, with 35% being specific to the slope and 19% shared with the intercept, summing to 54% (Figure 2). Additive genetic influences explained a substantial part of the variance of the baseline level but not of the slope.
Figure 2. Genetic and Environmental Influences on the Intercept and Slope of Inattention.
Observed mean values of inattention (squares) and model-fitted linear decrease (black line) are represented. The intercept (I), slope (S), and their loadings are indicated (slope loadings equal distance in years from the first measurement, divided by 10 to facilitate computations). The additive genetic influences (A), nonadditive genetic influences (D), and nonshared environment (E) standardized components of variance and 95% bootstrapped confidence estimates are provided for I and S (except for the nonsignificant dotted arrows). The width of the arrows is proportional to the effect.
The residuals were explained by the E term, including nonshared environmental influences as well as error variance (between 21% and 45%), with the rest being explained mostly by genetic influences. Detailed results are available in eTable 6 in the Supplement.
Discussion
This study examined the etiology of interindividual differences in the baseline levels and the developmental course of inattention and hyperactive/impulsive symptoms from childhood to adolescence. Interindividual differences in the overall decline in ADHD symptoms were explained by genetic and environmental influences that were largely distinct from those influencing the baseline level of symptoms. In addition, consistent with the literature, we found large genetic influences on ADHD symptoms with dominant and additive genetic influences for inattention symptoms and only additive genetic effects for hyperactive/impulsive symptoms.33
Developmental Course of Inattention and Hyperactivity/Impulsivity
At the phenotypic level, our results are consistent with clinical and population-based studies showing a substantial decrease in mean levels of hyperactive/impulsive symptoms with age.6,15–17 As compared with hyperactivity/impulsivity, inattention symptoms have been found to follow a less pronounced decline or be stable with age.2,6,15,18 In this study, we found that the decline in inattention symptoms was significant, even though it was clearly less pronounced than for hyperactivity/impulsivity. Although compatible with the extant literature, these findings may seem at odds with some recent population-based studies reporting increasing trajectories of inattention symptoms for subgroups of children.10,17,19 This apparent inconsistency stems from interindividual differences in the developmental course of symptoms, which can be illustrated by the distribution of rates of change in this study: despite the mean decline, a substantial percentage of the sample had increasing levels of inattention (eFigure in the Supplement). The age at onset of ADHD—postponed from 7 to 12 years in DSM-5—remains a controversial topic34 and may seem somewhat arbitrary in the face of the continuous change in symptoms described here. The increase in inattention symptoms for a subset of children in the population is consistent with the emergence of late-onset predominantly inattentive clinical cases, although evidence for late-onset cases is mixed.34 Alternatively, together with the decline in hyperactive/impulsive symptoms, this increase in inattention is consistent with the observed shift with advancing age from combined ADHD to predominantly inattentive.6
Genetic Effects on the Developmental Course of ADHD Symptoms
Meta-analyses have documented cross-sectional structural brain differences (eg, in basal ganglia) between children with ADHD and typically developing children and have suggested change in ADHD-related structures with advancing age.35,36 A recent longitudinal imaging study8 showed that interindividual differences in cortical thinning in the cingulate gyrus and medial prefrontal cortex in childhood and adolescence were associated with the clinical course of ADHD. Specifically, thickening or minimal thinning of these cortical regions occurred exclusively among patients with ADHD whose symptoms had decreased by adulthood to a level below the diagnosis threshold. Moreover, cortical change was independent of baseline symptom severity, revealing a specific relationship between the trajectories of cerebral cortical development in these areas and prognosis. Taken together, these and our findings suggest that the parallel developmental processes at the cortical and phenotypic levels might reflect specific genetic influences, mostly independent from those underlying the baseline status. The hypothesis of a specific genetic liability underlying both developmental processes is only one possible account for the findings and does not exclude complex explanations involving, for instance, gene-by-environment interactions. To test this hypothesis, future research could aim to identify genetic variants associated with the developmental course of ADHD symptoms and verify how these variants associate with cortical development (ie, whether the genetic effect on the developmental course of ADHD symptoms is mediated by cortical development).
Genome-wide association studies have not been successful so far in identifying specific genetic variants associated with ADHD.4 Our results show that some of these genetic variants should be expected to predict both the baseline status and the developmental course of ADHD symptoms, whereas others would predict only the latter. In other fields, studies have already uncovered genetic variants specifically associated with age-related systematic change (eg, obesity37). These studies37–40 also demonstrated that genetic variants may remain undetected when systematic change is not directly modeled. As such, when characterizing ADHD phenotype for genetic studies,41 it appears crucial to adopt a developmental perspective. Longitudinal monozygotic twin studies have shown enduring differences in ADHD symptoms and comorbidity between discordant twin pairs.42 However, identifying specific nonshared environmental factors contributing to these long-term differences has proven challenging, consistent with the general difficulty in identifying nonshared environmental influences.43–45
Limitations and Strengths
Mother ratings from childhood to adolescence constituted a coherent set of measures, which was essential to model systematic change with age. However, rater-related issues are important in twin studies and can influence heritability estimates and the type of genetic and environmental influences (eg, additive or dominant genetic effects).5,20,33,41 Furthermore, recent studies have focused on the transition from childhood to adulthood.5,20 Such studies present specific challenges as instruments and raters often change between childhood and adulthood. As such, although our cohort does not yet include adulthood data, it retains the following strengths: spanning a critical developmental period; distinguishing between both symptom domains (contrary to the aforementioned long-term studies); and using the same measure and the same rater throughout, which allowed us to fit a latent growth curve model. Given the common assumptions and limitations of twin models, caution must be applied when interpreting these findings (discussed further in eAppendix 2 in the Supplement). Finally, this study used dimensional measures of ADHD symptoms in a population-based sample. While this means that we captured interindividual differences across the population, our conclusions cannot be directly applied to clinical populations. The genetic etiology underlying clinical diagnoses of ADHD and ADHD symptoms in population-based samples may differ, although recent evidence points toward some overlap.46,47
Conclusions
A sharp general linear decrease in the levels of hyperactive/impulsive symptoms was observed from ages 8 to 16 years in this population-based sample of twins. A less pronounced decrease was observed for inattention symptoms. Important interindividual differences were detected (faster or slower decreases vs persistence, or even increases in inattention symptoms for a subset of children). These interindividual differences in the developmental course of symptoms were mostly explained by genetic influences, mostly independent from those influencing the baseline level of symptoms. Developmental models will be crucial in identifying genetic variants and specific environmental influences explaining why some children remit from ADHD, whereas others persist. The confirmation of large genetic influences on the developmental course of ADHD symptoms is important for both clinicians and patients. For clinicians, the maintenance or increase in symptoms (a decline being normative in the population) might represent a marker of vulnerability reflecting genetic liability and warrant closer follow-up. It also raises the question of the necessity to inform patients and their relatives about the higher risk of persistence in families of index cases with persistent symptoms.
Supplementary Material
Funding/Support
The Twins Early Development Study is supported by grant G0901245 (and previously G0500079) from the UK Medical Research Council. Dr Pingault is supported by Marie Curie Intra-European Fellowship 330699 from the European Commission. Dr Viding is supported by a Royal Society Wolfson Research Merit Award from the Royal Society and the Wolfson Foundation. Dr Plomin is supported by research professorship award G19/2 from the Medical Research Council and by advanced investigator award 295366 from the European Research Council.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Affiliations:Department of Psychology, Division of Psychology and Language Sciences, University College London, London, England (Pingault, Viding); MRC Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, King’s College London, London, England (Pingault, Viding, Greven, Plomin, Rijsdij); Department of Child and Adolescent Psychiatry, Charles Perrens Hospital and INSERM, The Bordeaux School of Public Health (Institut de Santé Publique, d’Epidémiologie et de Développement), Centre INSERM U897, Epidemiology-Biostatistics, University of Bordeaux, Bordeaux, France (Galéra); Department of Cognitive Neuroscience, Donders Institute for Brain, Cognition, and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands (Greven); Karakter Child and Adolescent Psychiatry University Center, Nijmegen, the Netherlands (Greven); Department of Psychology, Simon Fraser University, Burnaby, British Columbia, Canada (Zheng).
Author Contributions: Dr Pingault had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Pingault, Viding, Plomin, Rijsdijk.
Acquisition, analysis, or interpretation of data: Pingault, Viding, Galéra, Greven, Zheng, Plomin.
Drafting of the manuscript: Pingault, Viding, Rijsdijk.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Pingault, Rijsdijk.
Obtained funding: Pingault, Plomin.
Study supervision: Viding, Greven, Rijsdijk.
Conflict of Interest Disclosures: Dr Galéra reported receiving support from the industry to attend scientific congresses in the past. No other disclosures were reported.
Additional Contributions: We gratefully acknowledge the ongoing contribution of the participants in the Twins Early Development Study and their families.
References
- 1.Snowling M. Multiple perspectives on ADHD: implications for future research. J Child Psychol Psychiatry. 2009;50(9):1039–1041. doi: 10.1111/j.1469-7610.2009.02145.x. [DOI] [PubMed] [Google Scholar]
- 2.Willoughby MT. Developmental course of ADHD symptomatology during the transition from childhood to adolescence: a review with recommendations. J Child Psychol Psychiatry. 2003;44(1):88–106. doi: 10.1111/1469-7610.t01-1-00104. [DOI] [PubMed] [Google Scholar]
- 3.Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40(9):1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Merwood A, Asherson P. Attention deficit hyperactivity disorder: a lifespan genetic perspective. Adv Ment Health Intellect Disabil. 2011;5(4):33–46. doi: 10.1108/20441281111165599. [DOI] [Google Scholar]
- 5.Chang Z, Lichtenstein P, Asherson PJ, Larsson H. Developmental twin study of attention problems: high heritabilities throughout development. JAMA Psychiatry. 2013;70(3):311–318. doi: 10.1001/jamapsychiatry.2013.287. [DOI] [PubMed] [Google Scholar]
- 6.Willcutt EG, Nigg JT, Pennington BF, et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 2012;121(4):991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 8.Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74(8):599–606. doi: 10.1016/j.biopsych.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke B, Faraone SV, Asherson P, et al. International Multicentre Persistent ADHD Collaboration The genetics of attention deficit/hyperactivity disorder in adults: a review. Mol Psychiatry. 2012;17(10):960–987. doi: 10.1038/mp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pingault J-B, Côté SM, Vitaro F, Falissard B, Genolini C, Tremblay RE. The developmental course of childhood inattention symptoms uniquely predicts educational attainment: a 16-year longitudinal study. Psychiatry Res. 2014;219(3):707–709. doi: 10.1016/j.psychres.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Breslau N, Breslau J, Peterson E, et al. Change in teachers’ ratings of attention problems and subsequent change in academic achievement: a prospective analysis. Psychol Med. 2010;40(1):159–166. doi: 10.1017/S0033291709005960. [DOI] [PubMed] [Google Scholar]
- 12.Polderman TJC, Boomsma DI, Bartels M, Verhulst FC, Huizink AC. A systematic review of prospective studies on attention problems and academic achievement. Acta Psychiatr Scand. 2010;122(4):271–284. doi: 10.1111/j.1600-0447.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- 13.Pingault J-B, Côté SM, Lacourse E, Galéra C, Vitaro F, Tremblay RE. Childhood hyperactivity, physical aggression and criminality: a 19-year prospective population-based study. PLoS One. 2013;8(5):e62594. doi: 10.1371/journal.pone.0062594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SS, Hinshaw SP. Predictors of adolescent functioning in girls with attention deficit hyperactivity disorder (ADHD): the role of childhood ADHD, conduct problems, and peer status. J Clin Child Adolesc Psychol. 2006;35(3):356–368. doi: 10.1207/s15374424jccp3503_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57(11):1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Galéra C, Côté SM, Bouvard MP, et al. Early risk factors for hyperactivity-impulsivity and inattention trajectories from age 17 months to 8 years. Arch Gen Psychiatry. 2011;68(12):1267–1275. doi: 10.1001/archgenpsychiatry.2011.138. [DOI] [PubMed] [Google Scholar]
- 17.Larsson H, Dilshad R, Lichtenstein P, Barker ED. Developmental trajectories of DSM-IV symptoms of attention-deficit/hyperactivity disorder: genetic effects, family risk and associated psychopathology. J Child Psychol Psychiatry. 2011;52(9):954–963. doi: 10.1111/j.1469-7610.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- 18.Genolini C, Pingault J-B, Driss T, et al. KmL3D: a non-parametric algorithm for clustering joint trajectories. Comput Methods Programs Biomed. 2013;109(1):104–111. doi: 10.1016/j.cmpb.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Pingault J-B, Tremblay RE, Vitaro F, et al. Childhood trajectories of inattention and hyperactivity and prediction of educational attainment in early adulthood: a 16-year longitudinal population-based study. Am J Psychiatry. 2011;168(11):1164–1170. doi: 10.1176/appi.ajp.2011.10121732. [DOI] [PubMed] [Google Scholar]
- 20.Kan K-J, Dolan CV, Nivard MG, et al. Genetic and environmental stability in attention problems across the lifespan: evidence from the Netherlands twin register. J Am Acad Child Adolesc Psychiatry. 2013;52(1):12–25. doi: 10.1016/j.jaac.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Franke B, Vasquez AA, Johansson S, et al. Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology. 2010;35(3):656–664. doi: 10.1038/npp.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greven CU, Asherson P, Rijsdijk FV, Plomin R. A longitudinal twin study on the association between inattentive and hyperactive-impulsive ADHD symptoms. J Abnorm Child Psychol. 2011;39(5):623–632. doi: 10.1007/s10802-011-9513-7. [DOI] [PubMed] [Google Scholar]
- 23.Lacourse E, Boivin M, Brendgen M, et al. A longitudinal twin study of physical aggression during early childhood: evidence for a developmentally dynamic genome. Psychol Med. 2014;44(12):2617–2627. doi: 10.1017/S0033291713003218. [DOI] [PubMed] [Google Scholar]
- 24.Neale MC, McArdle JJ. Structured latent growth curves for twin data. Twin Res. 2000;3(3):165–177. doi: 10.1375/136905200320565454. [DOI] [PubMed] [Google Scholar]
- 25.Haworth CMA, Davis OSP, Plomin R. Twins Early Development Study (TEDS): a genetically sensitive investigation of cognitive and behavioral development from childhood to young adulthood. Twin Res Hum Genet. 2013;16(1):117–125. doi: 10.1017/thg.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conners CK. Conners’ Rating Scales–Revised: Technical Manual. New York, NY: Multi-Health System Inc; 2003. [Google Scholar]
- 27.Olsen JA, Kenny DA. Structural equation modeling with interchangeable dyads. Psychol Methods. 2006;11(2):127–141. doi: 10.1037/1082-989X.11.2.127. [DOI] [PubMed] [Google Scholar]
- 28.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria vs new alternatives. Struct Equ Modeling. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- 29.Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48(2):1–36. [Google Scholar]
- 30.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 31.Greven CU, Rijsdijk FV, Plomin R. A twin study of ADHD symptoms in early adolescence: hyperactivity-impulsivity and inattentiveness show substantial genetic overlap but also genetic specificity. J Abnorm Child Psychol. 2011;39(2):265–275. doi: 10.1007/s10802-010-9451-9. [DOI] [PubMed] [Google Scholar]
- 32.Plomin R, DeFries JC, Knopik VS, Neiderhiser JM. Behavioral Genetics. 6th ed. New York, NY: Worth; 2013. [Google Scholar]
- 33.Nikolas MA, Burt SA. Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: a meta-analysis. J Abnorm Psychol. 2010;119(1):1–17. doi: 10.1037/a0018010. [DOI] [PubMed] [Google Scholar]
- 34.Polanczyk GV, Moffitt TE. How evidence on the developmental nature of attention-deficit/hyperactivity disorder can increase the validity and utility of diagnostic criteria. J Am Acad Child Adolesc Psychiatry. 2014;53(7):723–725. doi: 10.1016/j.jaac.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(12):1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. 2011;168(11):1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- 37.Lasky-Su J, Lyon HN, Emilsson V, et al. On the replication of genetic associations: timing can be everything! Am J Hum Genet. 2008;82(4):849–858. doi: 10.1016/j.ajhg.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pingault J-B, Côté SM, Booij L, et al. Age-dependent effect of the MAOA gene on childhood physical aggression. Mol Psychiatry. 2013;18(11):1151–1152. doi: 10.1038/mp.2012.173. [DOI] [PubMed] [Google Scholar]
- 39.Charmantier A, Perrins C, McCleery RH, Sheldon BC. Age-dependent genetic variance in a life-history trait in the mute swan. Proc Biol Sci. 2006;273(1583):225–232. doi: 10.1098/rspb.2005.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi G, Rao DC. Ignoring temporal trends in genetic effects substantially reduces power of quantitative trait linkage analysis. Genet Epidemiol. 2008;32(1):61–72. doi: 10.1002/gepi.20263. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson J, Asherson P, Hay D, et al. Characterizing the ADHD phenotype for genetic studies. Dev Sci. 2005;8(2):115–121. doi: 10.1111/j.1467-7687.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 42.Lehn H, Derks EM, Hudziak JJ, Heutink P, van Beijsterveldt TCEM, Boomsma DI. Attention problems and attention-deficit/hyperactivity disorder in discordant and concordant monozygotic twins: evidence of environmental mediators. J Am Acad Child Adolesc Psychiatry. 2007;46(1):83–91. doi: 10.1097/01.chi.0000242244.00174.d9. [DOI] [PubMed] [Google Scholar]
- 43.Plomin R. Why are children in the same family so different? non-shared environment three decades later. Int J Epidemiol. 2011;40(3):582–592. doi: 10.1093/ije/dyq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burt SA, Larsson H, Lichtenstein P, Klump KL. Additional evidence against shared environmental contributions to attention-deficit/hyperactivity problems. Behav Genet. 2012;42(5):711–721. doi: 10.1007/s10519-012-9545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychol Bull. 2009;135(4):608–637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- 46.Groen-Blokhuis MM, Middeldorp CM, Kan K-J, et al. Psychiatric Genomics Consortium ADHD Working Group Attention-deficit/hyperactivity disorder polygenic risk scores predict attention problems in a population-based sample of children. J Am Acad Child Adolesc Psychiatry. 2014;53(10):1123–9.e6. doi: 10.1016/j.jaac.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Martin J, Hamshere ML, Stergiakouli E, O’Donovan MC, Thapar A. Genetic risk for attention-deficit/hyperactivity disorder contributes to neurodevelopmental traits in the general population. Biol Psychiatry. 2014;76(8):664–671. doi: 10.1016/j.biopsych.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.