Abstract
The parietal peritoneum (PP) is innervated by somatic and visceral afferent nerves. PP receives sensitive branches from the lower intercostal nerves and from the upper lumbar nerves. Microscopically, a dense network of unmyelinated and myelinated nerve fibers can be found all over the PP. The unmyelinated fibers are thin and are ending just underneath the PP. The myelinated fibers can penetrate the PP to reach the peritoneal cavity, where they lose their myelin sheath and are exposed to somatic and nociceptive stimuli. PP is sensitive to pain, pressure, touch, friction, cutting and temperature. Noxious stimuli are perceived as a localized, sharp pain. The visceral peritoneum (VP) itself is not innervated, but the sub-mesothelial tissue is innervated by the autonomous nerve system. In contrast to the PP, the visceral submesothelium also receives fibers from the vagal nerve, in addition to the spinal nerves. VP responds primarily to traction and pressure; not to cutting, burning or electrostimulation. Painful stimuli of the VP are poorly localized and dull. Pain in a foregut structure (stomach, duodenum or biliary tract) is referred to the epigastric region, pain in a midgut structure (appendix, jejunum, or ileum) to the periumbilical area and pain from a hindgut source (distal colon or rectum) is referred to the lower abdomen or suprapubic region. Peritoneal adhesions can contain nerve endings. Neurotransmitters are acetylcholine, VIP, serotonin, NO, encephalins, CGRP and substance P. Chronic peritoneal pain can be exacerbated by neurogenic inflammation, e.g. by endometriosis.
Keywords: enteric nervous system, innervation, myelinated fibers, peritoneum, unmyelinated fibers, visceral pain
Introduction
Gastrointestinal homeostasis, nutrition and health are dependent on the coordination between the intrinsic nervous system, functioning largely without information to the central nervous system (CNS), with the extrinsic innervation [1], which provides communication between the bowel and the CNS (reviewed in [2]). Aim of this review is to provide the reader with fundamental understanding of the innervation of the peritoneum, integrating textbook knowledge and recent advances in the field. Although this review is focusing on the neural anatomy and physiology of the peritoneum, it appears difficult to explore this topic without referencing simultaneously to the gastrointestinal neuroanatomy in general.
Historical overview
The first report concerning the presence of nerves in the peritoneal interstitium was made by Vater in 1741, who observed that the submesothelial connective tissue of cat mesentery contained oval corpuscles with a diameter of 1–2 mm [3]. Haller was the first to identify the presence of nerves in the submesothelial tissue in 1751. The corpuscles described by Vater, non-myelinated nerve endings with a sliced-onion appearance, were characterized as nerve endings by Paccini in 1830. Paccini found these corpuscles in the mesenterial peritoneum and in the visceral ligaments in humans, and identified correctly their function as baroreceptors. In 1857, Georg Meissner described the submucosal plexus, a ganglionated nerve network located between the mucosa and the bowel circular musculature [4]. In 1862, Auerbach, a neuropathologist from Breslau, used for the first time immunohistochemical techniques to stain nerves and described the plexus myentericus [5]. Ten years later, Ranvier described a network of nerve branches along the mesenteric arteries [6]. In 1881, Hirschsprung described a congenital disease characterized by the lack of ganglion cells in the Auerbach’s plexus [7]. In 1897, Robinson published the first textbook on the peritoneum and described this organ as being richly supplied with myelinated and nonmyelinated nerves [8].
Autonomic nervous system
The autonomic nervous system (ANS) is the part of the peripheral nervous system influencing the function of internal organs and their envelopes. Almost all tissues in the human body are traveled by numerous, very fine fibers of the ANS. One can distinguish between efferent and afferent fibers. The original cells of the efferent (motoric and secretory) fibers constitute heaps of nervous cells throughout the body that are enveloped by conjunctive tissue: the vegetative ganglia. The cellular bodies of the afferent (sensitive) fibers are located in the dorsal root ganglia.
Regulation of the ANS is largely unconscious and its role is to guarantee the inner homeostasis of the organism, in particular by regulating the organ functions depending on modifications in the external environment. Among others, ANS determines gastrointestinal motility, secretory activity, intestinal blood flow and visceral sensations (fullness, pain, etc.).
Homeostasis of the inner organs is provided by the interplay of two antagonistic systems, the sympathethic and the parasympathetic nervous systems (Figure 1). This division between sympathetic and parasympathetic systems concerns only the efferent motoric and secretory fibers, and not the afferent (viscero-sensitive) fibers. Anatomically, visceral afferents are distributed according to the type and function of the target organ. Spinal visceral afferent neurons project segmentally to the laminae I and V and deeper of the spinal dorsal horn [9]:
Figure 1:
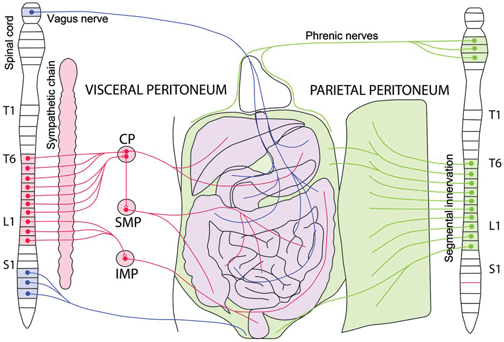
Innervation of the peritoneum.
The parietal peritoneum (green) is innervated by somatic and visceral afferent nerves and receives sensitive branches from the lower intercostal nerves and from the upper lumbar nerves. The diaphragm is innervated by the phrenic nerves (center) and by the lower intercostal nerves (periphery). The visceral peritoneum (VP) itself is not innervated, but the sub-mesothelial tissue is innervated by the autonomous nerve system (sympathetic: red; parasympathetic: blue). The visceral sub-mesothelium also receives fibers from the vagal nerve, in addition to the spinal nerves. CP: celiac plexus. SMP: superior mesenteric plexus. IMP: inferior mesenteric plexus.
-
–
stomach, small intestine or caecum: T4 to T12,
-
–
colon: T12 to L2 and L5 to S1,
-
–
mesentery: T5 to T12.
Parasympathetic nervous system
The parasympathetic vagus nerve is the 10th cranial nerve and controls the heart, the lungs and the viscera. The vagus nerve has both motoric (10–20 % of the fibers) and afferent sensitive (80–90 %) functions. Efferent axons from neurons in sacral dorsal root ganglia also follow the parasympathetic pelvic splanchnic nerves to supply the colon and rectum. While sympathetic and parasympathetic pathways overlap for the majority of the length of the bowel, the vagal afferents are thought to signal mainly from the upper gastrointestinal regions and the pelvic afferents from the colorectal region. Vagal afferent neurons project viscerotopically to the brain (precisely into the nucleus of the solitary tract in the medulla oblongata) via the nodose ganglia (reviewed in [2]).
Sympathetic nervous system
Axons from the thoracic and lumbar dorsal root ganglia travel via the sympathetic chain and grow into the digestive organs along the splanchnic nerves. The sympathetic nerves are located along the visceral arteries (not the veins), follow the peritoneal folds and the mesentery and converge into a larger network of nerves located in close vicinity to the abdominal aorta (Figure 2). The sympathetic nerves traverse the celiac plexus (to reach the esophagus, stomach and small intestine), the plexus mesenteric superior (to reach the small bowel) and the plexus mesenteric inferior (to reach the colon).
Figure 2:
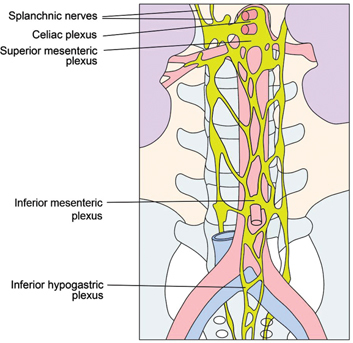
Sympathethical abdominal plexus, lower thoracic and abdominal part.
Adapted from [10].
The splanchnic nerves are paired visceral nerves carrying visceral efferent fibers as well as visceral afferent fibers from the gastrointestinal organs. Splanchnic nerves transport only sympathetic fibers. Pacinian corpuscles and free nerve endings in the walls of the viscera are the splanchnic afferent nerve receptors.
The celiac plexus is the largest of the autonomic plexuses, is situated at the level of the last thoracic and the first lumbar vertebra, and surrounds the celiac artery and the root of the superior mesenteric artery. The plexus is joined by the splanchnic nerves and some filaments from the vagus and phrenic nerves [11].
The lumbar splanchnic nerves originate from the lumbar part (L1 to L3) of the sympathetic trunk and travel to an adjacent plexus near the aorta. They contain preganglionic sympathetic and general visceral afferent fibers. Their synaptic site is located in the inferior mesenteric ganglion. The postsynaptic fibers innervate the smooth muscle and glands of the pelvic viscera including uterus, ovaries and rectum.
Enteric nervous system
In healthy subjects, most visceral afferent activity does not reach higher brain centers, except information regarding filling of the esophagus, stomach, rectum and bladder. In modern medicine, ANS has been complemented by a third anatomical and functional component, the intrinsic enteric nervous system (ENS). ENS is influenced by the sympathetic and parasympathetic nervous systems but can act independently from them. Since ENS regulates the functions of its target organ without input from the CNS, it is also called “the second brain” [12]. The high number of interconnected neurons explains the local autonomy of the gastrointestinal tract which function is preserved even after denervation.
The intrinsic ENS is a complex system of neural circuits controlling multiple functions, including bowel motricity, mucosal secretions, mesenteric blood flow and immune function. ENS consists of a mesh-like system of neurons derived from the neural crest cells [2]. Since the embryonic gut and its appendages arise as midline organs, their splanchnic innervation is bilateral, and accordingly, visceral pain is perceived in the midline.
Most enteric neurons involved in motor functions are located in the myenteric (Auerbach) plexus while many primary afferent neurons are located in the submucous (Meissner’s) plexus (Figure 3). The myenteric plexus provides motor innervation to both layers of the muscular bowel wall and carries both parasympathetic and sympathetic input. In contrast, the submucous plexus has solely parasympathetic fibers and provides secretory and motor innervation to the bowel mucosa. The submucous plexus is also the origin of primary afferent neurons, sensitive to chemical and mechanical stimuli (reviewed in 13). These viscero-sensitive fibers are myelinated and follow the sympathetic nerves to reach the spinal cord through the thoracic dorsal roots.
Figure 3:
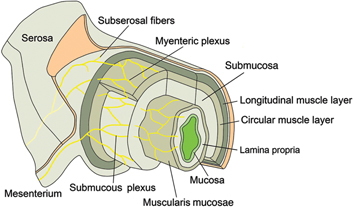
Innervation of the bowel and of the VP.
The myenteric plexus provides motor innervation to both muscular layers of the bowel and carries both parasympathetic and sympathetic input. The submucous plexus has only parasympathetic fibers and provides secretory and motor innervation to the bowel mucosa. Interneurons between both plexus represent the likely functional link between motor, secretory and vasomotor pathways. There are no nerve endings in the VP itself.
The dual projection of some of interneurons to both myenteric and submucous ganglia represents the likely functional link between motor, secretory and vasomotor pathways [14].
Electron microscopy of the myenteric plexus shows that the nerve endings loose their myelin sheath and constitute localized various swellings. These endings contain vesicles with clear or granulose content, which might detect loss of pressure at the surface of the parietal muscle cells. In general, nerve cells are distributed between muscle cells without direct membrane contact (and therefore differ from a synapse). Neurotransmitters are probable secreted by the nerve endings and impregnate diffusely the adjacent tissue. Vegetative nerve fibers have numerous axo-axonal synapses that can bring together sympathetic and parasympathetic axons [10].
Extrinsic innervation
The extrinsic sensory innervation is the information channel between the bowel and the CNS. During embryogenesis, axons from both the nodose ganglia and dorsal root ganglia grow into the gut to form the extrinsic innervation. Once they have reached the enteric organs, vagal sensory axons appropriate enteric cells and grow to provide innervation to four locations [2]:
-
–
Myelinated and unmyelinated fibers terminating in the submesothelial tissue directly under the bowel serosa,
-
–
Intramuscular arrays (IMA) located between the cells of the external muscle, probably stretch receptors,
-
–
Large intraganglionic laminar endings (IGLEs) associated with the connective tissue sheaths of ganglia [17]; tension in the gut wall activates IGLEs [15],
-
–
Sensory endings within the lamina propria of the mucosa; the sensory projections in the lamina propria correspond to functional mucosal receptors.
The extrinsic system consists of vagal and spinal sensory nerves [16], vagal and sacral parasympathetic motor axons, and axons from sympathetic neurons in prevertebral ganglia [17]. Receptors of the extrinsic nerves are sensitive to pressure, friction, cutting and burning and are therefore comparable to cutaneous receptors. Such sensory information is conveyed from the gastrointestinal organs to the CNS by two separate extrinsic pathways:
-
–
the vagus nerve, conveying the information directly from the bowel to the hindbrain,
-
–
spinal afferents transmitting sensory innervation indirectly through the dorsal root ganglia and the spinal cord [2]. Visceral sensory afferents are almost exclusively thinly myelinated Aδ-fibres and unmyelinated C-fibers [18].
The vagal and spinal visceral afferent neurons encode mechanical and chemical stimuli and relay these messages to the CNS. There are three main classes of afferent visceral nerves [19]:
-
–
low-threshold mechano-sensitive afferents responding to distension and contraction and other stimuli. These receptors are intensity encoding and respond to a range of innocuous to noxious stimuli. An important contrast with somatic nociception is the role of low-threshold Aβ-fibers, which only convey innocuous mechanical sensations in normal conditions,
-
–
specific chemo-sensitive afferents (probably only vagal),
-
–
high-threshold mechano-sensitive afferents are found in organs such as the esophagus, colon, ureter and uterus and respond only to noxious mechanical stimuli.
Viscera are also innervated by so-called silent nociceptors (mechanically insensitive afferents, MIAs) that can acquire mechano-sensitivity following inflammation [18].
Visceral nerves can encode sensations and/or reflexes by functionally specific sets of afferents (qualitative response), by intensity-coding of a single set of afferents (quantitative response) or by both, resulting into a wide range of distinct organ regulations. Pain elicited from visceral organs does not require obligatorily the activation of specific sets of “visceral nociceptors” and can be encoded by increased discharge intensity in spinal visceral afferents. Under pathological stress, sensitive pathways can also change their function: for example, chemo-sensitive spinal visceral afferents can be recruited to encode mechanosensitive information [18].
Sensitive visceral neurons project into the laminae I, II and V of the spinal root ganglia (see above). The projections are overlapping several spinal segments and extend medio-lateral over the whole width of the dorsal horn as well as to the contralateral horn. This viscero-sensitive signaling converges with somatic neurons receiving nociceptive input from corresponding dermatomes and myotomes. Therefore, visceral pain is referred to deep somatic tissues, to the skin and to other visceral organs (see below). This referred pain consists of spontaneous pain and mechanical hyperalgesia. These viscera-somatic interconnections can be sensitized or inhibited by spinal and supraspinal control systems, including the CNS. The viscero-somatic tract neurons project through the contralateral ventrolateral tract to the lower and upper brain stem, the hypothalamus and to various cortical areas (reviewed in 20).
Parietal peritoneum
The parietal peritoneum is sensitive to pain, pressure, touch, friction, cutting and temperature. It is innervated by the phrenic nerves and by the sensitive spinal (lower thoracic) viscero-somatic nerves.
The anterior and lateral surfaces of the diaphragmatic peritoneum receive sensitive branches from the intercostal nerves 6–8 [21]. In contrast, the central part of the diaphragm receives a sensitive branch from the phrenic nerve (C3-C5) and has a bilateral innervation by neurons from nodose and cervical dorsal root ganglia [22]. The remaining parietal peritoneum receives sensory nerves only from the (ipsilateral) dorsal root ganglia [23], and not from the vagal nerve. This innervation has the same segmental arrangement as the corresponding dermatomes (T6–T12). The T6 dermatome is in the xyphoid region, the T10 dermatome in the periumbilical region, the T12 dermatome extends in the lower abdomen down to the suprapubic area. Thus, parietal pain can be localized and usually lateralized to the left or to the right. The parietal peritoneum in the pelvis is mainly innervated by the obturator nerve, a branch of the lumbar plexus (L2–L4).
Anatomically, a network of nerve fibers covers the parietal peritoneum, which receives sensitive branches from the lower intercostal nerves and from the upper lumbar nerves. Microscopically, a dense nerve network of myelinated and unmyelinated fibers can be found in the parietal peritoneum. Afferent neural fibers are distributed all over the peritoneal surface, with a density of about 3 per square millimeter (in the rat). These fibers generally run straight and parallel to the intercostal nerves located in the abdominal muscles underlying the peritoneum. The unmyelinated fibers are thin (about 1 μm diameter) and their endings are ending just underneath the peritoneal cell layer. The myelinated fibers are often forming bundles composed of two or three fibers coming in contact with Schwann cells. In their further course, these fibers penetrate the mesothelial cell layer to reach the peritoneal cavity, where they lose their myelin sheath (Figure 4). The club-shaped endings of the nerves are covered with collagen fibers, contain many neurofilaments and a few mitochondria, but no synaptic vesicles. Since the sensory endings are located within the peritoneal cavity, they can enter in contact with peritoneal liquids and cells, and are probably sensitive to somatic or nociceptive stimuli [23].
Figure 4:
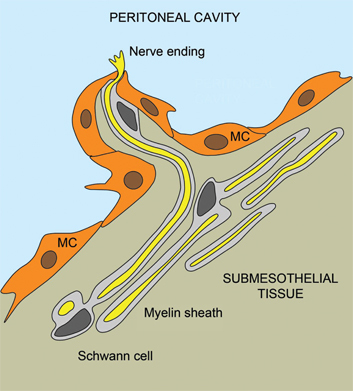
Innervation of the parietal peritoneum.
A schematic drawing of the nerve fibers in the parietal peritoneum. A bundle of myelinated fibers was seen in the subperitoneal space. The nerve fibers (yellow) were encircled with the myelin sheath (gray), having a nucleus of Schwann cell (dark grey). The nerve fibers penetrate the mesothelial cell (MC) layer and reach the peritoneal cavity. Adapted from [23].
Moreover, many nerve fibers in the peritoneum can be stained with antiserum against 200-kD neurofilament (a marker for mechano-sensory neurons) and their terminals are ending in the base of the dorsal horn, which is known to transmit proprioceptive information. Thus, the myelinated nerve network in the parietal peritoneum probably conveys mechanoreceptive information about distension and/or contraction of the abdominal wall [23].
Somatic, chemical or nociceptive stimuli can induce reflex reactions of the abdominal or pelvic muscles leading to involuntarily muscle contraction (e. g. “abdominal guarding” in the presence of bacterial peritonitis, or chronic pelvic pain in the presence of endometriosis).
Visceral peritoneum
The VP is innervated by the autonomous nerve system. Similar to the parietal peritoneum, the VP receives sensory nerves from the spinal nerves. However, in addition to the parietal peritoneum, the VP also receives innervation from the vagal nerve.
In a cadaveric study, unmyelinated nerve fibers were found both in the parietal and in the visceral fascia of the abdomen (defined as the connective tissue lying immediately underneath the mesothelium), whereas myelinated fibers were found only under the visceral mesothelium [24]. These myelinated nerve fibers are probably mechano-sensory neurons since, in the cat, they can be stained by an antiserum against the 200-kD neurofilament. Since this neurofilament is a marker for mechanoreceptors, the myelinated nerve network probably conveys information about distension and/or contraction of the peritoneum [23]. Clinical observations during hernia surgery in local anesthesia confirm that the VP is sensitive to stretching and tearing. Overdistension of a viscus leads to the sensation of pain. This was already documented by Lennander in 1906, who observed in the human patient that the VP is sensitive to strain, shift and stretching, but not to touch, pressure and thermal stimuli. Electric stimulation of the bowel serosa caused major contractions of the bowel, color fading/whitening but no pain [25].
Neurotransmitters
Chemical mediators of the ENS are acetylcholine, VIP, serotonin, nitric oxide (NO), encephalins, calcitonin gene-related peptide (CGRP) and substance P. Acetylcholine is an ester of acetic acid and choline and plays an important role both as an internal transmitter for the sympathetic nervous system and as the final product released by the parasympathetic nervous system [27]. Vasoactive intestinal peptide (VIP) is a 28 amino-acids peptide hormone belonging to the glucagon/secretin superfamily and is the ligand of class II G protein-coupled receptors [28]. Serotonin (5-hydroxytryptamin (5-HT) or enteramin) is an amine found in various organs, mostly (95 %) in the gastrointestinal tract, out of which 90 % in the enterochromaffin cells (as a tissue hormone) and 10 % in the nerve cells (as a neurotransmitter) [29]. Nitric oxide, a chemical compound (NO), is an important signaling molecule involved in many physiological and pathological processes, including vasodilation and neurotransmission. In various parts of the gastrointestinal tract, NO is an important non-adrenergic, non-cholinergic (NANC) neurotransmitter [29]. Encephalin is a peptide binding to the body’s opioid receptors and regulating nociception [30]. CGRP is a 34 amino-acids peptide produced in peripheral and central neurons and can function in the transmission of pain [31]. SP is an 11 amino-acids neuropeptide acting as a neurotransmitter and as a neuromodulator: release of SP is involved in neurogenic inflammation, which is a local inflammatory response to pathological stimuli [32].
Nerves in peritoneal adhesions
Both in the murine model [33] and in the human patient, myelinated and non-myelinated nerve fibers accompanied by Schwann cells were found to develop within peritoneal adhesions. These fibers were more abundant in the abdominal than in pelvic adhesions. Immunostaining was positive for all the neuronal markers examined, including synaptophysin, VIP and TH. Some adhesions displayed CGRP and SP, which are known as sensory nerve markers. Most nerve fibers were associated with blood vessels, were arranged parallel to the longitudinal axis of the adhesion, but had a random orientation with respect to the peritoneal surface [34].
Visceral pain
Visceral pain represents a major clinical problem, yet far less is known about its mechanisms compared to somatic pains, e. g. from cutaneous and muscular structures [18]. Visceral pain and discomfort are associated with excitation of thoracolumbar and sacral visceral afferents but usually not by the excitation of vagal afferents. Spinal visceral afferents are multimodal and can be activated by adequate mechanical and chemical stimuli.
Differences arise in the innervations of the visceral and parietal peritoneum, leading to differing patterns of sensation of painful stimuli. The VP receives its innervation from the ANS and responds primarily to traction and pressure or distension; painful stimuli are perceived as a poorly localized, dull pain. Pain in a foregut structure (stomach, duodenum or biliary tract) is referred to the upper abdomen (epigastric), pain in a midgut structure (appendix, jejunum, or ileum) to the periumbilical area and pain from a hindgut source (distal colon or rectum) is referred to the lower abdomen or suprapubic region. In contrast, the parietal peritoneum is innervated by both somatic and visceral afferent nerves. Therefore, noxious stimuli are perceived as a localized, sharp pain with rebound tenderness and are referred to as “peritonitis.” [35].
In the dorsal root ganglia, the splanchnic (intrinsic) and cerebrospinal (extrinsic) cell bodies are side by side. Their proximal fibers also terminate in close proximity within the spinal cord. The close relationship of these anatomic pathways may account for the fact that severe visceral pain, such as rapid distention of a viscus, may “spill over” into somatic segments (viscero-sensory and viscero-motor reflexes) in the absence of somatic nerve irritation [23]. Thus, disease of a visceral organ can cause pain or hyperesthesia in determined cutaneous areas. These remote cutaneous zones developing tenderness during visceral diseases (allodynia) have been described in the 1890s by Sir Henry Head, who also emphasized the existence of specific points within these zones, the “maximum points” [36]. These zones have approximately the same localization as the dermatomes which dorsal roots content the viscero-sensitive nervous fibers of the organ affected. Interestingly, this early clinical knowledge has been largely forgotten in Western medicine, but is actively used in Traditional Chinese Medicine (TCM).
Depending on the type of stimulus condition, different neural pathways are involved in chronic pain. Often pain overlaps between two organs because of dichotomy of primary afferent fibers innervating two pelvic organs and common convergence of two afferent fibers onto a spinal dorsal horn.
Visceral hypersensitivity can occur due to:
-
–
sensitization of primary sensory afferents innervating the viscera (e. g. by inflammation) or by recruitment of silent mechanosensitive afferents [20]
-
–
hyper-excitability of spinal ascending neurons (central sensitization) receiving synaptic input from the viscera, and
-
–
dysregulation of descending pathways that modulate spinal nociceptive transmission.
Examples of abdominal neurogenic inflammation are chronic pancreatitis pain or chronic pelvic pain in endometriosis. In both instances, neurogenic inflammation and development of inflammatory hyperalgesia probably require visceral afferents expressing transient receptor potential (TRP) channels TRPV1 and TRPA1 [37].
In endometriosis, release of inflammatory cytokines (in particular interleukins and prostaglandins) and nerve growth factors (NGF, NT-3) lead to a neogenesis of sensitive nerve fibers within the submesothelial tissue. The neurotransmitters SP and CGRP are liberated, leading to a local vasodilation, to chemotaxis of immune effector cells and to a further activation of inflammatory cytokines. Simultaneously, there is an increased local release of estrogens and semaphorine, an axonal growth cone guidance protein secreted by macrophages and acting as a short-range inhibitor. This leads to a reduction in the number of sympathetic nerve fibers (syNE) and to a depletion in sympathetic neurotransmitters, in particular norepinephrine, which act rather anti-inflammatory. Altogether, this process entertains chronic inflammation in the sub-mesothelial tissue. In addition, silent pain fibers (C-fibers) are activated through a local sensitization and carry the pain stimulus to the dorsal root ganglia. This activation of C-fibers appears to be enhanced by TRPV1, which is hyper-regulated in women with severe chronic pain [38]. TRPA1 and TRPV1 might also play an important role in the development of chemotherapy-related neuropathic pain [39].
Visceral pain is usually accompanied by autonomic and somatomotor reflexes, and associated with strong negative affective feelings. Visceral pain is primarily represented in the posterior dorsal insular cortex (primary interoceptive cortex). This cortex receives in primates its spinal synaptic inputs mainly from lamina I tract neurons (see above). The transmission of activity from visceral afferents to second-order spinal neurons is under control of endogenous anti- and pronociceptive control systems in the lower, upper brain stem and in the cerebral cortex (reviewed in [20]).
Conclusion
Modern publications on the innervation of the parietal and of the VP are relatively rare. The specific role of the peritoneum in visceral pain has not been defined precisely, although peritonitis or metastatic tumor invasion is life-threatening diseases. Neurogenic inflammation of the peritoneum, caused for example by endometriosis or by peritoneal adhesions, can also cause long-lasting, disabling symptoms. Further research in this area is needed.
Footnotes
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- 1.Langley JN. The autonomic nervous system, Part 1. W. Heffer. Cambridge, 1921.
- 2.Ratcliffe EM. Molecular development of the extrinsic sensory innervation of the gastrointestinal tract. Auton Neurosci. 2011;161:1–5. [DOI] [PMC free article] [PubMed]
- 3.Vater A. „De consensu partrium corporis humani“. Lerhmann (Eds.). Wittenberg, 1741.
- 4.Meissner G. Über die Nerven der Darmwand. Z Ration Med N F. 1857;8:364–66.
- 5.Auerbach L. Über einen Plexus myentericus. Morgenstern, editor. Breslau, 1862.
- 6.Ranvier LA. Recherches sur l’histologie et la physiologie des nerfs. Arch Physiol Norm Pathol. 1872;4:129–49.
- 7.Hirschsprung H. Stuhlträgheit Neugeborener in Folge von Dilatation und Hypertrophie des Colons. Jahrbuch Für Kinderheilkunde Und Physische Erziehung. Berlin. 1888;27:1–7.
- 8.Robinson B. The peritoneum. Keener WT, editor. Chicago, 1897:13.
- 9.Tanaka K, Matsugami T, Chiba T. The origin of sensory innervation of the peritoneum in the rat. Anat Embryol (Berl). 2002;205:307–13. [DOI] [PubMed]
- 10.Kahle W, Leonhardt H, Platzer W. Taschenatlas der Anatomie für Studium und Praxis, Bd. 3. Stuttgart: Thieme, 1998.
- 11.Williams PL, Warwick R, editors. Gray’s anatomy, 36th ed. Edinburgh: Churchill Livingstone, 1980:1132–34.
- 12.Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005;39:S184–93. [DOI] [PubMed]
- 13.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. [DOI] [PubMed]
- 14.Costa M, Brookes SJH, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut. 2000;47:15–19. [DOI] [PMC free article] [PubMed]
- 15.Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–68. [DOI] [PMC free article] [PubMed]
- 16.Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. [DOI] [PubMed]
- 17.Langley JN. The autonomic nervous system. Part 1. W. Heffer (Eds.). Cambridge, 1921.
- 18.Sikandar S, Dickenson AH. Visceral pain: the ins and outs, the ups and downs. Curr Opin Support Palliat Care. 2012;6:17–26. [DOI] [PMC free article] [PubMed]
- 19.Jänig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations [review]. Biol Psychol. 1996;42:29–51. [DOI] [PubMed]
- 20.Jänig W. Neurobiology of visceral pain. Schmerz. 2014;28:233–51. [DOI] [PubMed]
- 21.Körte W. Die Chirurgie des Peritoneums. Enke, editor. Stuttgart, 1927:16–19.
- 22.Tanaka K, Hayakawa T, Maeda S, Kuwahara-Otani S, Seki M. Distribution and ultrastructure of afferent fibers in the parietal peritoneum of the rat. Anat Rec (Hoboken). 2011;294:1736–42. [DOI] [PubMed]
- 23.Tanaka K, Kuwahara-Otani S, Maeda S, Minato Y, Yagi H. Possible role of the myelinated neural network in the parietal peritoneum in rats as a mechanoreceptor. Anat Rec (Hoboken). 2017;300:1662–69. [DOI] [PubMed]
- 24.Stecco C, Sfriso MM, Porzionato A, Rambaldo A, Albertin G, Macchi V, et al. Microscopic anatomy of the visceral fasciae. J Anat. 2017;231:121–28. [DOI] [PMC free article] [PubMed]
- 25.Lennander KG. Sensibilität der Bauchhöhle. Mitt Grenzgeb Med Chir. 1902;15:465.
- 26.Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci. 2000;915: 77–80. Review. [DOI] [PubMed]
- 27.Spohn SN, Mawe GM. Non-conventional features of peripheral serotonin signalling – the gut and beyond. Nat Rev Gastroenterol Hepatol. 2017;14:412–20. [DOI] [PMC free article] [PubMed]
- 28.Galligan JJ, Akbarali HI. Molecular physiology of enteric opioid receptors. Am J Gastroenterol Suppl. 2014;2:17–21. [DOI] [PMC free article] [PubMed]
- 29.Corazziari E. Role of opioid ligands in the irritable bowel syndrome [review]. Can J Gastroenterol. 1999;13 Suppl A:71A–5A. [DOI] [PubMed]
- 30.Bartho L, Benko R, Holzer-Petsche U, Holzer P, Undi S, Wolf M. Role of extrinsic afferent neurons in gastrointestinal motility. Eur Rev Med Pharmacol Sci. 2008;12 Suppl 1:21–31. Review. [PubMed]
- 31.Mashaghi A, Marmalidou A, Tehrani M, Grace PM, Pothoulakis C, Dana R. Neuropeptide substance P and the immune response. Cell Mol Life Sci. 2016;73:4249–64. [DOI] [PMC free article] [PubMed]
- 32.Sulaiman H, Gabella G, Davis C, Mutsaers SE, Boulos P, Laurent GJ, et al. Growth of nerve fibres into murine peritoneal adhesions. J Pathol. 2000;192:396–403. [DOI] [PubMed]
- 33.Sulaiman H, Gabella G, Davis C, Mutsaers SE, Boulos P, Laurent GJ, et al. Presence and distribution of sensory nerve fibers in human peritoneal adhesions. Ann Surg. 2001;234:256–61. [DOI] [PMC free article] [PubMed]
- 34.Robinson DR, Gebhart GF. Inside information: the unique features of visceral sensation. Mol Interv. 2008;8:242–53. [DOI] [PMC free article] [PubMed]
- 35.Beissner F, Henke C, Unschuld PU. Forgotten features of head zones and their relation to diagnostically relevant acupuncture points. Evid Based Complement Alternat Med. 2011;2011:240653. Epub 2010 Oct 19. DOI: 10.1093/ecam/nen088. [DOI] [PMC free article] [PubMed]
- 36.Schwartz ES, La JH, Scheff NN, Davis BM, Albers KM, Gebhart GF. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci. 2013;33:5603–11. [DOI] [PMC free article] [PubMed]
- 37.Mechsner S. Endometriose das verkannte Frauenleiden. Untersuchungen zum Verständnis der Pathogenese und der Schmerzentstehung. Habilitationsschrift. Berlin: Charité; 2010.
- 38.Billeter AT, Hellmann JL, Bhatnagar A, Polk HC, Jr. Transient receptor potential ion channels: powerful regulators of cell function. Ann Surg. 2014;259:229–35. [DOI] [PubMed]


