Abstract
Background
Low bone mineral density (BMD) is a characteristic feature of Beta thalassemia major (βTM) patients. Vitamin D is important for bone mineralization. Vitamin D receptors (VDR) genetic variants may be related to vitamin D status and BMD.
Objectives
To evaluate the effect of VDR genetic variants on vitamin D levels and BMD in βTM Egyptian patients supplemented with vitamin D.
Methods
This study was conducted on forty children with βTM and seventeen unrelated healthy sex and age-matched controls. Serum calcium, phosphorus, alkaline phosphatase, ferritin, and vitamin D were measured. VDR genetic variants (BsmI, TaqI, and FokI) were genotyped by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). BMD was measured by dual-energy X-ray densitometry (DEXA) of the lumbar spine.
Results
In βTM patients, 22.5% had insufficient, and 77.5% had sufficient levels of vitamin D, and no cases had vitamin D deficient. BMD Z score was significantly lower in βTM patients compared to controls (p<0.001). Osteopenia and osteoporosis of lumbar spines were observed in 70% and 22.5% of βTM patients respectively. BsmI bb and FokI Ff and ff genotypic variants were significantly associated with lower vitamin D and BMD Z score. No association was observed with TaqI genotypic variants.
Conclusions
Low BMD is prevalent in patients with βTM despite vitamin D supplementation. The BsmI bb, FokI Ff and ff genotypic variants of VDR can be considered as risk factors for the occurrence of osteoporosis in these children.
Keywords: Vitamin D, VDR genetic variants, BMD, Osteoporosis, DEXA, Thalassemia
Introduction
Thalassemia is one of the most common genetic diseases worldwide. 1 In Egypt, βTM is considered a major health problem. 2
Children with untreated or partially treated βTM die in the early decades of life from infection or heart failure. The mainstay of treatment is adequate blood transfusions with the prevention of post-transfusion iron overload which is incriminated in the etiology of multiple endocrine complications such as growth failure, delayed puberty, hypothyroidism, hypoparathyroidism. 3
Despite optimal conventional treatment and decline in endocrine complications, low bone mineral density (BMD), bone pain and fractures are common manifestations in patients with thalassemia. The etiology of bone disease in thalassemia is multifactorial including bone marrow expansion, inadequate physical activity, calcium and vitamin D deficiency, iron overload, the toxic effect of desferrioxamine and hypogonadism. 4,5
Symptoms of vitamin D deficiency are frequently confused with the symptoms of anemia in patients with βTM. However, improvement of vitamin D deficiency and bone diseases has been reported after treatment with vitamin D. 6–8
Vitamin D receptor (VDR) is a nuclear transcription factor that controls the action of vitamin D. It is encoded by a gene located on chromosome 12q12.14 and includes two promoter regions, eight protein-coding exons (from 2 to 9) and six untranslated exons (1a–1f). 9 The 1, 25(OH)D3 VDR complex can control the response to vitamin D through transcriptional or posttranscriptional processes. VDR genetic variations can modify the activity of VDR protein. Consequently, it may be related to the risk of bone disease in βTM. 10 However, its association with BMD has been established in a few different ethnic populations. 11,12
The present study aimed to evaluate the effect of VDR genetic variants on vitamin D serum levels and BMD in Egyptian patients with βTM on oral vitamin D supplementation.
Patients and Methods
The current case-control study was carried out on forty children with βTM recruited from Alexandria University Children’s Hospital. They were 20 males (50 %) and 20 females (50 %) with age ranging from 2 to 16 years. Seventeen unrelated healthy sex and age-matched children served as a control group. Patients with any renal, hepatic impairment, hypoparathyroidism or using medications affecting bone mineral metabolism (as glucocorticoids or anticonvulsant drugs) were excluded from the study. The legal guardians of both patients with thalassemia and controls had given informed consent after explaining the nature, steps, and aim of the study. The study was conducted with the approval of the Medical Ethics Committee of Alexandria Faculty of Medicine.
All patients were treated conventionally with regular blood transfusions in order to maintain pre-transfusion Hb levels above 9 g/dl, and adequate iron-chelation therapy with deferiprone, deferasirox or combined therapy with desferrioxamine. All patients were on oral vitamin D supplementation dose of 1000 IU daily.
Data collection
A full history was taken from all patients including duration of illness, the frequency of blood transfusion, iron chelation therapy: type and compliance, the presence of complications of thalassemia e.g. cardiac disease, liver disease, diabetes, the presence of complications that could be caused by vitamin D deficiency e.g. bone pain, or pathological fractures. All patients underwent thorough clinical examination including anthropometric measurements of weight and height with body mass index (BMI), height Z score and BMI Z score calculation, abdominal examination to detect hepatomegaly, tender liver, and splenomegaly, and musculoskeletal system examination to detect bone tenderness.
Blood sample collection
Peripheral blood samples were obtained by venipuncture using a sterile aseptic technique. About 5 milliliters of venous blood was withdrawn and divided into three vacutainer tubes. Three ml were delivered into a plain tube, for chemical investigation and vitamin D measurement. Two ml were divided equally into 2 EDTA tube for complete blood count (CBC) and DNA extraction for genotyping respectively.
Chemical analysis
Complete blood count was carried out for all study subjects on ADVIA 2120i Hematology System (Siemens Healthcare GmbH, Germany). All patients were evaluated biochemically for renal function, liver function tests, electrolytes including serum calcium (Ca), phosphorus (P) and alkaline phosphatase (ALP) on the Dimension® RxL Max® Integrated Chemistry System (Siemens Healthcare GmbH, Germany). Ferritin was measured on ADVIA Centaur XP Immunoassay System (Siemens Healthcare GmbH, Germany). Serum vitamin D (25(OH)D3) levels were measured by Enzyme-linked Fluorescent Assay (ELFA) technique on VIDAS (bioMérieux Clinical Diagnostics, France). A patient was considered to have vitamin D sufficiency if 25(OH)D3 (≥20) ng/ml, insufficiency if from 10 to 19 ng/ml and vitamin D deficiency if < 10 ng/ml. 13
VDR genotyping
Total genomic DNA was purified according to the manufacturer protocol using the QIAamp DNA Blood Mini Kit, (QIAGEN, Germany). The quantity and purity of DNA were assayed using a Nanodrop 2000 spectrophotometer (Thermo Scientific, USA). A260: A230 ratio greater than 1.6 and A260:280 ratios greater than 1.8 were considered indicators for highly pure DNA.
Vitamin D receptor genotyping regarding BsmI (rs1544410), TaqI (rs731236), FokI (rs2228570) single nucleotide polymorphisms was carried out by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique. The PCR reaction was carried out on the SimpliAmp Thermal Cycler (Applied Biosystems, USA). Two sequence-specific primers were used for each targeted fragment of VDR gene including intron 9 TaqI restriction site, intron 8 BsmI restriction site and intron 2 FokI restriction site as previously described. 14 The PCR primers were:
| BsmI(rs1544410) | Forward: 5′-CAA CCA AGA CTA CAA GTA CCG CGT CAG TGA-3′ |
| Reverse : 5′-AAC CAG CGG GAA GAG GTC AAG GG-3′ | |
| TaqI(rs731236) | Forward: 5′-CAG AGC ATG GAC AGG GAG CAA-3′ |
| Reverse: 5′-GCA ACT CCT CAT GGC TGA GGT CTC-3′ | |
| FokI(rs2228570) | Forward: 5′-AGC TGG CCC TGG CAC TGA CTC TGC TCT-3′ |
| Reverse: 5′-ATG GAA ACA CCT TGC TTC TTC TCC CTC-3′ |
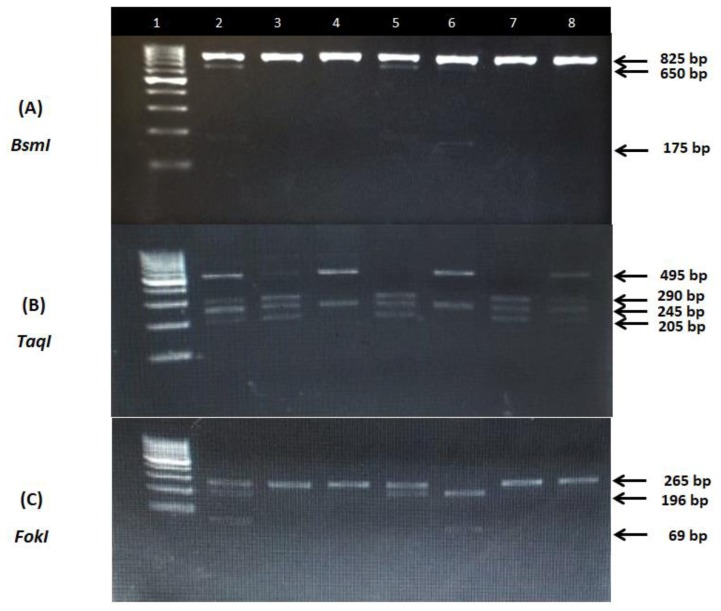
One hundred ng DNA was added to 12.5 ul of MyTaq Red Mix (Bioline, London, United Kingdom) and ten pmol of each primer in a total volume of 25 uL reaction mix. The thermal profile was 95°C for 1 minute as initial denaturation step, followed by 35 cycles each of 95°C for 15 seconds, 63°C for 15 seconds, and 72°C for 10 seconds for TaqI and BsmI. The annealing temperature for FokI was 85°C. The amplified products were digested by the New England Biolabs BsmI, TaqI, and FokI restriction endonucleases. BsmI and TaqI restriction endonucleases were incubated at 65°C for 15 minutes then inactivated at 80°C for 20 minutes. The FokI restriction endonuclease was incubated at 37°C for 15 minutes then inactivated at 65°C for 20 minutes. Digested products were electrophoretically separated electrophoresed through 2% agarose gels and 0.5× TBE buffer and visualized under UV light with ethidium bromide staining using Dolphin Doc gel documentation system (figure 1).
Figure 1.
Representative agarose gel electrophoresis illustrating PCR products for the VDR genetic variants. (A) BsmI variant: lane1, bp marker; lane 2,5,6, heterozygous subject, b allele cut with BsmI generating 650 and 175 bp fragments, B allele does not cut and is 825 bp; lane 3,4,7,8 homozygous BB subject. (B) TaqI variant: lane 1, bp marker; lane 2,8, heterozygous Tt subject, t allele cut with TaqI generating 290,245 and 205 bp fragments; lane 4,6,, homozygous TT subject with 495 and 245 bp bands; lane 3,5,7, homozygous tt subject with 290, 245 and 205 bands. (C) FokI variant: lane1, bp marker; lane 2,5, heterozygous subject, f allele cut with FokI generating 196 and 69 bp fragments, F allele does not cut and appears as 265 bp band; lane 3,4,7,8 homozygous FF subject; lane 6, homozygous ff subject with 196 anf 69 bp bands.
Bone mineral density measurement
Every patient underwent dual-energy X-ray absorptiometry (DEXA) scan using GE Lunar DPX Duo Bone Densitometer (GE Healthcare GmbH, Germany) of the lumbar spine (L1–L4). The BMD results were converted to age and gender-specific Z scores based on the normative reference data for BMD in Egyptian children. Patients were considered to be normal (Z score of −1 or higher), osteopenic (Z score between −1 and −2.5) or osteoporotic (Z score −2.5 or lower) based on WHO criteria. 15
Statistical analysis of the data
Data analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp).The Kolmogorov-Smirnov, Shapiro and D’agstino tests were used to verify the normality of distribution of variables. Comparisons between groups for categorical variables were assessed using the Chi-square test (Fisher or Monte Carlo). Student t-test was used to compare two groups for normally distributed quantitative variables while ANOVA was used for comparing between more than two groups and followed by Post Hoc test (Tukey) for pairwise comparison. Mann Whitney test was used to compare between two groups for not normally distributed quantitative variables while Kruskal Wallis test was used to compare more than two groups for not normally distributed quantitative variables and followed by Post Hoc test (Dunn’s) for pairwise comparison. Spearman coefficient was used to correlate between quantitative variables. Multivariable models were fit using logistic hazards models. The significance of the obtained results was judged at the 5% level.
Results
The characteristics of the studied subjects are summarized in table 1. The height Z score, weight Z score, and BMI Z score were significantly reduced in patients with βTM compared to controls (p=0.012, p<0.001 and p<0.001 respectively). Seven cases (70%) from βTM patients at pubertal age had hypogonadotrophic hypogonadism with delayed puberty which explains why some patients were extremely short in stature despite having normal growth hormone levels. Only one βTM patient had impaired glucose tolerance. The serum levels of phosphorus, alkaline phosphatase and ferritin were significantly higher in patients with βTM than the control group (p<0.001). On the other hand, the calcium level was significantly lower in the patients with βTM than the control group (p=0.004).
Table 1.
Characteristics of study Subjects.
| βTM patients (n= 40) | Control (n= 17) | p | |
|---|---|---|---|
| Age (years) | 8.96 ± 3.71 | 8.24±3.83 | 0.505 |
| Sex | |||
| Male | 20(50.0%) | 7(41.2%) | 0.542 |
| Female | 20(50.0%) | 10(58.8%) | |
| Height (Z score) | −0.85(−3.25 – 0.49) | −0.25(−0.82–0.01) | 0.022* |
| Weight (Z score) | −1.09(−3.15 – 0.91) | 0.07(−0.61–0.92) | 0.001* |
| BMI (Z score) | −0.81(−4.41 – 2.34) | 0.39(−0.02–1.05) | <0.001* |
| Ca (mg/dl) | 8.74 ± 1.22 | 9.38±0.46 | 0.006* |
| P (mg/dl) | 5.51 ± 0.70 | 4.60±0.38 | <0.001* |
| ALP (U/L) | 196.23 ± 70.70 | 64.18±19.19 | <0.001* |
| Ferritin (ng/dl) | 1816 (200–4672) | 64(7–80) | <0.001* |
| Vit D | 24.1(16.5–64.4) | 28.1(25.3–33.4) | 0.087 |
| Deficient (<10) n (%) | 0(0.0) | 0(0.0) | 0.046* |
| Insufficient (10 – 19) n (%) | 9(22.5) | 0(0.0) | |
| Sufficient (≥20) n (%) | 31(77.5) | 17(100.0) | |
| BMD (Z score) | −1.95(−6.0 – −1.0) | 0.90(−0.5 – 1.2) | <0.001* |
| Normal n (%) | 3(7.5) | 17(100.0) | <0.001* |
| Osteopenia n(%) | 28(70) | 0(0.0) | |
| Osteoporosis n(%) | 9(22.5) | 0(0.0) | |
| ALT (U/L) | 20.5(16.0–45.0) | 20.0(12.0–35.0) | 0.087 |
| AST (U/L) | 20.5(14.0–45.0) | 19.5(11.0–37.0) | 0.071 |
| Age at first transfusion (years) | 1.0(0.1–3.0) | – | – |
| Frequency of transfusional blood consumption /year | 12.0(10.0–24.0) | – | – |
Statistically significant at p ≤ 0.05
Vitamin D serum levels
In patients with βTM vitamin D serum levels ranged from 16.5–64.4 ng/ml with a median of 24.1 ng/ml while in the control group it ranged from 25.3–33.4 ng/ml with a median of 28.1 ng/ml. No significant difference was observed between both groups (p=0.087). In patients with βTM, nine patients (22.5%) had insufficient (10–19 ng/ml), and 31 patients (77.5%) had sufficient (≥20 ng/ml) serum levels of vitamin D while in the control group the 17 children included had sufficient vitamin D levels (p=0.046).
Bone Mineral Density (BMD) Z score
The BMD Z score showed a statistically significant reduction in patients with βTM as it ranged from −6.0 to −1.0 with median −1.95 compared to −1.0 to 1.3 with a median of 0.65 in the controls (p<0.001). Three patients (7.5%) had normal BMD Z score, 28 patients (70%) had osteopenia, and nine patients (22.5%) had osteoporosis. All subjects in the control group had normal BMD Z score (p<0.001). We reported a significant positive correlation between serum 25(OH) D3 levels and the Z score of BMD at the lumbar spine (r= 0.505, p= 0.001). Regarding the relations between clinical presentation of βTM patients with age and BMD, a statistically significant association was observed between bone tenderness and the age of βTM patients (p=0.016). No significant association could be detected regarding bone pain or fracture (table 2).
Table 2.
Associations between clinical presentation with age and BMD in βTM patients.
| Age (years) Mean ± SD. |
BMD (Z score) Median (Min. – Max.) |
||
|---|---|---|---|
| Bone pain | |||
| Absent | 7.9±3.2 | −2.0(−4.0 – −1.0) | |
| Present | 10.0±4.0 | −1.9(−6.0 – −1.1) | |
| p | 0.076 | 0.597 | |
| Fractures | |||
| Absent | 8.4±3.4 | −2.0(−4.0 – −1.0) | |
| Present | 10.9±4.3 | −1.8(−6.0 – −1.1) | |
| p | 0.076 | 0.172 | |
| Tenderness | |||
| Absent | 7.6±3.2 | −2.0(−4.0 – −1.0) | |
| Present | 10.4±3.7 | −1.9(−6.0 – −1.0) | |
| p | 0.016* | 0.839 |
Statistically significant at p ≤ 0.05.
VDR genotyping
The distribution of the genotypes of VDR gene polymorphism BsmI, TaqI, and FokI in the study subjects was by Hardy-Weinberg equilibrium. (p=0.647, 0.455 and 0.448 respectively). Studying the pattern of haplotypes inheritance in all studied children showed that the highest frequency was for BTF haplotype with a frequency of 42.5% followed by Btf, BtF, bTf, btF, btf, bTF, and BTf with a frequency of 15%, 12.5%, 10%, 7.5%, 6.3%, 5%, and 1.3% respectively. The association between the genotypes and alleles of VDR genetic variants with the clinical, biochemical parameters and BMD Z score is demonstrated in table 3, four respectively.
Table 3.
Association between the genotypes of BsmI, TaqI and FokI with different parameters in patients with βTM.
| BsmI | TaqI | FokI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| BB (n= 21) | Bb (n= 15) | bb (n= 4) | TT (n= 13) | Tt (n= 21) | tt (n= 6) | FF (n= 18) | Ff (n= 18) | ff (n= 4) | ||
| Sex | Male | 12 (57.1%) | 7 (46.7%) | 1 (25%) | 4 (30.8%) | 12 (57.1%) | 4 (66.7%) | 8 (44.4%) | 10 (55.6%) | 2 (50%) |
| Female | 9 (42.9%) | 8 (53.3%) | 3 (75%) | 9 (69.2%) | 9 (42.9%) | 2 (33.3%) | 10 (55.6%) | 8 (44.4%) | 2 (50%) | |
| p | 0.540 | 0.320 | 0.895 | |||||||
| Age (years) | Mean ± SD. | 9.2 ± 3.8 | 8.4 ± 3.7 | 9.8 ± 3.9 | 7.5b ± 3.2 | 9ab ± 3.8 | 12a ± 3 | 7.4b ± 3.2 | 11.1a ± 3.2 | 6.8ab ± 3.8 |
| p | 0.763 | 0.044* | 0.003* | |||||||
| Weight Z score | Median (Range) | −1 (−3.2 – 0.9) | −1.1 (−3.1 – 0.7) | −1.7 (−2.9 – −0.5) | −1.1 (−3.1 ± 0.7) | −0.9 (−2.6 – 0.9) | −1.9 (−3.2 – −0.7) | −0.5 (−3.2 – 0.9) | −1.7 (−3.1 ± 0.7) | −0.6 (−1.7 – 0.9) |
| P | 0.611 | 0.110 | 0.133 | |||||||
| Height Z score | Median (Range) | −0.3 (−2.6 – 0.3) | −1.3 (−2.9 – 0.5) | −1.3 (−3.3 – −0.9) | −1.3 (−2.9 – −0.1) | −0.3 (−2 – 0.5) | −1.1 (−3.3 – 0.2) | −0.3 (−2.6 – 0.5) | −1.3 (−3.3 – 0.3) | −0.7 (−1.4 – 0.2) |
| p | 0.120 | 0.222 | 0.093 | |||||||
| BMI Z score | Median (Range) | −0.7 (−4.4 – 1.5) | −0.2 (−2.7 – 2.3) | −1.1 (−1.4 – 0.5) | −0.1 (−2.7 ± 2.3) | −0.5 (−3.5 – 2.3) | −1 (−4.4 – −0.5) | −0.4 (−4.4 – 2.3) | −1 (−3.5 ± 0.3) | −0.3 (−1.3 – 1.5) |
| P | 0.865 | 0.364 | 0.379 | |||||||
| Ca (mg/dl) | Mean ± SD. | 9.1a ± 1.4 | 8.7ab ± 0.8 | 7.4b ± 0.1 | 8.7ab ± 0.9 | 8.5b ± 0.8 | 9.8a ± 2.2 | 8.6 ± 0.8 | 8.9 ± 1.3 | 8.9 ± 2.4 |
| p | 0.030* | 0.042* | 0.720 | |||||||
| P (mg/dl) | Mean ± SD. | 5.7 ± 0.6 | 5.4 ± 0.8 | 5 ± 0.7 | 5.5 ± 0.7 | 5.4 ± 0.7 | 5.9 ± 0.7 | 5.3 ± 0.8 | 5.8 ± 0.5 | 5.3 ± 1 |
| p | 0.140 | 0.350 | 0.156 | |||||||
| ALP (U/L) | Mean ± SD. | 178.5 ± 57.4 | 209.2 ± 76.4 | 240.5 ± 100.7 | 211.9 ± 90.5 | 186.9 ± 60.4 | 195 ± 60.9 | 183.1 ± 68.2 | 218.6 ± 75.2 | 154.8 ± 17.6 |
| p | 0.186 | 0.615 | 0.151 | |||||||
| Ferritin | Median (Range) | 2000 (200–4672) | 1500 (900–2732) | 2200 (1702–3935) | 1500 (900 – 3935) | 1700 (200 – 4672) | 2425.5(1000–3500) | 1630 (200 – 3186) | 2000 (706 – 4672) | 1351 (350 – 2000) |
| p | 0.292 | 0.200 | 0.227 | |||||||
| Vitamin D | Median (Range) | 27.1 (18.4–62.5) | 25.9 (17.1–64.4) | 17.6 (16.5–19.2) | 27.2 (18.4–55.1) | 23.5 (16.5–64.4) | 23.2 (16.7–28.8) | 28.4 (20.7–64.4) | 21.5 (16.7–41.4) | 21.1 (16.5–23.5) |
| p | 0.010* | 0.432 | 0.003* | |||||||
| Deficient | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | |
| Insufficient | 3a(14.3%) | 2a(13.3%) | 4b(100.0%) | 2(15.4%) | 5(23.8%) | 2(33.3%) | 0a(0.0%) | 7b(38.9%) | 2b(50.0%) | |
| Sufficient | 18a(85.7%) | 13a(86.7%) | 0b(0.0%) | 11(84.6%) | 16(76.2%) | 4(66.7%) | 18a(100.0%) | 11b(61.1%) | 2b(50.0%) | |
| p | 0.002* | 0.591 | 0.006* | |||||||
| Bone disease | Bone pain | 10 (47.6%) | 8 (53.3%) | 2 (50%) | 4a (30.8%) | 10a (47.6%) | 6b (100%) | 6 (33.3%) | 12 (66.7%) | 2 (50%) |
| 1.000 | 0.023* | 0.167 | ||||||||
| Fractures | 7 (33.3%) | 1 (6.7%) | 1 (25%) | 1a (7.7%) | 4a (19%) | 4b (66.7%) | 2 (11.1%) | 7 (38.9%) | 0 (0%) | |
| P | 0.150 | 0.020* | 0.102 | |||||||
| Tenderness | 10 (47.6%) | 7 (46.7%) | 2 (50%) | 3 (23.1%) | 11 (52.4%) | 5 (83.3%) | 6 (33.3%) | 12 (66.7%) | 1 (25%) | |
| p | 1.000 | 0.063 | 0.097 | |||||||
| BMD Z score | Median (Range) | −1.7 (−4 – −1) | −2 (−3 – −1.7) | −4 (−6 – −3.2) | −2 (−3.2 – −1.2) | −1.9 (−4 – −1) | −1.9 (−6 – −1.5) | −1.8 (−2.5 – −1) | −2 (−6 – −1.1) | −4 (−4 – −3) |
| p | 0.001* | 0.586 | 0.001* | |||||||
| Normal | 3a (14.3%) | 0a (0%) | 0a (0%) | 0 (0%) | 3 (14.3%) | 0 (0%) | 3a (16.7%) | 0a (0%) | 0a (0%) | |
| Osteopenia | 16a (76.2%) | 12a (80%) | 0b (0%) | 9 (69.2%) | 15 (71.4%) | 4 (66.7%) | 14a (77.8%) | 14a (77.8%) | 0b (0%) | |
| Osteoporosis | 2a (9.5%) | 3a (20%) | 4b (100%) | 4 (30.8%) | 3 (14.3%) | 3 (33.3%) | 1a (5.6%) | 4a (22.2%) | 4b (100%) | |
| p | 0.002* | 0.494 | 0.001* | |||||||
Common letters are not significant (i.e. Different letters are significant).
Statistically significant at p ≤ 0.05.
BsmI
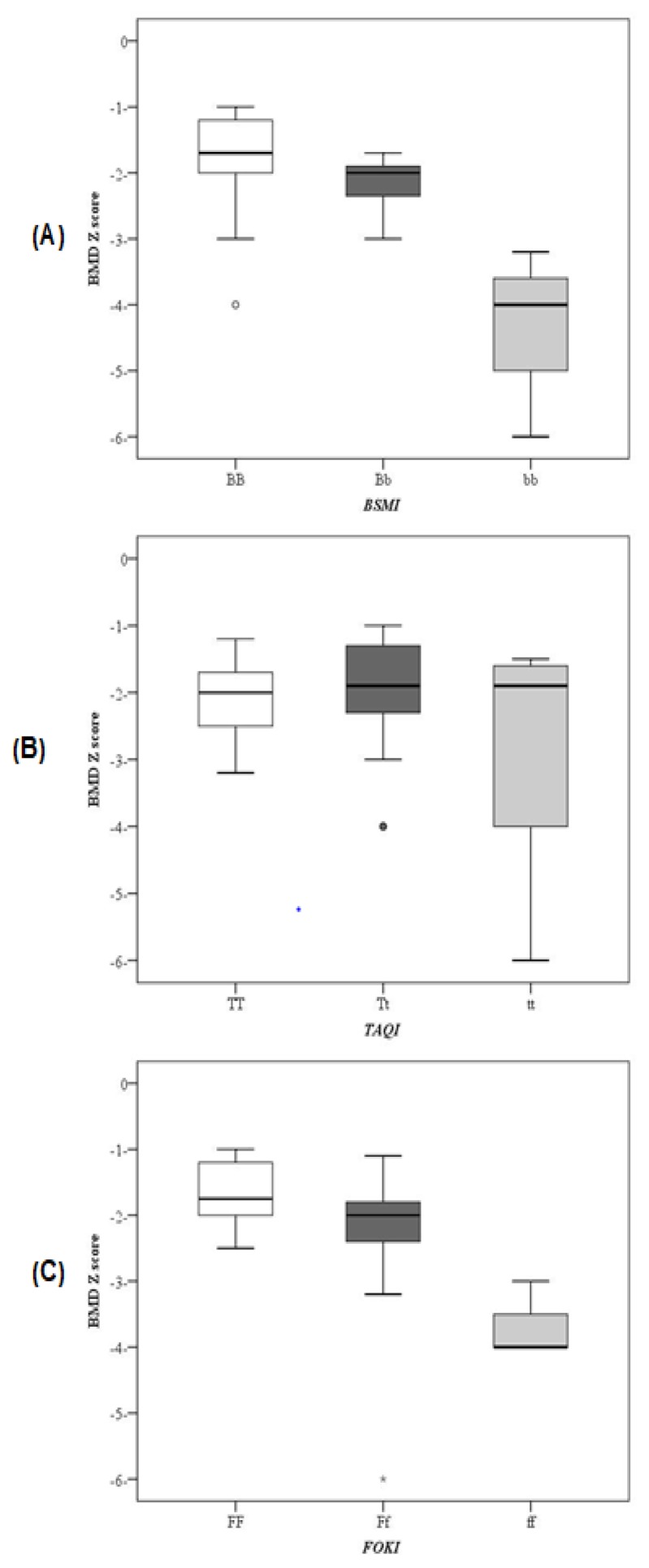
Twenty one βTM patients (52.5%) had BB genotype, 15 patients (37.5%) had Bb genotype and 4 patients (10%) had bb genotype. In the control group, 24 subjects (60%) had BB genotype, and ten subjects (40%) had Bb genotype. The B allele was found in 71.2 % of βTM patients and 80% of controls, while b allele frequency was 28.8% and 20% in the two groups respectively. No statistically significant differences could be detected between βTM patients and controls regarding the BsmI, genotypes or alleles frequencies. No statistically significant difference was found between different BsmI genotypes as regards the anthropometric measures or laboratory parameters except for serum calcium level which was significantly lower in bb genotype compared to BB and Bb genotypes (p=0.030). Thus, b allele was significantly associated with lower serum calcium levels compared to B allele (p=0.01). Vitamin D levels were significantly lower in BsmI bb genotype compared to Bb and BB genotypes (p=0.010). Moreover, the association of b allele with significantly lower vitamin D levels compared to B allele (p=0.006) confirmed these findings. Bone mineral density Z score differs significantly between the three genotypes (p=0.001) (figure 2). It was significantly lower in patients with bb genotype than Bb and BB genotypes. Osteoporosis was observed in 4 patients (100%) with bb genotype versus three patients 20% with Bb and two patients (9.5%) with BB genotypes (p=0.001). While osteopenia was found in 16 patients (76.2%) with BB, 12 patients (80%) with Bb and none with bb genotype (p=0.012), moreover, b allele was significantly associated with lower BMD Z score compared to B allele (p<0.001).
Figure 2.
BMD Z scores in different VDR genotypes of (A) BsmI (B) TaqI (C) FokI genetic variants.
TaqI
Thirteen patients (32.5%) with βTM had TT, 21 patients (52.5%) had Tt and 6 patients (15%) had tt genotype. In the control group, 18 subjects (45%) had TT, 18 subjects (45%) had Tt, and four subjects (10%) had tt genotype. The T allele frequency was 58.8% in βTM patients and 62.5% in controls. The t allele was found in 41.3% of βTM patients and 20% of controls. No statistically significant differences could be detected between both groups regarding the TaqI genotypes or alleles frequencies. No statistically significant differences were found between different TaqI genotypes or alleles regarding the anthropometric measures or BMD Z score (figure 2) and only calcium in laboratory parameters (p=0.042). However, the three TaqI genotypes showed significant differences in bone pain (p=0.023) and bone fractures (p=0.020). In patients with βTM bone pain was found in 100% of patients with tt genotype, compared to 47.6% of patients with Tt genotype and 30.8% of patients with TT genotype (p=0.023). Patients with a history of bone fractures were significantly higher in patients with tt genotype (66.7%) compared to Tt genotype (19%) and TT genotype (7.7%) of patients with TT genotype. The t allele was significantly associated with bone pain and tenderness (table 4).
Table 4.
Association between alleles of BsmI, TaqI and FokI with different parameters in patients with βTM.
| VDR genetic variants | BsmI | TaqI | FokI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Alleles | B | b | p | T | t | p | F | f | p | |
| Age (years) | Mean ± SD. | 9±3.7 | 8.9±3.6 | 0.913 | 8.2±3.5 | 10.1±3.7 | 0.021* | 8.6±3.6 | 9.7±3.8 | 0.198 |
| Sex | Male | 54.4% | 39.1% | 0.217 | 42.6% | 60.6% | 0.112 | 48.1% | 53.8% | 0.633 |
| Female | 45.6% | 60.9% | 57.4% | 39.4% | 51.9% | 46.2% | ||||
| Height Z score | Median (Range) | −0.3 (−2.9 – 0.5) | −1.3 (−3.3 – 0.5) | 0.031* | −0.9 (−2.9 – 0.5) | −0.8 (−3.3 – 0.5) | 0.681 | −0.7 (−3.3 – 0.5) | −1.2 (−3.3 ± 0.3) | 0.344 |
| Weight Z score | Median (Range) | −1 (−3.2 – 0.9) | −1.6 (−3.1 – 0.7) | 0.437 | −1 (−3.1 – 0.9) | −1.3 (−3.2 – 0.9) | 0.278 | −1 (−3.2 – 0.9) | −1.3 (−3.1 – 0.9) | 0.615 |
| BMI Z score | Median (Range) | −0.7 – −4.4 | −0.9 (−2.7 – 2.3) | 0.848 | −0.2 (−3.5 – 2.3) | −0.9 (−4.4 – 2.3) | 0.314 | −0.7 (−4.4 – 2.3) | −1 (−3.5 – 1.5) | 0.774 |
| Ca (mg/dl) | Median (Range) | 8.6(6.8–12.8) | 8.2(7.2–10.3) | 0.011* | 8.5(6.8–10.4) | 8.6(6.8–12.8) | 0.222 | 8.6(6.8–12.8) | 8.6(7.2–12.8) | 0.512 |
| P (mg/dl) | Median (Range) | 5.5(4.1–6.5) | 5.2(4.1–6.4) | 0.037* | 5.7(4.1–6.5) | 5.6(4.1–6.5) | 0.335 | 5.4(4.1–6.5) | 5.7(4.2–6.5) | 0.347 |
| ALP (U/L) | Median (Range) | 175(79–344) | 202(79–345) | 0.088 | 176(79–345) | 179(106–328) | 0.498 | 183.5(79–345) | 170(124–345) | 0.813 |
| S Ferritin | Median (Range) | 1700 (200–4672) | 1930 (900–3935) | 0.530 | 1660 (200–4672) | 2000 (200–4672) | 0.252 | 1815 (200–4672) | 1816 (350–4672) | 0.984 |
| Median (Range) | 26.7(17.1–64.4) | 20.7(16.5–64.4) | 0.006* | 26.7(16.5–64.4) | 23.7(16.5–64.4) | 0.222 | 27.2(16.7–64.4) | 21.5(16.5–41.4) | 0.001* | |
| Deficient | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | ||||
| Vitamin D | Insufficient | 14.0% | 43.5% | 0.004* | 19.1% | 27.3% | 0.392 | 13.0% | 42.3% | 0.003* |
| Sufficient | 86.0% | 56.5% | 80.9% | 72.7% | 87.0% | 57.7% | ||||
| Bone pain | 49.1% | 52.2% | 0.805 | 38.3% | 22 (66.7) | 0.012* | 44.4% | 61.5% | 0.152 | |
| Bone diseases | Fractures | 26.3% | 13% | 0.198 | 12.8% | 12 (36.4%) | 0.013 | 20.4%) | 26.9% | 0.511 |
| Tenderness | 47.4% | 47.8% | 0.970 | 36.2% | 21 (63.6%) | 0.015* | 24 (44.4%) | 53.8% | 0.430 | |
| Median (Range) | −1.9(−4-−1) | −2.4(−6–−1.7) | <0.001* | −2(−4–−1) | −1.9(−6–−1) | 0.754 | −1.9(−6–1) | −2.4(−6–1.1) | <0.001* | |
| Normal | 10.5% | 0% | 6.4% | 9.1% | 11.1% | 0% | ||||
| BMD Z score | Osteopenia | 77.2% | 52.2% | 0.002* | 70.2% | 69.7% | 0.869 | 77.8% | 53.8% | 0.002* |
| Osteoporosis | 12.3% | 47.8% | 23.4% | 21.2% | 11.1% | 46.2% | ||||
Statistically significant at p ≤ 0.05.
FokI
Eighteen βTM patients (45%) had FF genotype, 18 patients (45%) had Ff genotype, and four patients (10%) had ff genotype. In the control group, 24 subjects (60%) had FF genotype, 12 subjects (30%) had Ff genotype, and four subjects (10%) had ff genotype. The F allele was found in 67.5% of βTM patients and 75% of controls while f allele was found in 32.5% of βTM patients compared to 25% of controls. Patients harboring f allele had significantly lower vitamin D levels compared to the F allele (p=0.001). Subsequently, vitamin D levels were significantly higher in patients with FF compared to Ff and ff genotypes (p=0.003). Moreover, the f allele was significantly associated with lower BMD Z score (p<0.001). BMD Z score was significantly lower in ff compared to Ff and FF genotypes (p=0.001) (figure 2).
VDR allelic variants and osteoporosis
The VDR gene variants BsmI b allele and FokI f allele have been found be associated with increased risk for development of osteoporosis (OR, 6.398; 95% CI, 2.275–17.991; p<0.001 and OR= 6.353; 95% CI, 2.216–18.210; p=0.001 respectively) in patients with βTM. In multivariate analysis model for predicting osteoporosis, when considering age, sex, BMI Z score, Ca, P, ALP, vitamin D levels, and the studied three VDR allelic variants, the BsmI b allele and FokI f allele appeared to be independent predictors for osteoporosis in patients with βTM (OR, 4.677; 95% CI, 1.246–17.552; p=0.022 for BsmI b allele and OR, 4.581; 95% CI, 1.304–16.097; p=0.018 for FokI f allele) (table 5).
Table 5.
Univariate and multivariate analysis for the parameters predicating osteoporosis in total cases.
| Univariate | #Multivariate | |||
|---|---|---|---|---|
|
|
||||
| p | OR (95%C.I) | p | OR (95%C.I) | |
| Sex (Female) | 0.288 | 0.585(0.218–1.571) | ||
| Age (years) | 0.715 | 1.026(0.892–1.181) | ||
| BMI (Z score) | 0.240 | 0.805(0.561–1.156) | ||
| Vitamin D | 0.157 | 0.961(0.911–1.015) | ||
| Ca (mg/dl) | 0.122 | 0.636(0.358–1.128) | ||
| P (mg/dl) | 0.002* | 2.893(1.480–5.655) | 0.112 | 2.209(0.831 – 5.870) |
| ALP (U/L) | <0.001* | 1.011(1.005–1.017) | 0.057 | 1.008(1.000–1.016) |
| BsmI (b) | <0.001* | 6.398(2.275–17.991) | 0.022* | 4.677(1.246–17.552) |
| TaqI (t) | 0.964 | 0.977(0.358–2.671) | ||
| FokI (f) | 0.001* | 6.353(2.216–18.210) | 0.018* | 4.581(1.304–16.097) |
OR: Odd‘s ratio, C.I: Confidence interval,
All variables with p<0.05 was included in the multivariate,
Statistically significant at p ≤ 0.05
Discussion
Growth failure with osteoporosis remains frequent complication in patients with thalassemia especially with the marked improvement of their survival as a result of improvements in therapy. It is a challenge to understand the interactions between vitamin D status, genetic variants of VDR, and the risk of bone diseases development in βTM.
Vitamin D affects bone mineralization directly by genomic mechanism via VDR and indirectly through its stimulation of intestinal calcium and phosphorus absorption. 16 Vitamin D deficiency in patients with thalassemia is caused by decreased intake, lower sun exposure, defective skin synthesis; associated with jaundice, impaired absorption, or defective 25 hydroxylation of vitamin D in the liver due to hepatic siderosis. 8 In the present study, there was no significant difference in the serum vitamin D levels between βTM patients and controls. These findings were not surprising for us as our studied patients were receiving vitamin D supplementation as a part of their routine treatment regimen. Regarding vitamin D status, none of our patients had vitamin D deficiency, 22.5 % had vitamin D insufficiency, and 77.5% had sufficient vitamin D levels. On the other hand, Singh et al., 11 reported that 80.6% of patients with βTM had vitamin D deficiency. Also, Elhoseiny et al., 17 reported that vitamin D levels were significantly lower in Egyptian patients with βTM than controls with 60% of the studied patients had vitamin D deficiency. Moreover, El-Edel et al., 12 found sufficient levels of 25(OH)D3 in their thalassemic patients, however, it was lower among older patients, which may be explained by improvements in the use of effective chelating agents and good vitamin D supplementation that may lead to a normal vitamin D level in thalassemia patients.
Vitamin D is of large importance for homeostasis of calcium and bone metabolism. Many factors other than vitamin D deficiency play a role in causing hypocalcemia in patients with thalassemia such as hypoparathyroidism, decrease intake, impaired absorption and iron overload. 18 The present study reported lower serum calcium and higher serum phosphorus, alkaline phosphatase and ferritin in βTM patients compared to controls in spite of higher vitamin D levels in their serum.
The deficiency of calcium is highly prevalent in βTM patients as revealed by our results, which can lead to bone destruction in thalassemia patients by increasing the osteoclast function and/or reducing the osteoblast activity. 16 In the present study, only 7.5% of patients had normal BMD Z score, 70% had osteopenia, and 22.5% had osteoporosis. These results are similar to those of previous studies from India, 11 Israel 19 and Italy. 20 Moreover, In agreement with Singh et al., 11 we reported a significant positive correlation between serum 25(OH)D3 levels and the BMD Z score at the lumbar spine. These findings give additional evidence that vitamin D is an important factor that is required for bone development and preservation of bone mass.
Majority of vitamin D’s actions are mediated through binding to the nuclear hormone VDR which is a transcription factor regulating the expression of genes that mediate its biologic activity. 21 Upon binding to its ligand (1,25(OH)2D), VDR heterodimerizes with a retinoid-X receptor (RXR). Then, the VDR/RXR heterodimer binds to vitamin D responsive elements (VDREs) in the promotor of VDR target genes to exert its transcriptional regulatory effect. In humans, multiple allelic variants of the VDR gene have been identified with different distribution frequencies between race and ethnic groups. 22,23 Among more than 60 VDR variants, the most important are BsmI, and TaqI in the intron separating exon 8 and 9 and FokI variant in the translation start site. 16 Since VDR genetic variants affect the stability and activity of VDR mRNA and protein, it is likely that they influence the individual response to vitamin D treatment. Therefore, we tried to investigate the prevalence and the association of VDR genetic variants with different clinic-pathological parameters in patients with βTM.
Regarding BsmI genotypes distribution, the present study reported that 52.5% of patients had BB genotype, 37.5% of patients had Bb genotype and 10% had bb genotype. Elhoseiny et al., 17 reported similar results in the Egyptian patients. However, Ferrera et al., 20 reported different frequencies due to the different ethnicity of the studied subjects. In our study, 32.5% of patients had TaqI TT genotype, 52.5% had Tt genotype, and 15% had tt genotype. Also, 45% had FokI Ff genotype, and 10% had ff genotype. These results did not differ greatly from those reported by other studies on Egyptian patients 17 and other populations in Greek 24 or Italy. 20
Similar to Elhoseiny et al. 17 and Singh et al. 11 we couldn’t find any association between VDR genotypic variants and phosphorus, alkaline phosphatase or serum ferritin. On studying the relations between different genotypic variants and serum calcium level, the BsmI bb genotype was significantly associated with lower serum calcium levels compared to Bb and BB genotypes. Thus, the b allele is associated with low serum calcium. This datum is in contrast to the results of Elhoseiny et al. 17 who reported a significant association between BB genotype and lower calcium levels. Moreover, we couldn’t find any association between FokI genotypic variants and serum calcium levels.
In the current study, low 25(OH)D3 levels were significantly associated with the b allele. In addition, 25(OH)D3 was significantly lower in BsmI bb genotype compared to Bb and BB genotypes, which is in opposition to the results of Elhoseiny et al. 17 Furthermore, Singh et al. 11 couldn’t find any association between vitamin D levels and different VDR gene genotypes. Regarding FokI polymorphism, low levels of 25(OH)D3 were significantly associated with the f allele. We reported a significant association between ff genotype and lower 25(OH)D3 levels in thalassemic children. Our results were in agreement with those reported Elhoseiny et al. 17 and Dimitridou et al, 24 On the other hand, these results are in contrast to those reported by Smolders et al., 25 where the FF genotype was associated with lower 25(OH)D3 concentrations, but in patients with multiple sclerosis. Our results may be attributed to the functional nature of FokI variant in which the ff variant, initiation of translation occurs at the first ATG site, giving a long version of VDR protein with decreased transcriptional activity. Conversely, in the FF variant, translation begins at the second ATG site, resulting in a protein shortened by three amino acids, which has higher transcriptional activity and activation of target cells compared with full-length VDR protein. 26,27
Regarding the BMD Z score, our results revealed a significant association between BsmI b allele and FokI f allele and low BMD Z score. Furthermore, BMD Z score was significantly lower in bb genotype compared to Bb and BB genotypes of the BsmI variant. In addition, FokI ff and Ff genotypes had significantly lower BMD Z score than FF genotype. These results are in contrast to Ferrara et al. 20 and Singh et al. 11 as they reported a lower BMD of the lumbar spine with BsmI BB and FokI FF VDR genotypes. Also, El-Edel et al. 12 reported that β-thalassemia patients with the BB genotype had significantly lower BMD compared with those with bb or Bb genotypes. There was no significant association between different TaqI genotypes, alleles and BMD Z score in the present study which is concordance with finding reported by Singh et al. 11 However, these findings are in contrast to the findings of Vupputuri et al. 28 who showed a significant association of TaqI with BMD in Asian Indian adults.
It is worth mentioning that the multivariate model for prediction of osteoporosis revealed that both BsmI b allele and FokI f allele were found to be independent predictors for osteoporosis in βTM patients. This finding should be applied in the clinical settings of thalassemic patients’ management and risk assessment.
However, some limitations of the current study should be acknowledged. Due to financial limitations, we carried out the current study in a small sample size, and we did not study the relations between the causative mutation in the β globulin gene, VDR variants, and BMD. Moreover, larger multicenter studies to confirm our findings are warranted, as well as to investigate the possible etiologic links between VDR genetic variants and bone diseases in βTM in different ethnic populations.
VDR genotypes may potentially affect the individual’s response to vitamin D treatment, and consequently, vitamin D doses may need to be adjusted individually by increasing the dose of vitamin D supplementation according to the therapeutic response of each patient. This may vary depending upon the genetic makeup of each but further studies are needed to assess this.
Conclusions
BsmI bb and FokI Ff and ff genotypic variants of VDR can be considered as risk factors for the occurrence of osteoporosis in βTM children. Consequently, VDR genotyping may be used as an additional test in the assessment of children with thalassemia beside the biochemical levels of calcium and vitamin D to find out who is vulnerable to osteoporosis to be supervised with individually adjusted-dose of oral vitamin D supplementation.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Higgs DR, Engel JD, Stamatoyannopoulos G. Thalassaemia. Lancet. 2012;379(9813):373–383. doi: 10.1016/S0140-6736(11)60283-3. [DOI] [PubMed] [Google Scholar]
- 2.El-Shanshory M, Hagag A, Shebl S, Badria I, Abd Elhameed A, Abd El-Bar E, Al-Tonbary Y, Mansour A, Hassab H, Hamdy M, Alfy M, Sherief L, Sharaf E. Spectrum of Beta Globin Gene Mutations in Egyptian Children with beta-Thalassemia. Mediterr J Hematol Infect Dis. 2014;6(1):e2014071. doi: 10.4084/mjhid.2014.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kesse-Adu R, Howard J. Inherited anaemias: sickle cell and thalassaemia. Medicine. 41(4):219–224. doi: 10.1016/j.mpmed.2013.01.012. [DOI] [Google Scholar]
- 4.Voskaridou E, Terpos E. New insights into the pathophysiology and management of osteoporosis in patients with beta thalassaemia. Br J Haematol. 2004;127(2):127–139. doi: 10.1111/j.1365-2141.2004.05143.x. [DOI] [PubMed] [Google Scholar]
- 5.Borgna-Pignatti C, Gamberini MR. Complications of thalassemia major and their treatment. Expert Rev Hematol. 2011;4(3):353–366. doi: 10.1586/ehm.11.29. [DOI] [PubMed] [Google Scholar]
- 6.Soliman A, Adel A, Wagdy M, Al Ali M, ElMulla N. Calcium homeostasis in 40 adolescents with beta-thalassemia major: a case-control study of the effects of intramuscular injection of a megadose of cholecalciferol. Pediatr Endocrinol Rev. 2008;6(Suppl 1):149–154. [PubMed] [Google Scholar]
- 7.Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827–836. doi: 10.2147/TCRM.S3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soliman A, De Sanctis V, Yassin M. Vitamin d status in thalassemia major: an update. Mediterr J Hematol Infect Dis. 2013;5(1):e2013057. doi: 10.4084/mjhid.2013.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laczmanska I, Laczmanski L, Bebenek M, Karpinski P, Czemarmazowicz H, Ramsey D, Milewicz A, Sasiadek MM. Vitamin D receptor gene polymorphisms in relation to the risk of colorectal cancer in the Polish population. Tumour Biol. 2014;35(12):12397–12401. doi: 10.1007/s13277-014-2554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Yin X, Wang J, Xu D, Wang Y, Yang J, Tao Y, Zhang S, Feng X, Yan C. Associations between VDR Gene Polymorphisms and Osteoporosis Risk and Bone Mineral Density in Postmenopausal Women: A systematic review and Meta-Analysis. Sci Rep. 2018;8(1):981. doi: 10.1038/s41598-017-18670-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Singh K, Kumar R, Shukla A, Phadke SR, Agarwal S. Status of 25-hydroxyvitamin D deficiency and effect of vitamin D receptor gene polymorphisms on bone mineral density in thalassemia patients of North India. Hematology. 2012;17(5):291–296. doi: 10.1179/1607845412Y.0000000017. [DOI] [PubMed] [Google Scholar]
- 12.El-Edel RH, Ghonaim MM, Abo-Salem OM, El-Nemr FM. Bone mineral density and vitamin D receptor polymorphism in beta-thalassemia major. Pak J Pharm Sci. 2010;23(1):89–96. [PubMed] [Google Scholar]
- 13.Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, Hojsak I, Mihatsch W, Molgaard C, Shamir R, Turck D, van Goudoever J. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. 2013;56(6):692–701. doi: 10.1097/MPG.0b013e31828f3c05. [DOI] [PubMed] [Google Scholar]
- 14.Bid HK, Konwar R, Aggarwal CG, Gautam S, Saxena M, Nayak VL, Banerjee M. Vitamin D receptor (FokI, BsmI and TaqI) gene polymorphisms and type 2 diabetes mellitus: a North Indian study. Indian J Med Sci. 2009;63(5):187–194. doi: 10.4103/0019-5359.53164. [DOI] [PubMed] [Google Scholar]
- 15.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 16.Bikle DD. Vitamin D and bone. Curr Osteoporos Rep. 2012;10(2):151–159. doi: 10.1007/s11914-012-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elhoseiny SM, Morgan DS, Rabie AM, Bishay ST. Vitamin D Receptor (VDR) Gene Polymorphisms (FokI, BsmI) and their Relation to Vitamin D Status in Pediatrics betaeta Thalassemia Major. Indian J Hematol Blood Transfus. 2016;32(2):228–238. doi: 10.1007/s12288-015-0552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyal M, Abrol P, Lal H. Parathyroid and calcium status in patients with thalassemia. Indian J Clin Biochem. 2010;25(4):385–387. doi: 10.1007/s12291-010-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dresner Pollack R, Rachmilewitz E, Blumenfeld A, Idelson M, Goldfarb AW. Bone mineral metabolism in adults with beta-thalassaemia major and intermedia. Br J Haematol. 2000;111(3):902–907. [PubMed] [Google Scholar]
- 20.Ferrara M, Matarese SM, Francese M, Borrelli B, Coppola A, Coppola L, Esposito L. Effect of VDR polymorphisms on growth and bone mineral density in homozygous beta thalassaemia. Br J Haematol. 2002;117(2):436–440. doi: 10.1046/j.1365-2141.2002.03426.x. [DOI] [PubMed] [Google Scholar]
- 21.Pike JW. Genome-wide principles of gene regulation by the vitamin D receptor and its activating ligand. Mol Cell Endocrinol. 2011;347(1–2):3–10. doi: 10.1016/j.mce.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang Y, van Meurs JB, d’Alesio A, Jhamai M, Zhao H, Rivadeneira F, Hofman A, van Leeuwen JP, Jehan F, Pols HA, Uitterlinden AG. Promoter and 3′-untranslated-region haplotypes in the vitamin d receptor gene predispose to osteoporotic fracture: the rotterdam study. Am J Hum Genet. 2005;77(5):807–823. doi: 10.1086/497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338(2):143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Dimitriadou M, Christoforidis A, Fidani L, Economou M, Perifanis V, Tsatra I, Katzos G, Athanassiou-Metaxa M. Fok-I gene polymorphism of vitamin D receptor in patients with beta-thalassemia major and its effect on vitamin D status. Hematology. 2011;16(1):54–58. doi: 10.1179/102453311X12902908411878. [DOI] [PubMed] [Google Scholar]
- 25.Smolders J, Damoiseaux J, Menheere P, Tervaert JW, Hupperts R. Fok-I vitamin D receptor gene polymorphism (rs10735810) and vitamin D metabolism in multiple sclerosis. J Neuroimmunol. 2009;207(1–2):117–121. doi: 10.1016/j.jneuroim.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Whitfield GK, Remus LS, Jurutka PW, Zitzer H, Oza AK, Dang HT, Haussler CA, Galligan MA, Thatcher ML, Encinas Dominguez C, Haussler MR. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol. 2001;177(1–2):145–159. doi: 10.1016/S0303-7207(01)00406-3. [DOI] [PubMed] [Google Scholar]
- 27.Deng HW, Shen H, Xu FH, Deng HY, Conway T, Zhang HT, Recker RR. Tests of linkage and/or association of genes for vitamin D receptor, osteocalcin, and parathyroid hormone with bone mineral density. J Bone Miner Res. 2002;17(4):678–686. doi: 10.1359/jbmr.2002.17.4.678. [DOI] [PubMed] [Google Scholar]
- 28.Vupputuri MR, Goswami R, Gupta N, Ray D, Tandon N, Kumar N. Prevalence and functional significance of 25-hydroxyvitamin D deficiency and vitamin D receptor gene polymorphisms in Asian Indians. Am J Clin Nutr. 2006;83(6):1411–1419. doi: 10.1093/ajcn/83.6.1411. [DOI] [PubMed] [Google Scholar]