Abstract
Purpose
To compare the outcomes of robotic partial nephrectomy (RPN) with those of open PN (OPN) in patients with highly complex renal tumors defined as RENAL nephrometry score ≥ 10
Materials and methods
We analyzed clinical data from a total of 149 patients who underwent OPN or RPN for a highly complex renal mass at our institution between 2003 and 2017. Perioperative data, complication profiles, functional outcomes, pathologic variables, and oncologic outcomes were evaluated in both groups.
Results
The median (interquartile range, IQR) patient age was 52.0 (42.0–59.0) years, and the median (IQR) follow-up period was 30.0 (7.0–54.0) months. Among the patients, 64 (43.0%) and 85 (57.0%) underwent OPN and RPN, respectively. The RPN group showed higher rates of clinical T1b and ≥ T2 than the OPN group (p = 0.019). There were no significant differences between the groups in terms of intraoperative outcomes such as operation time, estimated blood loss, warm ischemic time, and transfusion. Notably, the RPN group showed significantly shorter length of hospital stay than the OPN group (p < 0.001). Regarding the complication profiles and renal functional outcomes, no significant differences were reported between the groups. The estimated glomerular filtration rate decline from baseline at the last follow-up showed no significant differences between the two groups (p = 0.351). Kaplan-Meier survival analysis also showed no significant differences in survival outcomes between the groups (log-rank test, all p > 0.05).
Conclusions
RPN performed in patients with highly complex renal tumors offers perioperative, functional, and oncologic outcomes comparable to those associated with OPN.
Introduction
The current consensus guidelines recommend partial nephrectomy (PN) to be the standard treatment option for clinical T1a renal tumors [1]. A previous prospective randomized phase III study demonstrated that PN offers functional outcomes (renal function preservation) better than and cancer control comparable to radical nephrectomy (RN) in “low-stage” tumors [2]. For the last decade, with the continued development and improvement of surgical techniques, there have been trends toward using PN over RN even in larger renal tumors (≥ clinical T1b) [3–6]. However, there are still no definite consensus guidelines regarding this [7, 8]; the American Urology Association has announced that RN is the standard of care for clinical T1b renal tumors, and that PN can be performed as an alternative standard therapy in only selected patients [8].
The surgical technique of PN has evolved gradually from open PN (OPN) to laparoscopic PN (LPN), and on to robotic PN (RPN). The use of RPN has been continuously increasing with the diffusion of the da Vinci Surgical System. Patel et al. [9] reported from their population-based analysis that the use of RPN has increased from 5 to 40% between 2008 and 2011. Subsequently, RPN has broadened the spectrum of indication in large and complex tumors with its advantage of being more convenient in conducting tumor excision and renorrhaphy [10–17]. However, the majority of studies have focused dominantly on tumor size as a surrogate marker for surgical difficulty [10–14].
The RENAL nephrometry score was developed as an assessment tool for predicting surgical complexity posed by postoperative complications or warm ischemic time (WIT) [18]. This system includes five domains: Radius, Exophytic/endophytic, Nearness to collecting system or sinus, Anterior/posterior, Location relative to polar lines. Even in small renal tumors, the degree of surgical difficulty is increased in the case of high RENAL score lesions (i.e. completely endophytic, close to collecting system, posterior, entirely between polar lines). However, studies on these issues are still lacking, especially in the field of RPN [15–17]. Thus, we aimed to compare the outcomes of RPN with those of OPN in patients with highly complex renal tumors represented by RENAL nephrometry score ≥ 10.
Materials and methods
Ethics statement
The Institutional Review Boards of the Seoul National University Bundang Hospital approved this study (Approval number: B-1805-466-102). As the present study was carried out retrospectively, written informed consent from patients was waived. Personal identifiers were completely removed and the data were analyzed anonymously. Our study was conducted according to the ethical standards recommended by the 1964 Declaration of Helsinki and its later amendments.
Study cohort
From June 2003 to March 2017, a total of 161 patients who underwent OPN or RPN for a highly complex renal mass (RENAL score ≥ 10) at our institution were included in this retrospective study. RPNs were performed after 2008 using the da Vinci Surgical System. Lymph node dissection was performed in the case of suspicious findings indicating lymph node invasion in preoperative imaging and/or intraoperative findings. Patients were excluded if they had non- renal cell carcinoma malignancies or metastatic disease. We also excluded the patients with a solitary kidney, multifocal tumors, von Hippel-Lindau syndrome, or OPN under hypothermia and cold ischemia. Subsequently, 7 patients of RPN group and 5 patients of OPN group were excluded; a total of 149 patients were included in final analysis.
Acquisition and definition of data
Clinical data in the prospectively maintained database of our institution were retrospectively reviewed. The RENAL nephrometry score was calculated by each physician as previously described [18]. The clinical variables measured as baseline characteristics according to the type of surgeries (OPN vs. RPN) included age, sex, body mass index (BMI), past medical history (including diabetes mellitus [DM], hypertension [HTN], and chronic kidney disease [CKD]), Eastern Cooperative Oncology Group performance status, American Society of Anesthesiologists scores, Charlson comorbidity index, laboratory data (including serum creatinine and an estimated glomerular filtration rate [eGFR] calculated by Modification of Diet in Renal Disease equation [19]), tumor laterality, clinical stage, and RENAL nephrometry score. Tumor size was determined as the longest diameter of each tumor in any single plane of the preoperative imaging study.
Variables for perioperative outcome analysis included operation time, estimated blood loss (EBL), WIT, length of hospital stay (LOS), intra-/postoperative transfusion, complication profiles including Clavien grade [20], and renal function changes. De novo CKD was defined as the development of stage ≥ 3 CKD with two consecutive values of eGFR < 60 ml/min/1.73m2 [21].
Pathological parameters including histological type according to the World Health Organization (WHO) classification system [22], pathologic stage according to the 7th edition of American Joint Committee guidelines [23], Fuhrmann nuclear grade, and positive surgical margin (PSM) were also evaluated.
Recurrence was defined as radiographically verified distant metastasis or local disease recurrence during the study period.
Follow-up protocol
According to the institutional standardized postoperative protocol, patients were generally followed-up after surgery at least every six months in the first year, annually over the next four years, and every two years thereafter. Follow-up protocols consisted of computed tomography (CT) or magnetic resonance imaging (MRI), bone scan, and chest radiography (and/or chest CT).
Recurrence-free survival (RFS) was defined as the interval between the date of surgery and the time of first tumor recurrence. The cause of death was determined by the responsible physicians and death certificates. Overall survival (OS) was calculated from the date of surgery to the date of last follow-up or death.
Statistical analyses
Clinicopathological characteristics were compared between the OPN and RPN groups using a chi-squared test for categorical variables, and an independent t-test or Mann-Whitney U test for continuous variables. Kaplan-Meier curve analysis was used to calculate the survival estimates for RFS and OS, and the log-rank test was used to conduct comparisons between the groups. All statistical analyses were performed using commercially available software (IBM SPSS Statistics ver. 21.0, Armonk, NY, USA) and two-sided p values < 0.05 were considered statistically significant.
Results
The median (IQR) patient age was 52.0 (42.0–59.0) years, and the median (IQR) follow-up period was 30.0 (7.0–54.0) months. At the last follow-up, there were three (2.0%) patients who had died of any cause (one, other cancer-related death [ampulla of vater cancer]; two, death with unknown cause from death certificates), and recurrence occurred in six (3.8%) patients overall. Among all patients, 64 (43.0%) underwent OPN and 85 (57.0%) underwent RPN.
Comparison of baseline characteristics
Comparative analysis results of the preoperative clinical features between the two groups are summarized in Table 1. The RPN group had a higher rate of clinical stage T1b and ≥T2 compared with the OPN group (p = 0.019). However, there were no significant differences in the other variables, notably in terms of preoperative renal function profile (serum creatinine and eGFR) and RENAL nephrometry score.
Table 1. Baseline characteristics.
| Variables | Median (interquartile range) or counts (%) | P | ||
|---|---|---|---|---|
| Total (N = 149) | OPN (N = 64) | RPN (N = 85) | ||
| Age, years | 52.0 (42.0–60.0) | 52.0 (40.5–60.5) | 53.0 (42.0–60.0) | 0.947 |
| Sex, male | 97 (65.1%) | 42 (65.6%) | 55 (64.7%) | 1.000 |
| BMI, kg/m2 | 24.7 (22.9–27.1) | 24.8 (22.9–26.5) | 24.7 (22.9–27.5) | 0.969 |
| ECOG score, ≥1 | 3 (2.0%) | 2 (3.1%) | 1 (1.2%) | 0.577 |
| ASA score, ≥2 | 4 (2.7%) | 2 (3.1%) | 2 (2.4%) | 0.897 |
| CCI score | 2 (1–3) | 2 (1–3) | 2 (1–2) | 0.642 |
| Diabetes mellitus, yes | 19 (12.8%) | 5 (7.8%) | 14 (16.5%) | 0.141 |
| Hypertension, yes | 53 (35.6%) | 20 (31.3%) | 33 (38.8%) | 0.389 |
| *CKD, stage≥3 | 6 (4.0%) | 3 (4.7%) | 3 (3.5%) | 1.000 |
| Preoperative creatinine, mg/dL | 0.88 (0.72–1.00) | 0.91 (0.77–1.00) | 0.86 (0.69–0.96) | 0.842 |
| Preoperative eGFR, ml/min/1.73 m2 | 85.6 (76.4–101.5) | 83.3 (74.2–98.5) | 87.1 (79.8–103.8) | 0.085 |
| Clinical stage | 0.019 | |||
| T1a | 78 (52.3%) | 42 (65.6%) | 36 (42.4%) | |
| T1b | 51 (34.2%) | 16 (25.0%) | 35 (41.2%) | |
| ≥ T2 | 20 (13.4%) | 6 (9.4%) | 14 (16.5%) | |
| RENAL score | 10.0 (10.0–10.0) | 10.1 (10.0–10.0) | 10.2 (10.0–10.0) | 0.346 |
| Laterality, Lt. | 85 (57.0%) | 36 (56.3%) | 49 (57.6%) | 0.869 |
ASA, American Society of Anesthesiologists; BMI, body mass index; CCI, Charlson comorbidity index; CKD, chronic kidney diseas; ECOG, Eastern Cooperative Oncology Group; eGFR, estimated glomerular filtration rate; OPN, open partial nephrectomy; RPN, robotic partial nephrectomy
*GFR < 60 ml/min/1.73m2
Comparison of perioperative outcomes
There were no significant differences in terms of intraoperative outcomes including operation time, EBL, WIT, and transfusion (Table 2). Notably, the RPN group showed significantly shorter LOS than the OPN group (median, 5 [RPN] vs. 7 [OPN] days, p < 0.001, Table 2). In complication profiles, the RPN group showed the lower major (Clavien grade 3–5) complication rates in the early (within three months of surgery) postoperative periods, but this trend was not statistically significant (9.4% [RPN] vs. 14.1% [OPN], p = 0.440, Tables 2 and 3). Regarding functional outcomes, the mean value of eGFR decline from baseline at the last follow-up showed no significant differences between the two groups (mean, 6.5 [RPN] vs. 3.8 [OPN] ml/min/1.73 m2, p = 0.351, Table 4). In addition, there was no significant difference in the development of de novo CKD (p = 1.000).
Table 2. Perioperative outcomes.
| Variables | Median (interquartile range) or counts (%) | P | |
|---|---|---|---|
| OPN (N = 64) | RPN (N = 85) | ||
| Operation time, min | 145 (105–180) | 150 (110–190) | 0.709 |
| Estimated blood loss, ml | 200 (100–300) | 200 (100–300) | 0.888 |
| Warm ischemic time, min | 21 (18–30) | 24 (19–34) | 0.147 |
| Transfusion | |||
| Intraoperative | 3 (4.8%) | 3 (3.5%) | 0.700 |
| Postoperative | 4 (6.3%) | 5 (5.9%) | 1.000 |
| Intraoperative complications | 4 (6.3%) | 8 (9.4%) | 0.556 |
| Postoperative complications | |||
| Overall (Clavien 1–5), n (%) | 15 (23.4%) | 16 (18.8%) | 0.544 |
| Major (Clavien 3–5), n (%) | 9 (14.1%) | 8 (9.4%) | 0.440 |
| Length of hospital stay, day | 7 (5–9) | 5 (5–7) | <0.001 |
| VAS score for pain in postoperative 1 day | 4 (4–5) | 4.5 (4–5) | 0.439 |
OPN, open partial nephrectomy; RPN, robotic partial nephrectomy; VAS, visual analogue scale
Table 3. Complication profiles.
| Variables | OPN (N = 64) | RPN (N = 85) | P |
|---|---|---|---|
| Intraoperative complications | 4 (6.3%) | 8 (9.4%) | 0.556 |
| Postoperative complications | |||
| Overall (Clavien 1–5), n (%) | 18 (28.1%) | 18 (21.2%) | 0.548 |
| Major (Clavien 3–5), n (%) | 12 (18.8%) | 10 (11.8%) | 0.438 |
| Details | |||
| Wound dehiscence | 3 | 2 | |
| Postoperative bleeding | 9 | 4 | |
| Pseudoaneurysm | 3 | 2 | |
| Pneumonia | 1 | - | |
| Atelectasis / Desaturation | 1 | - | |
| Pneumothorax | 1 | 2 | |
| Acute renal failure | - | 2 | |
| Ileus | - | 2 | |
| Urinary retention | - | 2 | |
| Other infection | - | 2 | |
Table 4. Renal functional outcomes.
| Variables | OPN (N = 64) | RPN (N = 85) | P |
|---|---|---|---|
| Preoperative creatinine, mg/dL | 0.91 (0.77–1.00) | 0.86 (0.69–0.96) | 0.842 |
| Preoperative eGFR, ml/min/1.73 m2 | 83.3 (74.2–98.5) | 87.1 (79.8–103.8) | 0.085 |
| *CKD, stage≥3 | 3 (4.7%) | 3 (3.5%) | 1.000 |
| Latest postoperative creatinine, mg/dL, median (IQR) | 0.92 (0.74–1.06) | 0.87 (0.71–1.05) | 0.662 |
| Latest postoperative eGFR, ml/min/1.73 m2, median (IQR) | 82.4 (69.9–92.3) | 84.8 (69.1–84.8) | 0.335 |
| +eGFR decline from baseline, mean (SD) | 3.8 (16.6) | 6.5 (18.0) | 0.351 |
| De novo CKD, stage≥3 | 2 (3.3) | 4 (4.9) | 1.000 |
| Follow-up duration, months, median (IQR) | 53.0 (33.3–81.0) | 15.0 (5.5–33.0) | <0.001 |
CKD, chronic kidney diseas; eGFR, estimated glomerular filtration rate; OPN, open partial nephrectomy; RPN, robotic partial nephrectomy
*GFR < 60 ml/min/1.73m2, + paired T-test
Comparison of pathologic and oncologic outcomes
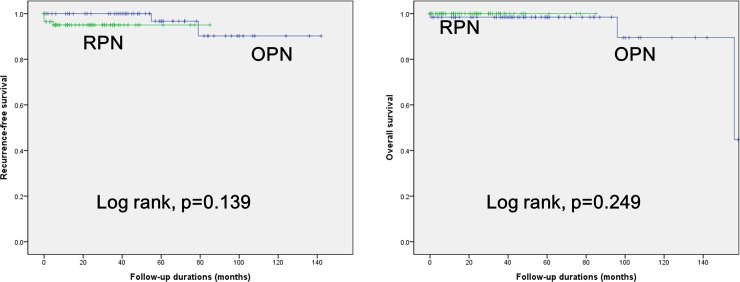
The RPN group showed a significantly larger pathologic tumor size than the OPN group (median, 4.3 vs. 3.1 cm, p = 0.014). There were no significant differences in the other pathologic outcomes including pathologic stage, Fuhrmann nuclear grade, and histologic subtypes (Table 5). There was no PSM in the RPN group. In addition, Kaplan-Meier survival analysis showed no significant differences in RFS and OS between the two groups (log-rank test, all p > 0.05, Fig 1).
Table 5. Pathologic and oncologic outcomes.
| Variables | Median (interquartile range) or counts (%) | P | |
|---|---|---|---|
| OPN (N = 64) | RPN (N = 85) | ||
| Tumor size (cm) | 3.1 (2.3–4.8) | 4.3 (2.9–5.2) | 0.014 |
| Pathological stage | 4 (6.3%) | 1 (1.2%) | 0.165 |
| pT1 | 57 (89.1%) | 74 (87.1%) | |
| pT2 | 3 (4.7%) | 10 (11.8%) | |
| pT3 | 4 (6.3%) | 1 (1.2%) | |
| Fuhrmann grade | 0.386 | ||
| ≤ 2 | 36 (56.3%) | 40 (47.1%) | |
| ≥ 3 | 28 (43.8%) | 45 (52.9%) | |
| Histological subtype | 0.203 | ||
| Clear cell | 50 (78.1%) | 65 (76.5%) | |
| Papillary | 3 (4.7%) | 1 (1.2%) | |
| Chromophobe | 3 (4.7%) | 10 (11.8%) | |
| Collecting duct | 0 (0%) | 0 (0%) | |
| Unclassified | 0 (0%) | 2 (2.4%) | |
| Benign | 8 (12.5%) | 7 (8.2%) | |
| Positive surgical margin | 1 (1.6%) | 0 (0%) | 0.432 |
| Recurrence | 2 (3.1%) | 4 (4.7%) | 0.306 |
| Overall mortality | 3 (4.7%) | 0 (0%) | 0.077 |
| Follow-up duration, months | 53.0 (33.3–81.0) | 15.0 (5.5–33.0) | <0.001 |
OPN, open partial nephrectomy; RPN, robotic partial nephrectomy
Fig 1.
Kaplan-Meier curve analysis of (A) recurrence-free survival and (B) overall survival according to the type of surgery performed.
Discussion
The RENAL nephrometry scoring system categorizes the complexity of renal masses. The score range of 4–6, 7–9, and ≥ 10 are deemed as low, moderate, and high complexity lesions, respectively [18]. Regarding complex renal tumors defined as RENAL nephrometry score ≥ 7, several previous studies reported that RPN offers perioperative, functional and oncological outcomes comparable to those associated with LPN or OPN [15–17]. Long et al. [16] compared perioperative outcomes between the LPN and RPN group for a complex renal tumors of RENAL nephrometry score ≥ 7 in a large single center cohort. Consequently, they found that LPN was associated with a higher conversion rate to RN (11.5% vs. 1%, p < 0.001) and a higher decrease in eGFR (-16.0% vs. -12.6%, p = 0.03). However, there were no significant differences in perioperative outcomes posed by WIT, EBL, transfusion rate, or complications between the two groups. In a very recent meta-analysis, Cacciamani et al. [24] reported that all perioperative, oncological, and survival outcomes were similar between OPN and RPN groups with similar RENAL nephrometry score. Importantly, in their sensitivity analyses focusing only on complex renal masses defined as RENAL nephrometry score ≥ 7, RPN had lesser EBL (Weighted mean difference [WMD], 66.32; 95% confidence interval [CI], 26.06–106.58; p = 0.001), fewer overall postoperative complications (Odd ratio [OR], 2.15; 95% CI, 1.40–3.29; p = 0.0004) and shorter LOS (WMD, 1.57, 95% CI, 1.04–2.09; p < 0.00001).
In a recent study from Cleveland Clinic, Garisto et al. [25] represented the first series comparing OPN (N = 76) vs. RPN (N = 203) for patients with RENAL nephrometry score ≥ 10, which is deemed as highly complex lesion [18]. The authors demonstrated that RPN was associated with a lower EBL (200 vs. 300ml, p < 0.001), shorter ischemic time (28 vs. 37 min, p < 0.001), lower intraoperative transfusion rates (3% vs. 15.8%, p < 0.001), and shorter LOS (3 vs. 5 days, p < 0.001) compared to OPN. Regarding renal functional outcomes, the median (IQR) value of eGFR at 3rd, 6th, and 12th postoperative months showed no significant differences between the two groups. Accordingly, CKD upstaging rates were comparable (44.3% [RPN] vs. 47.4% [OPN], p = 0.643). They also found no significant differences between the groups in survival outcomes including OS and RFS.
In the current study, we also found that the RPN group showed significantly shorter LOS (median, 5 vs. 7 days, p < 0.001, Table 2) in comparison with the OPN group. However, we found no significant differences between the groups in terms of intraoperative outcomes such as operation time, EBL, WIT, and transfusion rates (Table 1). Also, the mean value of eGFR decline at the last follow-up and the development of de novo CKD showed no significant differences between the two groups (Table 4).
Up to now, over 1000 cases of RPN have been performed in our institution, and recently, vast majority of renal tumor cases were performed by RPN (S1 Fig). In 2017, we conducted 90 cases of RN and 241 cases of PN; and PN comprised 222 cases of RPN, 9 cases of LPN, and 10 cases of OPN. With this broadening indication for RPN even in larger tumors, higher clinical stage tumors were dominantly included in the RPN groups. Consequently, current study showed a higher rate of clinical stage T1b and ≥ T2 in the RPN group compared to the OPN group (p = 0.019, Table 1). With this perspective, the median (IQR) WIT of the RPN group was slightly longer than that of the OPN group (median, 24 vs. 21 min, p = 0.147); this is significantly longer than the 17–20 min reported in previous RPN series [26–30]. In a previous study on Korean population, Kang et al. [27] reported that T1b cases showed longer operative times and WIT than T1a cases in 362 patients underwent RPN. Generally, in patients with highly complex tumors, such as those that make up our cohort, the longer WIT would be inevitable [12, 14, 31]; instead, it would be better to focus on the other outcomes such as renal functional outcomes and complication profiles. Importantly, there were no significant differences between the groups in terms of mean eGFR decline, De novo CKD, and intra/postoperative complication rates (Tables 2–4).
Regarding the pathologic and oncologic outcomes, we found no significant differences between the groups (Table 5 and Fig 1). There was no PSM in the RPN group. In fact, oncologic outcomes are of the most concern in the surgical approach to renal tumors. Several previous studies reported survival outcomes in the RPN group comparable or even superior to those in LPN or OPN groups in patients with complex renal tumors [11–17]. Cacciamani et al. [24] reported in their recent meta-analysis study that RPN was superior for PSM (OR, 1.73; p < 0.0001) and OS (OR, 2.98; p = 0.04). However, in the majority of studies, the follow-up duration was too short to draw definitive conclusions. Even in a recent study from Cleveland Clinic, the median follow-up period was only 25 months [25]. The current study also demonstrated that the RPN group had a significantly shorter follow-up duration than the OPN group (median, 15.0 vs. 53.0 months, p<0.001). Therefore, we could not draw definitive conclusions based on the present study. Long-term follow-up studies with larger sample sizes are needed to verify these results.
With the wide spread of RPN in field of renal tumors, the cost-effectiveness of RPN has been a source of unresolved debate [32, 33]. In a recent study, Buse et al. [32] described the results of cost-effectiveness analysis of RPN vs. OPN in US. The mean in‐hospital costs were $14,824 (95% CI, $13,368-$16,898) for RPN and $15,094 (95% CI, $13,491-$17,140) for OPN. Complications after RPN occurred in 23.3% (95% CI, 20.0–25.8%) and after OPN in 36.1% (95% CI, 35.6–36.6%) of the patients. In a sensitivity analysis, limited center experience was associated with relevant increase in RPN cost and consequently in low cost‐effectiveness. Accordingly, they concluded that RPN resulted in nominally lower cost but fewer perioperative complications than OPN, and RPN was not cost-effective in less experienced centers. In 2015, we also analyzed the difference between costs and utility after one year of RPN through incremental cost-effectiveness ratio (ICER) using a Decision Tree model [not published data]. Consequently, we found that ICER was 130 million KRW (willingness to pay [WTP]: 30 million KRW per 1 Quality-adjusted life year [QALY]); it is not cost-effective compared to laparotomy. However, as previously described, we found that the RPN group showed comparable or superior perioperative outcomes compared to the OPN group (Tables 2–4). From there results, we tentatively concluded that RPN should be considered especially in patients with highly complex renal tumors in high-volume centers.
The current study has several limitations. First, even with a large tertiary center cohort, the study population was still small due to the rarity of highly complex renal tumors (RENAL nephrometry score ≥ 10). Accordingly, the events of recurrence and mortality were rare, which prevented a clear analysis of RFS and OS. In addition, each case of RPN was performed after 2008 (toward the latter half of the study period); accordingly, the results may have been affected by each surgeon’s learning curve and RPN experience. In fact, the majority of cases were performed by two surgeons (S.E.L. and S.S.B.) with extensive and high-volume robotic experience; some of the cases (9.4%) were performed by a single low-volume surgeon. This surgeon-related factor may certainly have biased some part of the perioperative outcomes. Notably, in subsequent subgroup analysis of perioperative outcomes between high and low volume surgeons, there were significant differences between the groups in terms of operation time, EBL, WIT, and intraoperative transfusion rate (S1 Table). However, we found no significant changes even after excluding the data of a low volume surgeon in perioperative outcomes (S2 Table). Regarding this issue, it is important to note that these study findings should not be generalized to the entire urological community; RPN is reserved for experienced robotic surgeons and high-volume centers of excellence.
Conclusion
RPN performed in patients with highly complex renal tumors (RENAL nephrometry score ≥ 10) offers perioperative, functional, and oncologic outcomes comparable to those associated with OPN. Subsequently, we can extrapolate to suggest that the indication for RPN is broadening for all renal tumors, regardless of surgical difficulty. Longer follow-up studies with larger ample sizes and randomized controlled trials are awaited to verify these results.
Supporting information
(XLSX)
(TIF)
(PDF)
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913–24. 10.1016/j.eururo.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 2.Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, et al. A prospective, randomized EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011;59:543–52. 10.1016/j.eururo.2010.12.013 [DOI] [PubMed] [Google Scholar]
- 3.Hansen J, Sun M, Bianchi M, Rink M, Tian Z, Hanna N, et al. Assessment of cancer control outcomes in patients with high-risk renal cell carcinoma treated with partial nephrectomy. Urology 2012;80:347–53. 10.1016/j.urology.2012.04.043 [DOI] [PubMed] [Google Scholar]
- 4.Kopp RP, Mehrazin R, Palazzi KL, Liss MA, Jabaji R, Mirheydar HS, et al. Survival outcomes after radical and partial nephrectomy for clinical T2 renal tumours categorised by R.E.N.A.L. nephrometry score. BJU Int 2014;114:708–18. 10.1111/bju.12580 [DOI] [PubMed] [Google Scholar]
- 5.Long CJ, Canter DJ, Kutikov A, Li T, Simhan J, Smaldone M, et al. Partial nephrectomy for renal masses ≥7 cm: technical, oncological and functional outcomes. BJU Int 2012;109:1450–56. 10.1111/j.1464-410X.2011.10608.x [DOI] [PubMed] [Google Scholar]
- 6.Becker F, Roos FC, Janssen M, Brenner W, Hampel C, Siemer S, et al. Short-term functional and oncologic outcomes of nephron-sparing surgery for renal tumours ≥ 7 cm. Eur Urol 2011;59:931–7. 10.1016/j.eururo.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: kidney cancer, V.2.2011. National Comprehensive Cancer Network Web site. Available at: http://www.nccn.org; 2011 [accessed 06.07.11]. [DOI] [PubMed]
- 8.Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renalmass. J Urol 2009;182:1271–9. 10.1016/j.juro.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 9.Patel HD, Mullins JK, Pierorazio PM, Jayram G, Cohen JE, Matlaga BR, et al. Trends in renal surgery: robotic technology is associated with increased use of partial nephrectomy. J Urol 2013;189:1229–35. 10.1016/j.juro.2012.10.024 [DOI] [PubMed] [Google Scholar]
- 10.Ficarra V, Bhayani S, Porter J, Buffi N, Lee R, Cestari A, et al. Robot-assisted partial nephrectomy for renal tumors larger than 4 cm: results of a multicenter, international series. World J Urol 2012;30:665–70. 10.1007/s00345-012-0943-9 [DOI] [PubMed] [Google Scholar]
- 11.Brandao LF, Zargar H, Autorino R, Akca O, Laydner H, Samarasekera D, et al. Robot-assisted partial nephrectomy for ≥ 7 cm renal masses: a comparative outcome analysis. Urology 2014;84:602–8. 10.1016/j.urology.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 12.Malkoc E, Ramirez D, Kara O, Maurice MJ, Nelson RJ, Caputo PA, et al. Robotic and open partial nephrectomy for localized renal tumors larger than 7 cm: a single-center experience. World J Urol 2017;35:781–7. 10.1007/s00345-016-1937-9 [DOI] [PubMed] [Google Scholar]
- 13.Masson-Lecomte A, Yates DR, Bensalah K, Vaessen C, de la Taille A, Roumiguié M, et al. Robot-assisted laparoscopic nephron sparing surgery for tumors over 4 cm: operative results and preliminary oncologic outcomes from a multicenter French study. Eur J Surg Oncol 2013;39:799–803. 10.1016/j.ejso.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 14.Bertolo R, Autorino R, Simone G, Derweesh I, Garisto JD, Minervini A, et al. Outcomes of Robot-assisted Partial Nephrectomy for Clinical T2 Renal Tumors: A Multicenter Analysis (ROSULA Collaborative Group). Eur Urol 2018;74:226–32. 10.1016/j.eururo.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 15.Gupta GN, Boris R, Chung P, Linehan WM, Pinto PA, Bratslavsky G. Robot-assisted laparoscopic partial nephrectomy for tumors greater than 4 cm and high nephrometry score: feasibility, renal functional, and oncological outcomes with minimum 1 year follow-up. Urol Oncol 2013;31:51–6. 10.1016/j.urolonc.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long JA, Yakoubi R, Lee B, Guillotreau J, Autorino R, Laydner H, et al. Robotic versus laparoscopic partial nephrectomy for complex tumors: comparison of perioperative outcomes. Eur Urol 2012;61:1257–62. 10.1016/j.eururo.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Ma X, Huang Q, Du Q, Gong H, Shang J, et al. Comparison of robot-assisted and laparoscopic partial nephrectomy for complex renal tumours with a RENAL nephrometry score ≥7: peri-operative and oncological outcomes. BJU Int 2016;117:126–30. 10.1111/bju.13214 [DOI] [PubMed] [Google Scholar]
- 18.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182:844–53. 10.1016/j.juro.2009.05.035 [DOI] [PubMed] [Google Scholar]
- 19.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 2010;58:398–406. 10.1016/j.eururo.2010.06.032 [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol 2006;49:798–805. 10.1016/j.eururo.2005.11.035 [DOI] [PubMed] [Google Scholar]
- 23.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–74. 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 24.Cacciamani GE, Medina LG, Gill T, Abreu A, Sotelo R, Artibani W, et al. Impact of Surgical Factors on Robotic Partial Nephrectomy Outcomes: Comprehensive Systematic Review and Meta-Analysis. J Urol 2018;200:258–74. 10.1016/j.juro.2017.12.086 [DOI] [PubMed] [Google Scholar]
- 25.Garisto J, Bertolo R, Dagenais J, Sagalovich D, Fareed K, Fergany A, et al. Robotic versus open partial nephrectomy for highly complex renal masses: Comparison of perioperative, functional, and oncological outcomes. Urol Oncol. 2018;36:471.e1–471.e9 [DOI] [PubMed] [Google Scholar]
- 26.Arora S, Abaza R, Adshead JM, Ahlawat RK, Challacombe BJ, Dasgupta P, et al. 'Trifecta' outcomes of robot-assisted partial nephrectomy in solitary kidney: a Vattikuti Collective Quality Initiative (VCQI) database analysis. BJU Int 2018;121:119–23. 10.1111/bju.13967 [DOI] [PubMed] [Google Scholar]
- 27.Kang M, Gong IH, Park HJ, Sung HH, Jeon HG, Jeong BC, et al. Predictive Factors for Achieving Superior Pentafecta Outcomes Following Robot-Assisted Partial Nephrectomy in Patients with Localized Renal Cell Carcinoma. J Endourol 2017;31:1231–6. 10.1089/end.2017.0369 [DOI] [PubMed] [Google Scholar]
- 28.Hillyer SP, Bhayani SB, Allaf ME, Rogers CG, Stifelman MD, Tanagho Y, et al. Robotic partial nephrectomy for solitary kidney: a multi-institutional analysis. Urology 2013;81:93–7. 10.1016/j.urology.2012.08.055 [DOI] [PubMed] [Google Scholar]
- 29.Zargar H, Bhayani S, Allaf ME, Stifelman M, Rogers C, Larson J, et al. Comparison of perioperative outcomes of robot-assisted partial nephrectomy and open partial nephrectomy in patients with a solitary kidney. J Endourol 2014;28:1224–30. 10.1089/end.2014.0297 [DOI] [PubMed] [Google Scholar]
- 30.Tanagho YS, Kaouk JH, Allaf ME, Rogers CG, Stifelman MD, Kaczmarek BF, et al. Perioperative complications of robot-assisted partial nephrectomy: analysis of 886 patients at 5 United States centers. Urology 2013;81: 573–9. 10.1016/j.urology.2012.10.067 [DOI] [PubMed] [Google Scholar]
- 31.Volpe A, Blute ML, Ficarra V, Gill IS, Kutikov A, Porpiglia F, et al. Renal ischemia and function after partial nephrectomy: a collaborative review of the literature. Eur Urol 2015;68:61–74. 10.1016/j.eururo.2015.01.025 [DOI] [PubMed] [Google Scholar]
- 32.Buse S, Hach CE, Klumpen P, Schmitz K, Mager R, Mottrie A, et al. Cost-effectiveness analysis of robot-assisted vs. open partial nephrectomy. Int J Med Robot. 2018;14:e1920 10.1002/rcs.1920 [DOI] [PubMed] [Google Scholar]
- 33.Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Hu JC. Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol. 2012;187:1392‐1398. 10.1016/j.juro.2011.11.089 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(TIF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.