Abstract
Fatigue is a common symptom in many diseases and disorders and can reduce quality of life, yet lacks an adequate pharmacological intervention. To identify and develop such interventions, and to better understand fatigue, additional preclinical research is necessary. However, despite numerous mouse behavioral assays reportedly detecting fatigue-like behavior, the assumption that fatigue-like behavior is detected in many assays has not been validated through a cross-assay study. Thus, we modeled fatigue in mice by administering 5-fluorouracil, a chemotherapy drug known to cause fatigue in humans and fatigue-like behavior in mice, then evaluated its effects via voluntary wheel running activity (VWRA), locomotor activity in the open field test (OFT), immobility in the forced swim test (FST), and distance run in the treadmill fatigue test (TFT) and treadmill exercise capacity test. Additionally, taltirelin or methylphenidate was administered to alleviate fatigue-like behavior. As a result of 5-fluorouracil treatment, VWRA and the TFT were markedly reduced, indicating fatigue. The OFT, FST, and treadmill exercise capacity test, however, failed to detect fatigue-like behavior. Interestingly, both taltirelin and methylphenidate alleviated fatigue-like behavior in TFT. These data suggest that, of the current assays, only the TFT and VWRA should be expected to detect fatigue-like behavior. Moreover, this study provides additional evidence that taltirelin may provide a novel treatment for chemotherapy-induced fatigue and warrants further evaluation as an anti-fatigue therapeutic.
Keywords: open field, forced swim, exercise capacity, treadmill fatigue test, wheel running, fatigue model
1. Introduction
Fatigue is a symptom or major component of many diseases and disorders, including cancer, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME), and Sjögren’s syndrome (SS). Although the estimated prevalence of each is variable due to inconsistent diagnostic criteria, underdiagnosis, and overlap with other conditions, cancer-related fatigue (CRF) affects the majority of cancer patients [1–3] and CFS/ME and primary SS may each affect up to 3% of the population [4,5]. In these and other conditions, fatigue is not alleviated by rest and can decrease quality of life, the underlying causes of fatigue are yet unknown, and numerous comorbidities—such as anemia, depression, hypothyroidism, and immune system dysfunction—may contribute to or exacerbate patient fatigue. Furthermore, there is no FDA-approved drug treatment for fatigue. As such, research on fatigue is needed, but has numerous challenges to overcome.
The major goals of fatigue research are to understand the causes of fatigue, identify biomarkers, and develop new therapies. Research toward these goals requires preclinical models of fatigue, preclinical assays to measure fatigue-like behavior, and knowledge that fatigue-like behavior is being detected by the assay. Numerous models of fatigue are in use, including a variety of CRF models [6–9] as well as inflammation-induced fatigue models intended to recapitulate aspects of other disorders, such as CFS/ME and SS [10–12]. Although these models cannot fully replicate human fatigue, they can provide valuable tools for understanding potential causes of fatigue and identifying potential biomarkers and therapeutics.
In rodent models of fatigue, fatigue-like behavior has been reported using several behavioral assays. Voluntary wheel running activity (VWRA) is frequently used to measure fatigue-like behavior in rodents [13–17]. Various treadmill tests, including the treadmill fatigue test (TFT) developed by our laboratory [18], as well as variants developed by others [19,20], have been used to measure fatigue-like behavior. A treadmill is also used to measure physiological exhaustion via exercise capacity testing, which uses a different endpoint and testing protocol than the TFT, but whether or not fatigue influences the primary behavioral measure in this assay is unclear. The forced swim test (FST), typically used to assess depression-like behavior, has reportedly measured fatigue-like behavior in mice and rats [21–25]. Locomotor activity in the open field test (OFT) may provide a measure of fatigue-like behavior, although numerous other factors may influence this behavior. For instance, in a mouse model of interferon-αinduced fatigue, locomotor activity in the OFT is decreased, but the authors acknowledge this effect may not represent fatigue [26]. Additionally, in mouse models of “sickness behavior,” in which fatigue may contribute to the overall phenotype [17], exploration in the OFT is decreased [27,28], although this effect diminishes with increased mouse age [27]. The TFT, however, is the only one of these assays designed to measure fatigue, and it is not known if all of these assays will detect fatigue-like behavior in the same fatigue model.
The primary aim of this study was to evaluate the ability of different fatigue assays to detect fatigue-like behavior in mice. We focused on a single mouse model of fatigue, inducing fatigue via injections of a cytotoxic chemotherapy drug, 5-fluorouracil (5-FU). This model was selected because prior studies have shown it induces fatigue-like behavior as measured by VWRA [16,29] and, at a lower dose, 5-FU also induces fatigue-like behavior in the TFT [6]. Additionally, we tested two potential anti-fatigue therapeutics, taltirelin (TAL) and methylphenidate (MPH), to determine if any observed fatigue-like behavior was alleviated. TAL is a thyrotropin-releasing hormone (TRH) analog. TRH reduced CRF in a study of eight patients [30], but required i.v. administration of a 0.5 or 1.5 mg dose for this effect. TAL was selected for the current study because it has a more favorable pharmacokinetic profile than TRH and alleviates fatigue-like behavior, as determined using the TFT, in mouse models of chemotherapy-, irradiation-, or tumor-induced fatigue [6]. MPH is a psychostimulant that alleviates CRF in some patients in the clinic [31], but there is not a consistent anti-fatigue effect across patient populations. In the absence of an FDA-approved drug treatment for fatigue, however, the National Comprehensive Cancer Network recommends cautious use of psychostimulants as a pharmacological intervention for CRF [32]. MPH was therefore used in this study because of its potential as an anti-fatigue therapy in the clinic and as a comparator for TAL. After treatment to induce and alleviate fatigue, we evaluated mouse performance in multiple behavioral assays that reportedly measure fatigue in mice.
2. Materials and methods
2.1. Animals
Adult female C57BL/6NCr mice (7–8 weeks old at the start of experiments) were obtained from Charles River Laboratories (Frederick, MD). Female C57BL/6 mice were selected for 5-FU experiments as this strain and sex was used to establish the TFT [18] and in other studies using 5-FU to induce fatigue [16,29]. Mice were kept on a 12h:12h light cycle (lights off at 6 PM). Food and water were provided ad libitum. All experiments were performed with prior approval of the NIDDK Institutional Animal Care and Use Committee.
2.2. Drugs
TAL was purchased from Tocris (Minneapolis, MN) and Santa Cruz Biotechnology (Dallas, TX), MPH was generously provided by Dr. Jonathan Katz of the National Institute on Drug Abuse, 5-FU was purchased from APP Pharmaceuticals (Schaumburg, IL) and Fresenius Kabi (Lake Zurich, IL), ketamine was purchased from Putney (Portland, ME), and xylazine was purchased from Lloyd Laboratories (Shenandoah, IA). Drugs were prepared for a volume of 6 μL/g mouse body weight and injected intraperitoneally (i.p.). Each day of treatment, 5-FU was diluted with PBS to the necessary concentration. MPH was prepared fresh daily in PBS. TAL was dissolved in PBS and aliquots were stored at −20°C. TAL aliquots were thawed and brought to room temperature prior to injection.
2.3. Drug Treatment
To induce fatigue, 5-FU (60 mg/kg) was injected i.p. once daily (between 10 and 11 AM) for 5 consecutive days. To alleviate fatigue, mice were treated with one of three interventions: TAL, MPH, or PBS. TAL was injected i.p. at 1 mg/kg based on previous studies which demonstrated this to be a behaviorally active dose in rodents [6,33,34]; as such, alleviation of fatigue-like behavior should be detectable at this dose. MPH was injected i.p. at 2 (in the FST and treadmill experiments) or 6 mg/kg (in the VWRA and OFT experiments) as these doses have been previously shown to be behaviorally active in rodents [35–38]; as such, alleviation of fatigue-like behavior should be detectable at either dose of MPH. Interventions were injected once daily for 6 consecutive days (on the same 5 days as 5-FU injections, with an additional injection on the following day, 30 min prior to testing [or between 10 and 11 AM in the VWRA experiment]).
2.4. Voluntary Wheel Running Activity (VWRA)
Mice were individually housed in cages with free access to MiniMitter running wheels (Starr Life Sciences, Oakmont, PA). Wheel rotations were recorded via VitalView software (version 5.0, MiniMitter, Bend, OR). The timeline for the VWRA experiment is presented in Figure 1A. In short, we provided mice 7 days to acclimate to the running wheels and reach a consistent level of running activity. VWRA was measured for 4 additional days to establish a baseline for each subject. After completing the baseline measurement, subjects underwent treatment, as described above and in Figure 1A. VWRA was recorded for 6 days after the first day of treatment and is reported as the total number of counts occurring in each 24 h period, beginning at 6 AM. Each mouse was provided with approximately 10 g of standard chow per day in a small food-safe ceramic bowl on the floor of the cage. Any remaining food pellets or fragments were collected daily from each cage, weighed, and replaced with fresh pellets.
Figure 1. Timeline of experiments.
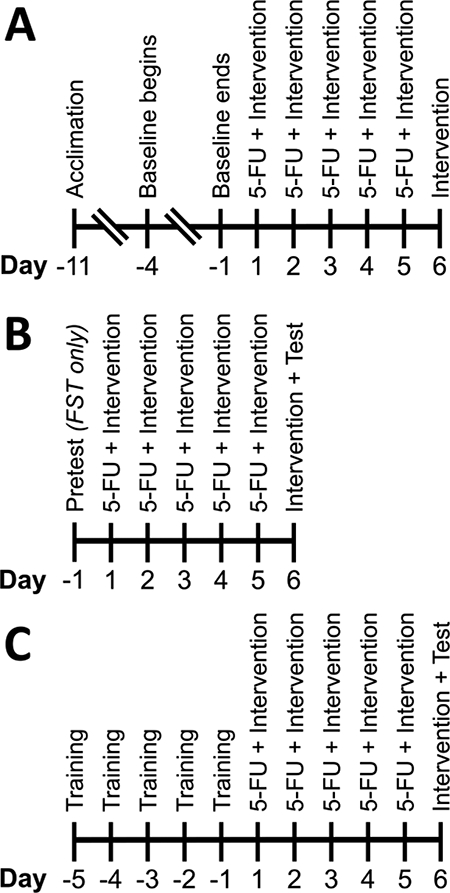
Experimental timelines for (A) voluntary wheel running activity, (B) open field and forced swim tests, and (C) treadmill exercise capacity and treadmill fatigue tests.
Data from two mice were excluded from our analysis; one mouse was euthanized due to illness prior to treatment and one died during the study.
2.5. Open Field Test (OFT)
The timeline for the OFT experiment is presented in Figure 1B. The OFT was performed by individually placing mice in 50 × 50 cm arenas (Stoelting Co., Wood Dale, IL) and allowing free exploration for a 30 min session. Mice were removed from the arena at the end of the session and the arena was cleaned with 70% isopropyl alcohol and allowed to dry before additional testing. Each session was recorded by an overhead video camera (model: DMK 21AUC03, Imaging Source, Charlotte, NC) and analyzed using ANY-maze software (version 4.99, Stoelting Co.). Following the study, mice were deeply anesthetized using ketamine/xylazine (100/10 mg/kg, i.p.) and whole blood was collected. A complete blood count was performed on each sample by the National Institutes of Health Clinical Center Hematology Service.
2.6. Forced Swim Test (FST)
The FST was performed using a modified version of the original protocol [39]. The timeline for the FST experiment is presented in Figure 1B. Mice underwent a swim pretest prior to treatment. For both the pretest and test, mice were individually placed in clear acrylic cylinders (interior Ø: 18.1 cm; height: 27.9 cm) filled to a height of 15 cm with tap water (temperature: 23.9 ± 1.7°C). After 8 min, mice were removed. Cylinders were emptied, rinsed, and refilled between swim sessions. One day after the pretest, subjects began 5-FU treatment, as described above. Swim tests were video recorded (Canon PowerShot ELPH 130 IS). Activity was tracked using EthoVision XT software (version 9.0.726, Noldus, Wageningen, The Netherlands). Minutes 2–8 of the pretest and test were analyzed with a mobility threshold of 5.25% and mobility averaged over 3 samples.
2.7. Treadmill Exercise Capacity Test
Treadmill training and testing was performed using a mouse treadmill (Columbus Instruments, Columbus, OH) at an incline of 10 degrees, with the shock grid delivering 1.22 mA electric shocks at 2 Hz. To determine the aerobic and anaerobic exercise capacity of mice receiving 5-FU, the treadmill exercise capacity test was performed. The timeline for the treadmill exercise capacity test experiment is presented in Figure 1C. For the exercise capacity test, the treadmill started at a speed of 10 m/min. The speed increased to 15 m/min at 10 min, 16.8 m/min at 15 min, and further increased by 1.8 m/min every subsequent 3 min. The shock grid, wire brush, tail tickling, and air puffs were used to motivate mice to run. Upon remaining on the shock grid for 5 consecutive seconds, the test was considered completed and mice were returned to their home cages.
Two mice were excluded from the final analysis; one mouse would not perform the training task and one mouse in the 5-FU+TAL group died prior to testing.
2.8. Treadmill Fatigue Test (TFT)
The TFT was performed per our published method [18]. The timeline for the TFT experiment is presented in Figure 1C. In contrast to the exercise capacity test, the experimenter did not interact with mice during testing. Upon remaining in the designated fatigue zone (i.e., the rear of the treadmill belt and/or the shock grid) for 5 consecutive seconds, the test was considered completed and mice were returned to their home cages.
2.9. Statistical Analyses
Analysis of variance (ANOVA) testing was performed using GraphPad Prism (version 7.02, GraphPad Software, La Jolla, CA). Where a significant main effect of treatment was observed via ANOVA, the following comparisons were made using post-hoc t-tests with a Holm-Bonferroni correction: PBS+PBS vs 5-FU+PBS, 5-FU+PBS vs 5-FU+TAL, and 5-FU+PBS vs 5-FU+MPH. For repeated measures, two-way ANOVAs were used. When a significant interaction between time and treatment was found, individual ANOVAs were performed for each time point, as described above. Results of statistical testing are presented in Supplementary Table 1. Data are presented as mean ± SEM.
3. Results
3.1. Effects of 5-FU treatment
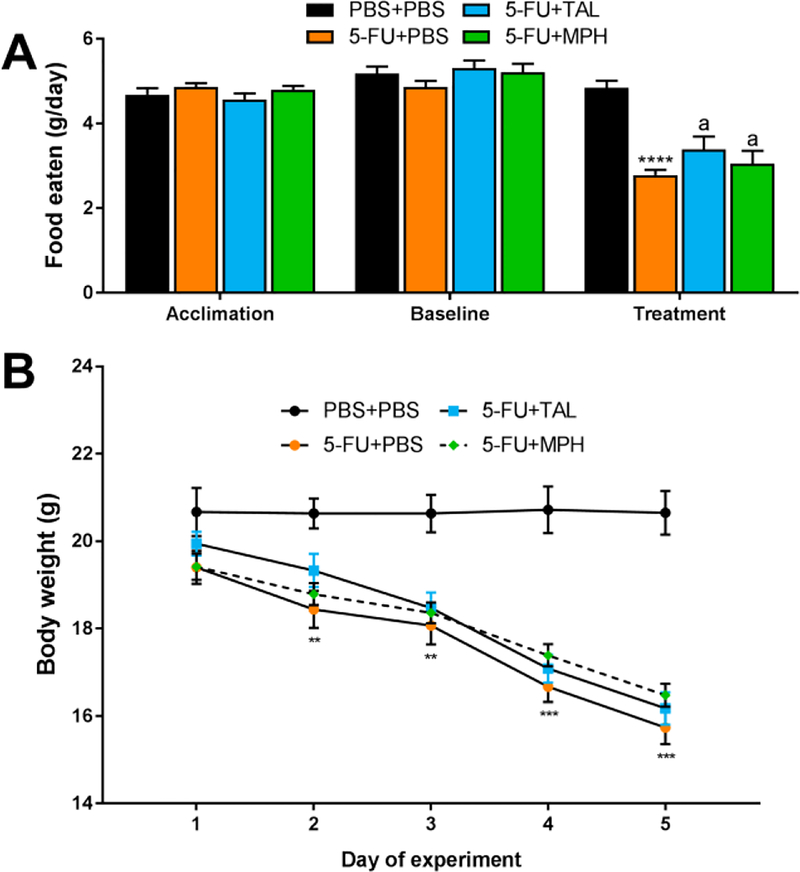
In addition to fatigue, 5-FU treatment had several noteworthy effects. In the VWRA experiment, food consumption and body weight were also recorded. Although mice ate similar amounts of food prior to drug treatment (i.e., during the acclimation and baseline periods), by the end of treatment, 5-FU-treated mice ate approximately forty percent less than controls (ANOVA, main effect of treatment: F(3,22) = 12.74, p < .0001) (Fig. 2A). Unsurprisingly, 5-FU-treated mice also displayed rapid, significant weight loss, losing several grams of body weight by the end of the experiment (Fig. 2B) (see Supplementary Table 1 for full statistical results). Similar weight loss effects were observed in all other experiments, with 5-FUtreated mice consistently displaying significant weight loss by the end of each experiment (Supplementary Table 1).
Figure 2. Effects of chemotherapy on food consumption and body weight.
(A) Average food eaten during acclimation (“Acclimation”) and baseline (“Baseline”) phases in the VWRA experiment and food eaten on day 5 of the experiment (“Treatment”). (B) Body weight of mice in the VWRA experiment over the 5 days of 5-FU treatment. Data represent mean±SEM of 6–7 mice per group. **p < .01, ***p < .001, ****p < .0001, 5-FU-PBS vs PBS+PBS, ap > .05 compared to 5-FU+PBS, t-test with Holm-Bonferroni correction
As expected from a cytotoxic chemotherapeutic agent, 5-FU caused a marked and statistically significant decrease in white blood cells and platelets and a slight, but significant, decrease in hematocrit (Table 1). Red blood cells, which have a slower turnover rate than white cells or platelets, showed a non-significant trend (ANOVA, main effect of treatment: F(3,16) = 2.859, p = .0697). Neither TAL nor MPH mitigated any of these effects (Fig. 2, Table 1).
Table 1.
Effects of chemotherapy and interventions on blood
| PBS+PBS n = 5 |
5-FU+PBS n = 5 |
5-FU+TAL n = 5 |
5-FU+MPH n = 5 |
|
|---|---|---|---|---|
| WBC count (K/μL) | 7.7 ± 0.8 | 2.1 ± 0.4*** | 1.4 ± 0.4 | 1.9 ± 0.2 |
| RBC count (M/μL) | 9.2 ± 0.3 | 8.3 ± 0.1 | 8.6 ± 0.3 | 8.2 ± 0.3 |
| Hematocrit (%) | 45 ± 0.9 | 39 ± 0.5** | 41 ± 1.4 | 40 ± 1.8 |
| Platelets (K/μL) | 761 ± 55 | 308 ± 60*** | 247 ± 44 | 322 ± 49 |
p < .01,
p < .001 compared to PBS+PBS, t-test with Holm-Bonferroni correction
3.2. Effects on VWRA
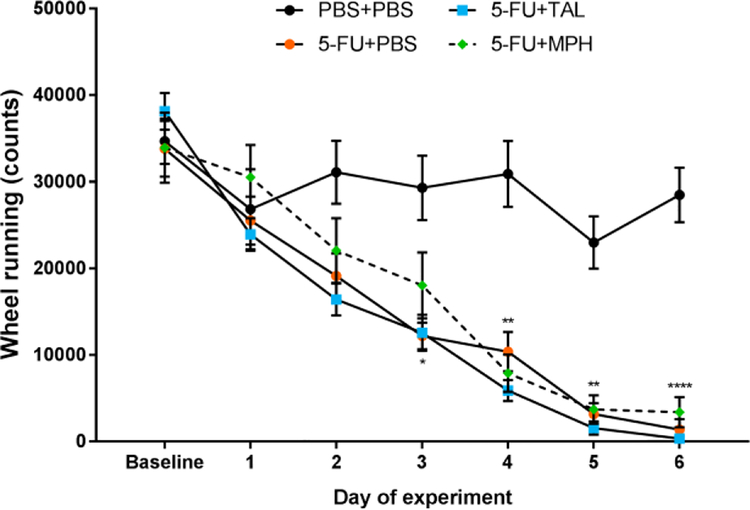
On the final day of the baseline period, prior to any treatment, VWRA was similar between groups (ANOVA, main effect of treatment: F(3,22) = 0.4452, p = .7231). After starting treatment, VWRA of 5-FUtreated mice markedly decreased, indicating fatigue-like behavior (Fig. 3). A two-way ANOVA of VWRA found a significant interaction between treatment and time (F(15,110) = 6.264, p < .0001) and ANOVAs on each day of treatment revealed significant treatment effects on days 2–6. Post-hoc analysis revealed VWRA was significantly decreased in 5-FU-treated mice on these days, but neither TAL nor MPH mitigated this effect (see Supplementary Table 1 for full statistical results).
Figure 3. Effects of chemotherapy on voluntary wheel running activity.
VWRA at baseline (the mean of 4 days of wheel running after mice acclimated to the running wheels) and daily during the treatment period (day 1–6). Data represent mean±SEM of 6–7 mice per group. Data from the PBS+PBS and 5-FU+PBS groups were previously published and are reproduced with permission from [18]. *p < .05, **p < .01, ****p < .0001, 5-FU+PBS vs PBS+PBS, t-test with Holm-Bonferroni correction
3.3. Effects on exploratory behavior in the OFT
In the OFT, treatment with 5-FU had no significant effect on most measures, but slightly decreased the speed of mice while mobile (Table 2). MPH and TAL, however, each significantly altered exploratory behavior in distinctly different manners (Table 2) (see Supplementary Table 1 for full statistical results). MPH-treated mice were more mobile and traveled a greater distance in the arena. TAL-treated mice spent less time mobile, but moved significantly faster; consequently, mice in the 5-FU+TAL group traveled a similar distance to the 5-FU+PBS group, but tended to spend more time in the edge of the arena.
Table 2.
Effects of chemotherapy and interventions in the open field
| PBS+PBS n = 5 |
5-FU+PBS n = 5 |
5-FU+TAL n = 5 |
5-FU+MPH n = 5 |
|
|---|---|---|---|---|
| Distance (m) | 129 ± 11 | 113 ± 7.0 | 94 ± 6.1 | 206 ± 23† |
| Time mobile (s) | 1289 ± 109 | 1267 ± 57.3 | 582 ± 63.6††† | 1847 ± 165† |
| Speed while mobile (m/s) | 0.10 ± 0.003 | 0.09 ± 0.003* | 0.17 ± 0.013†† | 0.11 ± 0.005† |
| Time in edge (%) | 91.3 ± 3.4 | 91.8 ± 1.3 | 99.3 ± 0.2 | 88.4 ± 1.9 |
p < .05 compared to PBS+PBS,
p < .05,
p < .01,
p < .001 compared to 5-FU+PBS, t-test with Holm-Bonferroni correction
3.4. Effects on behavior in the FST
In the FST pretest, no significant differences were observed in immobility (Table 3; Supplementary Table 1). In the test, after treatment, 5-FU had no effect on immobility on its own. TAL-treated mice, however, displayed markedly decreased immobility.
Table 3.
Effects of chemotherapy and interventions in the forced swim test
| PBS+PBS n = 8 |
5-FU+PBS n = 7 |
5-FU+TAL n = 7 |
5-FU+MPH n = 7 |
||
|---|---|---|---|---|---|
| Time | Pretest | 138 ± 26 | 126 ± 32 | 106 ± 26 | 113 ± 22 |
| Immobile (s) | Test | 172 ± 12 | 123 ± 20a | 37.1 ± 14† | 86.1 ± 23 |
p < .05 compared to 5-FU+PBS, t-test with Holm-Bonferroni correction;
p = .059 compared to PBS+PBS
3.5. Effects on distance run in the treadmill exercise capacity and the TFT
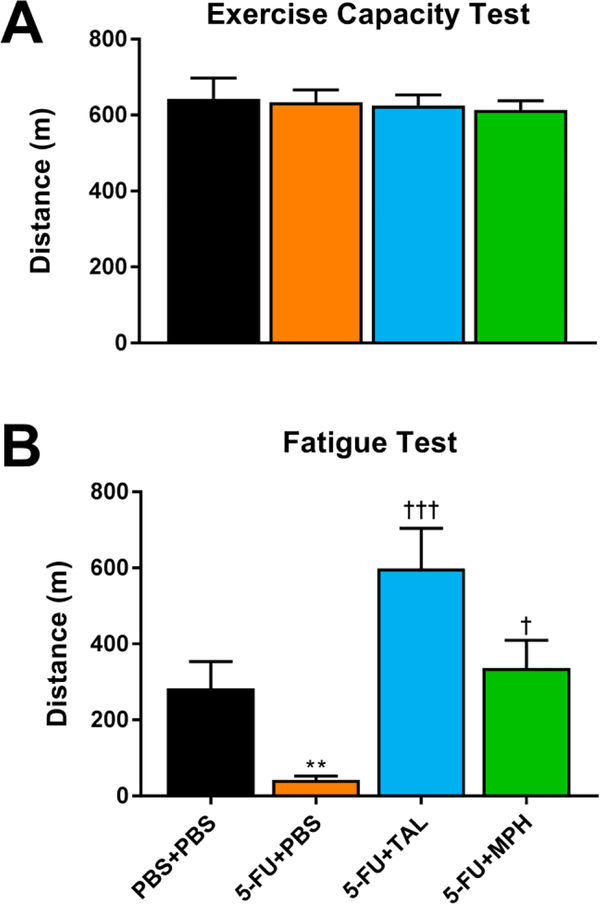
Despite the significant weight loss in 5-FU-treated mice (Supplementary Table 1), distance run in the treadmill exercise capacity test was not significantly affected by 5-FU treatment (ANOVA, main effect of treatment, F(3,42) = 0.1108, p = .9533) (Fig. 4A). In contrast, in the TFT, 5-FU-treated mice displayed significant fatigue, which TAL and MPH each reversed (ANOVA, main effect of treatment, F(3,44) = 9.587, p < .0001) (Fig. 4B).
Figure 4. Effects of chemotherapy and interventions on distance run in different treadmill tests.
Distance run by mice in the (A) treadmill exercise capacity test and (B) treadmill fatigue test. Data from the PBS+PBS and 5-FU+PBS groups in panel A were previously published and are reproduced with permission from [18]. Data represent the mean+SEM of 11–12 mice per group. **p < .01 compared to PBS+PBS, †p < .05, †††p < .001 compared to 5-FU+PBS, t-test with Holm-Bonferroni correction
4. Discussion
In the current study, we sought to detect fatigue-like behavior using a variety of mouse behavioral assays. We hypothesized that 5-FU treatment would induce fatigue-like behavior, as demonstrated by increased immobility in the FST; decreased locomotor activity in the OFT; decreased distance run in the treadmill exercise capacity test and TFT; and decreased VWRA. However, fatigue-like behavior was only detected by VWRA and the TFT. In the FST, the trend toward decreased immobility of 5-FU-treated mice is inconsistent with and cannot be regarded as fatigue-like behavior; this is in contrast to previous studies using FST immobility as a measure of fatigue that showed increased immobility, but provided no other measure of fatigue-like behavior than the FST [21–23]. Our findings in the OFT are consistent with prior studies using mouse models of chemotherapy- or immunologically-induced fatigue, which found no decrease in locomotor activity in the OFT in their fatigue models [7,40]. Insensitivity to fatigue-like behavior in the exercise capacity test is likely due to the liberal use of air puffs to motivate mice to continue running in this test; mice subjected to air puffs continued running for several minutes at a time after appearing fatigued. Moreover, although 5-FU caused weight loss, muscle loss did not appear to be an issue, based on gastrocnemius weight and preliminary body composition analysis (data not shown). As such, when motivated to run until physically incapable of running further, all mice ran a similar distance. The lack of effect using the treadmill exercise capacity test, compared to the clear effect of 5-FU observed in the TFT, also highlights the value of the TFT in detecting fatigue-like behavior.
We used 5-FU to induce fatigue-like behavior, which yielded a robust effect in two fatigue-sensitive assays. Given that 5-FU has been shown to induce fatigue-like behavior in mice in numerous studies [6,16,18,29,41], the TFT or VWRA have detected fatigue-like behavior in a variety of mouse fatigue models [6–8,11,13,16,18,27,42,43], and the current effects of 5-FU in the TFT and VWRA resemble previously-reported fatigue-like behavior, it is reasonable to conclude that the current treatment of 5-FU induced fatigue-like behavior and this was detected in the TFT and VWRA. The underlying causes of this fatigue-like behavior, however, and the means by which TAL or MPH alleviate it, are yet unknown. We observed that 5-FU administration in mice causes weight loss, decreased food consumption, and decreased water intake (data not shown), and it can increase inflammatory cytokine expression and production [44–45] and cause mucositis [45] and steatosis [46]. It is neither clear nor easy to delineate the extent to which any or all of these effects contribute to or influence fatigue-like behavior. However, as 5-FU is widely studied to understand its effects and their mechanisms, a future synthesis of the current study and other work using 5-FU may be able to provide novel insight into the mechanisms of fatigue induction by 5-FU and the apparent anti-fatigue effects of drugs like TAL and MPH. Moreover, a superior understanding of the mechanism of 5-FU-induced fatigue-like behavior in mice may also allow for a better assessment of how well the observed fatigue-like behavior corresponds to fatigue experienced by human patients.
TAL and MPH alleviated fatigue-like behavior in the TFT, but we did not observe this effect on VWRA. Although further study will be necessary to determine the causes of this difference between the assays, we hypothesize that the primary causes are differences in the nature of the behavior measured in each assay and the timing of drug administration and assay measurements. Mouse VWRA is a self-motivated, voluntary behavior that primarily occurs in the dark period, and has even been observed in wild mice [47]. While the motivation underlying this behavior is not entirely clear, effects of the current 5-FU treatment that TAL and MPH did not alleviate may have decreased mouse motivation to initiate VWRA. Mouse running in the TFT, in contrast, is not voluntarily initiated and provides external motivation to continue running. As such, TAL and MPH may alleviate fatigue-like behavior in the TFT because mice do not initiate the task and each intervention improves their ability to perform the task; future studies using a forced wheel running task may help test this hypothesis.
For consistency across all assays used in this study, TAL and MPH were injected during the light period. This may have contributed to the lack of observable anti-fatigue effect on VWRA. Administration during, or more proximal to, the dark period may yield an observable anti-fatigue effect; the wakefulness-promoting agent, modafinil, reportedly alleviates fatigue-like behavior in the VWRA when administered daily in the middle of the dark period [7]. In a pilot study, we found that TAL (1 mg/kg, i.p.), but not PBS, during the light period caused mice to be active, as determined by photobeam breaks, for 2–3 hours after injection (data not shown). Similarly, MPH administration (at 2, 5, or 10 mg/kg, i.p.) during the light period increases mouse locomotor activity for 1–2 hours [37]. A disruption of normal sleep by TAL or MPH, thus, may have interfered with detecting an anti-fatigue effect on VWRA. Lastly, it may also be that TAL or MPH, administered in the light period, did not produce anti-fatigue effects of sufficient duration to be observed in VWRA during the dark period.
The current study builds upon our prior work and further evaluates and compares TAL and MPH as anti-fatigue drugs in mouse models of fatigue. MPH has been tested in the clinic as a therapy for CRF and may reduce fatigue in some patients experiencing severe fatigue [31], but a recent meta-analysis of pharmacological interventions for CRF advises cautious use of MPH in CRF, given the potential for undesirable side-effects [48]. Thus, an effective anti-fatigue drug is still needed for many patients. We have previously found that TAL, but not MPH, increases distance run in the TFT in healthy, non-fatigued mice [6]. In the current study, we observed a similar effect in fatigued mice; compared to non-fatigued vehicle controls, the average MPH-treated mouse ran a similar distance, whereas the average TAL-treated mouse ran more than twice the distance. Additionally, while MPH had a locomotor stimulant effect in the OFT, TAL modulated exploratory activity in a distinctly different manner, indicating that the increased distance run by TAL-treated mice in the TFT is not simply due to a locomotor stimulant effect. Importantly, our current and previous findings highlight the differences between these drugs, and suggest that TAL alleviates fatigue via a substantially different mechanism than MPH. As such, TAL may be able to provide an effective fatigue therapy in a different or broader patient population than MPH.
5. Conclusion
The current study is the first to perform a cross-assay comparison of fatigue-like behavior and evaluate the ability of mouse behavioral assays to detect fatigue-like behavior in a mouse fatigue model. We found that the FST, OFT, and treadmill exercise capacity test were not sensitive to chemotherapy-induced fatigue. Consequently, these assays may not be useful tools for detecting fatigue-like behavior in mice. VWRA and the TFT were both sensitive to fatigue-like behavior, which reinforces their value as preclinical fatigue assays. Of these two assays, however, only the TFT also detected a reversal of fatigue-like behavior when putative anti-fatigue drugs were administered. Selection of mouse fatigue assays should be made with these findings in mind. Finally, our data suggest that TAL, or a similar drug, may offer a valuable fatigue therapeutic and warrants further evaluation.
Supplementary Material
Highlights.
5-fluorouracil induced fatigue and decreased food intake, body weight, and WBCs.
Fatigue was detectable using VWRA and TFT, but not OFT, FST, or exercise capacity.
Taltirelin alleviated fatigue in the TFT.
Methylphenidate alleviated fatigue in the TFT and increased locomotor activity.
Acknowledgments
The authors wish to thank Bruce Raaka, Ph.D., for assistance performing the forced swim tests, Michele Allen for technical assistance provided for the treadmill and open field tests, and Jan Linkenhoker, D.V.M., and the animal caretakers for their attentive care of mice used in these studies.
Funding:
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health [grant number 1Z01 DK011006].
Abbreviations:
- CIF
chemotherapy-induced fatigue
- CRF
cancer-related fatigue
- TFT
treadmill fatigue test
- OFT
open field test
- FST
forced swim test
- WBC
white blood cells
- RBC
red blood cells
- TAL
taltirelin
- MPH
methylphenidate
- 5-FU
5-fluorouracil
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none
References
- [1].Weis J Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Rev Pharmacoecon Outcomes Res 2011;11:441–6. doi: 10.1586/erp.11.44. [DOI] [PubMed] [Google Scholar]
- [2].Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Yennurajalingam S, et al. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer 2015;23:2461–78. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Johnston S, Brenu EW, Staines D, Marshall-Gradisnik S. The prevalence of chronic fatigue syndrome/ myalgic encephalomyelitis: a meta-analysis. Clin Epidemiol 2013;5:105–10. doi: 10.2147/CLEP.S39876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Patel R, Shahane A. The epidemiology of Sjögren’s syndrome. Clin Epidemiol 2014;6:247–55. doi: 10.2147/CLEP.S47399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dougherty JP, Wolff BS, Cullen MJ, Saligan LN, Gershengorn MC. Taltirelin alleviates fatigue-like behavior in mouse models of cancer-related fatigue. Pharmacol Res 2017;124:1–8. doi: 10.1016/j.phrs.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zombeck JA, Fey EG, Lyng GD, Sonis ST. A clinically translatable mouse model for chemotherapy-related fatigue. Comp Med 2013;63:491–7. [PMC free article] [PubMed] [Google Scholar]
- [8].Wolff BS, Renner MA, Springer DA, Saligan LN. A Mouse Model of Fatigue Induced by Peripheral Irradiation. J Vis Exp 2017. doi: 10.3791/55145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ray M, Rogers LQ, Trammell RA, Toth LA. Fatigue and sleep during cancer and chemotherapy: translational rodent models. Comp Med 2008;58:234–45. [PMC free article] [PubMed] [Google Scholar]
- [10].Littlefield AM, Setti SE, Priester C, Kohman RA. Voluntary exercise attenuates LPSinduced reductions in neurogenesis and increases microglia expression of a proneurogenic phenotype in aged mice. J Neuroinflammation 2015;12:138. doi: 10.1186/s12974-015-0362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moriya J, Chen R, Yamakawa J, Sasaki K, Ishigaki Y, Takahashi T. Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol Pharm Bull 2011;34:354–9. [DOI] [PubMed] [Google Scholar]
- [12].Matsumoto T, Takahashi H, Shiva D, Kawanishi N, Kremenik MJ, Kato Y, et al. The reduction of voluntary physical activity after poly I:C injection is independent of the effect of poly I:C-induced interferon-beta in mice. Physiol Behav 2008;93:835–41. doi: 10.1016/j.physbeh.2007.11.048. [DOI] [PubMed] [Google Scholar]
- [13].Renner M, Feng R, Springer D, Chen M-K, Ntamack A, Espina A, et al. A murine model of peripheral irradiation-induced fatigue. Behav Brain Res 2016;307:218–26. doi: 10.1016/j.bbr.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Grace PM, Loram LC, Christianson JP, Strand KA, Flyer-Adams JG, Penzkover KR, et al. Behavioral assessment of neuropathic pain, fatigue, and anxiety in experimental autoimmune encephalomyelitis (EAE) and attenuation by interleukin-10 gene therapy. Brain Behav Immun 2017;59:49–54. doi: 10.1016/j.bbi.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA. Development of a mouse model for assessing fatigue during chemotherapy. Comp Med 2011;61:119–30. [PMC free article] [PubMed] [Google Scholar]
- [16].Mahoney SE, Davis JM, Murphy EA, McClellan JL, Gordon B, Pena MM. Effects of 5fluorouracil chemotherapy on fatigue: role of MCP-1. Brain Behav Immun 2013;27:155–61. doi: 10.1016/j.bbi.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Katafuchi T, Kondo T, Yasaka T, Kubo K, Take S, Yoshimura M. Prolonged effects of polyriboinosinic:polyribocytidylic acid on spontaneous running wheel activity and brain interferon-alpha mRNA in rats: a model for immunologically induced fatigue. Neuroscience 2003;120:837–45. [DOI] [PubMed] [Google Scholar]
- [18].Dougherty JP, Springer DA, Gershengorn MC. The Treadmill Fatigue Test: A Simple, High-throughput Assay of Fatigue-like Behavior for the Mouse. J Vis Exp 2016. doi: 10.3791/54052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Azzinnari D, Sigrist H, Staehli S, Palme R, Hildebrandt T, Leparc G, et al. Mouse social stress induces increased fear conditioning, helplessness and fatigue to physical challenge together with markers of altered immune and dopamine function. Neuropharmacology 2014;85:328–41. doi: 10.1016/j.neuropharm.2014.05.039. [DOI] [PubMed] [Google Scholar]
- [20].Yoshizawa K, Yamane A, Nasu H, Suzuki H. Glucose prevents cisplatin-induced fatiguelike behavior in mice. PharmaNutrition 2018;6:107–12. doi: 10.1016/j.phanu.2018.06.001. [DOI] [Google Scholar]
- [21].Burne THJ, Johnston ANB, McGrath JJ, Mackay-Sim A. Swimming behaviour and postswimming activity in Vitamin D receptor knockout mice. Brain Res Bull 2006;69:74–8. doi: 10.1016/j.brainresbull.2005.10.014. [DOI] [PubMed] [Google Scholar]
- [22].Meeks A, Larson SJ. Evaluating fatigue in lupus-prone mice: preliminary assessments. Pharmacol Biochem Behav 2012;100:392–7. doi: 10.1016/j.pbb.2011.09.013. [DOI] [PubMed] [Google Scholar]
- [23].Tanaka M, Nakamura F, Mizokawa S, Matsumura A, Nozaki S, Watanabe Y. Establishment and assessment of a rat model of fatigue. Neurosci Lett 2003;352:159–62. [DOI] [PubMed] [Google Scholar]
- [24].Kumar A, Garg R, Kumar P. Nitric oxide modulation mediates the protective effect of trazodone in a mouse model of chronic fatigue syndrome. Pharmacol Rep 2008;60:664–72. [PubMed] [Google Scholar]
- [25].Cho SY, Lee JH, Song MJ, Park PJ, Shin ES, Sohn JH, et al. Effects of chitooligosaccharide lactate salt on sleep deprivation-induced fatigue in mice. Biol Pharm Bull 2010;33:1128–32. [DOI] [PubMed] [Google Scholar]
- [26].Dunn AL, Crnic LS. Repeated injections of interferon-alpha A/D in Balb/c mice: behavioral effects. Brain Behav Immun 1993;7:104–11. doi: 10.1006/brbi.1993.1011. [DOI] [PubMed] [Google Scholar]
- [27].Kinoshita D, Cohn DWH, Costa-Pinto FA, de Sá-Rocha LC. Behavioral effects of LPS in adult, middle-aged and aged mice. Physiol Behav 2009;96:328–32. doi: 10.1016/j.physbeh.2008.10.018. [DOI] [PubMed] [Google Scholar]
- [28].Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C). Brain Behav Immun 2007;21:490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- [29].Mahoney SE, Davis JM, Murphy EA, McClellan JL, Pena MM. Dietary quercetin reduces chemotherapy-induced fatigue in mice. Integr Cancer Ther 2014;13:417–24. doi: 10.1177/1534735414523315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kamath J, Feinn R, Winokur A. Thyrotropin-releasing hormone as a treatment for cancerrelated fatigue: a randomized controlled study. Support Care Cancer 2012;20:1745–53. doi: 10.1007/s00520-011-1268-8. [DOI] [PubMed] [Google Scholar]
- [31].Yennurajalingam S, Bruera E. Review of clinical trials of pharmacologic interventions for cancer-related fatigue: focus on psychostimulants and steroids. Cancer J 2014;20:319–24. doi: 10.1097/PPO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- [32].Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw 2015;13:1012–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Eto K, Kim SK, Nabekura J, Ishibashi H. Taltirelin, a thyrotropin-releasing hormone analog, alleviates mechanical allodynia through activation of descending monoaminergic neurons in persistent inflammatory pain. Brain Res 2011;1414:50–7.doi: 10.1016/j.brainres.2011.07.065. [DOI] [PubMed] [Google Scholar]
- [34].Thirunarayanan N, Nir EA, Raaka BM, Gershengorn MC. Thyrotropin-releasing hormone receptor type 1 (TRH-R1), not TRH-R2, primarily mediates taltirelin actions in the CNS of mice. Neuropsychopharmacology 2013;38:950–6. doi: 10.1038/npp.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding YS, et al. Dopaminetransporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology (Berl) 1999;146:93–100. [DOI] [PubMed] [Google Scholar]
- [36].Gaytan O, Ghelani D, Martin S, Swann A, Dafny N. Methylphenidate: diurnal effects on locomotor and stereotypic behavior in the rat. Brain Res 1997;777:1–12. [DOI] [PubMed] [Google Scholar]
- [37].Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, et al. Comparison between intraperitoneal and oral methylphenidate administration: A microdialysis and locomotor activity study. J Pharmacol Exp Ther 2000;295:51–7. [PubMed] [Google Scholar]
- [38].Yang PB, Swann AC, Dafny N. Psychostimulants given in adolescence modulate their effects in adulthood using the open field and the wheel-running assays. Brain Res Bull 2010;82:208–17. doi: 10.1016/j.brainresbull.2010.03.007. [DOI] [PubMed] [Google Scholar]
- [39].Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 1977;229:327–36. [PubMed] [Google Scholar]
- [40].Couch Y, Xie Q, Lundberg L, Sharp T, Anthony DC. A Model of Post-Infection Fatigue Is Associated with Increased TNF and 5-HT2A Receptor Expression in Mice. PLoS ONE 2015;10:e0130643. doi: 10.1371/journal.pone.0130643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Weymann KB, Wood LJ, Zhu X, Marks DL. A role for orexin in cytotoxic chemotherapyinduced fatigue. Brain Behav Immun 2014;37:84–94. doi: 10.1016/j.bbi.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Norden DM, Bicer S, Clark Y, Jing R, Henry CJ, Wold LE, et al. Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function. Brain Behav Immun 2015;43:76–85. doi: 10.1016/j.bbi.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol Res Nurs 2006;8:157–69. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- [44].Sakai H, Sagara A, Matsumoto K, Hasegawa S, Sato K, Nishizaki M, et al. 5-Fluorouracil induces diarrhea with changes in the expression of inflammatory cytokines and aquaporins in mouse intestines. PLoS ONE 2013;8:e54788. doi: 10.1371/journal.pone.0054788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li H-L, Lu L, Wang X-S, Qin L-Y, Wang P, Qiu S-P, et al. Alteration of Gut Microbiota and Inflammatory Cytokine/Chemokine Profiles in 5-Fluorouracil Induced Intestinal Mucositis. Front Cell Infect Microbiol 2017;7:455. doi: 10.3389/fcimb.2017.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sommer J, Mahli A, Freese K, Schiergens TS, Kuecuekoktay FS, Teufel A, et al. Analysis of molecular mechanisms of 5-fluorouracil-induced steatosis and inflammation in vitro and in mice. Oncotarget 2017;8:13059–72. doi: 10.18632/oncotarget.14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Meijer JH, Robbers Y. Wheel running in the wild. Proc Biol Sci 2014;281. doi: 10.1098/rspb.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tomlinson D, Robinson PD, Oberoi S, Cataudella D, Culos-Reed N, Davis H, et al. Pharmacologic interventions for fatigue in cancer and transplantation: a meta-analysis. Curr Oncol 2018;25:e152–67. doi: 10.3747/co.25.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.