Abstract
Background:
Natural gas drilling may pose multiple health risks, including congenital anomalies, through air pollutant emissions and contaminated water. Two recent studies have evaluated the relationship between natural gas activity and congenital anomalies, with both observing a positive relationship.
Objectives:
We aimed to evaluate whether residence near natural gas wells is associated with critical congenital heart defects (CCHD), neural tube defects (NTD), and oral clefts in Oklahoma, the third highest natural gas producing state in the US.
Methods:
We conducted a retrospective cohort study among singleton births in Oklahoma (n=476,600) to evaluate natural gas activity and congenital anomalies. We calculated an inverse distance-squared weighted (IDW) score based on the number of actively producing wells within a two-mile radius of the maternal residence during the month of delivery. We used modified Poisson regression with robust error variance to estimate prevalence proportion ratios (PPR) and 95% confidence intervals (CI) for the association between tertiles of natural gas activity (compared to no wells) and CCHD, NTD, and oral clefts adjusted for maternal education.
Results:
We observed an increased, though imprecise, prevalence of NTDs among children with natural gas activity compared to children with no wells (2nd tertile PPR: 1.34, 95% CI: 0.93, 1.93; 3rd tertile PPR: 1.20, 95% CI: 0.82, 1.75). We observed no association with CCHD or oral clefts overall. Specific CCHDs of common truncus, transposition of the great arteries, pulmonary valve atresia and stenosis, tricuspid valve atresia and stenosis, interrupted aortic arch, and total anomalous pulmonary venous connection were increased among those living in areas with natural gas activity compared to those living in areas without activity, though not statistically significant.
Discussion:
Our results were similar to previous studies for NTDs and specific CCHDs. Future directions include evaluating the association between specific phases of the drilling process and congenital anomalies to better refine the relevant exposure period.
Keywords: natural gas wells, congenital anomalies, air pollution
1. Introduction
Natural gas drilling may pose multiple health risks, including congenital anomalies, through air pollutant emissions and contaminated water (Adgate et al. 2014; Esswein et al. 2014; McKenzie et al. 2012; Webb et al. 2014). Through the various phases of natural gas drilling, including increased diesel traffic, use of generators, and the processes of drilling, hydraulic fracturing, and production, several categories of pollutants are emitted into the environment. These chemical hazards include particulate matter, polycyclic aromatic hydrocarbons (PAH), endocrine disrupting chemicals, heavy metals (including arsenic and manganese), and several air toxics (benzene, toluene, ethylbenzene, and xylene [BTEX]) among others, with physical, safety, and water scarcity hazards potentially associated with various health outcomes (Allshouse et al. 2017; Webb et al. 2017). Two recent studies have specifically evaluated the relationship between natural gas activity and congenital anomalies, with both observing a positive relationship (Ma et al. 2016; McKenzie et al. 2014). While McKenzie et al. (2014) worked with the Colorado Responds to Children with Special Needs registry, Ma et al. (2016) were limited by use of an ecologic design and birth certificates as the source of children with anomalies, which could underestimate the prevalence compared to a birth defects registry (Boulet et al. 2011; Zollinger et al. 2006).
The biologic mechanism by which emissions from natural gas drilling and production may cause congenital anomalies is unclear. However, studies have focused on the biologic mechanisms for chemicals emitted through oil and gas production to cause low birth weight, intrauterine growth restriction, and prematurity in infants (Kannan et al. 2006; Slama et al. 2008). In addition, several studies have reported adverse reproductive health outcomes among mouse offspring that ingested contaminated water with known endocrine disrupting chemicals produced during oil and gas drilling activities (Boule et al. 2018; Kassotis et al. 2015; Kassotis et al. 2016).
A potential biologic mechanism that appears to be more closely related to congenital anomalies is oxidative stress, leading to DNA damage and increasing the number of placental DNA adducts (Kampa and Castanas 2008; Kannan et al. 2006; Slama et al. 2008). Perera et al. (1999) reported that fetal tissue may be slower to metabolize toxic substances with evidence of DNA adducts from PAH in rodent fetal tissue, though levels of PAH were only one-tenth of those in the mother. Ren et al. (2011) observed a positive association between PAH measured in maternal placentas and neural tube defects (NTD) among their children after adjustment for maternal and child characteristics including folic acid supplementation (Odds Ratio [OR]: 6.0, 95% confidence interval [CI]: 2.0, 18.5). Benzene is known to cross the placenta, with levels in cord blood being equal to or greater than levels in the mother (Agency for Toxic Substances and Disease Registry 2007). Benzene has also been associated with NTDs in recent studies (Brender et al. 2002; Lupo et al. 2011; Swartz et al. 2015), though results are mixed (Janitz et al. 2018; Tanner et al. 2015). The nervous system may be more sensitive to the effects of air pollutants than other body systems due to the lack of blood brain barrier until after birth (Perera et al. 1999). Evidence for other types of congenital anomalies is lacking, but may follow similar pathways described for other birth outcomes (Webb et al. 2014; Webb et al. 2017).
In Oklahoma, the number of new oil or gas well completions increased from approximately 3,000 wells in 2000 to 4,700 wells in 2008, with a subsequent decrease in 2009 to approximately 2,500 wells (Murray 2013). The majority of these wells were vertically drilled, with horizontal drilling increasing over time, accounting for approximately 20% of wells in 2009. The authors also estimated that 44% of horizontally drilled wells used hydraulic fracturing based on reports to the Oklahoma Corporation Commission.
We aimed to evaluate whether residence near natural gas wells is associated with critical congenital heart defects (CCHD), NTDs, and oral clefts in Oklahoma, the third highest natural gas producing state in the US (U.S. Energy Information Administration 2017). Our study adds to the limited literature by conducting multiple sensitivity analyses, including evaluating natural gas well activity during the estimated month of conception and inclusion of ZIP centroidlevel geocodes, which have not been previously analyzed.
2. Methods
Study Design, Data Sources, and Outcomes.
We conducted a retrospective cohort study of live births in Oklahoma from January 1, 1997 through December 31, 2009 using data from the Oklahoma State Department of Health. Data on children with congenital anomalies were obtained from the Oklahoma Birth Defects Registry (OBDR), which is an active surveillance system that collects information on all congenital anomalies identified by birthing hospitals in Oklahoma. Congenital anomalies can be reported up to age 6 years (for the included birth years), but signs/symptoms of the anomaly must be present by age 2 years (Oklahoma State Department of Health 2018). We linked the records of children with congenital anomalies to birth certificates using Registry Plus™ Link Plus software v. 2.0 (CDC, Atlanta, GA), with 95% of records in OBDR linking to a birth certificate resulting in 26,023 children with anomalies and 629,430 children without anomalies. We obtained information on demographic and birth characteristics from the birth certificate for all children. We geocoded maternal residence at delivery from the birth certificate masked to congenital anomaly status using the Texas A&M Geocoding Service toolbox in ArcGIS v. 10.4 (Texas A&M University 2016) and using 2014 TIGER/Line files (U.S. Census Bureau 2017).
We excluded any children whose residence was outside of Oklahoma or not geocodable (N=7,606), plural births (N=17,932), and non-CCHD, NTDs, and oral clefts (N=22,225). Due to Tribal sovereignty, the Oklahoma Corporation Commission does not have jurisdiction over oil and gas production in the Osage Nation Reservation. Thus, we excluded children residing in Osage County in northeastern Oklahoma due to the lack of available oil and gas production records for this region (N=5,595). Furthermore, we excluded children whose residences geocoded to a ZIP code centroid (N=125,495), though these children were included in a sensitivity analysis to evaluate potential selection bias. Of those who geocoded to the ZIP code centroid, 42% were Post Office Boxes (N=53,210), 34% were rural routes (N=43,163), 22% were street addresses that were unable to be geocoded to the street level (N=28,144), and 0.8% had incomplete addresses with only a ZIP code available (N=978).
We included CCHDs, NTDs (spina bifida and anencephaly), and oral clefts (cleft lip and cleft palate) in our analysis (Table S1) (N=1,665 children). CCHDs are conditions that are often life-threatening within the first few weeks of life and are more validly reported to OBDR than other types of congenital heart defects (Mahle et al. 2009; Olney et al. 2015; Tanner et al. 2015). CCHDs include the following defects: common truncus/truncus arteriosus, transposition of the great arteries, double outlet right ventricle, Tetralogy of Fallot, single ventricle, pulmonary valve atresia and stenosis, tricuspid valve atresia and stenosis, Ebstein anomaly, hypoplastic left heart syndrome, coarctation of aorta, interrupted aortic arch, and total anomalous pulmonary venous connection.
Natural Gas Well Data.
We obtained historical data on producing natural gas wells from the Oklahoma Corporation Commission for each year of the study. These data contained information on the latitude and longitude of the well and monthly production levels of natural gas. However, we did not have information on whether the well was horizontally drilled or if hydraulic fracturing was used. We used the production levels to determine whether a natural gas well was actively producing and classified a well as active if natural gas production was reported during at least one month in a given year. We used an inverse distance-squared weighting (IDW) method to calculate the density of actively producing wells during the month of birth within a 2-, 5-, and 10-mile radius of the maternal residence at delivery using the following formula:
Where a=buffer distance (2-, 5-, 10-miles from birth residence), i=each well within the buffer, n=total number of wells within the buffer, and d=distance from birth residence to any actively producing natural gas well within the buffer. This method allows for assignment of a higher score to wells that were closer to the residence and a smaller score to wells that are farther away. Whitworth et al. (2018) and Whitworth et al. (2017) used this formula to calculate IDW at 0.5-, 2-, and 10-mile buffers, whereas McKenzie et al. (2014) and Stacy et al. (2015) used an IDW method without squaring the distance for all wells within the specified buffer (2-, 5-, and 10mile buffers in McKenzie et al. (2014) and 10-mile buffer in Stacy et al. (2015)). Using the squared distance allowed for higher IDW for wells close to the residence and lower IDW for wells further from the residence than would be calculated using the base (not squared) distance. Although we planned to additionally evaluate a 0.5-mile buffer around the birth residence based on previous studies indicating potential health hazards at this distance (McKenzie et al. 2012; Whitworth et al. 2017; Whitworth et al. 2018), the number of children with the included anomalies was too small for analysis at buffers less than 2 miles. Thus, our primary analysis used the 2-mile buffer, with sensitivity analyses evaluating 5- and 10-mile buffers.
The IDW summed well counts at the 2-mile buffer was classified into tertiles (1st: 0.251.88, 2nd: >1.88–9.00, 3rd: >9.00–47679.13) for comparison to those with no recorded wells within the buffer as the reference category. To evaluate specific CCHDs, NTDs, and oral clefts, we created a dichotomous variable to compare children with a birth residence near any actively producing natural gas well to those with no wells within a 2-mile buffer of the birth residence.
Statistical Analysis.
We used modified Poisson regression with robust error variance to calculate prevalence proportion ratios (PPR) and 95% confidence intervals (CI) for children with CCHD (N=874), NTDs (N=217), and oral clefts (N=603) using complete case analysis (29 of these children have more than one of the included anomalies). Children without any anomaly (N=474,935) were included as a comparison group for all three congenital anomaly categories. We used a directed acyclic graph (DAG) to determine the minimally sufficient set of confounding factors based on the literature (Figure S1, Table S2) (Casey et al. 2016; Ma et al. 2016; McKenzie et al. 2014; Stacy et al. 2015; Whitworth et al. 2017; Whitworth et al. 2018), which considered birth certificate variables of year of birth, sex, race/ethnicity (non-Hispanic [NH] white, NH African American, NH American Indian/Alaska Native, NH Asian/Pacific Islander, and Hispanic as reported by the mother), gestational age at delivery in weeks, birth weight in grams, urban/rural status of census block at delivery (based on the 2000 US Census), maternal factors of age at delivery in years, marital status (married/not married), prenatal care (yes/no), parity (number of previous live births), tobacco use during pregnancy (yes/no), and education (less than high school, completed high school, or more than high school) (as a surrogate for socioeconomic status [SES]). Our DAG analysis using DAGitty (Textor et al. 2011; Textor 2016) indicated we should control for either urbanization or maternal education (not both variables) in our multivariable model. It is unclear from the literature whether urbanization is a surrogate for natural gas exposure or SES, thus we chose to adjust our multivariable models for maternal education only (Tselios 2013).
We conducted the following sensitivity analyses: 1) evaluation of a fully adjusted model including all potential covariates noted above; 2) using buffers of 5- and 10-miles around the birth residence; 3) calculating the IDW using the base distance (not squared) for more direct comparison with the McKenzie et al. (2014) analysis at 2-, 5-, and 10-mile buffers; 4) including records geocoded to the ZIP code centroid; and 5) using wells producing during the estimated month of conception based on gestational age at delivery (N=462,654 children with reported gestational age at delivery). We conducted the analysis using estimated month of conception since congenital anomalies develop early in pregnancy (Czeizel et al. 2008; Selevan et al. 2000). Although we do not have information on residence at conception, we were able to determine the IDW for actively producing wells during an estimated month of conception using the month of birth and gestational age at delivery. Children missing gestational age at delivery from the birth record were excluded from this sensitivity analysis. To allow for a more direct comparison, we also conducted the analysis using the natural well activity during the month of birth excluding children with missing gestational age.
All analyses were conducted in SAS v. 9.4 and ArcGIS v. 10.4. We obtained Institutional Review Board approval from the University of Oklahoma Health Sciences Center and the Oklahoma State Department of Health.
3. Results
We observed differences in demographic and birth characteristics between children with specific anomalies and those without anomalies (Table 1). Notable differences include a higher frequency of males for children with CCHD and oral clefts compared to those without anomalies. Children with all anomaly types were more frequently born at an earlier gestational age and children with CCHD and NTD had a lower birthweight compared to children without anomalies.
Table 1.
Distribution of birth characteristics by congenital anomaly status, Oklahoma singleton births, 1997–2009.
| Critical Congenital Heart Defects (N=874) N (%) |
Neural Tube Defects (N=217) N (%) |
Oral Clefts (N=603) N (%) |
No Congenital Anomaly (N=474,935) N (%) |
|
|---|---|---|---|---|
| Sex | ||||
| Female | 369 (42.2) | 106 (48.8) | 236 (39.1) | 232,881 (49.0) |
| Male | 505 (57.8) | 111 (51.2) | 367 (60.9) | 242,046 (51.0) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (0.0) |
| Race/Ethnicity | ||||
| Non-Hispanic White | 576 (65.9) | 147 (67.7) | 419 (69.5) | 310,128 (65.3) |
| Non-Hispanic African American | 76 (8.7) | 18 (8.3) | 37 (6.1) | 50,986 (10.7) |
| Non-Hispanic American Indian | 84 (9.6) | 14 (6.5) | 60 (10.0) | 41,250 (8.7) |
| Non-Hispanic Asian/Unknowna | 29 (3.3) | <6b (1.8) | 20 (3.3) | 14,044 (3.0) |
| Hispanic | 109 (12.5) | 34 (15.7) | 67 (11.1) | 58,527 (12.3) |
| Maternal Age at Delivery (years) | ||||
| <20 | 107 (12.2) | 27 (12.4) | 94 (15.6) | 67,499 (14.2) |
| 20–34 | 671 (76.8) | 170 (78.3) | 459 (76.1) | 367,703 (77.4) |
| ≥35/Unknowna | 96 (11.0) | 20 (9.2) | 50 (8.3) | 39,733 (8.4) |
| Parity (Number of Previous Live Births) | ||||
| 0 | 358 (41.0) | 87 (40.1) | 220 (36.5) | 193,220 (40.7) |
| 1 | 255 (29.2) | 66 (30.4) | 193 (32.0) | 149,229 (31.4) |
| 2 | 162 (18.5) | 37 (17.1) | 110 (18.2) | 81,456 (17.2) |
| ≥3 | 99 (11.3) | 27 (12.4) | 80 (13.3) | 50,997 (10.7) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 33 (0.0) |
| Gestational Age at Delivery (weeks) | ||||
| <37 | 153 (17.5) | 66 (30.4) | 82 (13.6) | 37,846 (8.0) |
| 37–39 | 494 (56.5) | 119 (54.8) | 343 (56.9) | 261,327 (55.0) |
| ≥40 | 194 (22.2) | 25 (11.5) | 152 (25.2) | 161,879 (34.1) |
| Unknown | 33 (3.8) | 7 (3.2) | 26 (4.3) | 13,883 (2.9) |
| Birth Weight (grams) | ||||
| <1500 | 20 (2.3) | 31 (14.3) | 18 (3.0) | 4,598 (1.0) |
| 1500–2499 | 141 (16.1) | 34 (15.7) | 72 (11.9) | 23,940 (5.0) |
| 2500–3999 | 669 (76.5) | 142 (65.4) | 468 (77.6) | 406,342 (85.6) |
| ≥4000/Unknowna | 44 (5.0) | 10 (4.6) | 45 (7.5) | 40,055 (8.4) |
| Tobacco Use During Pregnancy | ||||
| Yes | 124 (14.2) | 34 (15.7) | 111 (18.4) | 72,299 (15.2) |
| No | 699 (80.0) | 173 (79.7) | 449 (74.5) | 370,793 (78.1) |
| Unknown | 51 (5.8) | 10 (4.6) | 43 (7.1) | 31,843 (6.7) |
| Maternal Education | ||||
| Less than High School | 204 (23.3) | 48 (22.1) | 146 (24.2) | 107,966 (22.7) |
| High School | 319 (36.5) | 77 (35.5) | 233 (38.6) | 165,269 (34.8) |
| More than High School/Unknowna | 351 (40.2) | 92 (42.4) | 224 (37.1) | 201,700 (42.5) |
| Urbanization | ||||
| Rural | 152 (17.4) | 45 (20.7) | 111 (18.4) | 76,881 (16.2) |
| Urban | 722 (82.6) | 172 (79.3) | 492 (81.6) | 398,054 (83.8) |
The Unknown category was combined with another category to ensure confidentiality following Oklahoma State Department of Health policies requiring suppression of categories with <6 observations.
Categories with <6 observations suppressed following Oklahoma State Department of Health policies (Neural Tube Defects column, Non-Hispanic Asian/Unknown row).
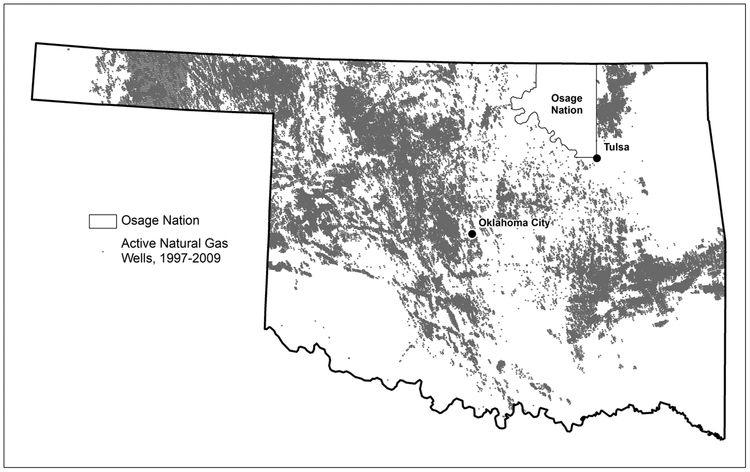
Producing natural gas well concentration from 1997–2009 was highest in the northwestern part of the state, with increased density also observed in southcentral and eastern Oklahoma (Figure 2). The geographic distribution of producing natural gas wells by year in Oklahoma was fairly constant (data not shown), with a total of 417,110 unique producing natural gas wells in Oklahoma over the study time period. The distribution of IDW well count indicates an increasing percentage of birth residences with no wells within the buffer as the buffer size decreases (Table S3).
Figure 2.
Distribution of producing natural gas wells in Oklahoma from 1997–2009 (excluding Osage Nation). Sources: Natural Gas Wells: Oklahoma Corporation Commission; Oklahoma Freeways: Federal Highway Administration, National Transportation Atlas.
We observed an increased prevalence of NTDs for those with natural gas activity in the 2nd (PPR: 1.34, 95% CI: 0.93, 1.93) and 3rd (PPR: 1.20, 95% CI: 0.82, 1.75) tertiles compared to birth residences with no wells in the 2-mile buffer after adjustment for maternal education (Table 2). However, the 95% confidence interval included the null value. We observed no association between natural gas activity at the 2-mile buffer and CCHDs or oral clefts. In an exploratory analysis, we evaluated the relationship between natural gas activity and specific CCHDs, NTDs, and oral clefts (Table 3). We observed an elevated prevalence of the following CCHDs among those with any natural gas activity compared to none, though the 95% confidence intervals included the null value: common truncus, transposition of the great arteries, pulmonary valve atresia and stenosis, tricuspid valve atresia and stenosis, interrupted aortic arch, and total anomalous pulmonary venous connection. We also observed an increased PPR for spina bifida and cleft palate only, though the results were not statistically significant.
Table 2.
Prevalence proportion ratios and 95% confidence intervals for the relationship between natural gas well activity within a 2-mile buffer of the birth residence and specific congenital anomalies (N=476,600).
| IDW Summed Well Count by Anomaly Type | N with Anomalies | Adjusteda PPR (95% CI) |
|---|---|---|
| Critical Congenital Heart Defects | 874 | |
| 0 | 494 | Reference |
| 0.25–1.88 | 132 | 1.02 (0.84, 1.24) |
| >1.88–9.00 | 128 | 1.01 (0.83, 1.23) |
| >9.00–47679.13 | 120 | 0.91 (0.75, 1.11) |
| Neural Tube Defects | 217 | |
| 0 | 114 | Reference |
| 0.25–1.88 | 28 | 0.89 (0.58, 1.37) |
| >1.88–9.00 | 39 | 1.34 (0.93, 1.93) |
| Oral Clefts | 603 | |
| 0 | 340 | Reference |
| 0.25–1.88 | 75 | 0.85 (0.67, 1.10) |
| >1.88–9.00 | 94 | 1.06 (0.84, 1.34) |
| >9.00–47679.13 | 94 | 1.03 (0.82, 1.29) |
IDW: Inverse Distance Weight, PPR: Prevalence proportion ratio, 95% CI: 95% confidence interval
Adjusted for maternal education
Table 3.
Association between natural gas well activity within a 2-miles of the birth residence and specific critical congenital heart defects, neural tube defects, and oral clefts (N=476,600).
| IDW Summed Well Count | N with Anomalies | Unadjusted PPR (95% CI) | Adjusteda PPR (95% CI) |
|---|---|---|---|
| Specific Critical Congenital Heart Defects | |||
| Common truncus | |||
| 0 | 24 | Referent | Referent |
| >0–47679.13 | 22 | 1.15 (0.65, 2.06) | 1.15 (0.64, 2.06) |
| Transposition of the great arteries | |||
| 0 | 64 | Referent | Referent |
| >0–47679.13 | 57 | 1.12 (0.78, 1.60) | 1.15 (0.80, 1.65) |
| Double outlet right ventricle | |||
| 0 | 20 | Referent | Referent |
| >0–47679.13 | 12 | 0.75 (0.37, 1.54) | 0.77 (0.38, 1.57) |
| Single ventricle | |||
| 0 | 37 | Referent | Referent |
| >0–47679.13 | 30 | 1.02 (0.63, 1.65) | 1.02 (0.63, 1.66) |
| Pulmonary valve atresia and stenosis | |||
| 0 | 22 | Referent | Referent |
| >0–47679.13 | 26 | 1.49 (0.84, 2.62) | 1.45 (0.82, 2.55) |
| Tricuspid valve atresia and stenosis | |||
| 0 | 17 | Referent | Referent |
| >0–47679.13 | 17 | 1.26 (0.64, 2.46) | 1.32 (0.66, 2.63) |
| Ebstein anomaly | |||
| 0 | 17 | Referent | Referent |
| >0–47679.13 | 12 | 0.89 (0.42, 1.86) | 0.95 (0.45, 2.00) |
| Hypoplastic left heart syndrome | |||
| 0 | 67 | Referent | Referent |
| >0–47679.13 | 39 | 0.73 (0.49, 1.09) | 0.76 (0.51, 1.13) |
| Coarctation of aorta | |||
| 0 | 126 | Referent | Referent |
| >0–47679.13 | 100 | 1.00 (0.77, 1.30) | 0.99 (0.76, 1.29) |
| Tetralogy of Fallot | |||
| 0 | 125 | Referent | Referent |
| >0–47679.13 | 83 | 0.84 (0.63, 1.10) | 0.85 (0.64, 1.12) |
| Interrupted aortic arch | |||
| 0 | 18 | Referent | Referent |
| >0–47679.13 | 23 | 1.61 (0.87, 2.98) | 1.57 (0.85, 2.92) |
| Total anomalous pulmonary venous connection | |||
| 0 | 34 | Referent | Referent |
| >0–47679.13 | 41 | 1.52 (0.96, 2.39) | 1.55 (0.98, 2.43) |
| Neural Tube Defects | |||
| Spina Bifida | |||
| 0 | 88 | Referent | Referent |
| >0–47679.13 | 85 | 1.21 (0.90, 1.64) | 1.22 (0.91, 1.65) |
| Anencephaly | |||
| 0 | 26 | Referent | Referent |
| >0–47679.13 | 18 | 0.87 (0.48, 1.59) | 0.88 (0.48, 1.61) |
| Oral Clefts | |||
| Cleft lip only | |||
| 0 | 122 | Referent | Referent |
| >0–47679.13 | 81 | 0.84 (0.63, 1.11) | 0.85 (0.64, 1.12) |
| Cleft palate only | |||
| 0 | 195 | Referent | Referent |
| >0–47679.13 | 174 | 1.12 (0.91, 1.38) | 1.12 (0.92, 1.38) |
IDW: Inverse Distance Weight, PPR: Prevalence proportion ratio, 95% CI: 95% confidence interval
Adjusted for maternal education
In our sensitivity analyses of 5- and 10- mile buffers around the birth residence and analyses adjusting for all potential covariates, we observed similar results as our primary analysis of a 2-mile buffer. However, the PPR for NTDs was attenuated at larger buffers and only elevated for the 3rd tertile after adjustment for maternal education (5-mile buffer PPR: 1.15, 95% CI: 0.81, 1.62; 10-mile buffer PPR: 1.19, 95% CI: 0.82, 1.73) (Table S4). When conducting analyses using the base distance (not squared) for all three buffers (Table S5), incorporating those who geocoded to the ZIP code centroid (Table S6), and evaluating actively producing wells during the estimated month of conception (Table S7), we observed little difference in the results compared to our primary analysis.
4. Discussion
We observed an increased PPR for the association between natural gas activity and NTDs among children in Oklahoma, though this was not statistically significant. This increased PPR was consistently observed in multiple sensitivity analyses. Our results support McKenzie et al. (2014), who observed an elevated odds of NTDs (OR: 1.9, 95% CI: 0.9, 4.3) among those with a natural gas activity in the 3rd tertile compared to no wells within 2 miles of the maternal residence at birth after adjustment for sex, parity, and maternal factors of age, ethnicity, smoking status, alcohol use, education, and elevation of residence. While results for 5- and 10mile buffers in our study were attenuated compared to the 2-mile buffer, this is also consistent with McKenzie et al. (2014) in their analysis of multiple buffers and may indicate higher potential exposure to air, water, or other contaminants at smaller distances from the residence (McKenzie et al. 2012; Whitworth et al. 2017; Whitworth et al. 2018). The authors restricted their analysis to rural children (small towns with <50,000 people), which differed from our population since we included births from the entire state. Although not statistically significant, McKenzie et al. (2014) observed a protective association among children with oral clefts (3rd v. 1st tertile OR: 0.8, 95% CI: 0.6, 1.2), which was not consistent with our results.
McKenzie et al. (2014) also reported an increased odds of congenital heart defects among those with natural gas activity in the 3rd tertile (OR: 1.3, 95% CI: 1.1, 1.5). The authors observed an elevated odds of several specific heart defects using a 10-mile buffer including ventricular septal defects (Adjusted OR: 1.5, 95% CI: 1.1, 2.1), pulmonary artery and valve defects (includes pulmonary valve atresia and stenosis and pulmonary artery anomalies combined) (Adjusted OR: 1.6, 95% CI: 1.1, 2.2) and tricuspid valve defects (includes tricuspid valve atresia and stenosis and Ebstein’s anomaly) (Adjusted OR: 4.2, 95% CI: 1.3, 13.0). Ventricular septal defects are not classified as CCHDs and, thus, were not evaluated in our study. At the 2-mile buffer using our definition of IDW, our results were consistent for pulmonary artery and tricuspid valve defects, though imprecise. Our study was the first to report an increased PPR for total anomalous pulmonary venous connection. Major differences between our analysis and that of McKenzie et al. (2014) include a focus on rural areas of Colorado and difference in the maximum IDW well score using the base distance (Colorado: 1,400, Oklahoma: 297 [Table S5]). The authors suggested that restriction to rural communities may limit the impact of urban air pollution on the study results. The difference in maximum IDW well count indicates more dense natural gas activity in rural Colorado than in Oklahoma and may indicate higher potential exposure in the rural Colorado population. Using the base distance would result in wells farther from the birth residence having a higher weight than those in our study. However, in our sensitivity analysis using this calculation for IDW (instead of the squared distance) at 2-, 5-, and 10-mile buffers, we observed little difference in our results.
Natural gas drilling has recently been evaluated with other perinatal outcomes, with mixed results (Casey et al. 2016; McKenzie et al. 2014; Stacy et al. 2015; Whitworth et al. 2017; Whitworth et al. 2018). A recent systematic review evaluated the relationship between oil and natural gas drilling and reproductive health (Balise et al. 2016). The authors concluded that based on existing evidence, oil and gas drilling activities are harmful for human reproductive health, with stronger evidence for miscarriage, prostate cancer, congenital anomalies, and decreased semen quality than other evaluated health outcomes.
Strengths of our study include the use of a population-based registry for both congenital anomalies and births. OBDR is an active surveillance system, which works with all birthing hospitals in Oklahoma to obtain information on congenital anomalies following the National Birth Defects Prevention Network guidelines (National Birth Defects Prevention Network 2004). We had information on all producing natural gas wells during the included birth years (excluding Osage Nation, see Methods). In addition, using the IDW method allowed us to retrospectively evaluate natural gas well activity within a specified buffer, which is individually calculated for each birth.
However, there are also limitations. One is the risk for survivor bias since not all children with anomalies will have survived to be included in our study and we only included live births in the analysis. This could potentially underestimate the association if natural gas activity is related to severe anomalies with high prenatal mortality. Because of small numbers of children with individual anomaly types, analyses at buffers smaller than 2 miles were infeasible for our study and limited our ability to evaluate tertiles of exposure for specific types of CCHDs, NTDs, and oral clefts. The inability to evaluate natural gas activity at smaller buffers may increase the risk of exposure misclassification. Because we did not conduct air sampling or collect biomarkers from mothers during pregnancy in our study, but instead, measured natural gas activity around the birth residence, there is a potential for misclassification. Additionally, mothers may not work near or in the home, which could result in an over or underestimate of the association depending on the location of the mother’s workplace during pregnancy. Furthermore, we only have information on residence at birth, not residence during early pregnancy, which is the critical window of development for congenital anomalies (Czeizel et al. 2008; Selevan et al. 2000). However, we re-evaluated the association with natural gas activity during the month of conception using gestational age at delivery and observed similar results to our primary analysis. For this sensitivity analysis, we excluded approximately 3% of births with missing gestational age, which resulted in essentially no change in the distribution of birth and demographic characteristics by anomaly type. While our study is at risk for misclassification due to residential mobility during pregnancy, a recent study of ambient benzene exposure and congenital anomalies indicated the misclassification is likely non-differential, resulting in bias towards the null (Lupo et al. 2010). Future studies should incorporate residential history into the exposure assessment to minimize this potential bias.
In conclusion, we observed an increased, though imprecise, prevalence of NTDs and several specific CCHDs among those with increased natural gas activity compared to those with no activity within a 2-mile buffer of the birth residence. While this study supports the results of previous studies, further evaluation of the relationship between natural gas wells and both NTDs and CCHDs is necessary. In addition, evaluating how emissions differ with each phase of the drilling and production process are essential to improve exposure assessment. In addition, pooling data from multiple states with high natural gas production will allow researchers to better evaluate specific types of congenital anomalies. Future studies should also consider sampling air pollutants and water contaminants emitted during the natural gas drilling and production process to better understand the distribution of pollutants and the potential for maternal exposure during pregnancy.
Supplementary Material
Figure S1. Directed acyclic graph for the relationship between natural gas well activity and congenital anomalies.
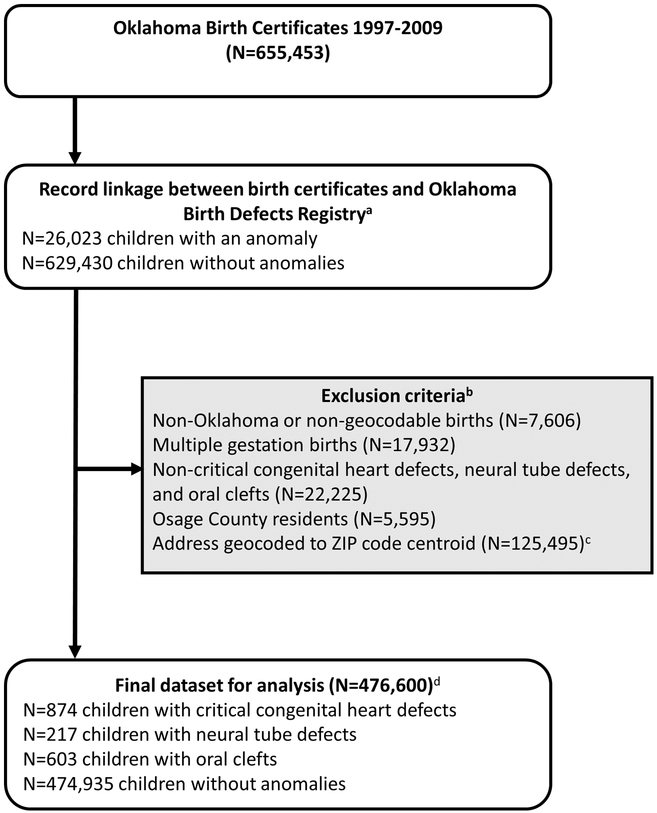
Figure 1.
Flow diagram describing study design and exclusion criteria. Footnotes: a95% of children in the Oklahoma Birth Defects Registry linked with a birth certificate. All birth records without a linked congenital anomaly record were classified as non-congenital anomalies. bExclusion criteria listed in order of exclusion. cWe conducted sensitivity analysis including zipcode level geocodes. d29 children have more than one included congenital anomaly.
Highlights.
Natural gas drilling may pose multiple health risks, including congenital anomalies, through air pollutant emissions and contaminated water
We observed an increased, though imprecise, prevalence of neural tube defects among children with natural gas activity compared to children with no wells
We observed no association with heart defects or oral clefts
Future directions include evaluation of specific phases of the drilling process
Acknowledgments
Funding: Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (U5GM104938) and an award from the National Institute on Minority Health and Health Disparities (R25MD011564) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH.
Abbreviations:
- BTEX
benzene, toluene, ethylbenzene, and xylene
- CCHD
Critical congenital heart defects
- CI
Confidence interval
- IDW
Inverse distance-squared weighted
- NTD
Neural tube defects
- OBDR
Oklahoma Birth Defects Registry
- PAH
polycyclic aromatic hydrocarbons
- PPR
Prevalence proportion ratio
- RUCA
Rural-Urban Commuting Area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adgate JL, Goldstein BD, McKenzie LM. 2014. Potential public health hazards, exposures and health effects from unconventional natural gas development. Environmental science & technology 48:8307–8320. doi: 10.1021/es404621d [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. 2007. Toxicological Profile for Benzene. Atlanta, GA: Available:https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=40&tid=14 [accessed October 19, 2018]. [PubMed] [Google Scholar]
- Allshouse WB, Adgate JL, Blair BD, McKenzie LM. 2017. Spatiotemporal Industrial Activity Model for Estimating the Intensity of Oil and Gas Operations in Colorado. Environ Sci Technol 51:10243–10250. doi: 10.1021/acs.est.7b02084 [DOI] [PubMed] [Google Scholar]
- Balise VD, Meng C-X, Cornelius-Green JN, Kassotis CD, Kennedy R, Nagel SC. 2016. Systematic review of the association between oil and natural gas extraction processes and human reproduction. Fertility and Sterility 106:795–819. doi: 10.1016/j.fertnstert.2016.07.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boule LA, Chapman TJ, Hillman SE, Kassotis CD, O’Dell C, Robert J, et al. 2018. Developmental Exposure to a Mixture of 23 Chemicals Associated With Unconventional Oil and Gas Operations Alters the Immune System of Mice. Toxicological Sciences 163:639–654. doi: 10.1093/toxsci/kfy066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet SL, Shin M, Kirby RS, Goodman D, Correa A. 2011. Sensitivity of Birth Certificate Reports of Birth Defects in Atlanta, 1995–2005: Effects of Maternal, Infant, and Hospital Characteristics. Public Health Rep 126:186–194. doi: 10.1177/003335491112600209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender J, Suarez L, Hendricks K, Baetz RA, Larsen R. 2002. Parental occupation and neural tube defect-affected pregnancies among Mexican Americans. Journal of Occupational and Environmental Medicine 44:650–656. doi: 10.1097/01.jom.0000023382.41727.7b [DOI] [PubMed] [Google Scholar]
- Casey JA, Savitz DA, Rasmussen SG, Ogburn EL, Pollak J, Mercer DG, et al. 2016. Unconventional Natural Gas Development and Birth Outcomes in Pennsylvania, USA. Epidemiology 27:163–172. doi: 10.1097/ede.0000000000000387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE, Puho EH, Acs N, Banhidy F. 2008. Use of specified critical periods of different congenital abnormalities instead of the first trimester concept. Birth Defects Research Part A: Clinical and Molecular Teratology 82:139–146. doi: 10.1002/bdra.20431 [DOI] [PubMed] [Google Scholar]
- Esswein EJ, Snawder J, King B, Breitenstein M, Alexander-Scott M, Kiefer M. 2014. Evaluation of some potential chemical exposure risks during flowback operations in unconventional oil and gas extraction: preliminary results. Journal of occupational and environmental hygiene 11:D174–184. doi: 10.1080/15459624.2014.933960 [DOI] [PubMed] [Google Scholar]
- Janitz AE, Dao HD, Campbell JE, Stoner JA, Peck JD. 2018. Association between benzene and congenital anomalies in Oklahoma, 1997–2009. Occupational and Environmental Medicine 75:822–829. doi: 10.1136/oemed-2018-105054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa M, Castanas E. 2008. Human health effects of air pollution. Environmental Pollution 151:362–367. doi: 10.1016/j.envpol.2007.06.012 [DOI] [PubMed] [Google Scholar]
- Kannan S, Misra DP, Dvonch JT, Krishnakumar A. 2006. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect 114:1636–1642. doi: doi: 10.1289/ehp.9081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis CD, Klemp KC, Vu DC, Lin CH, Meng CX, Besch-Williford CL, et al. 2015. EndocrineDisrupting Activity of Hydraulic Fracturing Chemicals and Adverse Health Outcomes After Prenatal Exposure in Male Mice. Endocrinology 156:4458–4473. doi: 10.1210/en.2015-1375 [DOI] [PubMed] [Google Scholar]
- Kassotis CD, Bromfield JJ, Klemp KC, Meng CX, Wolfe A, Zoeller RT, et al. 2016. Adverse Reproductive and Developmental Health Outcomes Following Prenatal Exposure to a Hydraulic Fracturing Chemical Mixture in Female C57Bl/6 Mice. Endocrinology 157:3469–3481. doi: 10.1210/en.2016-1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo PJ, Symanski E, Chan W, Mitchell LE, Waller DK, Canfield MA, et al. 2010. Differences in exposure assignment between conception and delivery: the impact of maternal mobility. Paediatr Perinat Epidemiol 24:200–208. doi: 10.1111/j.1365-3016.2010.01096.x [DOI] [PubMed] [Google Scholar]
- Lupo PJ, Symanski E, Waller DK, Chan W, Langlois PH, Canfield MA, et al. 2011. Maternal exposure to ambient levels of benzene and neural tube defects among offspring: Texas, 1999–2004. Environ Health Perspect 119:397–402. doi: 10.1289/ehp.1002212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z-Q, Sneeringer K, Liu L, Kuller LH. 2016. Time Series Evaluation of Birth Defects in Areas with and without Unconventional Natural Gas Development. Journal of Epidemiology and Public Health Reviews 1. doi: 10.16966/2471-8211.107 [DOI] [Google Scholar]
- Mahle WT, Newburger JW, Matherne GP, Smith FC, Hoke TR, Koppel R, et al. 2009. Role of Pulse Oximetry in Examining Newborns for Congenital Heart Disease: A Scientific Statement From the American Heart Association and American Academy of Pediatrics. Circulation 120:447–458. doi: 10.1161/circulationaha.109.192576 [DOI] [PubMed] [Google Scholar]
- McKenzie LM, Witter RZ, Newman LS, Adgate JL. 2012. Human health risk assessment of air emissions from development of unconventional natural gas resources. Sci Total Environ 424:79–87. doi: 10.1016/j.scitotenv.2012.02.018 [DOI] [PubMed] [Google Scholar]
- McKenzie LM, Guo R, Witter RZ, Savitz DA, Newman LS, Adgate JL. 2014. Birth outcomes and maternal residential proximity to natural gas development in rural Colorado. Environ Health Perspect 122:412–417. doi: 10.1289/ehp.1306722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KE. 2013. State-Scale Perspective on Water Use and Production Associated with Oil and Gas Operations, Oklahoma, U.S. Environmental Science and Technology 47:4918–4925. doi: 10.1021/es4000593 [DOI] [PubMed] [Google Scholar]
- National Birth Defects Prevention Network. 2004. Guidelines for Conducting Birth Defects Surveillance. Atlanta, GA:National Birth Defects Prevention Network, Inc; Available:https://www.nbdpn.org/guidelines.php [accessed October 19, 2018]. [Google Scholar]
- Oklahoma State Department of Health. 2018. Oklahoma Birth Defects Registry: Surveillance. Available: https://www.ok.gov/health/Community_&_Family_Health/Screening_&_Special_Services/Oklahoma_Birth_Defects_Registry/Surveillance/index.html [accessed June 11, 2018].
- Olney RS, Ailes EC, Sontag MK. 2015. Detection of critical congenital heart defects: Review of contributions from prenatal and newborn screening. Seminars in Perinatology 39:230–237. doi: 10.1053/j.semperi.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Jedrychowski W, Rauh V, Whyatt RM. 1999. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ Health Perspect 107 Suppl 3:451–460. doi: 10.1289/ehp.99107s3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren A, Qiu X, Jin L, Ma J, Li Z, Zhang L, et al. 2011. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proceedings of the National Academy of Sciences of the United States of America 108:12770–12775. doi: 10.1073/pnas.1105209108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. 2000. Identifying critical windows of exposure for children’s health. Environ Health Perspect 108 Suppl 3:451–455. doi: 10.1289/ehp.00108s3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama R, Darrow L, Parker J, Woodruff TJ, Strickland M, Nieuwenhuijsen M, et al. 2008. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect 116:791–798. doi: 10.1289/ehp.11074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy SL, Brink LL, Larkin JC, Sadovsky Y, Goldstein BD, Pitt BR, et al. 2015. Perinatal outcomes and unconventional natural gas operations in Southwest Pennsylvania. PLoS ONE 10:e0126425. doi: 10.1371/journal.pone.0126425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MD, Cai Y, Chan W, Symanski E, Mitchell LE, Danysh HE, et al. 2015. Air toxics and birth defects: a Bayesian hierarchical approach to evaluate multiple pollutants and spina bifida. Environmental health: a global access science source 14:16. doi: 10.1186/1476-069x-14-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JP, Salemi JL, Stuart AL, Yu H, Jordan MM, DuClos C, et al. 2015. Associations between exposure to ambient benzene and PM(2.5) during pregnancy and the risk of selected birth defects in offspring. Environmental Research 142:345–353. doi: 10.1016/j.envres.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Texas A&M University. 2016. Texas A&M GeoServices. Available: http://geoservices.tamu.edu/About/ [accessed May 12, 2017].
- Textor J, Hardt J, Knüppel S. 2011. DAGitty: A Graphical Tool for Analyzing Causal Diagrams. Epidemiology 22:745 10.1097/EDE.1090b1013e318225c318222be. [DOI] [PubMed] [Google Scholar]
- Textor J 2016. dagitty: Graphical Analysis of Structural Causal Models (R Software). v. 0.2–2 Available: https://CRAN.R-project.org/package=dagitty [accessed October 24, 2018].
- Tselios V 2013. Urbanization and Socioeconomic Status in the European Regions: The Role of Population Ageing and Capital City Regions. European Planning Studies 22:1879–1901. doi: 10.1080/09654313.2013.812063 [DOI] [Google Scholar]
- U.S. Census Bureau. 2017. TIGER/Line® Shapefiles and TIGER/Line® Files. Available: https://www.census.gov/geo/maps-data/data/tiger-line.html [accessed November 13, 2017].
- U.S. Energy Information Administration. 2017. Which States Consume and Produce the Most Natural Gas? Available: http://www.eia.gov/tools/faqs/faq.cfm?id=46&t=8 [accessed January 17, 2018].
- Webb E, Bushkin-Bedient S, Cheng A, Kassotis CD, Balise V, Nagel SC. 2014. Developmental and reproductive effects of chemicals associated with unconventional oil and natural gas operations. Rev Environ Health 29:307–318. doi: 10.1515/reveh-2014-0057 [DOI] [PubMed] [Google Scholar]
- Webb E, Moon J, Dyrszka L, Rodriguez B, Cox C, Patisaul H, et al. 2017. Neurodevelopmental and neurological effects of chemicals associated with unconventional oil and natural gas operations and their potential effects on infants and children. Rev Environ Health. doi: 10.1515/reveh-2017-0008 [DOI] [PubMed] [Google Scholar]
- Whitworth KW, Marshall AK, Symanski E. 2017. Maternal residential proximity to unconventional gas development and perinatal outcomes among a diverse urban population in Texas. PLoS ONE 12:e0180966. doi: 10.1371/journal.pone.0180966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth KW, Kaye Marshall A, Symanski E. 2018. Drilling and Production Activity Related to Unconventional Gas Development and Severity of Preterm Birth. Environ Health Perspect 126:037006. doi: 10.1289/ehp2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger TW, Przybylski MJ, Gamache RE. 2006. Reliability of Indiana birth certificate data compared to medical records. Annals of Epidemiology 16:1–10. doi: 10.1016/j.annepidem.2005.03.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Directed acyclic graph for the relationship between natural gas well activity and congenital anomalies.