Abstract
Oceanic islands can be relatively isolated from overfishing and pollution sources, but they are often extremely vulnerable to climate and anthropogenic stress due to their small size and unique assemblages that may rely on a limited larval supply for replenishment. Vulnerability may be especially high when these islands bear permanent human populations or are subjected to regular or intermittent fishing. Since the late 1970's, Brazil has been establishing marine protected areas (MPAs) around its four oceanic island groups, which concentrate high endemism levels and are considered peripheral outposts of the Brazilian Biogeographic Province. In 2018, the Brazilian legally marine protected area increased >10-fold, but most of the ~1,000,000 km2 of MPAs around Brazil's oceanic islands are still unknown and unprotected. Here, we provide the first detailed quantitative baseline of benthic reef assemblages, including shallow and mesophotic zones, of the Fernando de Noronha Archipelago (FNA). The archipelago is partially protected as a no-take MPA and recognized by the UNESCO as a World Heritage Site, but also represents the only Brazilian oceanic island with a large permanent human population (3,000 people), mass tourism (up to 90,000 people per year) and a permanent small-scale fishing community. The influence of depth, wave exposure, and distance from the island and shelf edge on the structure of benthic assemblages was assessed from benthic photoquadrats obtained in 12 sites distributed in the lee and windward shores of the archipelago. Unique assemblages and discriminating species were identified using Multivariate Regression Trees, and environmental drivers of dominant assemblages’ components were evaluated using Boosted Regression Trees. A total of 128 benthic taxa were recorded and 5 distinct assemblages were identified. Distance to the insular slope, depth and exposure were the main drivers of assemblages’ differentiation. Our results represent an important baseline for evaluating changes in benthic assemblages due to increased local and global stressors.
Introduction
The Brazilian Biogeographic Province (BBP) is a secondary biodiversity centre in the Atlantic Ocean that extends along the eastern South American margin from the Amazon mouth (04°N) south to Santa Catarina (29°S) [1]. The BBP is closely related to the Caribbean Biogeographic Province and encompasses ~690,000 km2 of continental shelf (<100 m depth), as well as four peripheral outposts in oceanic archipelagos with high endemism levels: St. Peter and St. Paul’s Archipelago (SPSPA), Fernando de Noronha Archipelago (FNA), Rocas Atoll (ROA), and Trindade/Martin Vaz Archipelago (TMVA) [2]. The ecological relevance of these oceanic islands motivated the declaration of no-take marine protected areas (ntMPA) in ROA (1978, 35,186.41 km2) and FNA (1988, 10,929.47km2), as well as a multiple-use MPA (muMPA) in FNA, ROA and SPSPA (1986, 154,409.03km2). In 2018, muMPAs and ntMPAs were declared in SPSPA (400,000 and 40,000 km2, respectively) and TMVA (400,000 and 60,000 km2), this latter also covering the adjacent Columbia Seamount. Together, the MPAs around Brazil’s oceanic archipelagos cover 809,429.4 km2 under multiple-use and 116,418.5 km2 under no-take regimes, but most of their areas is below 100 m depth and remains to be characterized even in terms of bottom type and oceanographic forcing [3].
With the exception of SPSPA, which drops steeply into the deep sea, rhodolith beds dominate the relatively flat insular shelves and adjacent seamounts’ tops surrounding all of these island groups [4, 5]. Benthic assemblages of shallow (< 30 m depth) and mesophotic (30–90 m) reefs of ROA [6–8], SPSPA [9, 10] and TMVA [11] have already been characterized, but comprehensive quantitative information is still lacking for FNA [12], the only Brazilian oceanic island with significant human population and infrastructure (3,000 inhabitants).
Oceanic islands can be relatively isolated from overfishing and pollution, but they are often extremely vulnerable due to their small size, especially when they bear permanent human populations or are subjected to regular or intermittent fishing [13]. Mesophotic benthic assemblages may harbour unique biodiversity and ecological characteristics [14–16], and have been regarded as less susceptible to disturbances (the ‘deep reef refugia’ hypothesis [17, 18]). However, mesophotic reefs tend to be neglected in assessments and monitoring programs, and their responses to global changes and local impacts are still controversial [19, 20, 21]. Thus, comprehensive baseline assessments of the shallow and mesophotic reefs of the Brazilian oceanic islands [4, 8, 9, 11, 22] are essential for long-term monitoring aiming to assess the ecological processes underlying reef dynamics [23, 24] and MPA effectiveness.
The spatial and temporal dynamics of benthic assemblages are influenced by abiotic (e.g, depth, wave exposure and bottom inclination) and ecological drivers (e.g., competition, predation, recruitment), as well as by anthropogenic forcing (e.g. overfishing, pollution) [25, 26]. At mesophotic depths, wave exposure, temperature and light availability decrease sharply, with clear effects on benthic assemblages [9, 27, 28]. Distance offshore also influences the structure of benthic assemblages due to land-sourced nutrification and turbidity gradients, and also by constraints in larval supply. Deeper offshore sites may also be affected by nutrient-rich and colder upwelling waters enriched in zooplankton [29]. The release of top-down control due to overfishing also promotes cascading effects and acute changes in benthic assemblages [30]. Therefore, the influence abiotic and biotic drivers is hard to disentangle and often results in complex spatial and temporal patterns [31, 32]. Multivariate Regression Trees (MVRT) are a powerful tool to deal with assemblage data, as they account for non-linear relationships, continuous and categorical data, missing values, imbalance and complex interactions. They can identify discrete assemblages along environmental gradients and are easy to interpret [31, 32]. In this context, we used MVRT to identify different benthic reef assemblages and their main drivers across a wide depth gradient (5–50 m) in the FNA. In addition, univariate patterns of the most abundant species/functional groups were also described. The results obtained here constitute a powerful baseline that will help understand possible global and local impacts on reef assemblages and support management and conservation actions in this important South Atlantic Oceanic reefs.
Material and methods
Study area
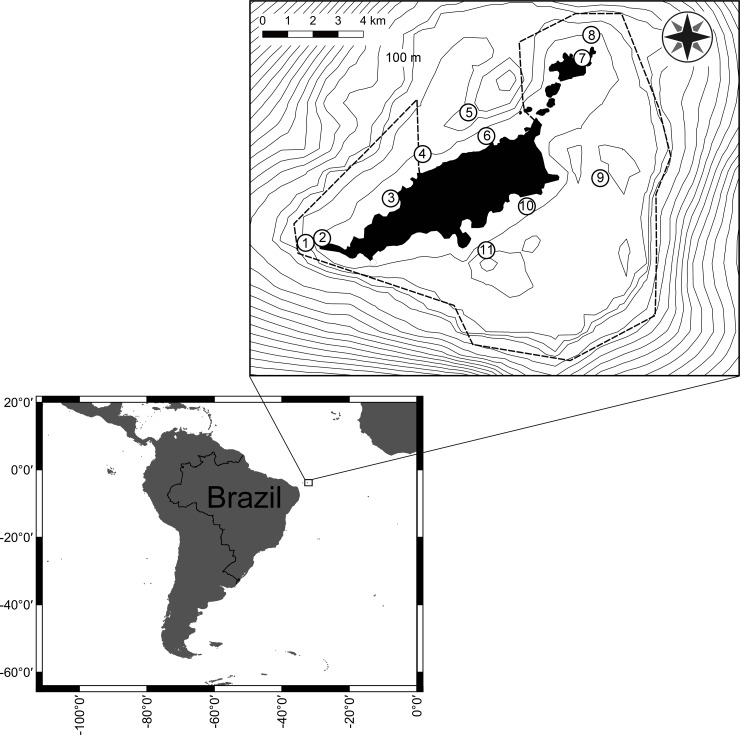
Fernando de Noronha is a volcanic archipelago located on the relatively flat top of a seamount within the Fernando de Noronha Ridge (Fig 1). The region is under the influence of the west-flowing, warm (26–27 C°), saline (36°/oo), and oligotrophic Equatorial Current [33]. It encompasses 20 islands and islets that total ~27 km2, and ~190 km2 of insular shelf (<80m) dominated by rhodolith beds, with soft sediments nearshore [5]. Rocky reefs occur largely as the islands' shore in depths of up to 60 m, and also as a few isolated outcrops across the shelf. The upper slope (90–70 m depth) is rocky and steep, with low sediment accumulation. Carbonate framework dominated by coralline algae occurs as incipient fringes in the shallower part of the islands (<20m) and around outcrops, especially on the windward shore [5, 34, 35]. Climate is tropical with predominance of SE/E trade winds during the summer. The leeward (NW) shore is known as “mar de dentro”, while the windward (SE) shore is known as “mar de fora” (Fig 1). There are only a few intermittent freshwater streams, but FNA encompasses the only mangrove habitat in Brazilian oceanic islands (0.01 km2) [12].
Fig 1. Map of the Fernando de Noronha Archipelago.
Sampling sites: 1 –Cabeço da Sapata (CBS), 2 –Caverna da Sapata (CVA), 3 –Baía dos Golfinhos (BG), 4 –Laje Dois Irmãos (LDI), 5 –Cabeço dois Irmãos (CDI), 6 –Morro de Fora (MF), 7 –Buraco do Inferno (BI), 8 –Cabeço das Cordas (CC), 9 –Pedras Secas (PS), 10 –Ilha do Frade (IF) and 11 –Cabeço Submarino (CSU).
Sampling
We used SCUBA with mixed-gas to sample 12 sites in the lee- and windward shores of FNA, in November 2011, covering different exposures, depths, and distances offshore and to the slope (Fig 1). Benthic assemblages were characterized using haphazardly placed photo-quadrats (n = 221) in five depth strata: 0–10, 10–20, 20–30, 30–40, 40–50 m (n = 5–15 per stratum). After the divers have reached the depth strata, photo quadrats were placed by them non-intentionally in intervals of 10 diver fin kicks. Photo quadrats were composed of a mosaic of 15 high-resolution digital images totalling 0.3 m2, see Magalhães et al. [9] for details. Relative cover was estimated through the identification of organisms at the lowest possible taxonomic level below 300 randomly distributed points per quadrat using the Coral Point Count with Excel Extensions Software [36]. Turf algae were defined as “<5 cm high multispecies assemblages structured by short algae, mostly with filamentous or smaller-sized corticated morphology” cf. [9, 37].
Hydrodynamism was evaluated from weight loss of gypsum balls attached to a vertical line and a buoy for 24h. A total of 60 units were deployed at the windward (n = 30) and leeward shores (n = 30) (Fig 1). Once wind is always stronger in the windward portion of the FNA, gypsum balls were deployed only in October 2011, a month that represents intermediate conditions between summer swells and winter relatively calmer seas. The units were weight before and after deployment to the nearest 0.01g [38, 39].
Data analyses
Differences in weight loss of gypsum units between sites were tested using Analysis of Variance (ANOVA) followed by Tukey post-hoc tests [40]. Multivariate Regression Trees (MVRT) were used to identify unique benthic assemblages and the main environmental drivers leading to their differentiation. A MVRT is built by repeatedly splitting groups of samples aiming at producing nodes as homogeneous as possible regarding the response variable. Homogeneity is measured by finding the best split that minimizes the sums of squares about the multivariate mean within each node [32]. The MVRT approach makes no assumptions about the relationships between dependent and independent variables and an optimal tree is selected based on its predictive accuracy through cross validation. Accuracy is inferred from the cross-validation correlation, which varies from zero (poor predictor) to one (excellent predictor) [32]. Another approach is to make a PCA with different MVRT solutions in order to identify the one best explaining variability in the data. For the MVRT analysis, unidentified species within major taxa (e.g. sponges) were pooled into a single category.
Boosted Regression Trees (BRT) were used to understand drivers of abundance of the most common benthic organisms (> 2% relative cover) and the ones contributing to splitting (i.e. assemblage differentiation) on the MVRT model, with 10 taxa/functional groups retained for the final analyses (see Results). The BRT models were built following the procedures of [31]. The basic BRT approach consists on the combination of a large number of simple regression trees (in which predictions are based on recursive binary splits) using the technique of boosting in order to improve model accuracy. At each step, a new tree is fitted for the residuals from the previous model aiming at reducing the loss function. The process is stochastic, as only a subset of the original data, set at random, is used at each step. The most important attributes of BRT models are bag-fraction, learning rate and tree complexity. Bag-fraction is the proportion of data selected to fit a tree at each step (e.g. a bag-fraction of 0.5 means that 50% of the data are selected at random to fit a tree). The learning rate determines the contribution of each tree to the overall model explanation, while the tree complexity represents the number of nodes (splits) of each tree [31, 32]. To avoid model overfitting and attain highest accuracy, as indicated by lowest values of cross-validation deviance and standard error, optimal BRT models were selected by examining all possible combinations of values for bag-fraction (0.5 and 0.75), learning rate (0.001, 0.005, 0.01 and 0.05) and tree complexity (1 to 5) cf. [31].
All analyses were carried out in R (version 3.2.5; R Core Development Team 2014; available at http://www.R-project.org). Data from this work was made available for public access through the Dryad platform (http://datadryad.org/).
Results
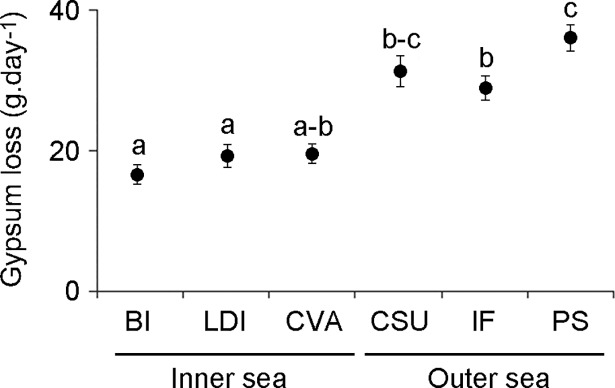
As expected, higher turbulence (as inferred from gypsum ball mass loss) was recorded in the SE side of the FNA than in the NW side (ANOVA: p < 0.05) (Fig 2). A total of 128 benthic taxa belonging to eight major groups (cyanobateria, fleshy macroalgae, turf algae, crustose calcareous algae, Porifera, Hydrozoa, Zoanthidea, Anthozoa and Ascidiacea) were recorded. Porifera was the most speciose group, with 69 infrageneric taxa, followed by fleshy macroalgae (34 taxa). Seven species of scleractinian corals were also recorded (S1 Table). Most abundant benthic organisms (> 2% of total benthic cover) were as follows: the scleractinian coral Montastrea cavernosa (11.6% of total benthic cover), crustose calcareous algae (CCA; 9.5%), the algae Dictyopteris jamaicensis (8.8%) and Canistrocarpus cervicornis (8.7%), sediment (7.1%), Caulerpa ssp. (6.2%), turf algae (5.2%), the sponge Monanchora arbuscula (4.8%), Jania/Amphiroa algae (4.4%) and the fire-coral Millepora alcicornis (2.3%).
Fig 2. Mass loss (mean ± SE) of gypsum units deployed in the inner (sheltered / NW) and outer (exposed / SE) sides of the FNA.
Letters indicate differences detected by Tukey post hoc test. Codes for sampling stations as in Fig 1.
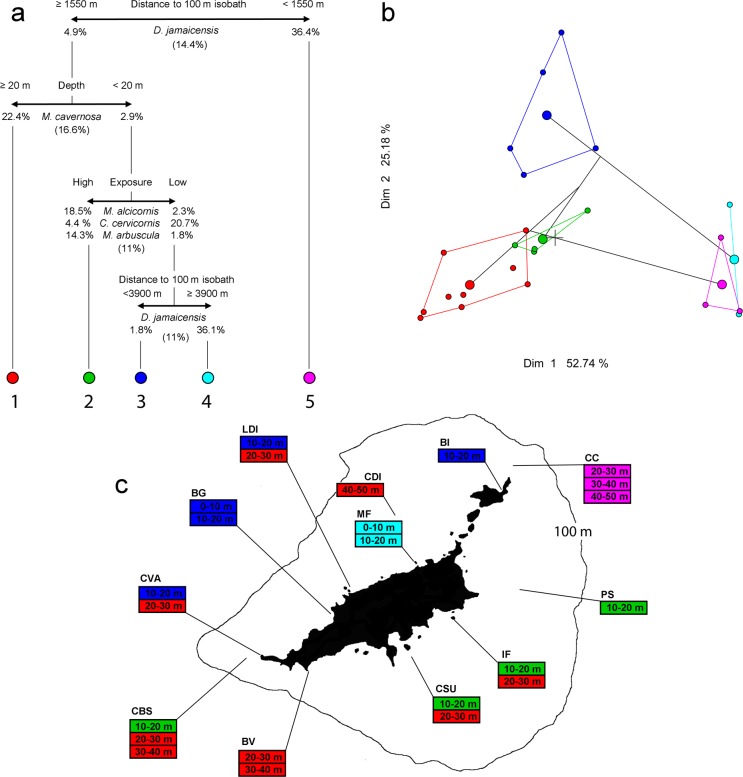
The best MVRT (cross-validated relative error = 0.47 and cross-validation correlation = 0.53) identified five distinct benthic assemblages within the FNA (Fig 3A). The first two axes of the PCA calculated considering these five assemblages explained 77.9% of total variability in the data (Fig 3B). The first split of the MVRT separated a group of four assemblages characterized by low Dictyopteris jamaicensis cover (<4.9%) from a single assemblage characterized by high D. jamaicensis cover (>36.4%) (Fig 4). This latter assemblage was found at all three depth strata (20–50 m) from the Cabeço das Cordas site. The main environmental driver responsible for this first split was the distance from the insular shelf break (i.e. 100 m isobath). Cabeço das Cordas was the closest site to the insular shelf break (< 1550 m), while all other assemblages/sites were located > 1550 m from deep reefs. The second split distinguished a relatively deep assemblage (20–50 m) characterized by high M. cavernosa cover (mean 22.4%) (located in both, the leeward and windward) from three shallow assemblages in the inner and outer sea, all of them with low M. cavernosa cover (2.9%) (Fig 4). This latter group was further subdivided according to exposure, with one assemblage composed by one homogeneous assemblage of shallow high exposed reefs (10–20 m) in the outer sea (i.e. surge zone) and two other assemblages in shallow (0–20 m) low exposed reefs of the inner sea. Reefs in the surge zone of the outer sea were dominated by M. alcicornis and M. arbuscular. The two shallow assemblages in the inner sea were characterized by low (1.8%) and high (36.1%) D. jamaicensis cover, the former in sites closer to deep reefs and the latter in the a single site closest to the island (Morro de Fora) (Fig 3C).
Fig 3. Benthic reef assemblages of the Fernando de Noronha Archipelago.
(A) Multivariate Regression Tree (MVRT) for benthic assemblages of the Fernando de Noronha Archipelago, off NE Brazil. Environmental drivers for each split are given in the upper portion, while organisms responsible for the split and the relative contribution of the split to total model variation explained are given in the lower portion. (B) Principal Component Analysis (PCA) plot showing separation between the five distinct assemblages identified by the MVRT. (C) Spatial distribution of the five typical assemblages according to depth strata and sampling sites. Codes for sampling stations as in Fig 1.
Fig 4. Relative abundance (mean ± SE) of species/functional groups responsible for splits (i.e. differentiation between assemblages) in the MVRT model in the five typical assemblages.
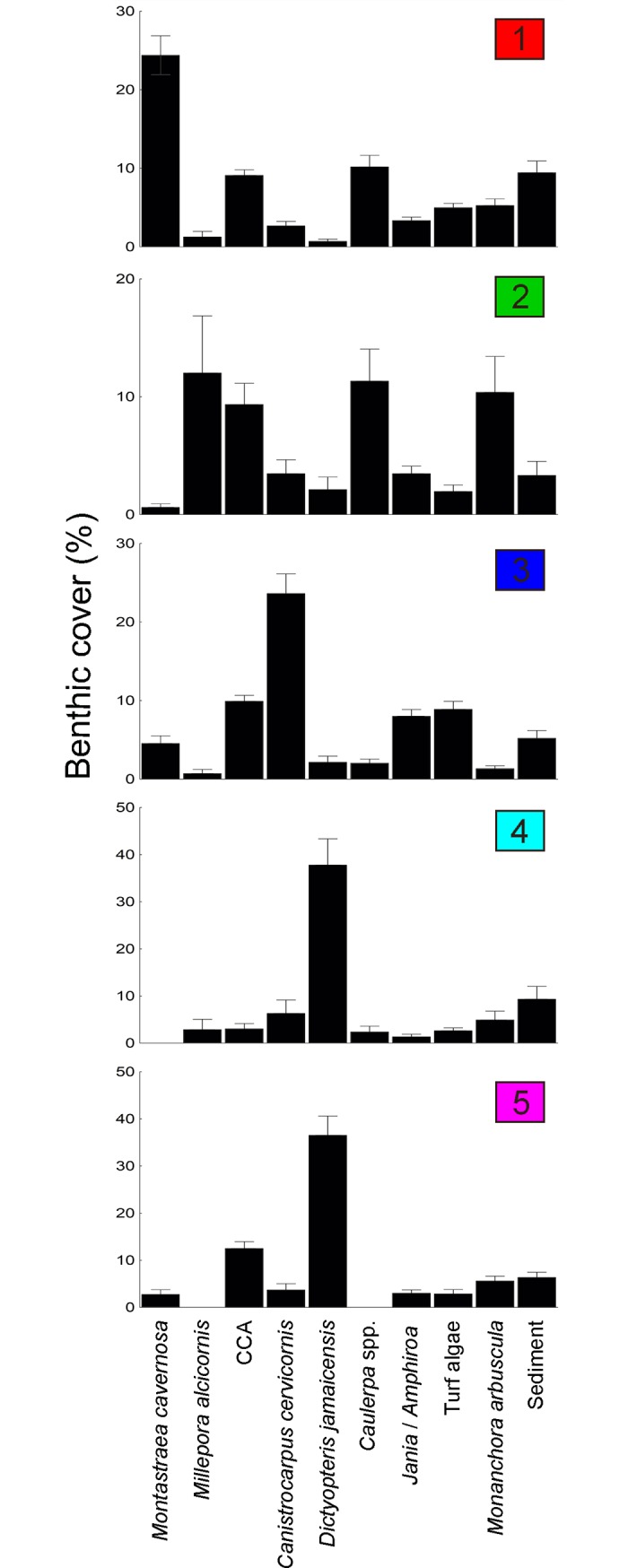
All species/groups representing > 2% of total benthic cover are shown. Colour pattern follows Fig 2. Note differences in scale of y axis.
The main environmental drivers, spatial and bathymetric distribution of assemblages and the relative cover of dominant and typical taxa/functional groups of each of the five benthic assemblages within the FNA are summarized in Figs 3 and 4.
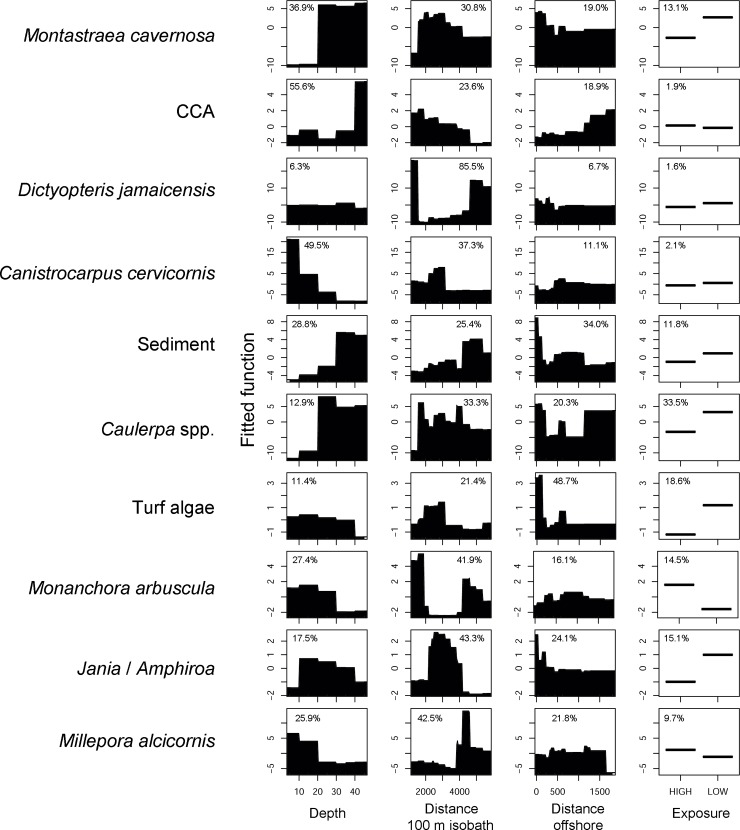
The BRT models supported the patterns observed in the MVRT, but added more detail to the main environmental drivers shaping the abundance and spatial distribution of the most abundant components of benthic assemblages (Fig 5 and Table 1). Depth was the most important predictor for M. cavernosa, CCA and C. cervicornis. M. cavernosa dominates in depths > 20 m and CCA is most abundant in depths greater than 40. On the other hand C. cervicornis dominates reefs shallower than 20 m. Distance to deep reefs was the main environmental driver for variation in abundance of D. jamaicensis, M. arbuscula, Jania/Amphiroa and M. alcicornis. Abundance of the former two species was lower at intermediate distances, and the opposite pattern was recorded for Jania / Amphiroa. Millepora alcicornis was more abundant in reefs > 4000 m from deep reefs.
Fig 5. Partial dependence plots obtained with Boosted Regression Trees using four predictors (depth, distance from 100 m isobath, distance offshore and exposure).
Relative contributions (%) of explanatory variables are given. Taxa/functional groups are shown in decreasing order of abundance. Only the most abundant taxa/functional groups (> 2% of total benthic cover) and the ones responsible for differentiating benthic assemblages (see Fig 2A) are given. Y axes are centered to have zero mean over the data distribution.
Table 1. Optimal settings and predictive performance of boosted regression tree (BRT) analyses used for modeling spatial patterns in abundance (relative cover) of dominant benthic organisms/functional groups (> 2% of total benthic cover; decreasing order of abundance) in the Fernando de Noronha Archipelago, off NE Brazil. Optimal settings: bf–bag fraction, lr–learning rate, tc–tree complexity.
| BRT model | Optimal settings | Number of trees | Cross Validation Deviance (± SE) | Cross Validation Correlation (± SE) | ||
|---|---|---|---|---|---|---|
| bf | lr | tc | ||||
| Montastraea cavernosa | 0.5 | 0.001 | 4 | 4550 | 201.4 (23.0) | 0.66 (0.02) |
| Crustose Calcareous Algae | 0.75 | 0.001 | 3 | 6200 | 41.4 (4.7) | 0.46 (0.03) |
| Dictyopteris jamaicensis | 0.5 | 0.005 | 5 | 2050 | 123.8 (21.6) | 0.80 (0.03) |
| Canistrocarpus cervicornis | 0.75 | 0.001 | 4 | 4950 | 96.8 (23.0) | 0.73 (0.05) |
| Sediment | 0.75 | 0.005 | 4 | 5500 | 76.1 (12.9) | 0.59 (0.10) |
| Caulerpa spp. | 0.75 | 0.001 | 5 | 3000 | 91.9 (10.8) | 0.52 (0.05) |
| Turf algae | 0.75 | 0.001 | 2 | 4350 | 29.3 (3.8) | 0.51 (0.06) |
| Monanchora arbuscula | 0.75 | 0.001 | 4 | 4650 | 47.2 (8.0) | 0.53 (0.07) |
| Jania / Amphiroa | 0.75 | 0.001 | 4 | 3450 | 18.8 (2.8) | 0.53 (0.08) |
| Millepora alcicornis | 0.75 | 0.005 | 4 | 6500 | 65.0 (29.2) | 0.54 (0.10) |
Distance offshore was the most important predictor for turf algae and sediment abundance, with both dominating in reefs < 200 m from the island. Exposure was the main environmental driver for Caulerpa spp. cover, which showed higher abundance on low exposed areas, with minor effects on other organisms (Fig 5).
Discussion
This is the first comprehensive quantitative assessment of benthic rocky reef assemblages of the FNA since the seminal work by [12]. The main methodological difference between [12] and this one is that the former focused on shallow reefs (< 30 m depth), while we focused on a broader depth gradient (0–50 m). Eston et al [12] describe benthic assemblages strikingly dominated by fleshy macroalgae (50–100% cover), particularly Dictyota spp., Dictyopteris spp. and Sargassum spp., in their three shallow stations (Buraco do Inferno, Carreiro da Pedra and Desembarque sites). Within these three stations, samples were obtained in platforms < 10 m depth and maximum depths of < 15 m (see Figs 3–5 in [12]). The only site in which [12] found a relatively high cover of Porifera and scleractinian corals (particularly M. cavernosa) was the deepest site sampled by them (Caverna da Sapata, 30 m maximum depth). The Caverna da Sapata site was also sampled here, with the identification of two distinct assemblages: one between 10–20 m dominated by the fleshy macroalga C. cervicornis (formerly Dictyota cervicornis) and another between 20–30 m dominated by M. cavernosa. Thus, the general pattern of fleshy macroalgae dominance (particularly Dictyopteris jamaicensis and C. cervicornis) in shallow stations (< 20 m) and dominance of M. cavernosa in deeper stations (20–50 m) recorded here, broadly agrees with patterns described by [12]. However, only a systematic monitoring program may help to elucidate possible temporal shifts in the structure of benthic reef assemblages of the FNA and the present work represents an important baseline for future comparisons.
Turf and fleshy macroalgae are dominant components of shallow benthic rock reef assemblages of the four Brazilian oceanic islands [4, 6, 7, 9, 11, 12]. Algal predominance may be driven by overfishing of herbivores and eutrophication, being thus considered as a symptom of reef degradation [30, 41, 42]. However, there are indications that natural factors, such as high light intensity and low herbivory levels, may lead to algal predominance in some habitats [43, 44]. For the FNA there is no indication of overfishing, as most of the Archipelago is fully protected and herbivorous fishes are abundant [35]. Future studies may clarify if natural factors and/or pollution (sewage) are driving high macroalgae abundance. In addition, using Pacific and Caribbean reefs as global baselines may have several limitations, as they are, most likely, not representative of the reefs of other regions [9, 44]. In fact, algal beds have already been recognized as important nursery habitats for some reef fishes in NE Brazil [45]. High algal abundance in inner-shelf reefs of the FNA could be driven by low herbivory intensity and indeed biomass of large herbivorous fishes is relatively low on shallow reefs of the FNA [35]. Unfortunately, cross-shelf patterns in herbivory intensity per se were never measured in continental and insular Brazilian reef ecosystems. Experimental studies may help to elucidate the relative contribution of top down (herbivory) and bottom-up (light and nutrient availability) processes leading to high turf and macroalgae abundance in Brazilian oceanic islands.
Five distinct benthic assemblages were identified in the MVRT. Distance to the break of the insular shelf (100 m isobath), depth and exposure were the main environmental drivers leading to assemblages’ differentiation. A “typical” cross-shelf pattern was recorded, with high turf and macroalgae abundance in shallow inner-shelf reefs, and high abundance of reef building organisms (particularly crustose calcareous algae and the scleractinian coral Montastraea cavernosa) in deeper outer-shelf reefs [29]. The BRT models corroborated the assemblage-level cross-shelf pattern recorded in the MVRT, as depth and distance offshore were among the most important predictors for most species/functional groups. Sediment was particularly abundant in sites close to the island (< 200 m), suggesting sediment may facilitate algae abundance and negatively influence CCA and coral abundance on inner-shelf reefs of the FNA. The site closest to deep reefs (Cabeço das Cordas) harboured a unique benthic assemblage across a broad depth gradient (20–50 m) characterized by the highest CCA cover within the FNA (see Fig 4). Lower water temperatures due to upwelling, steeper slopes, less sediments, and distance from the island are among the alternative non-exclusive hypotheses that may explain the influence of proximity to deep reefs on benthic assemblages. Proximity to the continental shelf break has been previously demonstrated to influence the dynamics of reef fish assemblages of the Abrolhos Bank, Brazil [46], highlighting the importance of including this variable in future studies on reef communities.
Shallow stations (10–20 m depth) off the exposed side of the FNA harbour a typical assemblage characterized by relatively high abundance of the Caulerpa spp., the fire-coral Millepora alcicornis and the sponge Monanchora arbuscula. In addition, one algal species (Turbinaria turbinata) occurred exclusively in the 10–20 m depth stratum of Pedras Secas, the second most abundant organism in this site (18.3% relative cover). Turbinaria turbinata is typical of turbulent waters [47] and indeed Pedras Secas was the site with highest turbulence within the FNA (see Fig 2). Caulerpa spp. (particularly C. racemosa var. peltata) were major components of shallow (5–10 m depth) benthic assemblages of the St. Peter and St. Paul’s Archipelago, which are also subjected to strong turbulence [9]. The latter results corroborate the hypothesis that the Caulerpa genus can survive under high disturbance regimes [48]. High ecological versality and ability to thrive in shallow high hydrodynamical environments are also known for M. alcicornis [49] and M. arbuscula [50].
Despite logistical challenges, the assessment of mesopohotic reefs of geographically isolated oceanic sites in Brazil increased sharply in the last decade, e.g., [5, 6, 7, 8, 9, 11, 22, 50]. Although the widely accepted shallower limit of the mesophotic zone is 30 m [27, 28], results obtained here indicate a single benthic assemblage in the upper mesophotic zone of the FNA between 20–50 m (note the lower limit of this assemblage may be deeper, as no samples were obtained > 50 m depth). Upper mesophotic reefs of the FNA were dominated by the scleractinian coral M. cavernosa. This same coral species is the most abundant scleractinian of the largest coral reef complex in the South Atlantic (Abrolhos, central Brazilian coast), corroborating the idea that it is the current major reef-building species in the SW Atlantic [51] and highlighting the importance of further studies focusing on its conservation status. In the Caribbean Montastraea reefs support high biodiversity and a large suite of ecosystem processes and services [52]. Montastraea cavernosa is recognized as a sediment resistant species [53] and is associated with both shallow high turbidity coastal reefs [51] and low light sediment-free mesophotic reefs [11, 28]. Another important aspect of mesophotic reefs of the FNA was the relatively high richness of Porifera species. Most sponge species recorded here (67 taxa), including possible new occurrences and species (see S1 Table), occurred between 40–50 m. This is noteworthy, as the FNA was already previously recognized as hosting the highest richness of Porifera among Brazilian oceanic islands [54].
This study adds to the knowledge about Brazilian oceanic islands providing important baseline quantitative data for shallow and mesophotic benthic reef assemblages of the FNA. Although protected from fishing, the growing population of the FNA (from about 2000 to 6000 people in the last 20 years) may lead to potential indirect local impacts (e.g. pollution). In addition, the global trend of increased sea surface temperature and acidity [55–56] highlights the importance of implementing a long-term monitoring program focusing on reef assemblages of the FNA.
Supporting information
The “X” denotes presence of the biological groups.
(XLSX)
Acknowledgments
We thank PARNAM Fernando de Noronha and APA Fernando de Noronha/Rocas/São Pedro e São Paulo/ICMBio for providing research permits, Alice Grossman, Leonora Fritzsche, Atlantis Divers, for logistical assistance and data collection, and Tito Lotufo for Tunicate identification. Financial support was provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq (grant 447101/2014-5 to GHPF). We acknowledge individual grants from CNPq (to GHPF, GMAF, RLM and RBFF), from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (to GMAF), and from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (to ZM and FCM). The manuscript was improved by comments provided by Peter Nelson and other anonymous referees.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was supported by Grant 447101/2014-5 to GHPF - Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq; Individual grants from CNPq (to GHPF, GMAF, RLdM and RBFF), from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (to GMAF), and from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (to ZM and FCM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pinheiro HT, Bernardi G, Simon T, Joyeux JC, Macieira RM, Gasparini JL, et al. Island biogeography of marine organisms. Nature. 2017; 549:82–85 10.1038/nature23680 [DOI] [PubMed] [Google Scholar]
- 2.Floeter SR, Rocha LA, Robertson DR, Joyeux JC, Smith‐Vaniz WF, Wirtz P et al. Atlantic reef fish biogeography and evolution. J Biogeogr. 2008; 35:22–47 [Google Scholar]
- 3.Giglio VJ, Pinheiro HT, Bender MG, Bonaldo RM, Costa-Lotufo LV, Ferreira CEL, et al. Large and remote marine protected areas in the South Atlantic Ocean are flawed and raise concerns: Comments on Soares and Lucas. 2018; Marine Policy; 96:13–17. [Google Scholar]
- 4.Pereira-Filho GH, Amado-Filho GM, Moura RL, Bastos AC, Guimarães SMPB, Salgado LT et al. Extensive Rhodolith Beds Cover the Summits of Southwestern Atlantic Ocean Seamounts. J Coast Res 2012; 279:261–269 [Google Scholar]
- 5.Amado-Filho GM, Pereira-Filho GH, Bahia RG, Abrantes DP, Veras PC, Matheus Z. Occurrence and distribution of rhodolith beds on the Fernando de Noronha Archipelago of Brazil. Aquatic Bot. 2012; 101: 41–45 [Google Scholar]
- 6.Fonseca AC, Villaça R, Knoppers B. Reef flat community structure of Atol das Rocas, northeast Brazil and southwest Atlantic. J Mar Biol 2012: Article ID 179128 [Google Scholar]
- 7.Longo GO, Morais RA, Martins CDL, Mendes TC, Aued AW, Cândido DV et al. Between-habitat variation of benthic cover, reef fish assemblage and feeding pressure on the benthos at the only atoll in South Atlantic: Rocas Atoll, NE Brazil. PloS ONE 2015; 10:e0127176 10.1371/journal.pone.0127176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amado-Filho GM, Moura RL, Bastos AC, Francini-Filho RB, Pereira-Filho GH, Bahia RG et al. Mesophotic ecosystems of the unique South Atlantic atoll are composed by rhodolith beds and scattered consolidated reefs. Mar. Biodivers. 2016; 46: 933–936 [Google Scholar]
- 9.Magalhães GM, Amado-Filho GM, Rosa MR, Moura RL, Brasileiro PS, Moraes FC et al. Changes in benthic communities along a 0–60 m depth gradient in the remote St. Peter and St. Paul Archipelago (Mid-Atlantic Ridge, Brazil). Bull Mar Sci 2015; 91:377–396 [Google Scholar]
- 10.Rosa MR, Alves AC, Medeiros DV, Coni EOC, Ferreira CM, Ferreira BP et al. Mesophotic reef fish assemblages of the remote St. Peter and St. Paul’s Archipelago, Mid-Atlantic Ridge, Brazil. Coral Reefs 2016; 35:113–123 [Google Scholar]
- 11.Pereira-Filho G, Amado-Filho G, Guimarães S, Moura RL, Sumida P, Abrantes DP et al. Reef fish and benthic assemblages of the Trindade and Martin Vaz island group, Southwestern Atlantic. Braz J Oceanogr. 2011; 59:201–212 [Google Scholar]
- 12.Eston VR, Migotto AE, Oliveira-Filho EC, Rodrigues SA, Freitas JC. Vertical Distribution of benthic marine organisms on rocky coasts of the Fernando Noronha Archipelago (Brazil). Bol Inst Oceanogr 1986; São Paulo 34: 37–53 [Google Scholar]
- 13.Edgar GJ, Bates AE, Bird TJ, Jones AH, Kininmonth S, Stuart-Smith RD, et al. New approaches to marine conservation through the scaling up of ecological data, Annual Review of Marine Science 2016; 8:435–461. 10.1146/annurev-marine-122414-033921 [DOI] [PubMed] [Google Scholar]
- 14.Hinderstein LM, Marr JCA, Martinez FA, Dowgiallo MJ, Puglise KA, Pyle RL. Introduction to mesophotic coral ecosystems: characterization, ecology, and management. Coral Reefs 2010; 29:247–251 [Google Scholar]
- 15.Harris PT, Bridge TCL, Beaman RJ, Webster JM, Nichol SL, Brooke BP. Submerged banks in the Great Barrier Reef, Australia, greatly increase available coral reef habitat. ICES J Mar Sci 2013; 70:284–293 [Google Scholar]
- 16.Kane C, Kosaki RK, Wagner D. High levels of mesophotic reef fish endemism in the northwestern Hawaiian Islands. Bull Mar Sci 2014; 90:693–703 [Google Scholar]
- 17.Lesser MP, Slattery M, Leichter JJ. Ecology of mesophotic coral reefs. J Exp Mar Biol Ecol. 2009; 375:1–8 [Google Scholar]
- 18.Bongaerts P, Sampayo EM, Hoegh-Guldberg O. Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs 2010; 29: 309–327 [Google Scholar]
- 19.Bak RPM, Nieuwland G, Meesters EH. Coral reef crisis in deep and shallow reefs: 30 years of constancy and change in reefs of Curacao and Bonaire. Coral Reefs 2005; 24:475–479 [Google Scholar]
- 20.Lesser MP, Slattery M Invasive lionfish causes a phase shift to algal dominated communities at mesophotic depths on a Bahamian coral reef. Biol Invasions 2011; 13:1855–1868 [Google Scholar]
- 21.Rocha LA, Pinheiro TH, Shepherd B, Papastamatiou YP, Luiz OJ, Pyle RL, et al. Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 2018; 361:281–284 10.1126/science.aaq1614 [DOI] [PubMed] [Google Scholar]
- 22.Meirelles PM, Amado-Filho GM, Pereira-Filho GH, Pinheiro HT, Moura RL, Joyeux JC., et al. Baseline assessment of mesophotic reefs of the Vitória-Trindade seamount chain based on water quality, microbial diversity, benthic cover and fish biomass data. PloS ONE 2015; 10:e0130084 10.1371/journal.pone.0130084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes TP, Bellwood DR, Baird AH, Brodie J, Bruno JF, Pandolfi J. Shifting base-lines, declining coral cover, and the erosion of reef resilience: comment on Sweatman. Coral Reefs 2011; 30(3): 653–660 [Google Scholar]
- 24.Sandin SA, Smith JE, DeMartini EE, Dinsdale EA, Donner SD, Friedlander AM. Baselines and degradation of coral reefs in the northern Line Islands. PLoS ONE 2008; 3:e1548 10.1371/journal.pone.0001548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCook L, Jompa J, Diaz-Pulido G. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 2001; 19:400–417 [Google Scholar]
- 26.Sandin SA, McNamara DE Spatial dynamics of benthic competition on coral reefs Oecologia 2012; 168:1079–1090 10.1007/s00442-011-2156-0 [DOI] [PubMed] [Google Scholar]
- 27.Lesser MP, Slattery M, Leichter JJ. Ecology of mesophotic coral reefs. J Exp Mar Biol Ecol. 2009; 375:1–8 [Google Scholar]
- 28.Kahng SE, Spalding HL, Brokovich E, Wagner D, Weil E, Hinderstein L, et al. Community ecology of mesophotic coral reef ecosystems. Coral Reefs 2010; 29:255–275 [Google Scholar]
- 29.Wismer S, Hoey AS, Bellwood DR. Cross-shelf benthic community structure on the Great Barrier Reef: relationships between macroalgal cover and herbivore biomass. Mar Ecol Progr Ser 2009; 376: 45–54 [Google Scholar]
- 30.Mumby PJ, Steneck RS. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends in Ecology and Evolution 2008; 23(10):555–563 10.1016/j.tree.2008.06.011 [DOI] [PubMed] [Google Scholar]
- 31.Elith J, Leathwick JR, Hastie T A. Working guide to boosted regression trees. J Anim Ecol 2008; 77:802–813 10.1111/j.1365-2656.2008.01390.x [DOI] [PubMed] [Google Scholar]
- 32.De'Ath G. Multivariate regression trees: a new technique for modeling species–environment relationships. Ecology 2002; 83:1105–1117 [Google Scholar]
- 33.Düing W, Ostapoff F, Merle J (eds). Physica1 oceanography of the tropical Atlantic during GATE. 1980; Miami, FIa, University of Miami, ll7p. [Google Scholar]
- 34.Pereira-Filho GH, Francini-Filho RB, Pierozzi I Jr, Pinheiro HT, Bastos AC, Moura RL, et al. Sponges and fish facilitate succession from rhodolith beds to reefs. Bulletin of Marine Sciences. 2015; 91(1):45–46 [Google Scholar]
- 35.Krajewski JP & Floeter SR. Reef fish community structure of the Fernando de Noronha Archipelago (Equatorial Western Atlantic): the influence of exposure and benthic composition. Env Biol Fishes 2011; 92:25 [Google Scholar]
- 36.Kohler KE, Gill SM. Coral Point Count with Excel extensions (CPCe): A visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci. 2006; 32:1259–1269 [Google Scholar]
- 37.Wilson SK, Bellwood DR, Choat JH, Furnas MJ. Detritus in the epilithic algal matrix and its use by coral reef fishes. Oceanogr Marine Biol 2003; 41:279–310 [Google Scholar]
- 38.Foster MS, Dean TA, Deisher LE. Subtidal techniques In: Litter M, Litter DS (eds) Handbook of phicological methods. Ecological field methods: macroalgae. Cambridge University Press; 1985; pp 199–231 [Google Scholar]
- 39.Fulton CJ, Bellwood DR. Wave‐induced water motion and the functional implications for coral reef fish assemblages. Limnol Oceanogr 2005; 50:255–264 [Google Scholar]
- 40.Zar JH Biostatistical Analysis. 4th ed Prentice-Hall, New Jersey; 1999 [Google Scholar]
- 41.Bellwood DR, Hughes TP, Folker C, Nyström M Confronting the coral reef crisis. Nature 2004. 429: 827–833 10.1038/nature02691 [DOI] [PubMed] [Google Scholar]
- 42.Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Current Biol 2007; 17(4):360–365 [DOI] [PubMed] [Google Scholar]
- 43.Francini-Filho RB, Ferreira CM, Coni EOC, Moura RL, Kaufman L. Foraging activity of roving herbivorous reef fish (Acanthuridae and Scaridae) in eastern Brazil: influence of resource availability and interference competition. J Mar Biol Assoc 2010; UK 90:481–492 [Google Scholar]
- 44.Bruno JF, Precht WF, Vroom PS, Aronson RB. Coral reef baselines: how much macroalgae is natural? Mar Pull Bull. 2014; 80:24–29 [DOI] [PubMed] [Google Scholar]
- 45.Chaves LTC, Pereira PHC, Feitosa JLL. Coral reef fish association with macroalgal beds on a tropical reef system in North-eastern Brazil. Mar Freshwater Res. 2013; 64:1101–1111 [Google Scholar]
- 46.Francini-Filho RB, Moura RL. Dynamics of fish assemblages on coral reefs subjected to different management regimes in the Abrolhos Bank, eastern Brazil. Aquatic Conserv Mar Freshwater Ecosys. 2008; 18:1166–1179 [Google Scholar]
- 47.Littler DS, Littler MM Caribbean Reef Plants. Off Shore Graphics, Inc; 1st ed Washington DC; 542p 2000. [Google Scholar]
- 48.Balata D, Piazzi L, Rindi F. Testing a new classification of morphological functional groups of marine macroalgae for the detection of responses to stress. Mar Biol. 2011; 158:2459–2469 [Google Scholar]
- 49.Leão ZMAN, Kikuchi RKP, Testa V. Corals and coral reefs of Brazil 1st ed In: Cortés J (ed) Latin American Coral Reefs. Elsevier, Amsterdam, pp 9–52; 2003. [Google Scholar]
- 50.Moraes FC. Esponjas das ilhas oceânicas brasileiras. 1st ed Museu Nacional: Rio de Janeiro; 2011. [Google Scholar]
- 51.Francini-Filho RB, Coni EOC, Meirelles PM, Amado-Filho GM, Thompson FL, Pereira-Filho GH et al. Dynamics of coral reef benthic assemblages of the Abrolhos Bank, eastern Brazil: Inferences on natural and anthropogenic drivers. PLoS ONE 2013; 8:e54260 10.1371/journal.pone.0054260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chollett I, Mumby PJ. Predicting the distribution of Montastraea reefs using wave exposure. Coral Reefs 2012; 31:493–503 [Google Scholar]
- 53.Lasker HR. Sediment rejection by reef corals: the roles of behavior and morphology in Montastraea cavernosa (Linnaeus). J Exp Mar Biol Ecol. 1980; 47: 77–87 [Google Scholar]
- 54.Moraes F, Muricy G. A new species of Stoeba (Demospongiae: Astrophorida) from oceanic islands off north-eastern Brazil. J. Mar. Biol. Ass. 2007; 87: 1387–1393 [Google Scholar]
- 55.Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003; 301:929–933 10.1126/science.1085046 [DOI] [PubMed] [Google Scholar]
- 56.Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E. Coral reefs under rapid climate change and ocean acidification. Science 2007; 318:1737–1742 10.1126/science.1152509 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The “X” denotes presence of the biological groups.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.