Abstract
Wolf spiders are abundant and voracious predators at the soil-plant interface in cotton crops. Among other prey, they attack late-instar larvae of the cotton bollworm Helicoverpa spp., an economically important pest. Consequently, wolf spiders in transgenic Bt cotton could provide significant biological control of Bt-resistant Helicoverpa larvae that descend to the soil to pupate. The predator-prey interactions between wolf spiders and Helicoverpa could, however, be constrained by the presence of alternative prey and intraguild predators. This study used laboratory enclosures to analyse the effect of alternative prey on predatory selection of the wolf spider Tasmanicosa leuckartii Thorell. The prey included another wolf spider Hogna crispipes Koch (potential intraguild predator), the ground cricket Teleogryllus commodus Walker (minor pest), and Helicoverpa armigera larvae (major pest). We tested if encounter rates, prey vulnerability, and prey nutritional content influenced the likelihood that a prey was attacked. In three-way food webs, Tasmanicosa encountered and attacked Teleogryllus and Helicoverpa in similar frequencies. However, in the presence of a competing intraguild predator and potential prey (Hogna) in a four-way food web, Tasmanicosa did not always attack Teleogryllus at first encounter, but still attacked Helicoverpa at each encounter. Helicoverpa (protein-poor) and Hogna (protein-rich) were consumed by Tasmanicosa in similar proportions, suggesting that Tasmanicosa might benefit from nutrient balance as an outcome of diverse prey in this food web. As Teleogryllus (protein rich) escapes quicker than Helicoverpa and Hogna, Hogna may be an easier protein-rich option than Teleogryllus. Field surveys showed that while Teleogryllus was the most common prey, wolf spiders feed on diverse insect taxa, as well as other spiders. That Tasmanicosa readily attacked Helicoverpa larvae in the presence of alternative prey is an encouraging result that supports the potential of Tasmanicosa predation to assist in the control of Bt-resistant Helicoverpa larvae and thereby inhibit the proliferation and spread of resistance.
Introduction
Interactions between a predator and its prey rarely occur in isolation. Instead, predatory interactions usually occur within food webs that also involve primary producers, alternative prey, and other predators. When multiple prey are present, predation outcomes may be driven by prey availability (passive selection) or by predator-prey interactions upon prey encounter [1]. Variables influencing passive selection of prey include abundance, prey dispersion [2] and camouflage [3]. After encounter, predation outcomes may be determined by the prey’s ability to escape [4, 5], prey defences [5], prey size [6–8], predator hunger state [4, 9], or prey nutritional content [10, 11]. Predators may not attack prey that are difficult to catch and subdue, or impose risk of injury or toxicity. Optimal foraging theory combines these factors on predation outcomes, and posits that after predators encounter prey they should aim to maximize their energy intake [12] while minimizing the risks and energy spent in prey capture [13]. Understanding the mechanisms that mediate predation tendencies is crucial to predict the structure and function of food webs.
In agricultural food webs, generalist predators do not target pest prey exclusively, yet these predators are still an important component of integrated pest management [14]. Prey diversity can be essential to sustain generalist predator populations; when pest densities are low, predators may rely on alternative prey to sustain growth and survival, allowing the predator population to be maintained [15]. In some cases, a diet consisting of only single pest prey may be nutritionally deficient and can drastically reduce the survival of generalist predators [16]. As a result, foraging on diverse prey may enable predators to balance nutrient intake [17–19]. Alternative prey therefore may be essential for predators to thrive in agricultural systems. Because the presence of alternative prey may influence predator-prey interactions and food web dynamics, it is important to understand how the presence of alternative prey might affect the biological control of pest species.
Abundance of alternative prey may influence whether a generalist predator feeds on a particular pest predominantly or switches prey preference [20]. Interference of the biological control of pests due to the presence of alternative prey has been reported in several studies; for example, in the presence of Collembola (alternative prey), spiders may kill fewer pest aphids [15, 21, 22]. In other cases, generalist predators can still effectively suppress pest populations despite the availability of diverse prey. For example, the wolf spider Hogna sp. continues feeding on cucurbit pests, even when alternative prey are available [23]. Predators may also respond differently to different types of alternative prey. For instance, the wolf spider Pardosa prativaga consumes fewer aphids when vinegar flies (Drosophila spp.) are available, but does not change its predation rate on aphids when collembola are available [24]. The interactions between generalist predators, pest prey, and alternative prey are difficult to predict and vary among species and agricultural systems.
In cotton agroecosystems, biological control by predators can help suppress pest populations. For example, a predator complex of spiders, predacious bugs, lacewings, and predacious beetles are important for the biocontrol of whitefly Bemisia tabaci, and the mirid bug Lygus [25–27]. Similarly, the ladybeetle Coccinella septempunctata can be a key factor to supress populations of cotton aphids [28]. The bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae; referred to hereafter as Helicoverpa) is a key pest of particular importance in cotton [29]. Genetically modified Bt-cotton produces toxins that destroy the gut lining of Lepidoptera, and was introduced to control the larvae of Helicoverpa spp. [30, 31]. However, the long term viability of Bt cotton is constantly threatened by the potential of Helicoverpa to develop resistance to Bt-toxins [32]. Bt-resistant larvae, like all Helicoverpa, descend from the cotton plants to pupate underground, and later emerge as moths that convey resistance traits to the next generation. After foraging on cotton, these larvae are exposed to predators on the plant-soil interface. By killing larvae of Helicoverpa as they descend to pupate, or later as they emerge as moths, ground predators present in cotton crops can inhibit the proliferation of Bt-resistant genes in Helicoverpa populations, as it has been shown with other Lepidoptera pests [33]. Because Bt cotton fields require fewer insecticide spray applications than conventional cotton, they can harbour a diverse and sizeable arthropod community [34, 35]. This arthropod diversity may serve as alternative prey to sustain predator populations in Bt cotton fields.
In the present study, we examine the predator-prey interactions of four arthropods commonly found on the soil surface of cotton fields in New South Wales, Australia. Wolf spiders (Araneae: Lycosidae) are commonly classified as generalist predators, and will readily accept crickets, Lepidoptera larvae, and other wolf spiders as prey [36]. The wolf spiders Tasmanicosa leuckartii (Thorell) and Hogna crispipes Koch; (referred to hereafter as Tasmanicosa and Hogna) are abundant in cotton fields, with Hogna representing approximately 35% and Tasmanicosa representing approximately 12% of the wolf spider community [37]. Both spider species attack late-instar Helicoverpa larvae as they descend from the plant to pupate in the soil, and emerging adults on the soil [38, 39]. The ground cricket Teleogryllus commodus Walker (Orthoptera: Gryllidae; referred to hereafter as Teleogryllus) is also commonly found in cotton fields, and has been reported to be an occasional pest in cotton when they are abundant. Adults and late-instar nymphs can be early-season pests, as they feed on the leaves and stems of cotton seedlings [40]. This ground food web includes therefore two abundant predators in Bt cotton fields (Tasmanicosa and Hogna), an abundant but economically minor cotton pest (Teleogryllus), and a less abundant, yet economically important cotton pest (Helicoverpa).
Here, we evaluate how interactions between Tasmanicosa, Hogna, Teleogryllus and Helicoverpa influence predation outcomes. First, we test the hull hypothesis that prey attack probability is independent of prey first encounter. If Tasmanicosa is a generalist predator in this food web with no prey preference for Hogna, Teleogryllus or Helicoverpa, we then expect that the first prey encountered will be the first prey attacked. Additionally, we investigate whether prey protein and lipid contents of Hogna, Teleogryllus, and Helicoverpa influence prey selection in Tasmanicosa.
Materials and methods
Collection of spiders and Teleogryllus
This study was carried out at the Australian Cotton Research Institute (ACRI; 33°S, 149°E), near Narrabri, New South Wales, Australia. Adult males, females and late-instar juveniles of the wolf spiders Tasmanicosa (cephalothorax width = 7.1 ± 1.2 mm; mean ± SD); and Hogna (cephalothorax width 5.7 ± 1.1 mm; mean ± SD) were collected in and around Bt-cotton fields after sunset from December until March 2014. Because Teleogryllus nymphs shelter in soil cracks and are difficult to collect from cotton fields, Teleogryllus nymphs (body length = 12.8 ± 2.4 mm, mean ± SD) were collected around the buildings at ACRI. Spiders and crickets were found by visual search using a headlamp (Petzl Tikka, 140 lumens), and collected manually in clear 70 ml cylindrical plastic containers. After collecting, all spiders and crickets were brought to the laboratory. Spider cephalothorax width and cricket body length were measured to the nearest 0.01 cm using a manual caliper (resolution 0.01 cm). Each spider and cricket was weighed to the nearest 0.01 g using a digital scale (Sartorius model A200S, Gottingen, Germany).
Collected spiders were housed in clear plastic containers (220 mm height x 228 mm length x 228 mm width, 8.5 L, Décor Tellfresh superstorer, NSW, Australia; referred to hereafter as ‘spider container’) containing 2 L of moist soil, and were kept in a controlled environment room (24.4 ± 0.5 oC, mean ± SD) with a L14:D10 photoperiod. Each container had a retreat in each of two opposite corners comprising a hole in the soil approximately 2 cm deep and 1 cm diameter. The retreat was partially covered by a 3 x 3 cm sheet cut from the bark of a ‘Paper Bark tree’ (Melaleuca sp.). All spiders were isolated by placing an opaque PVC pipe (100 mm diameter x 150 mm height) over each spider’s retreat until experiments started. Teleogryllus were kept in the same 70mL plastic clear containers used for collection. Each spider and Teleogryllus were kept in the laboratory in their respective containers for ~ 20 h before being used in food web experiments, and during this period no food or water were supplied. All alive spiders and crickets were released in the fields after trials.
Helicoverpa predation
This experiment assessed whether Tasmanicosa kills both Helicoverpa larvae reared in artificial diet (used in food web experiments) and larvae reared in cotton plants (field scenario). Larvae of Helicoverpa armigera were supplied by the Commonwealth Scientific and Industrial Research Organization (CSIRO) Agriculture Flagship Bt Resistance Monitoring Group. Larvae were reared in individual wells in trays with soy and agar diet [41, 42]; referred to as ‘diet-reared’ larvae), and kept in a controlled environment room (24.4 ± 0.5 oC, mean ± SD) with a L14:D10 photoperiod. A separate group of larvae were reared from neonates on plant material (referred to as ‘plant-reared’ larvae). Plant-reared larvae were placed in individual wells in trays containing a mixture of cotton leaves, flowers, and squares (Sicot 71 conventional, non-Bt, RRF). Larvae were transferred into new trays with fresh plant material every two days. Both diet-reared and plant-reared larvae were maintained until they reached 5th instar (the last instar on the plant before burrowing underground to pupate) and were then weighed to the nearest 0.01 g (body weight = 0.40 ± 0.07 g, mean ± SD) using a digital scale (Sartorius model A200S).
To assess whether spiders kill both plant-reared and diet-reared larvae, one Tasmanicosa was paired simultaneously with one diet-reared 5th instar larva and one plant-reared 5th instar larva (N = 10). To distinguish larvae, both were marked with different colour dyes (HCA Colours, Kingsgrove NSW, Australia; VM311 Pink and VM315 Orange), alternating colours in different trials (so that five plant-reared larvae and five diet-reared larvae had pink dye, and five of each had orange dye). Larval mortality from predation was recorded after 24 h.
First encounter and first attack in three-way and four-way food webs
To determine the effect of alternative prey and intraguild predators on the first prey attacked by Tasmanicosa, three-way (Helicoverpa–Teleogryllus–Tasmanicosa; N = 27) and four-way (Helicoverpa–Teleogryllus–Hogna–Tasmanicosa; N = 23) food webs were set up in which animals were present together in the same spider container. All food web experiments were carried out in the same controlled environment room described above (see Collection of spiders and Teleogryllus). Due to the high mortality and low sample size of plant-reared Helicoverpa larvae, all food web experiments were carried out using diet-reared Helicoverpa larvae. Approximately 30 minutes after the dark phase commenced in the controlled environment room, one 5th instar Helicoverpa larva and one Teleogryllus nymph were placed inside a container housing either (1) one Tasmanicosa; or (2) one Tasmanicosa and one Hogna. Because we were interested in analysing the predatory behaviour of Tasmanicosa as a focal predator, Hogna was always smaller than Tasmanicosa to avoid bi-directional intraguild predation. PVC pipes were immediately removed to allow spiders to explore and hunt. Continuous video recording began immediately after Helicoverpa and Teleogryllus were released, and ended 24 h later. The recording system comprised a 1/3” CCD monochromatic infra-red camera (CCS- Sony Go Video, Sony) with a 4 mm C mount lens positioned above each container, which recorded to a 2TB DVR4-100 hard drive recorder. One infrared illuminator (IR-covert, 940 nm) was placed 10 cm to the side of each spider container. To improve video contrast, each animal was dusted with fluorescent dye (HCA Colours Australia, Kingsgrove, NSW; VM311 Pink for Helicoverpa larvae and Teleogryllus, VM315 for Hogna, and VM317 Yellow for Tasmanicosa). A previous study showed that dust dyes did not affect predatory behaviour [39]. For each trial, we recorded which prey item was first encountered (physical contact between predator and prey) and first attacked by Tasmanicosa (the spider lunging towards the prey).
Nutritional analysis
To determine the relationship between prey protein and lipid content, and predation outcomes in food webs, a protein and lipid analysis was carried out in Tasmanicosa, Hogna, Teleogryllus and Helicoverpa. Because spiders were caught from the field 24 h before experiments and were not offered prey before trials, we made the assumption that spiders were responding to the Helicoverpa offered to them as if the prey were sourced from the field, and would not respond differently to a diet-reared Helicoverpa. Therefore, we assumed that any link between predation outcomes and nutrient content would be based on the typical protein and lipid contents of prey in the field. Juvenile Tasmanicosa (N = 8), juvenile Hogna (N = 12), field-collected Teleogryllus (N = 12), diet-reared Helicoverpa (N = 21), and plant-reared Helicoverpa (N = 12) were collected for nutritional analysis. Tasmanicosa, Hogna and Teleogryllus were collected from the same fields described above, and immediately frozen at -20°C for nutritional analysis. Diet-reared and plant-reared Helicoverpa larvae were reared as described above, and frozen at -20°C once they reached 5th instar.
Protein and lipid analyses were performed following protocols previously tested in arthropods [43, 44]. After freezing, each arthropod was dried in an oven at 60 oC for 48 h before lipid and protein analysis. Lipid content was measured gravimetrically by submerging each dried arthropod in chloroform for 24 h, discarding the chloroform, repeating for another 24 h, and then drying again. The lipid content was estimated by taking the difference in the dry weight of samples before and after soaking them in chloroform. Protein was extracted from ground sub-samples (3–5 mg) using 0.1 M NaOH and heat (90°C for 30 minutes) after which samples were centrifuged and the supernatant was collected for analysis. Protein content was then measured using the Bradford Assay modified for use in 96 well microplates following manufacturer’s instructions (protein assay kit #500–001, Bio-Rad, Hercules CA, USA). We analysed each sample in triplicate and all samples were run together on the same plate reader (Biotek EL808, Vinooski, VT, USA) with a calibration curve created using a protein standard (bovine gamma globulin, Bio-Rad #500–001).
Wolf spider prey in cotton fields
A field survey was carried out to determine common prey of wolf spiders in a mixed cotton field. This survey was carried out between 26 February and 7 March 2015, between the ‘peak flower’ and ‘open boll’ stage of cotton growth [45], which is the period when adult wolf spiders are most abundant in cotton fields at ACRI [37]. Surveys were carried out on dry nights, as wolf spiders remain in their burrows during rain (personal observation). Visual surveys took place around the edge of a triangular shaped cotton field 240m wide (= 240 rows: 1 cotton row/ m) with row lengths ranging from 60 m to 160 m. This mixed cotton field comprised Bt, non-Bt, and pigeon pea refuge plantings. Even though wolf spiders are abundant at this stage of the season, the plant canopy already covered the plot rows, precluding visual search within the crop. Therefore, only the 3 m around the edges of the plant rows of the field were surveyed for wolf spiders for 2hrs after sunset. Nocturnal wolf spiders hunt more actively immediately after sunset, and feeding tends to decline over the following hours [46]. During surveys, for every spider with a cephalothorax width greater than approximately 3.5mm we recorded its species, and whether it was holding a prey in its chelicerae. Because juvenile Tasmanicosa and Hogna can have similar cephalothorax patterns and are difficult to differentiate in the field, all juvenile spiders without a characteristic adult or subadult pattern were classified as “other Lycosidae”. All spiders holding a prey were captured and taken to the laboratory for prey identification.
Statistical analysis
A test of independence with post-hoc adjusted residual z values was used to determine if frequencies of first attack were independent of first encounter for Tasmanicosa towards Hogna, Teleogryllus and Helicoverpa. Percent protein and percent lipid in arthropods were analysed for normality using a Shapiro-Wilk test, log-transformed where necessary and three extreme outlier values were removed (based on biologically unrealistic protein values that could only reflect errors) to meet the assumptions of parametric testing. A multivariate analysis of variance (MANOVA) was used to test for differences in percent lipid and protein between diet-reared Helicoverpa, plant-reared Helicoverpa, field-collected Teleogryllus, and field- collected Hogna and Tasmanicosa, using dry body mass as a covariate. Post-hoc least significant differences (LSD) were carried out to determine differences in percent lipid and percent protein separately between each arthropod. All statistical analyses were carried out using SPSS [47].
Ethics statements
Animal research: no humans, vertebrates, or cephalodods were used in this study
Field studies: Prey field observation and collection was done at the Australian Cotton Research Institute; no permits were required for CSIRO staff (M. Whitehouse and D. Rendon).
Results
Helicoverpa predation
At the end of Helicoverpa predation trials, all spiders had killed both the plant-reared and the diet-reared larvae. In six out of ten trials, Tasmanicosa killed plant-reared Helicoverpa first (regardless of coloured dye dust), no larvae were rejected, and both Helicoverpa larvae were completely consumed.
First encounter and first attack in three-way and four-way food webs
In three-way food webs, there were no differences in the proportions of first encounters between Tasmanicosa and Teleogryllus or Helicoverpa (Pearson χ2 = 0.30, df = 1, p = 0.58; Fig 1). Frequency of first attack was not random, and was associated with frequency of first encounter (χ2 = 11.81, df = 1, p < 0.01; Fig 1); Tasmanicosa attacked more frequently the first prey encountered, regardless of whether it was Teleogryllus or Helicoverpa. After being first encountered, 90% of Helicoverpa (N = 10) and 75% of Teleogryllus (N = 12) were immediately attacked by Tasmanicosa.
Fig 1. Percentages of first encounter (dashed arrows) and first attack (solid arrows) of Tasmanicosa in a three-way videorecorded food web arena.
Direction of arrows point to the predator that made the encounter/attack, thicker arrows represent higher proportions. Percentages indicate cases in which prey was encountered first (out of n = 27), or attacked first (out of n = 27).
Similarly, in four-way food webs, there were no differences in the proportions of first encounters between Tasmanicosa and Hogna, Teleogryllus, or Helicoverpa (Pearson χ2 = 2.40, df = 2, p = 0.30, cases in contingency table = 40; Fig 2). Hogna attacked and killed Teleogryllus and Helicoverpa each 34.7% of the time, but this did not affect the proportion of first encounter of Telegogryllus and Helicoverpa by Tasmanicosa. Frequency of Tasmanicosa first attack was not random, and was associated with frequency of first encounter (χ2 = 11.35, df = 4, p = 0.023; Fig 2). Post-hoc tests revealed that Helicoverpa and Hogna were often attacked first by Tasmanicosa, even when they were not the first prey encountered (all z < 1.96), while Teleogryllus was not always attacked when it was encountered first (adjusted residual z = 2.9).
Fig 2. Percentages of first encounter (dashed arrows) and first attack (solid arrows) of Tasmanicosa and Hogna in a four-way videorecorded food web arena.
Direction of arrows point to the predator that made the encounter/attack, thicker arrows represent higher proportions. Percentages indicate cases in which prey was encountered first (out of n = 23), or attacked first (out of n = 23).
No prey escaped a spider attack, and all attacks resulted in the spider completely consuming the prey. When attacked by a spider, Helicoverpa displayed behaviours such as biting, bobbing its body from side to side, ejecting faeces and regurgitating. Larger Helicoverpa larvae sometimes lifted an attacking spider off the ground. None of these Helicoverpa behaviours were life threatening for a spider, and no spider died from attacks on Helicoverpa, or retreated after initiating attack. When in contact with or being seized by a spider, Teleogryllus exhibited behaviours such as kicking and head-butting. No spiders died from injuries inflicted by Teleogryllus. Teleogryllus usually jumped away when coming into contact with a spider, or even when a spider moved within a few centimetres. Hogna usually ran away after detecting an approaching Tasmanicosa, but never counter-attacked or bit, and were quickly seized by larger spiders. No Tasmanicosa died from attacking Hogna.
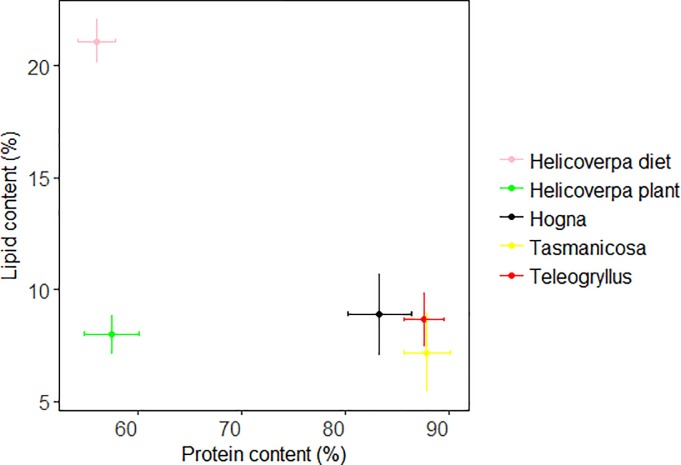
Lipid and protein content
Comparing protein and lipid contents of all arthropods, we found that protein content was lowest in plant-reared Helicoverpa, and was similar among field-collected Teleogryllus, Hogna and Tasmanicosa. (Wilks Lambda = 0.07, F = 32.69, df = 3, 39, p<0.01, post-hoc LSD; Fig 3). Lipid contents were not different among plant-reared Helicoverpa, and field-collected Teleogryllus, Hogna and Tasmanicosa (Wilks Lambda = 0.07, F = 1.02, df = 3, 39, p = 0.39).
Fig 3. Percent body content of protein and lipid in four arthropods (mean and SD), caught near cotton fields, reared on cotton plant material, or reared on soy and agar diet.
Diet changed lipid but not protein contents in Helicoverpa; diet-reared Helicoverpa had higher lipid content than plant-reared Helicoverpa (Wilks’ Lambda = 0.19, F = 91.13, df = 1, 31, p < 0.01; Fig 3), whereas diet-reared Helicoverpa had similar protein content as plant-reared Helicoverpa (Wilks’ Lambda = 0.19, F = 0.23, df = 1, 31, p = 0.63).
Wolf spider prey in cotton fields
A total of 597 wolf spiders were observed in the fallow 3 m beyond the plant rows of the cotton fields, and 18 spiders were recorded holding prey (3.0%; Table 1). Some prey items (including Lepidoptera) could not be identified to species because they had been masticated by the spider. The most common prey was the ground cricket Teleogryllus commodus (33% of all prey observed). Two spiders were observed consuming another spider, confirming the occurrence of intraguild predation in the field.
Table 1. Wolf spider prey observed in visual surveys around edges of cotton fields.
|
Tasmanicosa leuckartii (N = 114) |
Hogna crispipes (N = 90) |
Other Lycosidae (N = 393) |
|
|---|---|---|---|
| Orthoptera | |||
| Gryllidae: Teleogryllus commodus | 0 | 1 | 5 |
| Dermaptera | |||
| Labiduridae: Labidura truncate | 1 | 0 | 2 |
| Lepidoptera moth | 0 | 0 | 2 |
| Lepidoptera caterpillar | 0 | 1 | 0 |
| Lycosidae | 0 | 0 | 2 |
| Coleoptera | |||
| Scarabeidae: Mimadoretus sp. |
0 | 0 | 1 |
| Hymenoptera | |||
| Formicidae: Iridiomyrmex sp. |
0 | 0 | 1 |
| Unknown | 0 | 0 | 2 |
Discussion
In the present study, we observed that in the presence of alternative prey, Helicoverpa was still targeted as prey by Tasmanicosa. Predation outcomes can result from a combination of prey driven (passive selection) or predator driven (active selection) variables.
Wolf spiders are considered generalist predators that exhibit little prey selectivity, tending to feed according to availability. Wolf spiders respond principally to prey movement [48], and have been assumed to attack whenever a moving suitable prey is detected and comes within reach [49]. From three-way and four-way food web trials, we observed that Tasmanicosa encountered all prey at a similar rate. However, in four-way webs, Tasmanicosa did not always attack Teleogryllus at first encounter. This mismatch may be influenced by both the presence of an intraguild predator (Hogna), and the prey’s defence mechanisms. Prey encounters [50] or prey abundance [22] are not always the decisive factor for predation tendencies in hunting spiders. Other intrinsic characteristics of available prey should be taken into account to understand the factors that drive frequency of predation, such as prey’s defences or ability to escape.
Because antipredatory behaviour and prey choice are not mutually exclusive in determining predation outcomes, disentangling their confounding effects is not straightforward. In some cases, passive selection mechanisms underlie what might seem to be a predator’s active choice. For example, predatory midge larvae (Chaoborus) appear to select prey that are medium-sized, but this size selection is in fact confounded by the rate of prey encounter and capture success, thereby indicating a combination of passive prey selection and active predator choice [2]. Other studies support the hypothesis of active prey choice regardless of passive selection variables. For example, predatory mirids selected two-spotted mites over phytoseiid mites regardless of how easily they are found and captured, suggesting that mirids actively choose prey based on nutritional benefits [5]. In four-way food webs, Tasmanicosa did not always attack Teleogryllus after first encounter. This suggests that predation outcomes were not always determined by rate of encounter, and that prey vulnerability and the predator’s response to such vulnerability can mediate the food web connections between Tasmanicosa, Hogna, Teleogryllus and Helicoverpa.
Potential risks and costs associated with attacking prey can be an important predictor of predatory decisions. Risks could involve physical injury or death due to counter attack, while costs involve energetic expenditure for subduing prey such as chasing and restraining. In the present trials, Helicoverpa and Teleogryllus exhibited defence behaviours against Tasmanicosa. Since Tasmanicosa was never physically injured while attempting to subdue Teleogryllus and Helicoverpa, it could be assumed that under the circumstances and prey sizes used for this study, Teleogryllus and Helicoverpa pose a similarly low risk to Tasmanicosa. Additionally, the body-bobbing behaviour of Helicoverpa might intensify the spider’s attack behaviour as a visual stimulus [51]. Being venomous predators themselves, spiders should pose the highest risk for predation. However, in four-way food webs Hogna did not counter-attack as a defence mechanism, and was usually attacked after first encounter. In fact, in the presence of Hogna, Tasmanicosa did not always attack Teleogryllus at first encounter, suggesting that Tasmanicosa might benefit from instead consuming a competitor intraguild predator [52].
The ability of prey to escape and how a predator responds to escaping prey likely underlie predation outcomes. Compared to Helicoverpa and Hogna, Teleogryllus is a very mobile prey. After encounter, Tasmanicosa might not even have the chance to attempt an attack if Teleogryllus quickly jumps away. Dangles et al. (2006) found that wood crickets (Nemobius) could detect a wolf spider (Pardosa) 5mm away and still escape [53]. Additionally, attack success was correlated to prey distance and attack velocity, but wolf spiders did not modify their attack velocity depending on prey distance. Some crickets can also detect chemical cues from wolf spiders and modify their behaviour to avoid predation [54], and therefore crickets might not need to see or touch the spider to escape. From a predator’s perspective, Tasmanicosa had a 100% success rate at killing Teleogryllus after attacking in an enclosed container, but Teleogryllus might be better at escaping attacks in field conditions. If Tasmanicosa had experienced failed attacks towards Teleogryllus in the field, it is possible that spiders chose not to attack Teleogryllus immediately after encounter to avoid wasting energy in failed predation attempts. Thus, both Teleogryllus’ ability to escape and Tasmanicosa hesitance to attack a quick prey can mediate predation outcomes in this food web. In the presence of slower prey such as Helicoverpa and Hogna, Teleogryllus is relatively costly and difficult to pursue, and may therefore represent a less vulnerable prey to Tasmanicosa than Helicoverpa and Hogna. This may reduce Tasmanicosa’s tendency to pursue Teleogryllus, particularly as Hogna had a similar protein and fat content to Teleogryllus.
Spider nutrition also has the potential to influence prey selection, yet few studies have explored the links between spider nutrition and food webs [55]. Nutrient intake by predators is important to regulate development, health, and reproduction [56], therefore prey selectivity in hunting spiders may be mediated by nutrient optimization and toxin aversion [57]. Some studies have argued that predatory arthropods are limited by nitrogen intake which is necessary for building proteins [17, 58]. From this perspective, spiders might benefit more by consuming prey with higher nitrogen content, such as other predators or omnivores [59], as nitrogen content enhances growth rates and survival in spiders [60], and herbivores tend to have lower protein content [61]. However, this nitrogen-limitation view has been challenged [62], arguing that the predator’s life stage, the way the predator differentially extracts nutrients, and the value of other macronutrients such as lipids and carbohydrates have a stronger effect on nutrient-mediated arthropod food webs. In our study, we did not control for hunger state, as spiders were not offered any prey before trials. Hunger state can also influence prey selection outcomes, and future studies should explore how spiders’ starvation levels or body nutrient contents directly relate to predation outcomes.
Even though food web trials were carried out using diet-reared Helicoverpa, our first experiment with Helicoverpa predation suggests that Tasmanicosa did not discriminate between diet-reared or plant-reared Helicoverpa. Tasmanicosa did not reject protein-poor prey (Helicoverpa) in the presence of protein-rich prey (Teleogryllus and Hogna). By having lower protein and similar lipid contents as Hogna, Helicoverpa might not seem to provide a nutritional advantage to Tasmanicosa, especially considering that the gut of Helicoverpa in the field is likely to have a high content of plant cellulose, a carbohydrate indigestible to the spider. Welches et al. (2016) found that spiders often choose to pursue low-quality aphid prey, even when high-quality alternative prey are available [63]. Furthermore, there is evidence that wolf spiders (Lycosa helluo) develop quicker and survive longer on a diet of mixed arthropods [64]. Spiders might benefit from varied proportions of different amino acids and essential micronutrients rather than just bulk protein [65]. Greenstone (1979) found that the wolf spider Pardosa ramulosa preys on three different species of flies (Diptera: Ephydra, Trichocorixa and Aedes) in quantities enabling the proportions of essential amino-acids reflect those present in the spider’s haemolypmph [66]. Helicoverpa contains essential amino acids and digestible carbohydrates [67] which can contribute to a balanced nutrient intake. Other spiders can represent as much as 38% of a wolf spider’s mixed diet (for a review, see [52]), yet a diet consisting solely of conspecifics can be detrimental to spider development. For example, Oelbermann and Scheu (2002) found that lycosid spiderlings fed only spiders died sooner than spiderlings fed fruit flies or aphids, suggesting that conspecifics, despite their high protein content, still lack essential nutrients for development and survival [68]. Hence, similar predation rates on Helicoverpa and Hogna could represent diet mixing by Tasmanicosa to diversify their diet.
Field surveys showed that wolf spiders do feed on a diverse diet, composed of various insect orders. In the field, Teleogryllus was the most common prey. In this study, we did not quantify the abundance of each insect taxa, therefore we cannot determine if the frequency of prey attacked was linked to prey encounter in the field. Other studies suggest that spiders diversify their diet independently of prey availability, as some prey items are overrepresented in the diet relative to their abundance [69]. Our spider enclosure studies suggest that wolf spiders would likely feed on Helicoverpa, even if Teleogryllus is more common, and Helicoverpa is scarcer in cotton fields. The relationship between prey availability and prey selection in this cotton food web warrants further study.
From a pest control perspective, the results of the present study show that Tasmanicosa still kills Helicoverpa even when other common prey are available. In an agricultural landscape dominated by Bt-cotton, Helicoverpa larvae are less commonly encountered than are other wolf spiders or Teleogryllus. Predators often become more adept at killing and handling common prey, which may lead to development of a preference over unfamiliar prey [20]. However, our results indicate that Tasmanicosa would likely kill upon encounter any rare Helicoverpa larva that has succeeded in developing on Bt cotton in the same field as many other more common prey. The presence of various alternative prey ensures that there is an abundant population of spiders that can contribute to eliminate any Bt survivor larva. The results of this study thereby support the value of Tasmanicosa as an effective predator that can contribute to the control of Bt-resistance in Helicoverpa.
Acknowledgments
Thanks to Jess Hawley for providing technical assistance in lipid and protein analysis, and to Tracey Parker and Dr. Sharon Downes for providing Helicoverpa armigera from their laboratory colonies.
Data Availability
All data are available in Dryad, doi:10.5061/dryad.pm8p741.
Funding Statement
This project was funded by a Macquarie University Post-graduate Research fund. D. Rendon was supported by an Endeavour Post-graduate scholarship (Australia Awards). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sih A, Christensen B. Optimal diet theory: when does it work, and when and why does it fail? Anim Behav. 2001;61:379–90. [Google Scholar]
- 2.Pastorok RA. Prey vulnerability and size selection by chaoborus larvae. Ecology. 1981;62(5):1311–24. [Google Scholar]
- 3.Endler JA. A predator's view of animal colour patterns. Evol Biol. 1978;11:319–64. [Google Scholar]
- 4.Lang A, Gsodl S. Prey vulnerability and active predator choice as determinants of prey selection: a carabid beetle and its aphid prey. J Appl Entomol. 2001;125(1–2):53–61. [Google Scholar]
- 5.Provost C, Lucas E, Coderre D. Prey preference of Hyaliodes vitripennis as an intraguild predator: Active predator choice or passive selection? Biol Control. 2006;37(2):148–54. [Google Scholar]
- 6.Turesson H, Persson A, Bronmark C. Prey size selection in piscivorous pikeperch (Stizostedion lucioperca) includes active prey choice. Ecol Freshw Fish. 2002;11(4):223–33. [Google Scholar]
- 7.Bence JR, Murdoch WW. Prey size selection by the mosquitofish—relation to optimal diet theory. Ecology. 1986;67(2):324–36. [Google Scholar]
- 8.Downes SJ. Size-dependent predation by snakes: selective foraging or differential prey vulnerability? Behav Ecol. 2002;13(4):551–60. [Google Scholar]
- 9.Molles MC Jr., Pietruszka RD. Prey selection by a stonefly: the influence of hunger and prey size. Oecologia. 1987;72(3):473–8. 10.1007/BF00377582 [DOI] [PubMed] [Google Scholar]
- 10.Simpson SJ, Sibly RM, Lee KP, Behmer ST, Raubenheimer D. Optimal foraging when regulating intake of multiple nutrients. Anim Behav. 2004;68:1299–311. [Google Scholar]
- 11.Schmidt JM, Sebastian P, Wilder SM, Rypstra AL. The nutritional content of prey affects the foraging of a generalist arthropod predator. Plos One. 2012;7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krebs JR. Optimal foraging: decision rules for predators. Behav Ecol. 1978:23–63. [Google Scholar]
- 13.Sih A. Optimal behavior can foragers balance 2 conflicting demands. Science. 1980;210(4473):1041–3. 10.1126/science.210.4473.1041 [DOI] [PubMed] [Google Scholar]
- 14.Symondson WOC, Sunderland KD, Greenstone MH. Can generalist predators be effective biocontrol agents? Ann Rev Entomol. 2002;47:561–94. [DOI] [PubMed] [Google Scholar]
- 15.Harwood JD, Sunderland KD, Symondson WOC. Prey selection by linyphiid spiders: molecular tracking of the effects of alternative prey on rates of aphid consumption in the field. Mol Ecol. 2004;13(11):3549–60. 10.1111/j.1365-294X.2004.02331.x [DOI] [PubMed] [Google Scholar]
- 16.Harwood JD, Phillips SW, Lello J, Sunderland KD, Glen DM, Bruford MW, et al. Invertebrate biodiversity affects predator fitness and hence potential to control pests in crops. Biol Control. 2009;51(3):499–506. [Google Scholar]
- 17.Matsumura M, Trafelet-Smith GM, Gratton C, Finke DL, Fagan WF, Denno RF. Does intraguild predation enhance predator performance? A stoichiometric perspective. Ecology. 2004;85(9):2601–15. [Google Scholar]
- 18.Toft S, Li D, Mayntz D. A specialized araneophagic predator's short-term nutrient utilization depends on the macronutrient content of prey rather than on prey taxonomic affiliation. Physiol Entomol. 2010;35(4):317–27. [Google Scholar]
- 19.Mayntz D, Raubenheimer D, Salomon M, Toft S, Simpson SJ. Nutrient-specific foraging in invertebrate predators. Science. 2005;307(5706):111–3. 10.1126/science.1105493 [DOI] [PubMed] [Google Scholar]
- 20.Murdoch WW. Switching in general predators experiments on predator specificity and stability of prey populations. Ecol Monogr. 1969;39(4):335–54. [Google Scholar]
- 21.Gavish-Regev E, Rotkopf R, Lubin Y, Coll M. Consumption of aphids by spiders and the effect of additional prey: evidence from microcosm experiments. Biocontrol. 2009;54(3):341–50. [Google Scholar]
- 22.Kuusk A-K, Ekbom B. Lycosid spiders and alternative food: Feeding behavior and implications for biological control. Biol Control. 2010;55(1):20–6. [Google Scholar]
- 23.Wise DH, Moldenhauer DM, Halaj J. Using stable isotopes to reveal shifts in prey consumption by generalist predators. Ecol Appl. 2006;16(3):865–76. [DOI] [PubMed] [Google Scholar]
- 24.Madsen M, Terkildsen S, Toft S. Microcosm studies on control of aphids by generalist arthropod predators: Effects of alternative prey. Biocontrol. 2004;49(5):483–504. [Google Scholar]
- 25.Vandervoet TF, Ellsworth PC, Carriere Y, Naranjo SE. Quantifying conservation biological control for management of Bemisia tabaci (Hemiptera: Aleyrodidae) in cotton. J Econ Entomol. 2018;111(3):1056–68. 10.1093/jee/toy049 [DOI] [PubMed] [Google Scholar]
- 26.Hagler JR, Blackmer F. Identifying inter- and intra-guild feeding activity of an arthropod predator assemblage. Ecol Entomol. 2013;38(3):258–71. [Google Scholar]
- 27.Hagler JR. An immunological approach to quantify consumption of protein-tagged Lygus hesperus by the entire cotton predator assemblage. Biol Control. 2011;58(3):337–45. [Google Scholar]
- 28.Xia JY, Wang J, Cui JJ, Leffelaar PA, Rabbinge R, van der Werf W. Development of a stage-structured process-based predator-prey model to analyse biological control of cotton aphid, Aphis gossypii, by the sevenspot ladybeetle, Coccinella septempunctata, in cotton. Ecological Complexity. 2018;33:11–30. [Google Scholar]
- 29.Fitt G, Wilson L, Kelly D, Mensah R. Advances with integrated pest management as a component of sustainable agriculture: the case of the australian cotton industry. Integrated Pest Management: Dissemination and Impact, Vol 2 2009:507–24. [Google Scholar]
- 30.Whitehouse MEA, Wilson LJ, Fitt GP, Constable GA, editors. Integrated pest management and the effects of transgenic cotton on insect communities in Australia: Lessons from the past and future directions. 3rd International symposium of biological control of arthropods; 2009; Christchurch, New Zealand.
- 31.Wilson LJ, Whitehouse MEA, Herron GA. The management of insect pests in Australian cotton: an evolving story. Annu Rev Entomol. 2018;63:215–37. 10.1146/annurev-ento-020117-043432 [DOI] [PubMed] [Google Scholar]
- 32.Downes S, Mahon R. Evolution, ecology and management of resistance in Helicoverpa spp. to Bt cotton in Australia. J Invertebr Pathol. 2012;110(3):281–6. 10.1016/j.jip.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Chen M, Collins HL, Onstad DW, Roush RT, Zhang Q, et al. Natural enemies delay insect resistance to bt crops. Plos One. 2014;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitehouse MEA, Wilson LJ, Davies AP, Cross D, Goldsmith P, Thompson A, et al. Target and nontarget effects of novel "triple-stacked" bt-transgenic cotton 1: canopy arthropod communities. Environ Entomol. 2014;43(1):218–41. 10.1603/EN13167 [DOI] [PubMed] [Google Scholar]
- 35.Whitehouse MEA, Wilson LJ, Fitt GP. A comparison of arthropod communities in transgenic Bt and conventional cotton in Australia. Environ Entomol. 2005;34(5):1224–41. [Google Scholar]
- 36.Nentwig W. Non-webbuilding spiders—prey specialists or generalists. Oecologia. 1986;69(4):571–6. 10.1007/BF00410365 [DOI] [PubMed] [Google Scholar]
- 37.Rendon D, Whitehouse MEA, Hulugalle NR, Taylor PW. Influence of crop management and environmental factors on wolf spider assemblages (Araneae: Lycosidae) in an australian cotton cropping system. Environ Entomol. 2015;44(1):174–85. 10.1093/ee/nvu025 [DOI] [PubMed] [Google Scholar]
- 38.Rendon D, Hagler JR, Taylor PW, Whitehouse MEA. Integrating immunomarking with ecological and behavioural approaches to assess predation of Helicoverpa spp. larvae by wolf spiders in cotton. Biol Control. 2018;122:51–9. [Google Scholar]
- 39.Rendon D, Whitehouse MEA, Taylor PW. Consumptive and non-consumptive effects of wolf spiders on cotton bollworms. Entomol Exp Appl. 2016;158(2):170–83. [Google Scholar]
- 40.Johnson ML, Pearce S, Wade M, Davies AP, Silberbauer L, Gregg P, et al. Review of beneficials in cotton farming systems. NSW Australia: Cotton Research and Development Corporation, 2000. [Google Scholar]
- 41.Downes S, Parker TL, Mahon RJ. Frequency of Alleles Conferring Resistance to the Bacillus thuringiensis Toxins Cry1Ac and Cry2Ab in Australian Populations of Helicoverpa punctigera (Lepidoptera: Noctuidae) From 2002 to 2006. J Econo Entomol. 2009;102(2):733–42. [DOI] [PubMed] [Google Scholar]
- 42.Teakle RE, Jensen JM. Heliothis punctiger. Handbook of insect rearing Volume 2 1985:313–22. [Google Scholar]
- 43.Barry KL, Wilder SM. Macronutrient intake affects reproduction of a predatory insect. Oikos. 2013;122: 1058–64. [Google Scholar]
- 44.Hawley J, Simpson SJ, Wilder SM. Effects of prey macronutrient content on body composition and nutrient intake in a web-building spider. PLoS one. 2014;9(6): e99165 10.1371/journal.pone.0099165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Constable GA, Shaw AJ. Temperature requirements for cotton. NSW Agriculture and Fisheries, 1988. [Google Scholar]
- 46.Hayes JL, Lockley TC. Prey and nocturnal activity of wolf spiders (Araneae, Lycosidae) in cotton fields in the delta region of Mississippi. Environ Entomol. 1990;19(5):1512–8. [Google Scholar]
- 47.IBM. SPSS Statistics for Windows. 20.0 ed Armonk, NY: IBM; 2011. [Google Scholar]
- 48.Persons MH, Uetz GW. The effect of prey movement on attack behavior and patch residence decision rules of wolf spiders (Araneae: Lycosidae). J Insect Behav. 1997;10(5):737–52. [Google Scholar]
- 49.Edgar WD. Prey and predators of the wolf spider Lycosa lugubris. J Zool. 1969;159(4):405–11. [Google Scholar]
- 50.Brechbuehl R, Casas J, Bacher S. Diet choice of a predator in the wild: overabundance of prey and missed opportunities along the prey capture sequence. Ecosphere. 2011;2(12). [Google Scholar]
- 51.Bardwell CJ, Averill AL. Effectiveness of larval defenses against spider predation in cranberry ecosystems. Environ Entomol. 1996;25(5):1083–91. [Google Scholar]
- 52.Hodge MA. The implications of intraguild predation for the role of spiders in biological control. J Arachnol. 1999;27(1):351–62. [Google Scholar]
- 53.Dangles O, Ory N, Steinmann T, Christides JP, Casas J. Spider's attack versus cricket's escape: velocity modes determine success. Anim Behav. 2006;72:603–10. [Google Scholar]
- 54.Storm JJ, Lima SL. Predator-naive fall field crickets respond to the chemical cues of wolf spiders. Can J Zool. 2008;86(11):1259–63. [Google Scholar]
- 55.Wilder SM. Spider Nutrition: an integrative perspective. Advances in insect physiology, Vol 40: Spider Physiology and Behaviour. 2011;40:87–136. [Google Scholar]
- 56.Jensen K, Mayntz D, Toft S, Raubenheimer D, Simpson SJ. Prey nutrient composition has different effects on Pardosa wolf spiders with dissimilar life histories. Oecologia. 2011;165(3):577–83. 10.1007/s00442-010-1811-1 [DOI] [PubMed] [Google Scholar]
- 57.Toft S. Prey choice and spider fitness. J Arachnol. 1999;27(1):301–7. [Google Scholar]
- 58.Fagan WF, Denno RF. Stoichiometry of actual vs. potential predator-prey interactions: insights into nitrogen limitation for arthropod predators. Ecol Lett. 2004;7(9):876–83. [Google Scholar]
- 59.Denno RF, Fagan WF. Might nitrogen limitation promote omnivory among carnivorous arthropods? Ecology. 2003;84(10):2522–31. [DOI] [PubMed] [Google Scholar]
- 60.Okuyama T. Growth of a jumping spider on nitrogen enriched prey. Acta Arachnologica. 2008;57(1):47–50. [Google Scholar]
- 61.Wilder SM, Norris M, Lee RW, Raubenheimer D, Simpson SJ. Arthropod food webs become increasingly lipid-limited at higher trophic levels. Ecol Lett. 2013;16(7):895–902. 10.1111/ele.12116 [DOI] [PubMed] [Google Scholar]
- 62.Wilder SM, Eubanks MD. Might nitrogen limitation promote omnivory among carnivorous arthropods? Comment. Ecology. 2010;91(10):3114–7. [DOI] [PubMed] [Google Scholar]
- 63.Welch KD, Whitney TD, Harwood JD. Non-pest prey do not disrupt aphid predation by a web-building spider. Bull Entomol Res. 2016;106(1):91–8. 10.1017/S0007485315000875 [DOI] [PubMed] [Google Scholar]
- 64.Uetz GW, Bischoff J, Raver J. Survivorship of wolf spiders (Lycosidae) reared on different diets. J Arachnol. 1992;20(3):207–11. [Google Scholar]
- 65.Mayntz D, Toft S. Nutrient composition of the prey's diet affects growth and survivorship of a generalist predator. Oecologia. 2001;127(2):207–13. 10.1007/s004420000591 [DOI] [PubMed] [Google Scholar]
- 66.Greenstone MH. Spider feeding behavior optimizes dietary essential amino-acid composition. Nature. 1979;282(5738):501–3. [Google Scholar]
- 67.Lawo NC, Wackers FL, Romeis J. Characterizing indirect prey-quality mediated effects of a Bt crop on predatory larvae of the green lacewing, Chrysoperla carnea. J Insect Physiol. 2010;56(11):1702–10. 10.1016/j.jinsphys.2010.06.012 [DOI] [PubMed] [Google Scholar]
- 68.Oelbermann K, Scheu S. Effects of prey type and mixed dites on survival, growth and development of a generalist predator, Pardosa lugubris (Araneae: Lycosidae). Basic Appl Ecol. 2002;3(3):285–91. [DOI] [PubMed] [Google Scholar]
- 69.Whitney TD, Sitvarin MI, Roualdes EA, Bonner SJ, Harwood JD. Selectivity underlies the dissociation between seasonal prey availability and prey consumption in a generalist predator. Mol Ecol. 2018;27(7):1739–48. 10.1111/mec.14554 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in Dryad, doi:10.5061/dryad.pm8p741.