Abstract
The peritoneal cavity (cavum peritonei) is incompletely divided into spaces and recessus (or fossae), which are playing an important role in health and disease. Peritoneal subspaces are determined by the parietal attachments of the abdominal organs, the ligaments and mesenteries. These include the splenorenal, the falciform, the triangular, the gastrosplenic, the phrenicocolic and the gastrocolic ligaments; the greater omentum and the lesser omentum (formed by the gastrohepatic and hepatoduodenal ligaments); the small bowel mesenterium and the mesocolon. These ligaments and mesenteries divide the peritoneal cavity into several distinct anatomic and functional regions. The supramesocolic compartment is divided into a bilateral subphrenic space and a subhepatic space continuing into the lesser sac (bursa omentalis). The inframesolic compartment is divided into a left and right region by the mesentery. The right paracolic gutter communicates with the pelvis and with the right suphrenic space. The left paracolic gutter is separated from the left subphrenic space by the phrenocolic ligament. The peritoneal space is virtual, is completely occupied by the intraabdominal organs and can only be visualized by radiological means in the presence of air (organ perforation), liquid (ascites, pus, bile, gastrointestinal fluids) or tumor invasion. Peritoneal morphology has numerous pathophysiological implications: it impacts on the propagation of intraabdominal infections, determines the spreading of peritoneal metastasis and can cause bowel volvulus. Internal hernias can arise at the junction between intraperitoneal and extraperitoneal bowel segments, in particular into the left paraduodenal recessus. Knowledge of peritoneal morphology is a precondition for developing locoregional therapeutic strategies in peritoneal disease and for effective peritoneal dialysis.
Keywords: anatomy, morphology, pathophysiology, peritoneum
Introduction
The peritoneal cavity (cavum peritonei) is the portion of the abdominal cavity delineated by the peritoneum. Beyond this cavity is placed the subperitoneal (or retroperitoneal) space. The peritoneal cavity is delineated by the parietal peritoneum and the visceral peritoneum and is a closed anatomic space. An open anatomic communication with the external environment is only present in women through the genital canal (continuum of the tubes, uterus and vagina) [1]. This communication is indeed a precondition for the transport and the fecondation of ovula. In the presence of sexually transmitted diseases, pathogens might migrate along this path into the peritoneal space and cause a tubo-ovarial abscess or peritonitis [2].
Visualization of the peritoneal space
Under physiological conditions, the peritoneal space is completely occupied by the intraabdominal organs. Thus, the peritoneal space is virtual and cannot be visualized by radiological means. This space only becomes apparent under pathological conditions, in the presence of gas, liquid or tumor.
Pneumoperitoneum
The presence of free air within the peritoneal space (“pneumoperitoneum”) is always abnormal and is usually caused by perforation of a hollow organ. Expansion of a gas within the peritoneal cavity, e.g. following a perforation of a gastric ulcer, is independent from gravity. Radiological features of hollow organ perforation include the presence of free gas or fluid within the supra- and/or inframesocolic compartments, segmental bowel wall thickening, bowel wall discontinuity, stranding of the mesenteric fat and abscess formation [3]. Historically, induction of a pneumoperitoneum with 500 to 1,000 mL air 24 h before plain abdominal X-ray was used until the 1960s as an aid to visualize indirectly the abdominal organs [4].
Liquid
Under physiological conditions, a volume of about 1 L of peritoneal fluid is produced within 24 h [5]. This peritoneal fluid is reabsorbed by the subperitoneal lymphatic channels. Only a capillary film of serous fluid (approximately 50–100 mL) separates the parietal and visceral layers of peritoneum from one another and lubricates the peritoneal surfaces [6]. This volume increases considerably under pathological conditions such as portal hypertension, portal venous thrombosis, malignant ascites, peritonitis, etc.. The peritoneal reflections that form the peritoneal ligaments, mesenteries, and omenta and the natural flow of peritoneal fluid determine the route of spread of intraperitoneal fluid and disease processes within the abdominal cavity [7]. Starting from the organ of origin, the flow of the pathological liquids within the peritoneal cavity is directed by the body position, the anatomy of the ligaments and mesenteries and intraabdominal pressure gradients (Figure 1B). As a rule, secondary to gravity, intraperitoneal fluid flows predominantly into the decline regions of the abdomen. In the upright position, most fluid accumulates in the pelvis, whereas in the supine position most fluid is found in the right and left paracolic gutter and in the pelvis [8].
Figure 1:
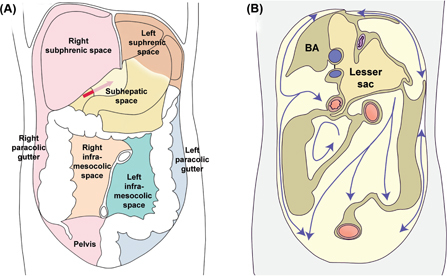
The peritoneal cavity is divided into several anatomic compartments by the ligaments and mesenteries.
(A) Peritoneal spaces, organs in place. The supramesocolic compartment is divided into a bilateral subphrenic space and a subhepatic space continuing into the lesser sac. The inframesolic compartment is divided into a left and right region by the mesentery. (B) The movement of fluids within the peritoneal cavity is determined by the peritoneal compartments. Note the large volume and the anatomic boundaries of the lesser sac. Yellow: parietal areas covered with peritoneum. Brown: bare parietal areas. BA: bare area of the right diaphragm. Adapted from Ref. (8).
Tumor invasion
Malignant tumor (peritoneal metastasis, peritoneal carcinomatosis, malignant peritoneal mesothelioma, primary adenocarcinoma of the peritoneum) or tumors of intermediate malignity (pseudomyxoma peritonei) can also invade the peritoneal spaces and make them visible by radiological means (CT-scan, MRI, etc.). Exfoliated tumor cells are transported throughout the peritoneum by physiological peritoneal fluid and disseminate within the abdominal cavity. Extensive seeding of the peritoneal cavity by tumor cells is often associated with ascites, particularly in advanced, high-grade serous ovarian carcinomas [5].
Divisions and compartments of the peritoneal cavity
The peritoneal cavity (cavum peritonei) is incompletely divided into spaces and fossae (or recesses), which are playing an important role in the circulation of the intraperitoneal fluid and the spreading, respectively limitation of infections. The anatomy of the peritoneal spaces is determined by the parietal attachments of the organs. Peritoneal ligaments are double layers or folds of peritoneum that support a structure within the peritoneal cavity; omentum and mesentery are specifically named peritoneal ligaments. Most abdominal ligaments arise from the ventral or dorsal mesentery [7]. They include the triangular ligament, the falciform ligament, the splenorenal ligament, the gastrosplenic ligament, the phrenicocolic ligament, the gastrocolic ligament, the greater omentum, the lesser omentum (formed by the gastrohepatic ligament and hepatoduodenal ligament), and the transverse mesocolon [9].
The transverse colon and mesocolon are the major landmarks dividing the peritoneal cavity into supramesocolic and inframesocolic compartments. On the anterior aspect of the liver, the supramesolic compartment is divided into the left and tight subphrenic spaces by the falciform ligament. The subhepatic space, including the lesser sac, is located under the liver. The inframesocolic compartment is subdivided by the root of the small intestine mesentery into the right and the left inframesocolic space and into the pelvis. These spaces are illustrated in Figure 1.
Right subphrenic space (Figure 1A, pink)
The right subphrenic space is located underneath the right diaphragm. It does not communicate with the left subphrenic space since the falciform ligament attaches ventrally to the anterior abdominal wall and divides the supramesocolic compartment. The right subhepatic space continues caudally lateral to the liver to the right paracolic gutter, located between the ascending colon and the lateral abdominal wall.
Subhepatic space (Figure 1A, yellow)
The right subhepatic space continues medially through the foramen of Winslow (epiploic foramen) to the lesser sac (bursa omentalis). The organs surrounding the lesser sac are the spleen on the left, the stomach and duodenum anterior and right, the transverse colon anterior, and the pancreas on its posterior aspect (Figures 1B and 2).
Figure 2:
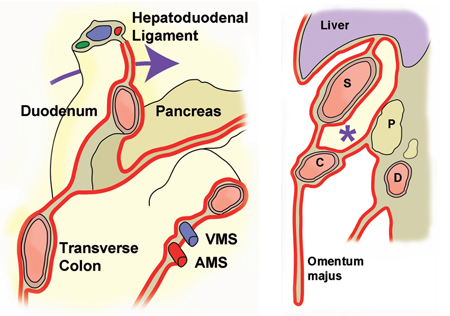
Anatomy of the lesser sac.
The left panel shows the insertions of the transverse mesocolon and of the mesentery and their anatomic relationship with the duodenum. The arrow shows the foramen Winslow behind the hepatoduodenal ligament. VMS: superior mesenteric vein. AMS: superior mesenteric artery. The right panel shows a sagittal section of the lesser sac (*). S: stomach, C: colon, D: duodenum.
The lesser sac is a potentially large cavity with various recesses into the subphrenic area cranially and into the greater omentum caudally (Figure 3). The left gastric artery, when it arises from the celiac artery, divides the lesser sac into a superior and inferior fossa, forming the gastropancreatic plica. The superior recess (a), located to the left of the gastropancreatic plica, is the most voluminous of the recesses and extends between the caudate lobe of the liver and the diaphragm. Further recesses are the inferior duodenal fossa (b), the retroduodenal fossa (c) and the paraduodenal fossa (d).
Figure 3:
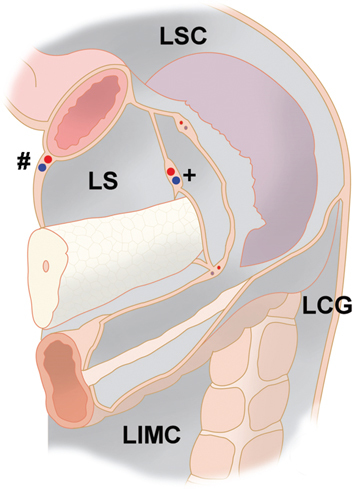
Peritoneal compartments in the left upper abdomen (detailed view).
The left subphrenic space is separated from the left paracolic gutter by the phrenicocolic ligament, en extension of the greater omentum. Note the virtual space between stomach and the spleen: the superficial peritoneal layer contains the short gastric vessels, the posterior peritoneal layer contains the splenic vessels. Abbreviations: LS: lesser sac; LSC: left subphrenic compartment; LCG: left colic gutter; LIMC: left inframesocolic compartment. #: gastro-omental vessels; +: splenic vessels.
Left subphrenic space (Figure 1A, brown)
The left subphrenic space is separated from the left paracolic gutter by the phrenicocolic ligament and the right subphrenic recess by the falciform ligament (Figure 3). This space contains the gastrohepatic fossa, the gastrosplenic recessus and the splenorenal recessus located in the dorsal part. The splenorenal fossa extends anteriorly and medially behind the tail of the pancreas. The splenorenal fossa is in continuity with the left subphrenic space but is separate from the lesser sac. Posteriorly, the falciform ligament is in continuity with the left and right triangular ligaments. Together with the ligamentum falciforme, the triangular ligaments are suspending the liver and attach on the bare area of the diaphragm. The left triangular ligament is formed by the fusion of the inferior and superior reflections of the coronary ligaments. It is short and does not compartmentalize the left subphrenic space. The right triangular ligament is formed by the fusion of the superior and inferior reflections of the right coronary ligament. Unlike the left triangular ligament, the right triangular ligament is long and separates the right subphrenic space from the right subhepatic space [7], forming the Morison pouch.
On the basis of theoretical morphological knowledge, it can be postulated that a gastric cancer will spread into the left rather than into the right subphrenic space. Recently, results of peritoneal cytology in three abdominal compartments (left subphrenic space, and right subhepatic space and pelvis) were compared in 1,039 patients with primary gastric adenocarcinoma; 11 % patients had at least one positive cavity. Positive cytology in more than one compartment was significantly associated with a poorer prognosis [10]
Greater omentum
Whereas the mesocolon transversum divides the peritoneal cavity in the sagittal plane, the greater omentum divides this cavity in the frontal plane. The greater omentum is formed by 4 adherent serosal layers and is covering anteriorly the most part of the inframesocolic compartment [11]. It originates on the greater gastric curvature and is vascularized by branches of the right and left gastroepiploic arteries. It crosses the transverse colon and descends in front of the abdominal viscera, occasionally down to the symphysis. The omental portion between the stomach and the colon is called the gastrocolic ligament, and the portion below the colon is the apron [12]. The omentum is attached to various organs. On the left side, an extension of the gastrocolic ligamentum may lie over the anterior surface of the stomach, or over the anterior part of the spleen. The omentum forms the ligament between the spleen and stomach (gastrosplenic ligament) and attaches the spleen to the dorsal abdominal wall. Although the greater omentum is mobile and can patch on several organs in case of disease, it is not able to move actively. However, the greater omentum is able to encapsulate intraperitoneal collections of pus as well as infarcted organs [13].
Lesser omentum
The lesser omentum attaches the small curvature of the stomach and the duodenal bulb to the inferior aspect of the liver. The lesser omentum is formed by the gastrohepatic and hepatoduodenal ligaments, which are in anatomic continuity. The hepatoduodenal ligament contains the portal vein, the hepatic artery and the common bile duct. The gastrohepatic ligament contains the coronary vein and left gastric artery; it is very thin and translucid; however, in an autopsy series, normal anatomy was found in only 9 %, the remaining presenting some variations from this, many of direct surgical importance, in particular the presence of one or two aberrant hepatic arteries in 37 % of cases [14].
Left inframesocolic space (Figure 1A, green)
The left infracolic space is located between the small bowel mesentery and the mesentery of the colon descendens and of the sigmoid colon. The sigmoid mesentery is located obliquely in front of the iliosacral joint and this mesentery has a remarkable degree of mobility so that this bowel segment can be found in various locations within the peritoneal cavity. Because of this mobility, the sigmoid mesentery can also rotate along its own vascular axis, creating a sigmoid volvulus [15].
Right inframesocolic space (Figure 1A, beige)
The right infracolic space is limited by the caecum, the ascending colon, the mesoappendix and by the small bowel mesentery to the left. The ileum and the appendix always have a mesenterium. The caecum and the ascending colon are only partially covered by the peritoneum and their posterior aspect is often in direct contact with the posterior abdominal wall (fixed caecum). However, it is not rare to observe a true caecal mesentery, in such case the caecum is partially covered by the peritoneum. The caecum can then be very mobile and, as the colon sigmoid, it can rotate on its root, creating a volvulus [16].
The visceral peritoneum represents about 70 % of the total peritoneal surface. Since the mesojejunum and mesoileum build the largest part of the visceral peritoneum, a bowel perforation into the right or left inframesocolic space always results clinically into a severe, diffuse peritonitis. In the presence of a diffuse peritonitis, abdominal lavage is a common surgical practice, but few studies have been conducted to assess its efficacy at removing cells from the abdominal cavity, particularly during laparoscopic surgery [17]. Abdominal lavage has also be proposed as a mean of reducing postoperative tumor recurrence, and its favorable role has been confirmed in a recent meta-analysis [18]. However, washing for peritoneal lavage is likely to be limited to a limited space, in particular when it is performed laparoscopically without opening the ligaments and mesenteries.
Left and right paracolic gutters
The lateral recesses of the pelvis merge cranially to the right and left paracolic gutters. The right paracolic gutter is a relatively wide space between the ascending colon and the lateral abdominal wall. It is in continuity with the right subphrenic space and continues cranially lateral to the liver to the right hemidiaphragm. Therefore, an infection can progress cranially along the right paracolic gutter up to the liver. The posterior access to the right diaphragm is narrowed by the nude area and forms the hepatorenal fossa (or Morison pouch). The left paracolic gutter is a narrow space lateral to the descending colon and this gutter is limited cranially by the phrenicocolic ligament, so that there is no anatomic continuity with the left subphrenic space.
Purulent peritonitis from acute left colon diverticulitis is a relatively common presentation of diverticular disease. Historically the treatment was surgical removal of the segment involved (so-called Hartmann procedure). Nowadays, therapy is depending on the severity of diverticulitis, as defined by the Hinchey classification [19]. In particular, laparoscopic peritoneal lavage has been proposed as a lesser invasive treatment option in Hinchey stage 3. A recent meta-analysis showed that in acute perforated diverticulitis with purulent peritonitis laparoscopic lavage is comparable to sigmoid resection in term of mortality but it is associated with a significantly higher rate of reoperations and a higher rate of intra-abdominal abscess [20]. It is unlikely to find an explanation for these contrasting results in various severity grades, since all patients had a Hinchey stage III. Another, more likely explanation would be different locations in the colon sigmoid (proximal vs. distal, medial vs. lateral, anterior vs posterior) determining spreading into specific morphologic spaces. Knowledge and integration of anatomic criteria could further refine the current tailored approach of severe diverticulitis (resection vs. drainage vs lavage).
Pelvic space
The pelvic space is the most caudal space in the peritoneal cavity. The pelvis contains anteriorly the urinary bladder, most part of which is covered by the peritoneum. In women, the uterus and the tubes are located within a large transverse peritoneal fold dividing the pelvis into an anterior and posterior part. The pelvic space is divided ventrally by the remnant of the urachus (median umbilical ligament), the obliterated umbilical arteries (medial umbilical ligament), and the lateral umbilical ligaments (inferior epigastric vessels) into five fossae: the right and left lateral and medial inguinal fossae and the supravesical fossa. The peritoneal fossae of the pelvis continue laterally as the paravesical fossae, and dorsally in the male as the rectovesical fossa and in the female as the cul-de-sac (Douglas pouch) and the uterovesical fossa. The pelvic peritoneal space is usually occupied by small bowel loops [21].
Metastatic spreading is varying with tumor histology: whereas adenocarcinoma predominantly metastasizes to the liver, mucinous colorectal cancers and signet-ring cancers more frequently develop peritoneal metastases [22]. Metastatic spread is also varying with the primary tumor localization, with a high rate of abdominal metastases in colon cancer patients, whereas rectal cancer patients more often develop metastases at extra-abdominal sites (liver and lung) [23]. Colon cancer may metastasize within the abdominal cavity, giving rise to transcoelomic ovarian metastases, which are frequently detected together with peritoneal metastases. Peritoneum and ovaries are often considered a continuum and have a common embryologic origin (intermediate plate of the mesoderm), which differs from the digestive tube (lateral plate of the mesoderm). Ovarian metastases are substantially more common among women with colon than rectal cancer. Colorectal metastasis to the ovary is bilateral in 43 % of cases [24].
This anatomic continuity between the right colon and the pelvis also plays an important role in the case of female infertility. In a large case-control study, whereas no excess risk of tubal infertility was associated with a simple appendectomy without rupture. Ruptured appendix increases the relative risk of tubal infertility [25]. In cohort studies investigating risk factors for infertility, the strongest correlation (OR 7.2) with anamnestic factors was a history of complicated appendicitis [26].
Peritoneal recessus and fossae
Besides the compartments defined above, organs, mesenteries, vascular folds and traction folds build into several larger and less pronounced peritoneal cul-de-sacs (fossae, recessus and/or fovea). For example, the peritoneal recessus (Figure 3) beyond the gastrosplenic ligament and ahead of the splenorenal ligament is a frequent route for subperitoneal spread of pancreatitis-related fluid. The splenorenal ligament is the most dorsal aspect of the dorsal mesentery. It contains the pancreatic tail and splenorenal collateral vessels in patients with portal hypertension [7].
Rarely, these recessus can be the origin and the localization of internal abdominal hernias. Internal hernias are rare with a postmortem incidence below 1 % [27] and are in an estimate of 0.2 and 4.1 % the cause of all intestinal obstructions [28].
Left paraduodenal recessus (Figure 4A)
The ascending duodenum emerges beyond the upper part into the peritoneal space, giving rise to the proximal jejunum. At this place is located a peritoneal pocket, the left paraduodenal recessus. The left paraduodenal recessus can be divided into smaller fossae: the superior duodenal fossa, the inferior duodenal fossa, the retroduodenal fossa and the paraduodenal fossa (d).
Figure 4:
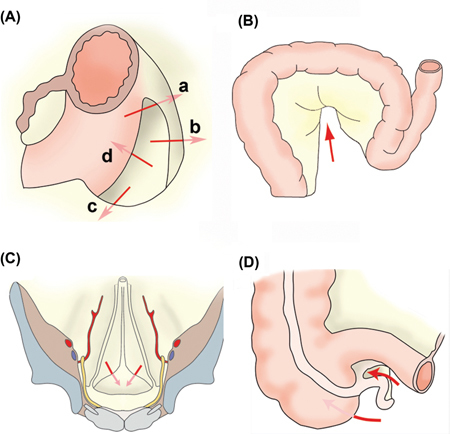
Mesenteries and peritoneal ligaments build into several larger and less pronounced peritoneal cul de sacs (called fossae, recessus and/or fovea).
(A) Left paraduodenal recessus, located at the duodenojejunal angle where the duodenum is entering again the peritoneal cavity, and subdivided into: a) the superior duodenal fossa, b) the inferior duodenal fossa, c) the retroduodenal fossa and d) the paraduodenal fossa. (B) The sigmoid recessus is found on the outside of the colon sigmoideum, approximately in its middle (arrow). (C) The supravesical fossae are located between the remnants of the urachus and the left or right umbilical artery. (D) The recessus ileocaecalis superior and inferior are located at the junction of the ileum with the caecum.
Sigmoidal recessus (Figure 4B)
The fairly constant, but sometimes only indicated, sigmoid recessus is found on the outside of the colon sigmoideum, approximately in its middle. Hernias of the sigmoid mesocolon account for 6 % of internal hernias [29]. Hernias of the sigmoid mesocolon can be classified into intersigmoid, transmesosigmoid and intramesosigmoid hernias [30].
Supravesical recessus (Figure 4C)
The supravesical fossa is a triangular area bounded laterally and above by median and medial umbilical ligaments, and below by the peritoneal reflection that passes from the anterior abdominal wall to the dome of the bladder [31].
Recessus ileocaecalis (Figure 4D)
At the junction of the ileum with the caecum are the recessus ileocaecalis superior and inferior. The upper pocket is formed by the ileum, caecum, and a pleural fold, which runs from the caecum to the mesenteric root. The recessus inferior is formed by the ileum and caecum as well as by the ileocaecalis peritoneal fold tight between the two organs. Retrocaecal hernias have been described early [32] but their incidence is very rare with a handful cases published over a century. About 10–15 % of internal hernias are accounted for by pericaecal hernias. Types of pericaecal hernias include: ileocolic, retrocaecal, ileocaecal and paracaecal. Occurrence of such hernia is favorized by imperfect accolement of the caecum to the posterior abdominal wall during the fetal development [31].
Conclusions
The compartments of the peritoneal cavity play an important role in health and disease and are determined by the abdominal organs, the ligaments and mesenteries. Peritoneal morphology has an impact on the propagation and encapsulation of intraabdominal infections, determines the spreading pattern of peritoneal metastasis and can be the origin of bowel volvulus. Internal hernias can arise at the junction between intraperitoneal and extraperitoneal bowel segments, in particular into the left paraduodenal recessus.
Peritoneal compartments might determine the accuracy of peritoneal lavage for diagnosis a subclinical tumor spreading within the abdomen. Detection of free tumor cells might be different from place to place and may also vary depending on different cancer origins (stomach, colon, ... ) and cancer types (adenocarcinoma, mucinous, signet-ring). The number of positive cavities may indicate the stage of transcoelomic tumor spread and predict the prognosis of patients with positive peritoneal washing cytology [33]. Thus, in the absence of macroscopic peritoneal lesions, the surgeon should be encouraged to perform several random biopsies and cytology.
Neoadjuvant intraperitoneal chemotherapy combined with systemic chemotherapy (NIPS) has been proposed as a method to reduce the extent of peritoneal metastasis before cytoreductive surgery [34]. In analogy, postoperative intraperitoneal chemotherapy protocols have been introduced recently to prevent locoregional recurrence in T4 colon cancer [35]. Clearly, efficacy of these approaches will depend on the adequate distribution of the chemotherapeutic solution into the various abdominal compartments in order to reach all diseased peritoneal surfaces. It is well documented that specific area in the peritoneum are not treated using a single-port chemotherapy. Thus, implantation of two intraperitoneal catheters, for example one in the supramesocolic compartments and the second one into the inframesocolic compartment might improve homogeneity of drug distribution. However, it has to be considered that the rate of catheter-linked complication, which is already significant with a single intraperitoneal catheter [36], might further increase.
At the present stage of science, it is not possible to offer responses to all the questions raised above. However, knowledge and consideration of peritoneal morphology remains a precondition to translate successfully novel diagnostic and therapeutic approaches into clinical practice. Specifically, a detailed knowledge of the peritoneal morphology will be helpful to diagnose rare causes of intestinal obstruction, to better understand spreading of peritoneal metastasis, to drain effectively intraperitoneal fluid collections, to develop locoregional therapeutic strategies such as intraperitoneal chemotherapy, and to further improve available peritoneal dialysis protocols.
Acknowledgments
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- 1.Standring S, editor. Gray’s anatomy, 41st ed. London: Elsevier, 2015.
- 2.Chappell CA, Wiesenfeld HC. Pathogenesis, diagnosis, and management of severe pelvic inflammatory disease and tuboovarian abscess. Clin Obstet Gynecol 2012;55:893–903. [DOI] [PubMed]
- 3.Singh JP, Steward MJ, Booth TC, Mukhtar H, Murray D. Evolution of imaging for abdominal perforation. Ann R Coll Surg Engl 2010;92:182–8. [DOI] [PMC free article] [PubMed]
- 4.Truelove SC, Lumsden K. Diagnostic pneumoperitoneum. Br Med J 1955;2:585–8. [DOI] [PMC free article] [PubMed]
- 5.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010;177:1053–64. [DOI] [PMC free article] [PubMed]
- 6.Kim S, Kim TU, Lee JW, Lee TH, Lee SH, Jeon TY, et al. The perihepatic space: comprehensive anatomy and CT features of pathologic conditions. Radiographics 2007;27:129–143. [DOI] [PubMed]
- 7.Tirkes T, Sandrasegaran K, Patel AA, Hollar MA, Tejada JG, Tann M, et al. Peritoneal and retroperitoneal anatomy and its relevance for cross-sectional imaging. Radiographics 2012;32:437–51. [DOI] [PubMed]
- 8.Petermann J. Die Chirurgie des Bauchfells und des Netzes. In: Kirschner M, Nordmann O, editors. Die Chirurgie. Berlin: Urban und Schwarzenberg, 1927:127–201.
- 9.Meyers MA, Charnsangavej C, Oliphant M., editors. Meyers’ dynamic radiology of the abdomen, 6th ed. New York: Springer, 2011:23–34.
- 10.Kano Y, Kosugi S, Ishikawa T, Otani T, Muneoka Y, Sato Y, et al. Prognostic significance of peritoneal lavage cytology at three cavities in patients with gastric cancer. Surgery 2015;158:1581–9. [DOI] [PubMed]
- 11.Körte W. Die Chirurgie des Peritoneums. Stuttgart: Enke, 1927:10–13.
- 12.Liebermann-Meffert D. Topographical relations. In: Liebermann-Meffert D, Whte H, editors. The greater omentum. Berlin: Springer, 1983:3–5.
- 13.Renzi E, Boeri G. Das Netz als Schutzorgan. Berl Klin Wschr 1903;40:773–75.
- 14.Weiglein AH. Variations and topography of the arteries in the lesser omentum in humans. Clin Anat 1996;9:143–50. [DOI] [PubMed]
- 15.Osiro SB, Cunningham D, Shoja MM, Tubbs RS, Gielecki J, Loukas M. The twisted colon: a review of sigmoid volvulus. Am Surg 2012;78:271–9. [PubMed]
- 16.Pirró N, Corroller LE, Solari C, Merad A, Sielezneff I, Sastre B, et al. Cecal volvulus: anatomical bases and physiopathology. Morphologie 2006;90:197–202. [DOI] [PubMed]
- 17.Brundell SM, Tucker K, Chatterton B, Hewett PJ. The effect of lavage on intraabdominal cell burden. Surg Endosc 2002;16:1064–7. [DOI] [PubMed]
- 18.Coccolini F, Catena F, Glehen O, Yonemura Y, Sugarbaker PH, Piso P, et al. Effect of intraperitoneal chemotherapy and peritoneal lavage in positive peritoneal cytology in gastric cancer. Systematic review and meta-analysis. Eur J Surg Oncol 2016;42:1261–7. [DOI] [PubMed]
- 19.Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg 1978;12:85–109. [PubMed]
- 20.Ceresoli M, Coccolini F, Montori G, Catena F, Sartelli M, Ansaloni L. Laparoscopic lavage versus resection in perforated diverticulitis with purulent peritonitis: a meta-analysis of randomized controlled trials. World J Emerg Surg 2016;30:42. [DOI] [PMC free article] [PubMed]
- 21.Platzer W, editor. Taschenatlas der Anatomie, 9th ed. Stuttgart: Thieme, 2005.
- 22.Hugen N, van de Velde CJ, de Wilt JH, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol 2014;25:651–7. [DOI] [PMC free article] [PubMed]
- 23.Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep 2016;15:29765. [DOI] [PMC free article] [PubMed]
- 24.Lash RH, Hart WR. Intestinal adenocarcinomas metastatic to the ovaries. A clinicopathologic evaluation of 22 cases. Am J Surg Pathol 1987;11:114–21. [DOI] [PubMed]
- 25.Mueller BA, Daling JR, Moore DE, Weiss NS, Spadoni LR, Stadel BV, et al. Appendectomy and the risk of tubal infertility. N Engl J Med 1986;315:1506–8. [DOI] [PubMed]
- 26.Luttjeboer FY, Verhoeve HR, van Dessel HJ, van der Veen F, Mol BW, Coppus SF. The value of medical history taking as risk indicator for tuboperitoneal pathology: a systematic review. BJOG 2009;116:612–25. [DOI] [PubMed]
- 27.Dhillon A, Farid SG, Dixon S, Evans J. Right salpingo-ovarian and distal ileal entrapment within a paracaecal hernia presenting as acute appendicitis. Int J Surg Case Rep 2013;4:1127–9. [DOI] [PMC free article] [PubMed]
- 28.Martin LC, Merkle EM, Thompson WM. Review of internal hernias: radiographic and clinical findings. Am J Roentgenol 2006;186:703–717. [DOI] [PubMed]
- 29.Harrison OJ, Sharma RD, Niayesh MH. Early intervention in intersigmoid hernia may prevent bowel resection-A case report. Int J Surg Case Rep 2011;2:282–4. [DOI] [PMC free article] [PubMed]
- 30.Benson JR, Killen DA. Internal hernias involving the sigmoid mesocolon. Ann Surg 1964;159:382–384. [DOI] [PMC free article] [PubMed]
- 31.Sozen I, Nobel J. Inguinal mass due to an external supravesical hernia and acute abdomen due to an internal supravesical hernia: a case report and review of the literature. Hernia 2004;8:389–92. [DOI] [PubMed]
- 32.Coley WB, Hoguet JP. Retrocaecal internal hernia. Ann Surg 1929;90:765–8. [PMC free article] [PubMed]
- 33.Homma Y, Ushida S, Yamada M, Kobayashi H, Suzuki K. Positive peritoneal washing cytology in multiple cavities can predict poor prognosis of advanced gastric cancer patients. Ann Surg Oncol 2010;17:455–60. [DOI] [PubMed]
- 34.Yonemura Y, Ishibashi H, Hirano M, Mizumoto A, Takeshita K, Noguchi K, et al. Effects of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy and neoadjuvant intraperitoneal/systemic chemotherapy on peritoneal metastases from gastric cancer. Ann Surg Oncol. 2016. Aug 9. Epub ahead of print. [DOI] [PubMed]
- 35.Sugarbaker PH. Improving oncologic outcomes for colorectal cancer at high risk for local-regional recurrence with novel surgical techniques. Expert Rev Gastroenterol Hepatol 2016;10:205–13. [DOI] [PubMed]
- 36.Helm CW. Ports and complications for intraperitoneal chemotherapy delivery. BJOG 2012;119:150–9. [DOI] [PubMed]


