Abstract
Background: Multimodal therapeutic strategies have improved the outcome of peritoneal metastases (PM). However, objective assessment of therapy response remains difficult in PM, since radiological studies have a poor accuracy for low-volumetric disease. There is an obvious need for a histological gold standard allowing assessment of tumor response to treatment in PM.
Content: We propose to perform peritoneal punch biopsies with a diameter of 3 to 5 mm in all four abdominal quadrants. We propose a four-tier Peritoneal Regression Grading Score (PRGS), defined as Grade 1: complete response (absence of tumor cells), Grade 2: major response (major regression features, few residual tumor cells), Grade 3: minor response (some regressive features but predominance of residual tumor cells), Grade 4: no response (tumor cells without any regressive features). Acellular mucin and infarct-like necrosis should be regarded as regression features. We recommend reporting the mean and the worst value of the regression grades obtained. When complete tumor response is suspected intraoperatively, a peritoneal cytology should be sampled.
Summary: A generic, unique score for the assessment of histological tumor response to chemotherapy in PM makes sense because of the clinical impact of histological response to therapy and because the organ of metastasis (peritoneum) is the same. By adopting PRGS, different centers will be able to use a uniform terminology and grading that will allow meaningful comparison of their results.
Outlook: PRGS has now to be validated in several gastrointestinal and gynecological cancer types and may be useful both in clinical and research settings.
Keywords: peritoneal matastasis, peritoneal regression grading score, regresion
Introduction
Tumor regression grading (TRG) scores are widely used in the neoadjuvant setting for primary tumors. For example, commonly used TRGs for upper gastrointestinal carcinomas are the Mandard grading and the Becker grading system and for rectal cancer the Dworak or the Rödel grading system [1, 2]. These systems are similar but not entirely identical regarding the specific criteria, ranking and number of categories. The lack of standardization is a critical issue in tumor regression grading because it prevents comparison between different research studies from different histological tumor origins. This leads to uncertainties and delays in assessment of efficacy of novel therapeutic strategies.
In the metastatic setting, previous published studies in the field are limited to histological response evaluation of initially unresectable colorectal liver metastases (CRLM). In this setting, major histological response has been recognized as a favorable prognostic factor after induction therapy [3–5]. This response was categorized using three different grading systems: TRG, mTRG and the system proposed by the group of MD Anderson [3].
Multimodal therapeutic strategies have improved the outcome of patients with peritoneal metastases (PM). For example, a survival advantage has been demonstrated by combining intraperitoneal and intravenous chemotherapy in ovarian cancer [6]. Combining cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has improved the outcome of this disease [7, 8]. Neoadjuvant strategies might further improve results of CRS and HIPEC [9]. In the palliative setting, Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) might be a promising approach [10].
However, objective assessment of therapy response remains difficult in PM, since radiological studies have a poor accuracy for low-volumetric disease. Smaller peritoneal metastases are difficult to detect by imaging techniques, and variations in their volume are even more difficult to measure. Neither computed tomography (CT) nor magnetic resonance imaging are reliable predictors of tumor “stickiness” or overwhelming small bowel or mesenteric involvement [11]. Thus, peritoneal metastases are often classified as “nonmeasurable disease” and considered ineligible for response evaluation. As a result, these patients are not included in randomised studies [12].
A handful studies have assessed histological tumor regression for therapy response and/or prognostic assessment in PM. For example, in a retrospective Japanese study on 142 patients having received induction chemotherapy before CRS and HIPEC for colorectal PM, pathological response had a prognostic significance [13]. Prognostic significance of histological response in PM of colorectal origin was confirmed recently in another French study on 142 patients [9]. The cumulative 5-years survival rates were 75% and 57% for patients with complete and major response, respectively, and histological tumor response was the sole independent predictor of survival in multivariate analyses. Further data on histological response after induction chemotherapy are available in mucinous adenocarcinoma of appendiceal origin. In a series of 34 patients, 10 (29%) having received preoperative systemic chemotherapy followed by HIPEC, had a complete or near complete histological response and patients showing complete response had a better overall survival [14].
In the palliative setting, first data on tumor response of PM after PIPAC reported large areas of devitalized tumor with mucin pools in a patient with diffuse carcinomatosis from an appendiceal signet ring cell carcinoma [15]. These first results have been reproduced in three retrospective studies evaluating histological tumor response after PIPAC in ovarian [10], colorectal [16], and gastric cancer [17]. One of the studies just mentioned was a regulatory phase-2 study on the efficacy of PIPAC with low-dose cisplatin and doxorubicin in platin-resistant, recurrent ovarian cancer [10].
Taken together, these data support the need for a histological gold standard allowing assessment of tumor response to chemotherapy in PM. Biopsy specimens for such histological assessment can be obtained during staging laparoscopy, a common procedure in PM. A standardized tumor regression grading system is needed in order to categorize the regressive changes after chemotherapy treatment and/or to investigate these changes for potential prognostic significance. This information should indeed be as objective as possible, using standardized, determinable and reproducible criteria.
Moreover, standard sampling procedures have to be determined for peritoneal metastases. Such standards have already been developed in several specialties, for example punch biopsies in dermatology and gynecology, or multiple biopsies in prostate cancer. In a similar way, the quality, number and size of the specimens available for analysis will influence the results.
Finally, agreement has to be established on the interpretation of the results. For example, tumor regression grade might vary among different peritoneal biopsies obtained at different peritoneal sites. It has to be determined if the prognostically most useful information is achieved by selecting the best, the strongest or weakest regression, or if a median regression grade is prognostically more useful.
We propose standard operating procedures for peritoneal sampling and introduce a novel Peritoneal Regression Grading Score (PRGS) for reporting the regressive changes in the obtained biopsies and/or surgical specimens.
Content
1. Recommendations for peritoneal sampling
1.1 During laparotomy or laparoscopy, the peritoneal cavity should be explored thoroughly and the Peritoneal Carcinomatosis Index (PCI) [18] should be documented.
Comment: placement of the camera access port in the abdominal midline might be advantageous [19]. The staging procedure should be videorecorded to allow later comparisons, or documented with intraoperative pictures during open surgery.
1.2 At least 4 biopsies should be taken at suspect localizations (typically in the right upper quadrant, right lower quadrant, left upper quadrant and left under quadrant), typically from tumor nodules.
Comment: usually, biopsies are taken from the parietal peritoneum. In the presence of adherences, it might be impossible to obtain samples from each quadrant. Biopsies from the visceral peritoneum might induce a risk of postoperative hollow organ perforation. Using narrow-band imaging and image enhancement software might increase accuracy of biopsies [20].
1.3 Peritoneal biopsies should have a diameter of at least 3 mm, ideally 5 mm. The use of a punch biopsy device is recommended to generate standardized samples.
Comment: the sample morphology is decisive for proper histopathological analysis. The biopsy should contain both the mesothelial and the submesothelial layers. Peritoneal samples are directional and the relationship surface/depth can influence results, in particular for quantitative pharmacological analyses.
1.4 Additionally, a local peritonectomy of several square centimeters should be taken.
Comment: a larger sample is needed in order to increase the accuracy of negative (tumor-free) biopsies for documenting complete tumor regression.
1.5 Representative samples should be taken from surgical specimen.
Comment: in the case of cytoreductive surgery, representative samples should be taken from each resected organ. The analysis of all tumor nodules is not feasible in clinical routine and is not required to assess diagnosis, extent and regression grading. Only appropriate selection is mandatory.
1.6 Research samples should be obtained from the biopsies above.
Comment: it can be based on formalin-fixed, parafin embedded tumor tissue and/or frozen tissue. In any case the presence or absence of tumor has to be validated by a pathologist and the preservation of sufficient tumor tissue for routine diagnosis and assessment of PRGS has the absolute priority. Whether to divide the research samples into two parts and to send half of the sample to the routine diagnostic laboratory and the other half to the research laboratory/biobank or to send all samples directly to the diagnostic laboratory and then to decide which sample to use for research, is an individual decision for each institution.
1.7 In the cases a negative peritoneal histology is suspected, a peritoneal cytology is recommended.
Comment: after induction therapy or palliative chemotherapy, no vital tumor cells might be documented in the peritoneal biopsies and in the local peritonectomy sample. In this case, another three-step sections is recommended to confirm complete response. In the presence of tumor scarring or in the absence of macroscopic peritoneal lesions, sampling of peritoneal fluid for cytological analysis is advisable.
2. Recommendations for fixation and staining
2.1 For sample fixation, the use of 10% buffered formalin is recommended for a 24–48 hours period of time.
Comment: sample fixation should be appropriate for reliable histopathological analysis and tumor regression grading. Optimal fixation is mandatory to preserve antigenicity for immunohistochemistry and DNA integrity for molecular studies.
2.2 Samples should be embedded in paraffin using a controlled temperature.
Comment: adequate embedding is needed in order to perform a reliable histopathological analysis. This step is also important if ancillary studies, such as immunohistochemistry and molecular testing, are further needed.
2.3 Research samples are typically shock-deep frozen in liquid nitrogen in the operating room or rapidly after receipt at the pathology laboratory.
Comment: for research samples, warm ischemia time should be reduced to a minimum. After aliquoting, samples should be adequately labeled and shock-frozen. Then, research samples should be stored in liquid nitrogen or at -80 °C. Continuity of the cold chain should be documented.
2.4 Standard staining should be hematoxylin-eosin (HE).
Comment: HE staining is performed according to usual protocols. Additional staining might be useful but is so far not necessary for PRGS.
2.5 Immunohistochemical testing or molecular investigation may be needed in particular situations.
Comment: immunohistochemistry may be helpful for diagnosis, discrimination of histiocytes or mesothelial reactive cells from alterated tumor cells, for example in patients with gastric adenocarcinoma of the diffuse type. In gastric cancer, also determination of Her-2/neu status might be required. Moreover, in PM of colorectal origin, determination of RAS and/or microsatellite instability status might be needed.
3. Proposal of a novel Peritoneal Regression Grading Score (PRGS)
No histological regression grading has been validated so far in the setting of PM. In order to evaluate the efficacy of an increasing number of multimodal therapeutic strategies in PM, such a standardized grading system is urgently needed. Previous work has documented that 4-tier tumor regression grading systems are more reproducible than 5-tier systems [1], which is why we propose to use a 4-tier regression grading system in PM.
3.1 Tissue changes before and after therapies represent the key elements to evaluate the effect of induction or palliative treatment.
Comment: determination of a tumor regression grading score is facilitated by the availability of repeated biopsies in the same individual, allowing direct comparison before and after therapies. Such a procedure is not always applicable, for example in patients pretreated with systemic chemotherapy who have not been biopsied prior to the latter.
3.2 Macroscopic features of regression of PM are progressive sclerosis and flattening, as well as disparition of neovessels.
Comment: before chemotherapy, PM nodules appear macroscopically ill-delineated and their consistence is soft, although interstitial tumor fluid pressure might be elevated. PM nodules with mucinous and/or signet-ring histology can have a «marmelade-like» aspect, adhering to the parietal and visceral peritoneum. Under therapy, PM nodules typically develop a glassy aspect with clear-cut limits and they may then be hard at palpation. Later on, in the case of response, PM nodules progressively flatten but do not vanish. Flat, white scars remain, which might be difficult to distinguish from tumor nodules, rendering determination of a Peritoneal Carcinomatosis Index (PCI) difficult.
The first implantation of PM is usually observed in the immediate vicinity of a superficial peritoneal vessel. Later on, these PMs are supplied by an important network of neovessels with atypical morphology. Typically, these vessels are broad, tortuous and can show typical “lakes”, possibly caused by blood extravasation [20]. In the course of therapy, these neovessels might regress or eventually completely disappear.
3.3 Histological features of regression of PM are disappearance of tumor cells and progressive fibrosis. They can be unique or combined with acellular mucin pools and/or infarct-like necrosis.
Comment: tumor regression results in partial or complete disappearance of malignant cells and replacement of the tumor by fibrous or fibro-inflammatory granulation tissue and/or mucinous acellular pools and/or infarct-like necrosis.
Residual tumor cells are hyperchromic and usually show nuclear atypia (karyorrhexis, pyknosis, or enlargement of nuclei). Giant cells may also be present. Apoptotic figures are frequent, mitoses are rare. Tumor cells can show eosinophilic or vacuolated cytoplasm, and can show oncocytic features. These alterations may be quite localized, with histologically typical areas of cancer infiltrates immediately adjacent to marked cytopathic atypical cells [1].
Replacement of tumor cells by fibrotic scars is defined by the appearance of fibroblasts and collagen bundles of various abundance, associated or not with elastotic areas. However, identification of fibrotic tissue as an authentic feature of response can be challenging when, this response is weak and overlaps with normal stromal tissue. In this respect, the presence of foamy macrophages has been shown to be associated with regression due to previous cytotoxic treatment [5, 21], while stromal changes like fibrosis and granulating inflammation following endogenous tumor necrosis can also be observed in untreated carcinomas [22].
Necrosis of PM can be induced by chemotherapy, but is sometimes also found as a sign of ischemia in the centre of untreated, centimetric lesions. “Dirty” necrosis, containing nuclear debris in a patchy distribution, should not be considered as a feature of response. In contrast, infarct-like-necrosis (ILN), defined by confluent areas of eosinophilic cytoplasmic remnants surrounded by a rim of fibrosis and often associated with foamy macrophages, microcalcifications, and cholesterol clefts, is a typical feature of response to chemotherapy [5, 21, 34].
In analogy, mTRG also integrates ILN as an additional feature of therapy response in liver metastases [5]. In this setting, ILN is significantly associated with the use of angiogenesis inhibitors and was not observed in CRLM after surgery alone [4, 21, 23]. The increasing use of angiogenesis inhibitors in metastatic colorectal, gastric, and ovarian cancer is a good reason to integrate ILN in the assessment of tumor response of PM.
The presence of mucin represents a further challenge in assessing response of PM to chemotherapy. Mucin pools have been already reported after neoadjuvant chemoradiation of locally advanced rectal cancer and oesophageal cancer, in 13% to 30% of the cases [24–28]. Mucin pools have been integrated as features of response in various classifications used in the neoadjuvant setting [29, 30]. They can be distinguished from untreated mucinous adenocarcinomas by several clues. First, treated tumors usually showed a mucin component larger than 50% of the whole tumor, sometimes reaching 100%, and thus corresponding to a complete response. Second, post-treatment mucin pools are less basophilic than untreated colloid carcinomas. Third, small areas of fibrosis within the mucin pools can be present, representing combined features of histological response in the same area. In the literature, complete response with acellular mucin pools is associated with an excellent prognosis, both in oesophageal and rectal cancer [24, 25, 28,].
Mucin is frequently seen in peritoneal histology. In the absence of prior chemotherapy, mucin is mainly observed as part of mucinous and signet ring carcinomas often associated with a poor prognosis [31]. Mucin is also present in low grade appendical mucinous neoplasms (LAMNs), tumors with a relatively good prognosis [32]. These tumors can particularly contain acellular mucin pools after chemotherapy, as described above [24, 25, 28,].
3.4 Definition of the Peritoneal Regression Grading Score (PRGS).
There is an obvious need for a regression grading system in PM. This system should include only clear and well-defined criteria in order to achieve a high reproducibility between different biopsies, surgical specimens, and investigators. Since 4-tier tumor regression grading systems have been shown to be more reproducible than 5-tier systems, we decided to propose a Peritoneal Regression Grading Score (PRGS) with only 4 ranked categories [1, 2]. This number of categories appeared to us meaningful and sufficient to evaluate tumor response in clinical practice. In analogy to previous work [3], and since tumor regression is defined as partial or complete disappearance of malignant cells and their replacement by fibrous or fibro-inflammatory tissue, we decided to include both of these criteria in the classification. In addition and as mentioned above, we will consider acellular mucin pools and ILN as features of response [5]. (Figure 1A, B)
Figure 1:
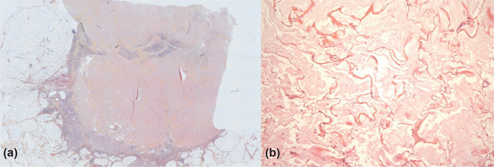
(A) Infarct-like necrosis in a peritoneal metastasis from a colorectal carcinoma: large and well limited necrotic eosinophilic areas. (B) Colloid response in peritoneal metastasis from a colorectal carcinoma: acellular mucin pools without tumor cells.
The proposed PRGS identifies four categories on the basis of the presence of residual tumor cells and the extent of regression features. PRGS 1 corresponds to a complete regression with absence of tumor cells; PRGS 2 to major regression features with only a few residual tumor cells; PRGS 3 to minor regression with predominance of residual tumor cells and only few regressive features; PRGS 4 to no response to therapy where the tumor cells are not accompanied by any regressive features. PRGS categories are exemplified in Table 1 (Figure 2).
Table 1:
Definition of the peritoneal regression grading score (PRGS).
| Grade | Peritoneal regression grading score (PRGS) | |
| Tumor cells | Regression features | |
| PRGS 1–complete response | No tumor cells | Abundant fibrosis and/or acellular mucin pools and/or infarct-like necrosis |
| PRGS 2–major response | Regressive changes predominant over tumor cells | Fibrosis and/or acellular mucin pools and/or infarct-like necrosis predominant over tumor cells |
| PRGS 3–minor response | Predominance of tumor cells | Tumor cells predominant over fibrosis and/or acellular mucin pools and/or infarct-like necrosis |
| PRGS 4–no response | Solid growth of tumor cells (visible at lowest magnification) | No regressive changes |
Figure 2:
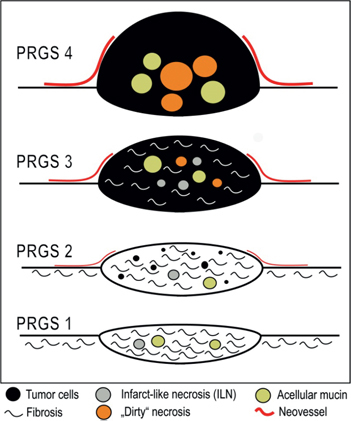
Schematic representation of the peritoneal regression grading score (PRGS).
3.5 Initial histology of PM should not determine tumor grading but initial Peritoneal Regression Grading Score (PRGS).
Comment: most patients with peritoneal metastases have been treated with systemic chemotherapy at the time of first peritoneal biopsy, so that determination of tumor grading would be meaningless. Moreover, initial biopsies allow objective documentation of tumor response to previous (systemic) chemotherapy (Figure 3).
Figure 3:
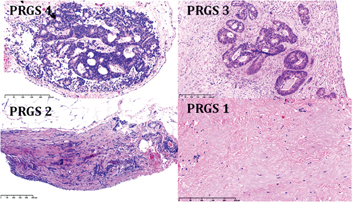
Examples of regression patterns according to the peritoneal regression grading score (PRGS)-after systemic chemotherapy.
PRGS 1 and 2 from PM of gastric cancer and PRGS 3 and 4 from PM of CRC.
3.6 The adjacent, tumor-free peritoneum might develop treatment-associated changes.
Comment: in parallel to tumor tissue, the adjacent, “normal” peritoneum can undergo morphological changes as a response of the membrane to non-physiological solutions [33]. These changes may include acute and subacute inflammation as well as development of fibrosis. It has been suggested that intraperitoneal chemotherapy first leads to an unspecific chemical peritonitis during the first days after application and that drug-specific cytotoxicity develops at a later stage, during the weeks following therapy. Changes in the adjacent peritoneum may comprise variability in nuclear/cytoplasmic ratio, nuclear pleomorphism, density of chromatin, and the presence of prominent, multiple or irregular nucleoli [1]. These therapy-induced changes in the adjacent, non-neoplastic tissue can be difficult to distinguish from regressive changes in the PM (Figure 4).
Figure 4:
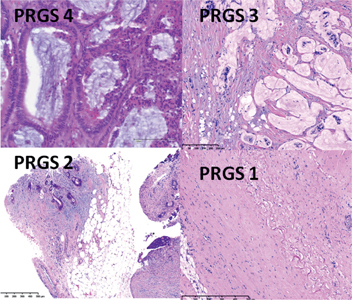
Examples of regression patterns according to the Peritoneal Regression Grading Score (PRGS)-after intraperitoneal chemotherapy- in these examples Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC).
PRGS 1: Peritoneal metastasis of duodenal mucinous adenocarcinoma after two PIPAC applications. PRGS 2: Peritoneal metastasis of a colorectal adenocarcinoma after two PIPAC applications. PRGS 3: Peritoneal metastasis of duodenal mucinous adenocarcinoma after one PIPAC application. PRGS 4: Peritoneal metastasis of a colorectal adenocarcinoma after one PIPAC application.
4. Interpretation of the Peritoneal Regression Grading Score
In order to be able to use a uniform terminology that will allow meaningful comparison of results, there must also be an agreement on the interpretation of the grade of tumor regression observed at microscopy.
4.1 Multiple peritoneal biopsies may show different PRG-scores.
Comment: from most PM patients we treat multiple tumor biopsies are taken (after staging laparoscopy or after surgical cytoreduction) and these biopsies may show different grades of regression. There is no consensus whether to consider only the highest (worst) PRGS or to calculate the mean PRGS.
At present we do not know if differences in PRGS obtained in different biopsies from the same patient have biological (i. e. due to different tumor clones as confounders) or therapeutic, in particularly for cell-cycle dependant drugs or because of inhomogeneous drug distribution, significance. This semi-quantitative assessment may, however, be helpful for the improvement of our understanding of the PM biology related to response to chemotherapy. We propose at present time to report the highest as well as the mean PRGS. These recommendations are preliminary and need to be validated in adequate prospective studies.
4.2. In case of tumor-free peritoneal punch biopsies and tumor-free local peritonectomy, the results of CT-scan and cytology have to be considered in order to define “complete response” after therapy.
Comment: it is difficult to operate with categories such as complete response or regression in a patient with metastatic disease but, if all 4 peritoneal punch biopsies and also the local peritonectomy are tumor-free, the question is how to interpret such findings. All clinical, radiological, histological, and cytological information has to be considered. The combination of the following findings is defined as a complete clinical response:
-
–
CT scan: no target lesion can be assessed and no ascites is documented,
-
–
Peritoneal biopsies: n=4, all biopsies are tumor-free,
-
–
Centimetric local peritonectomy complete regression of tumor,
-
–
Peritoneal cytology: no malignant cells [35].
4.3 In cases where the Peritoneal Regression Grading Score cannot be determined, PM should be classified as PRGSx.
Comment: enteroparietal adhesions may prevent access to the abdomen, prohibiting sampling of peritoneal biopsies. In the absence of representative tumor material, tumor response should be classified as PRGSx.
4.4 At present, the prognostic significance of the PRGS has not been determined.
Comment: determining tumor regression grading in addition to radiological studies for prognostic assessment and/or for determining therapy response is a new approach in PM. The proposed PRGS represents at the present stage a draft based on the best available evidence and personal experience of the authors, obtained in primary tumors, parenchymatous metastases and PM. The PRGS now needs to be validated in PM from various origins (e. g. ovarian, gastric, pancreatic, colorectal cancer etc.), histological subtypes (e. g. carcinoma, mesothelioma etc…) and in various indications (in particular neoadjuvant and palliative settings). This validation process represents a significant challenge that can only be mastered by collaborative efforts between different groups.
Summary
In contrast to several primary tumors in the neoadjuvant setting and hepatic metastases of colorectal cancer, no tumor regression grading has been validated so far in PM. There is an urgent need for a gold standard for therapy assessment in PM. A generic, unique score determining tumor regression after chemotherapy in PM makes sense, because of the clinical impact of histological response to therapy in various pathologies, and because the organ of metastatic spread (the peritoneum) remains the same. In the absence of such a standard, we now propose first recommendations for sampling, tumor regression grading, and interpretation of the microscopic findings in PM. At least 4 peritoneal punch biopsies of 3 to 5 mm in diameter should be taken in all 4 abdominal quadrants, plus a centimetric local peritonectomy. The novel, Peritoneal Regression Grading Score (PRGS) includes the following 4 categories: Grade 1: complete response (absence of tumor cells), Grade 2: major response (major regression features, few residual tumor cells), Grade 3: minor response (some regressive features but predominance of residual tumor cells), Grade 4: no response (tumor cells without any regression features). Acellular mucin and infarct-like necrosis should be regarded as regression features. We recommend reporting the median and the worst value of the regression grades obtained. When complete tumor response is suspected intraoperatively, a peritoneal cytology should be sampled.
Outlook
Definition of the PRGS is expected to be a dynamic process, aiming at bringing together pathologists and clinicians from different countries and having the common aim of improving the therapy and the prognosis of PM. Only the adoption of common standards will allow meaningful comparison and exchange of data, which is a precondition for successful research on PM in the future. The proposed PRGS is intended to speed up and scale up this research, by facilitating prognostic, therapeutic, and theranostic studies in PM.
Author contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- 1.Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment. Front Oncol 2013 7;3:262. [DOI] [PMC free article] [PubMed]
- 2.protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. College of American Pathologists 2016. http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/cp-colon-16protocol-3400.pdf.
- 3.Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol 2007;18:299–304. [DOI] [PubMed]
- 4.Blazer DG, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 2008;26:5344–51. [DOI] [PubMed]
- 5.Chang HHL, Leeper WR, Chan G, Quan D, Driman DK. Infarct-like necrosis: a distinct form of necrosis seen in colorectal carcinoma liver metastases treated with perioperative chemotherapy. Am J Surg Pathol 2012;36:570–6. [DOI] [PubMed]
- 6.Gould N, Sill MW, Mannel RS, Thaker PH, DiSilvestro PA, Waggoner SE, et al. A phase I study with an expanded cohort to assess feasibility of intravenous docetaxel, intraperitoneal carboplatin and intraperitoneal paclitaxel in patients with previously untreated ovarian, fallopian tube or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2012;127:506–10. [DOI] [PMC free article] [PubMed]
- 7.Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FA. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2005;12:65–71. [DOI] [PubMed]
- 8.Goéré D, Malka D, Tzanis D, Gava V, Boige V, Eveno C, et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg 2013;257:1065–71. [DOI] [PubMed]
- 9.Passot G, You B, Boschetti G, Fontaine J, Isaac S, Decullier E, et al. Pathological response to neoadjuvant chemotherapy: a new prognosis tool for the curative management of peritoneal colorectal carcinomatosis. Ann Surg Oncol 2014;21:2608–14. [DOI] [PubMed]
- 10.Tempfer CB, Winnekendonk G, Solass W, Horvat R, Giger-Pabst U, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: A phase 2 study. Gynecol Oncol 2015;137:223–8. [DOI] [PubMed]
- 11.Lambert LA. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin 2015;65:284–98. [DOI] [PubMed]
- 12.Klaver YL, Hendriks T, Lomme RM, Rutten HJ, Bleichrodt RP, de Hingh IH. Intraoperative versus early postoperative intraperitoneal chemotherapy after cytoreduction for colorectal peritoneal carcinomatosis: an experimental study. Ann Surg Oncol 2012;19:475–82. [DOI] [PubMed]
- 13.Yonemura Y, Canbay E, Ishibashi H. Prognostic factors of peritoneal metastases from colorectal cancer following cytoreductive surgery and perioperative chemotherapy. ScientificWorldJournal 2013 Apr 18;2013:978394. [DOI] [PMC free article] [PubMed]
- 14.Bijelic L, Stuart OA, Sugarbaker P. Adjuvant bidirectional chemotherapy with intraperitoneal pemetrexed combined with intravenous Cisplatin for diffuse malignant peritoneal mesothelioma. Gastroenterol Res Pract 2012;2012:890450. [DOI] [PMC free article] [PubMed]
- 15.Solass W, Kerb R, Mürdter T, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014;21:553–9. [DOI] [PMC free article] [PubMed]
- 16.Demtröder C, Solass W, Zieren J, Strumberg D, Giger-Pabst U, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis 2015 Sep 24. doi: 10.1111/codi.13130. [Epub ahead of print]. [DOI] [PubMed]
- 17.Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg 2016;20:367–73. [DOI] [PMC free article] [PubMed]
- 18.Sugarbaker PH. Management of peritoneal metastases – Basic concepts. J BUON 2015 May;20 Suppl 1:S2-11. Review. PubMed PMID: 26051329. [PubMed]
- 19.Leblanc E, Sonoda Y, Narducci F, Ferron G, Querleu D. Laparoscopic staging of early ovarian carcinoma. Curr Opin Obstet Gynecol 2006;18:407–12. [DOI] [PubMed]
- 20.Kikuchi H, Kamiya K, Hiramatsu Y, Miyazaki S, Yamamoto M, Ohta M, et al. Laparoscopic narrow-band imaging for the diagnosis of peritoneal metastasis in gastric cancer. Ann Surg Oncol 2014;21:3954–62. [DOI] [PubMed]
- 21.Bibeau F, Gil H, Castan F, et al. Comment on “Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab”. Br J Cancer 2013;109:3127–9. [DOI] [PMC free article] [PubMed]
- 22.Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521–30. [DOI] [PubMed]
- 23.Schirripa M, Loupakis F, Pollina L, et al. Reply: Comment on “Histopathologic evaluation of liver metastases from colorectal cancer patients treated with FOLFOXIRI plus bevacizumab”. Br J Cancer 2013;109:3129–30. [DOI] [PMC free article] [PubMed]
- 24.Chirieac LR, Swisher SG, Correa AM, et al. Signet-ring cell or mucinous histology after preoperative chemoradiation and survival in patients with esophageal or esophagogastric junction adenocarcinoma. Clin Cancer Res 2005;11:2229–36. [DOI] [PubMed]
- 25.Shia J, McManus M, Guillem JG, et al. Significance of acellular mucin pools in rectal carcinoma after neoadjuvant chemoradiotherapy. Am J Surg Pathol 2011;35:127–34. [DOI] [PubMed]
- 26.Shia J, Guillem JG, Moore HG, et al. Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol 2004;28:215–23. [DOI] [PubMed]
- 27.Smith KD, Tan D, Das P, et al. Clinical significance of acellular mucin in rectal adenocarcinoma patients with a pathologic complete response to preoperative chemoradiation. Ann Surg 2010;251:261–4. [DOI] [PubMed]
- 28.Lim S-B, Hong S-M, Yu CS, et al. Prevalence and clinical significance of acellular mucin in locally advanced rectal cancer patients showing pathologic complete response to preoperative chemoradiotherapy. Am J Surg Pathol 2013;37:47–52. [DOI] [PubMed]
- 29.Fernández-Aceñero MJ, Granja M, Sastre J, García-Paredes B, Estrada L. Prognostic significance of tumor regression in lymph nodes after neoadjuvant therapy for rectal carcinoma. Virchows Arch 2016 Jan 11. [Epub ahead of print]. [DOI] [PubMed]
- 30.Chang F, Deere H, Mahadeva U, George S. Histopathologic examination and reporting of esophageal carcinomas following preoperative neoadjuvant therapy: practical guidelines and current issues. Am J Clin Pathol 2008;129:252–62. [DOI] [PubMed]
- 31.Pelz JO, Stojadinovic A, Nissan A, Hohenberger W, Esquivel J. Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis. J Surg Oncol 2009;99:9–15. [DOI] [PubMed]
- 32.Carr NJ, Cecil TD, Mohamed F, Sobin LH, Sugarbaker PH, González-Moreno S, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the peritoneal surface oncology group international (PSOGI) modified Delphi process. Am J Surg Pathol 2016;40:14–26. [DOI] [PubMed]
- 33.Onishi A, Akimoto T, Morishita Y, Hirahara I, Inoue M, Kusano E, et al. Peritoneal fibrosis induced by intraperitoneal methylglyoxal injection: the role of concurrent renal dysfunction. Am J Nephrol 2014;40:381–90. [DOI] [PubMed]
- 34.Aloysius MM, Zaitoun AM, Beckingham IJ, et al. The pathological response to neoadjuvant chemotherapy with FOLFOX-4 for colo- rectal liver metastases: a comparative study. Virchows Arch 2007;451:943–8. [DOI] [PubMed]
- 35.Michael CM, Davidson B. Pre-analytical issues in effusion cytology. Pleura and Peritoneum 2016;1:45–56. [DOI] [PMC free article] [PubMed]


