Abstract
Fin whales (Balaenoptera physalus) have a global distribution, but the population inhabiting the Gulf of California (GoC) is thought to be geographically and genetically isolated. However, their distribution and movements are poorly known. The goal of this study was to describe fin whale movements for the first time from 11 Argos satellite tags deployed in the southwest GoC in March 2001. A Bayesian Switching State-Space Model was applied to obtain improved locations and to characterize movement behavior as either “area-restricted searching” (indicative of patch residence, ARS) or “transiting” (indicative of moving between patches). Model performance was assessed with convergence diagnostics and by examining the distribution of the deviance and the behavioral parameters from Markov Chain Monte Carlo models. ARS was the predominant mode behavior 83% of the time during both the cool (December-May) and warm seasons (June-November), with slower travel speeds (mean = 0.84 km/h) than during transiting mode (mean = 3.38 km/h). We suggest ARS mode indicates either foraging activities (year around) or reproductive activities during the winter (cool season). We tagged during the cool season, when the whales were located in the Loreto-La Paz Corridor in the southwestern GoC, close to the shoreline. As the season progressed, individuals moved northward to the Midriff Islands and the upper gulf for the warm season, much farther from shore. One tag lasted long enough to document a whale’s return to Loreto the following cool season. One whale that was originally of undetermined sex, was tagged in the Bay of La Paz and was photographed 10 years later with a calf in the nearby San Jose Channel, suggesting seasonal site fidelity. The tagged whales moved along the western GoC to the upper gulf seasonally and did not transit to the eastern GoC south of the Midriff Islands. No tagged whales left the GoC, providing supporting evidence that these fin whales are a resident population.
Introduction
Population ecology has traditionally focused on understanding temporal fluctuations of animal abundance, but how animals move over time is fundamental for understanding population processes, and it is still relatively poorly understood[1]. Movement is an essential component in the life history and habitat use of individuals [2]. Movement is defined as a change in the spatial location of an individual in time, driven by processes that act across multiple spatial and temporal scales, as a strategy for locating suitable breeding or feeding habitats, moving toward or away from conspecifics, or just to relocate [3,4]. Understanding animal movement is also important for wildlife conservation planners, who are interested in maintaining the connectivity between designated nature reserves across large geographic areas [5].
The development of Argos Platform Terminal Transmitter (PTT) technology for satellite telemetry [6,7] has played an important role in movement and migration studies for many species around the world, especially thanks to advances in spatio-temporal data collection methods [5]. While the deployment of tracking devices can impact the welfare of equipped animals, and both tags and deployment efforts can be expensive [8–10], the benefits often outweigh these costs. Through the use of telemetry, it is currently possible to track and record data about an animal’s survival, reproduction, behavior, and physiology [8]. Recent tags characterize the horizontal and vertical movements of individuals while recording their physiological state, as they travel across entire continents or to the most remote regions of the world´s oceans [11–14]. Despite the iconic status of baleen whales in conservation, our knowledge about their movements is still scarce for many species due to the remoteness or seasonal inaccessibility of their habitats. In some cases their abundance is low, further complicating data collection [15–17].
Understanding the causes of movement has been identified as one of the key challenges in the ecology of marine megafauna [18]. Fortunately, the development of electronic tags and statistical methods to analyze animal movement has improved baleen whale ecology research substantially [19–22]. Among these, Bayesian switching state-space modeling (SSM) has been used to analyze animal movement from satellite telemetry [23–26] and extended to make inferences about behavioral modalities, enabling a better understanding of the interaction between an animal’s behavior and its environment [24,26,27].
The distribution and population structure of the globally distributed fin whales (Balaenoptera physalus) appears to be more complex than previously thought. Their migration patterns do not conform to seasonal movement from summer feeding grounds to winter breeding grounds traditionally posited for baleen whales [28,29]. Some fin whale populations stray from the traditional whale migration framework by occurring in isolation, like those from the Mediterranean Sea [29–31] and the Gulf of California (GoC) [32,33].
Genetic and acoustic evidence suggests that fin whales from the GoC constitute a unique and apparently isolated population in the Eastern North Pacific Ocean [34,35]. Existing evidence indicates an increased presence in winter and spring in some areas of the GoC, possibly related to prey availability, and a subsequent decline during the summer when their primary prey is least abundant, suggesting some seasonal movements for this population within the GoC [33,36]. Additionally, occasional temporal and spatial overlap in song types with other populations suggests an exchange from the adjacent Eastern North Pacific [36–38]. Available data neither support nor refute the hypothesis that fin whales are residents of the GoC. Sighting data from the southern GoC and the Pacific coast of Baja California give no indication that fin whales migrate between the GoC and the Pacific Ocean [36]. This is the main reason previous researchers have recommended satellite tagging in addition to genetic and photo-identification techniques as valuable tools for examining this hypothesis and for better describe their movements [39].
Information about the distribution and general movements of fin whales in the GoC is limited and comes primarily from the 1990s. Most recent publications have focused on genetics and acoustics, but do not address the movement patterns of fin whales within the GoC. The goal of this study is to fill this knowledge gap by describing fin whale movements and their inferred behavior in the GoC in space and time, using the first satellite telemetry data gathered on this supposedly resident population.
Materials and methods
Data
Ethics statement
Tagging occurred under the permit Oficio No. DOO 02–0427 from the Mexican Secretaría de Medio Ambiente y Recursos Naturales. The study also was conducted under U.S. National Marine Fisheries Service (NMFS) Permit No. 369–1440, authorizing close approach and deployment of implantable satellite tags on large whales, which are protected by the 1972 Marine Mammal Protection Act and the 1973 Endangered Species Act in the United States. All tagging procedures described in this permit, and used in this manuscript, were subjected to an internal NMFS and external review by veterinarians and other marine mammal researchers prior to approval. In addition, this study was carried out in strict accordance with the policies and guidelines of the Oregon State University Institutional Animal Care and Use Committee (IACUC), composed of veterinarians and other university administrators, under IACUC Permit No. 2284. IACUC acceptance assures that the research follows its guidelines for humane care and use while meeting its objectives to reduce, replace, and refine the use of animals in research.
Study area
The GoC is a semi-enclosed sea adjacent to the Eastern North Pacific Ocean, renowned for its high productivity and biological diversity [40–44]. It is located between 107–115°W and 23–32°N, with a length of 1,100 km and a width ranging from 108 to 234 km [45]. The GoC is characterized by six basins with depths exceeding 2,000 m, and containing steep slopes, narrow and wide continental shelves, numerous islands, and coastal lagoons [46]. Two distinct seasons can be identified in the GoC: a highly productive cool season during the northern hemisphere's winter and spring, and a less productive warm season in the boreal summer and autumn [44,47,48].
Satellite tag deployment
Fieldwork took place from 25–31 March 2001 in the southwestern GoC, along the east coast of the Baja California Peninsula, Mexico. Of 11 Argos-monitored radio tags, 10 were deployed on the east side of Carmen Island, off Loreto, and one was deployed in the Bay of La Paz. All tagged individuals were adults without calves and of unknown sex. Tags were deployed from a small vessel (~7 m) using a modified air-powered system [19,49], and placed as close to the midline of the body as possible. Tags were designed for nearly complete implantation into the blubber layer, and consisted of a Telonics ST-15 UHF Argos transmitter and two Duracell 2/3 A lithium batteries housed in a stainless steel cylinder (19 cm long by 1.9 cm in diameter). Details of tag design, construction, and attachment are described in Mate et al. [19]. To prolong battery life, the transmitters were programmed for continuous transmission for 4 hours every day during the time that satellites passed overhead for the first 90 days, and then for 4 hours every other day thereafter.
Argos data processing
Tagged whales were tracked using the Argos satellite-based system that assigns a quality to each location, depending, among other things, on the number and temporal distribution of transmissions received per satellite pass (50). The accuracy associated with each Argos satellite location is reported as one of seven possible location classes (LC), in descending order of accuracy: 3, 2, 1, 0, A, B, Z and ranging from less than 200 m (LC 3) to greater than 5 km (LC B) [50,51]. Prior to analysis, Argos locations were filtered by LC as follows. First, locations of class Z were removed because of the unbounded errors associated with this class. Lower-quality LCs (LC 0, A, or B) were not used if they were received within 20 min of higher-quality locations (LC 1, 2, or 3). Finally, duplicate locations were discarded.
Modeling
State-space modeling
The Bayesian SSM developed by Jonsen et al. [24–26] was applied to the filtered Argos locations for each track, using the R programming language v. 2.12.1 [52] and WinBUGS v. 1.4.3 [53]. The SSM is a time-series model that allows unobservable, true states to be inferred from observed data, while accounting for errors arising from imprecise observations and from stochasticity in the process being studied [23].
The model provided regularized tracks for each tagged whale with one estimated location per day, after accounting for Argos satellite location errors (based on Vincent et al., [51] and the movement dynamics of the animals. The SSM ran two Markov chain Monte Carlo (MCMC) simulations each for 30,000 iterations, where the first 10,000 iterations were discarded as a burn-in, and the remaining iterations were thinned by removing every fifth one [20,24]. This process left two 4,000-iteration long chains for analysis.
Analysis of movement behavior
Behavioral switching models classify animal movement behavior into coarse modes at temporal scales greater than the minimum observed sampling interval, based on fundamental differences in movement inferred from the observed locations. These tools are useful in identifying when and where animals engage in different activities (e.g., searching, foraging, resting, migrating [8,11,22].
Included in our SSM model implementation was the classification of locations into two behavioral modes: transiting (mode 1) and area-restricted searching (ARS; mode 2). These were based on mean turning angles (θ) and autocorrelation in speed and direction (γ). Even though only two behavioral modes were modeled, the posterior means of the MCMC samples provided continuous values between 1 and 2 [20,24]. As in Jonsen et al. [26] and Bailey et al. [20], we classified observations with posterior means greater than 1.75 as ARS behavior, and observations with posterior means lower than 1.25 as transiting behavior [21,22]. Locations with posterior mean values in between these cutoffs were labeled “uncertain” [24,26,27].
MCMC convergence assessment
Considering the small number of tagged individuals, we checked each track thoroughly to ensure all available tracks were accurately represented in our models for subsequent behavioral interpretation. We used the deviance and behavior parameters of MCMC convergence as indicator metrics to assess model convergence through a series of checks on the SSM output as follows:
The MCMC runs were visually verified for random travel along the sampling space and compared between the two generated chains [54,55].
Comparative trends in the cumulative averages and standard deviations for three parameters (deviance, θ, and γ) were visually examined to verify that the chains stabilized and to compare the relative performance between the two chains.
The resulting distributions of each parameter were visually inspected to identify anomalies like bimodality or incomplete tails, which would indicate departures from normality [56].
To more thoroughly assess the performance of each modeled track, we derived six additional metrics from the Argos data: a) the mean number of filtered locations per day, b) the standard deviation of the number of filtered locations per day, c) the input:output ratio of Argos:SSM locations, d) the total number of tracking days, e) the total number of Argos locations filtered, and f) the ratio of total number of Argos locations filtered to the total number of tracking days. We used simple linear regression modeling in the R software to determine whether any of these track-level metrics influenced the respective deviance (i.e., -2 × log-likehood + a standardizing factor) from the SSM [54,55,57].
Post-processing
SSM locations occurring on land
Due to the long and narrow geometry of the GoC as well as the presence of numerous islands, a number of estimated SSM locations occurred on land (since the SSM method is not land aware). To ensure the SSM tracks did not cross over land, we developed an ad-hoc method to move these locations over water. For this purpose, we used the 95% credible limits in longitude and latitude provided by the SSM respectively as the semi-major and semi-minor axes of an ellipse around each land-based SSM location (with the premise that a location can fall anywhere inside this ellipse with 95% probability) through a custom script in Python v.2.7 [58,59] For each ellipse, the land-based portion was deleted and the centroid of the remaining ocean-based portion was calculated and replaced for each land location in the track, while retaining the behavioral mode originally estimated for the land-based locations by the SSM. A final set of SSM tracks was built after the land-based locations were corrected, from which speed and distance between location pairs were calculated by behavioral mode in Python [59].
Seasonality
The ocean surface conditions of the GoC are dominated by atmospheric forcing and ocean dynamics. Higher temperatures are found at the head and the mouth of the gulf during the summer while lower temperatures are found in the northern half and around the Midriff Islands throughout the year. As a result, two climatic seasons exist: a cool season from December to May and a warm season from June to November [41,44,48,60–63]. Considering this, the resulting tracks were split according to the timing of the cool and warm seasons to understand how fin whale movements were distributed seasonally in time and space. Maps of the movements and inferred behavioral mode where created for each season in ArcGIS v.10.3 [64].
Results
A total of 11 satellite tags were deployed in 2001. One tag (PTT 23033) did not send any valid location and another one (PTT 10840) only transmitted five locations, so both were excluded from further analyses. The remainder of the tags (n = 9) transmitted a total of 607 locations (after excluding LC Z). Mean track duration was 70 days (± SD 61 days). The mean number of locations per day was 1.7 (± SD 0.6 locations per day) (Table 1).
Table 1. Summary of tracking data for 11 Argos-monitored radio tags deployed on fin whales in the Gulf of California in 2001.
For each SSM modeled track, D is the deviance and DIC is the deviance information criterion.
| PTT | Transmission date | Tracking days | Number of locations | SSM results | |||
|---|---|---|---|---|---|---|---|
| Deployed | Last message | Total Locs | Locs/day | D | DIC | ||
| 829 | 2001-Mar-26 | 2001-Apr-30 | 35 | 8 | 1.2 | -184 | -179 |
| 830 | 2001-Mar-31 | 2001-May-27 | 57 | 32 | 1.3 | -64.3 | -19.7 |
| 849 | 2001-Mar-30 | 2001-Sep-22 | 176 | 242 | 1.9 | -1176 | -922 |
| 824 | 2001-Mar-31 | 2001-Apr-20 | 20 | 51 | 2.6 | -51.4 | -20.1 |
| 833 | 2001-Mar-27 | 2001-Apr-28 | 32 | 78 | 2.6 | -188 | -139 |
| 834 | 2001-Mar-22 | 2001-Apr-11 | 20 | 13 | 1.1 | -111 | -79.5 |
| 836 | 2001-Mar-26 | 2001-Aug-26 | 153 | 50 | 1.1 | 62.92 | 126.1 |
| 840* | 2001-Mar-26 | 2001-Aug-05 | 10 | 5 | NA | NA | NA |
| 843 | 2001-Mar-26 | 2001-Apr-21 | 26 | 31 | 2.1 | -143 | -111 |
| 23033* | 2001-Mar-31 | 2001-Apr-10 | 10 | 0 | NA | NA | NA |
| 23038 | 2001-Mar-27 | 2001-Jul-23 | 118 | 96 | 1.5 | -542 | -390 |
* These tags were excluded from analysis due to too few data points.
MCMC convergence assessment
The distribution of the deviance and the movement parameters (θ and γ) in the MCMC chains indicated that these parameters were generally well-behaved for all tracks except PTT 829, which only had eight Argos locations (see S1 File).
The linear regression of the deviance on the derived track metrics indicated that only the total number of locations per track had a reasonable explanatory power on the deviance value (R2 = 0.847, F = 38.95, p < 0.0004). The other metrics did not have a significant relationship to the deviance (Fig 1). As a result, the SSM output for PTT 829 was discarded (see S1 File).
Fig 1. The relationship between the deviance and the derived per-track metrics.
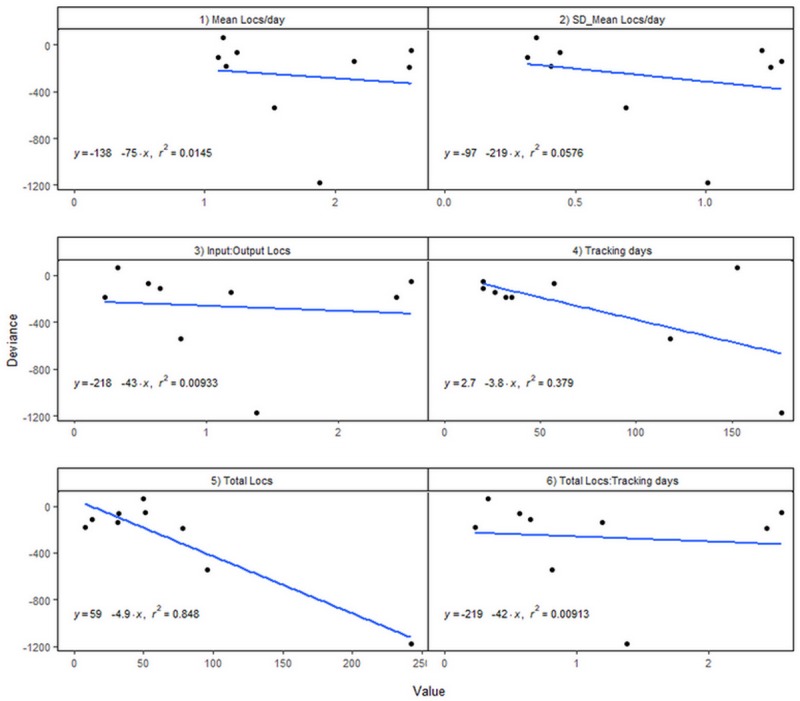
a) mean number of locations (Locs) per day; b) SD of mean number of locations per day; c) the ratio of Argos:SSM locations; d) total number of tracking days; e) total number of locations per track; and f) the ratio of total number of Argos locations to the total number of tracking days.
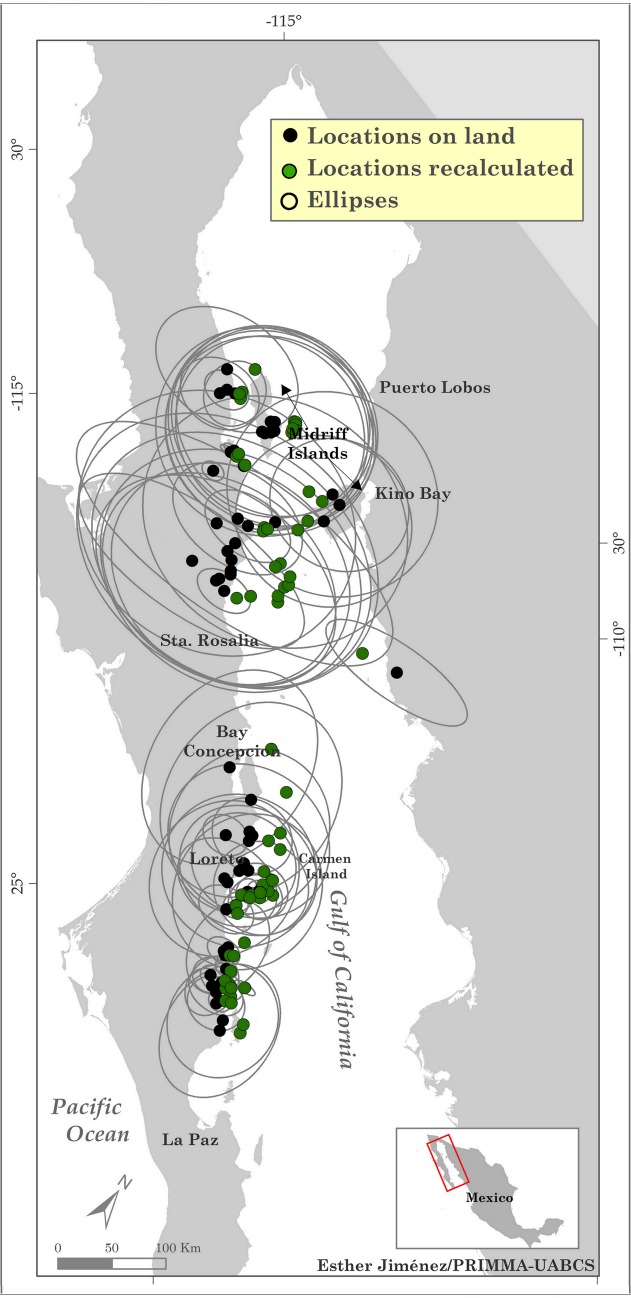
The remaining eight good Argos tracks generated 562 SSM locations, of which 11% occurred on land (Fig 2).
Fig 2. Correction of land-based SSM locations.
Ellipses representing the SSM 95% credible limits in longitude and latitude are shown in black. Black dots are locations on land, and green dots are the respective corrected locations.
Behavioral inferences
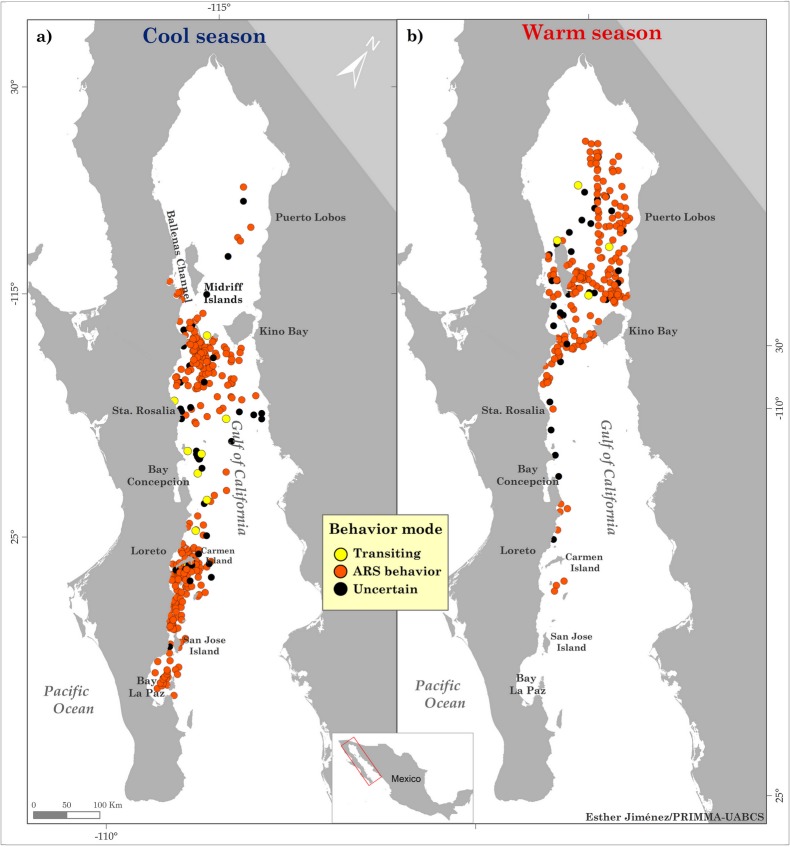
Behavioral modes inferred by the SSM indicated that ARS behavior was dominant for both the cool (83% of the SSM locations) and the warm (84% of the SSM locations) seasons during the monitoring period. Transiting behavior occurred for 3–4% of the SSM locations and uncertain behavior corresponded to 14% of the SSM locations for each season. Spatially, ARS behavior during the cool season was located mainly in two areas: 1) Loreto-La Paz Corridor, where the inshore habitats were the principal habitat for fin whales, and 2) the area between Santa Rosalia and the southern Midriff Islands. The space in between areas (1) and (2) contained tracks of fin whales exhibiting uncertain and transiting modes, indicating that the whales were not using these areas for long periods, but merely passing through (Fig 3, left). During the warm season, tracking data showed the preferred areas were in the center of the GoC, with ARS behavior occurring around Midriff Islands and along the coast of Sonora State (from Tiburon Island to Puerto Peñasco). Transiting and uncertain behaviors were also exhibited around the Midriff Islands (Fig 3, right).
Fig 3. Spatial distribution of the classification of behavioral mode by season for fin whale SSM locations.
Left is the cool season (from December to May; n = 333) and right is the warm season (from June to November; n = 229).
On average, fin whales traveled 25 km per day (range: 22.94–28.31 km), with a mean speed of 1.06 km/h. The minimum total tracked distance was 1,034 km and the maximum was 13,199 km (Table 2). During ARS mode, travel speed was slower (mean = 0.84 km/h) than during transiting mode (mean = 3.48 km/h).
Table 2. Mean distances and speeds traveled between consecutive daily SSM locations for eight fin whales tracked in the Gulf of California in 2001.
| PTT | Distance (km) | Speed (km/h) | Speed by behavioral mode (km/h) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Total track | Mean | SD | Min | Max | Transiting | ARS | Uncertain | |
| 824 | 27.89 | 22.8 | 0.66 | 115.4 | 6136.6 | 1.16 | 0.95 | 0.03 | 4.81 | NA | 1.01 | 0.25 |
| 830 | 23.51 | 14.68 | 2.57 | 64.68 | 1034.55 | 0.98 | 0.61 | 0.11 | 2.69 | NA | 0.96 | 1.58 |
| 833 | 27.34 | 22.06 | 0.66 | 115.4 | 6861.25 | 1.14 | 0.92 | 0.03 | 4.81 | NA | 0.96 | 0.21 |
| 834 | 26.33 | 21.76 | 0.66 | 115.4 | 7108.39 | 1.1 | 0.91 | 0.03 | 4.81 | NA | 0.5 | 1.18 |
| 836 | 23.38 | 21.36 | 0.07 | 129 | 9867.44 | 0.97 | 0.89 | 0 | 5.38 | 5.09 | 0.66 | 0.06 |
| 843 | 22.94 | 21.18 | 0.07 | 129 | 10252.9 | 0.96 | 0.88 | 0 | 5.38 | ND | 0.65 | 0.37 |
| 849 | 28.31 | 23.29 | 0.66 | 115.4 | 5718.87 | 1.18 | 0.97 | 0.03 | 4.81 | 2.54 | 1.1 | 0.55 |
| 23038 | 23.49 | 21.23 | 0.07 | 129 | 13199.65 | 0.98 | 0.88 | 0 | 5.38 | 2.5 | 0.88 | 0.03 |
| Mean | 25.4 | 0.68 | 114.16 | 7522.46 | 1.06 | 0.03 | 4.76 | 3.38 | 0.84 | 0.53 | ||
Seasonality
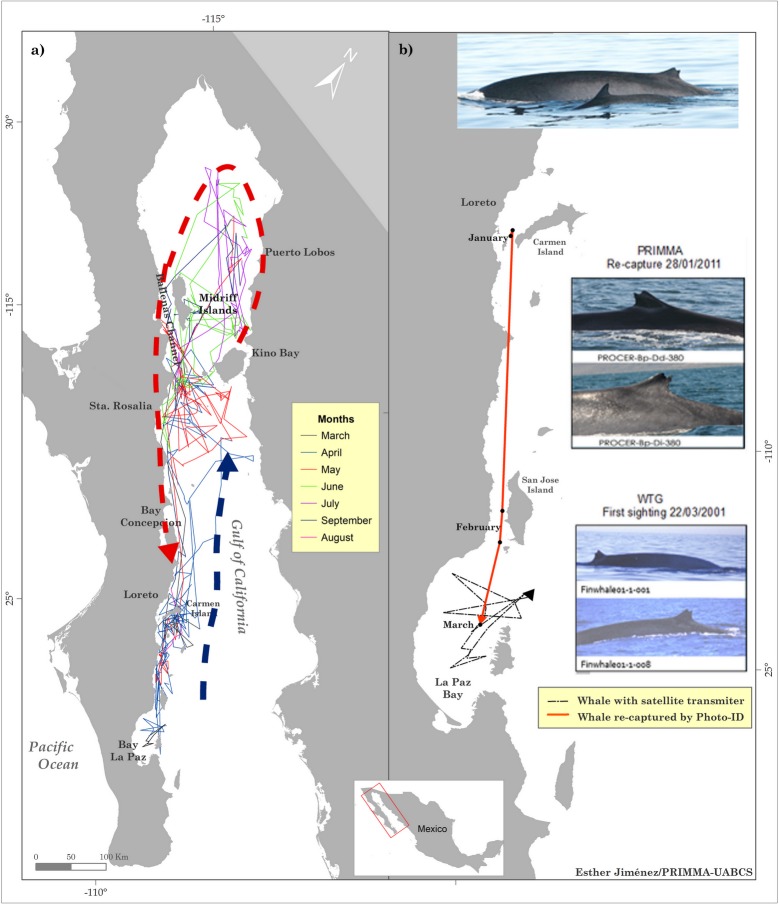
All the tags transmitted into the cool season. Two whales (PTT 10824 and 10833) moved between Loreto and San Jose Island during March and April and then their tags stopped transmitting. The individual tagged in the Bay of La Paz (PTT 10834) stayed in the area until April, when the tag stopped transmitting. The individual with the PTT 830 moved northward towards Santa Rosalia, where that tag also stopped transmitting. The rest of the whales (PTT 849, 10836 and 23088) migrated northward between April and May along the western margin of the GoC, and continued to send data up until the warm season (Fig 4A).
Fig 4.
a) Seasonality of movements of eight fin whales tagged in March 2001 from SSM-derived locations. Portions of the tracks associated to specific months, the dashed lines represent the purported migratory pattern; b) the black line indicates the whale with tag PTT 10834 tagged and photographed in the Bay of La Paz by the Whale Telemetry Group (WTG), Marine Mammal Institute, Oregon State University during 2001. The orange line indicates the photographic re-capture location of this whale in 2011 by Programa de Mamíferos Marinos (PRIMMA/UABCS), Universidad Autónoma de Baja California Sur, Mexico, in the company of a calf.
During the warm season, only three individuals moved northward past Santa Rosalia and into the upper GoC through the Midriff Islands. These tags lasted for more than 100 days into the cool season and just one whale (PTT 849) moved southward in the GoC, ending up off Loreto (Fig 4A; see S2 File for individual maps of each track). A photograph of the whale with tag PTT 10834 (tagged in Bay of La Paz) was taken by the Whale Telemetry Group (WTG) at Oregon State University’s Marine Mammal Institute at the moment of tagging (in 2001), and it was matched with the fin whale photographic catalog from Programa de Investigación de Mamíferos Marinos from the Universidad Autónoma de Baja California Sur (PRIMMA/UABCS). The matching photograph, collected in the nearby San Jose Channel, was dated 28 January 2011 (10 years later), in which the whale was accompanied by a calf, evidence that the tagged individual was female (Fig 4B).
Discussion
MCMC convergence assessment
SSM analysis of animal telemetry data has been used in various ways, including to filter error-prone Argos [50] and light-based [65] locations, or to estimate unobserved behavioral states (e.g., 27,30). Indeed, SSMs have become a prominent tool in the study of animal movement [66]. However, SSMs cannot always deal with some of the issues inherent to telemetry data from marine animals, like temporal gaps in the reception of Argos locations, tracks of short duration, or unclassifiable movement behavior. Exploration of the MCMC convergence and the distribution of the deviance and the behavior parameters, was a useful way to decide to keep or discard tracks with small numbers of locations. Tracks with few Argos locations that were spread over many days led to problems of stability and convergence in all MCMC parameters. This information was confirmed by observed departures from normality of the distribution of the deviance and the behavior parameters and by the regression analyses. For this reason, the transmission schedule programmed into Argos-monitored tags needs to weigh battery preservation considerations against performance in analytical methods like SSMs to avoid erroneous interpretations about movement and inferred behavior.
Movements and behavioral inference
The present study represents the first attempt to examine the movements and behavior of fin whales in the GoC through satellite telemetry. Despite the small sample size, the information on movement data is valuable for understanding how fin whales move, use, and connect the areas along the GoC. Our findings support the general movements in time and space previously described based on photo-identification studies in the GoC. They also support the previous notion that the population does not leave the GoC, and contributes to fine-scale knowledge about the extent to which fin whales use their habitat. This information could provide the baseline for developing conservation and management efforts for this resident population.
We identified two principal destinations for fin whales in the GoC for their seasonal migration, the Loreto-La Paz Corridor and the Midriff Islands, which both showed a high percentage of ARS behavior (83–84%) for each season. ARS behavior mostly has been related to feeding activities due to the animals occupying patchily distributed areas of sufficiently abundant prey. This is because shifting between the patches increases search effort and thus decreases the likelihood of securing enough food [66]. During the cool season, fin whales travel northward along the western margin of the GoC, where environmental conditions are dominated by a combined north-south current [44,47,48,67] and outgoing tidal currents acting to aggregate euphausiids (Nyctiphanes simplex), which are the principal prey for fin whales in the GoC [68–70]. This season is also the breeding season for the euphausiids, producing more than 80% of the calyptopis larval phase in the mid-southern GoC at Carmen Island (off Loreto), San Jose Island, and the Baja California Peninsula [69,71,72]. In contrast, during the warm season, this area is mainly where the food concentrations fall due to temperature changes.
The seasonal movements showed the fin whales reduced their activity and spent time in certain habitats in the GoC. Even though there is a general lack of knowledge about how fin whales use the GoC, most of the data published refers to the Midriff Islands, where fin whales have been observed in feeding activity. However, the species could have a wider distribution to the south [39,71,73,74]. The results of this study effectively show that there are more places than in the northern GoC where fin whales are distributed during the cool season than had been previously documented. Also, in these newly observed areas, the whales exhibited the same types of behavior as in the northern feeding areas [39,71,75]. Our results are in broad agreement with seasonal movements mentioned by other authors who coincidentally recorded fin whales in the Loreto-La Paz Corridor [36] with high prey biomass known to be important for fin, blue (Balaenoptera musculus), and Bryde's whales (Balaenoptera edeni) [69,76–78]. This habitat overlap during the cool season is strongly driven by the prey species, even though these whale species are all in this krill-based foraging ground during winter and spring [20,79].
On the other hand, it is important to note that December to February is the reproductive season for fin whales. Even though the ARS mode behavior is assumed to represent feeding activity, it could also be a proxy for other social interactions [66] that relate to reproductive behavior since the data are being collected in seasonally reproductive areas.
Concerning the warm season, the tagged whales moved to the northern GoC and ARS behavior also occurred in this area, especially in the vicinity of the Midriff Islands. We suggest this movement is also related to prey distribution because this area is one of the two places (Bay of La Paz being the other place) in the GoC where euphasiids can avoid the highest temperatures of the GoC during the summer. By the end of the summer, their highest densities start to fall [80,81]. Some authors have suggested that to deal with the decline in euphausiids, fin whales exploit other potential seasonal prey source [71]. While the euphasiids decrease in biomass, that of the Pacific sardine (Sardinops sagax) increases in this area, [82] and during the autumn the sardines migrate to the south to Loreto along the western margin of the GoC. Through isotope analyses, this hypothesis has been investigated, finding that fin whales switch prey during the summer, resulting in an increase in δ15N for the euphausiid Nyctiphanes simplex in skin samples during the summer [83]. Comparing skin samples from fin and Bryde´s whales the similarity in δ15N values also coincides with the known icthyophagous habits of the Bryde’s whale [84]. Considering all this, we suggest that fin whale movements in the GoC are adapted in time and space to coincide with high densities of both euphausiid and fish prey types.
For the warm season, we suggest the main activity of fin whales, as evidenced by ARS behavior distributions, is indicative of foraging activity. At this time, their distribution is consistent with the distribution of prey species as described by other authors [33]. Also, the tracking data collected during the warm season was outside the mating and calving season for this species [85–87]. Similar behavior has been documented for other resident fin whale populations such as the Mediterranean population, which primarily exhibits ARS behavior throughout the year as they move between potential feeding areas [31,88,89]. These cases are not unique among balaenopterids, as there are other non-migratory populations found in the tropics, subtropics, and enclosed seas, such as Bryde’s whales [76] and one distinct population segment of humpback whales (Megaptera novaeangliae) in the Arabian Sea [88], which is able to subsist on year-round productivity.
The satellite telemetry data obtained in this study revealed five important details about movements fin whales in the GoC: 1) the tagged individuals did not move toward the mouth, much less leave the GoC, supporting the hypothesis of its residence. 2) The movements of the tagged whales were related in time and space to the distribution of their prey. 3) Fin whale movement behavior was consistent with foraging activity year round, as is the case for the isolated population in the Mediterranean Sea [90]. 4) No tagged whales moved to the eastern margin of the GoC to the south of the Midriff Islands (this was only observed in the upper GoC). 5) The photographic recapture of the whale with tag PTT 10384 10 years later in the company of a calf in the nearby San Jose Channel, suggests seasonal site fidelity to the southern GoC and a possible calving area during the cool season.
Finally, the fin whale movements documented in this study overlapped with anthropogenic activity in the GoC, mainly traffic by fishing and tourism vessels [91]. The main risks of this overlap include entanglements in fishing gear, and harassment by boats that approach too closely [92]. The intensity and spatial extent of these threats to marine mammals in the GoC is unclear, but sightings of entangled cetaceans or individuals with ship strike marks have been increasing in recent years in Mexico [91]. The results obtained in this study will help better identify areas where interactions between fin whales and threats need to be monitored and evaluated to make better decisions regarding development plans and to manage responses to human activities [93]. Also, we suggest a greater sampling effort during the warm season in the north of the GoC to gain a better understanding of fin whale movements during this time for which data remain scarce.
Supporting information
(PDF)
(PDF)
Acknowledgments
We thank the U.S. Office of Naval Research for sustaining support of tag development under a series of grants. Barbara Lagerquist and Mary Lou Mate of the Whale Telemetry Group, Marine Mammal Institute, Oregon State University, assisted with tagging fieldwork, while Martha Winsor and Thomas Follett provided valuable technical and analytical assistance. In particular, we thank Thomas Follett for data curation, for developing the methodology for recalculating the land-based SSM locations, and for writing the Python script to implement it. We also thank Jorge Acevedo for his comments on an early draft and Kerri Seger for reviewing the English version. The manuscript was greatly improved by comments from two anonymous reviewers.
Abbreviations
- ARS
area-restricted searching
- d
day
- DIC
Deviance Information Criterion
- GoC
Gulf of California
- LC
location class
- Locs
locations
- MCMC
Markov chain Monte Carlo
- PTT
Platform Terminal Transmitter
- SSM
state-space model
Data Availability
The data used in this study are available on Movebank (movebank.org, study name "Fin Whales Gulf of California 2001") and are published in the Movebank Data Repository under DOI: http://doi.org/10.5441/001/1.65h5s5p2.
Funding Statement
This study was funded by private donors to Oregon State University’s Endowed Marine Mammal Program. This work was also funded by Consejo Nacional de Ciencia y Tecnologia (CONACYT) from Mexico. The institution gave the scholarship No. 199425 for Doctorate Program and Scholarship No. 001507 for internship at Marina Mammals Institute at Oregon State University.
References
- 1.Turchin P. Quantitative analysis of movement: measuring and modeling population redistribution in animals and plants. Sinauer Associates, Sunderland, Mass; 1998. [Google Scholar]
- 2.Dingle H, Drake VA. What Is Migration?. Bioscience. 2007;57: 113–121. 10.1641/B570206 [DOI] [Google Scholar]
- 3.Okubo A, Levin SA. Diffusion and Ecological Problems: Modern Perspectives Second Edi Antman S, Sirovic L, Marsden JE, Wiggins S, editores. New York: Springer Science & Business; 2001. 10.1007/978-1-4757-4978-6 [DOI] [Google Scholar]
- 4.Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, et al. A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci. 2008;105: 19052–19059. 10.1073/pnas.0800375105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kays R, Crofoot MC, Jetz W, Wikelski M. Terrestrial animal tracking as an eye on life and planet. Science (80-). 2015;348: aaa2478 10.1126/science.aaa2478 [DOI] [PubMed] [Google Scholar]
- 6.Fancy SG, Pank FL, Douglas DC, Curby CH, Garner GW, Amstrup SC, et al. Satellite telemetry: a new tool for wildlife research and management. FISH Wildl Serv Washint DC. Washington, D. C.; 1988;No. FWS-PU: 61.
- 7.Cohn JP. Tracking wildlife: High-tech devices help biologists trace the movements of animals through sky and sea. Bioscience. 1999;49: 12–17. 10.1525/bisi.1999.49.1.12 [DOI] [Google Scholar]
- 8.Walker KA, Trites AW, Haulena M, Weary DM. A review of the effects of different marking and tagging techniques on marine mammals. Wildl Res. 2012;39: 15–30. 10.1071/WR10177 [DOI] [Google Scholar]
- 9.Hoel K, Barret RT, Boe KE, Lydersen C, Swenson JE. Risk assessment concerning the welfare of certain free-ranging wild mammals and birds subjected to marking. 2013. [Google Scholar]
- 10.Solsona Berga A, Wright AJ, Galatius A, Sveegaard S, Teilmann J. Do larger tag packages alter diving behavior in harbor porpoises?. Mar Mammal Sci. 2015;31: 756–763. 10.1111/mms.12179 [DOI] [Google Scholar]
- 11.Shaffer SA, Tremblay Y, Weimerskirch H, Scott D, Thompson DR, Sagar PM, et al. Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. Proc Natl Acad Sci. 2006;103: 12799–12802. 10.1073/pnas.0603715103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponganis PJ, Meir JU, Williams CL. In pursuit of Irving and Scholander: a review of oxygen store management in seals and penguins. J Exp Biol. 2011;214: 3325–3339. 10.1242/jeb.031252 [DOI] [PubMed] [Google Scholar]
- 13.McDonald BI, Ponganis PJ. Insights from venous oxygen profiles: Oxygen utilization and management in diving California sea lions. J Exp Biol. 2013;216: 3332–3341. 10.1242/jeb.085985 [DOI] [PubMed] [Google Scholar]
- 14.Hussey NE, Kessel ST, Aarestrup K, Cooke SJ, Cowley PD, Fisk AT, et al. Aquatic animal telemetry: A panoramic window into the underwater world. Science (80-). 2015;348: 1255642 10.1126/science.1255642 [DOI] [PubMed] [Google Scholar]
- 15.Montgomery S. Workshop to assess possible systems for tracking large cetaceans held at Seattle, Washington on 24–26 February 1987. Workshop report (No. PB-87-182135/XAB; OCS/MMS-87/0029). Mar Mammal Comm Washington, DC (USA). United States; 1987;
- 16.Bograd SJ, Block BA, Costa DP, Godley BJ. Biologging technologies: New tools for conservation. Introduction. Endanger Species Res. 2010;10: 1–7. 10.3354/esr00269 [DOI] [Google Scholar]
- 17.Thomisch K. Distribution patterns and migratory behavior of Antarctic blue whales. Berichte zur Polar und Meeresforschung Reports polar Mar Res. 2017;707: 1–194. 10.2312/BzPM_0707_2017 or 10.2312/BzPM_0707_2017 [DOI] [Google Scholar]
- 18.Hays GC, Ferreira LC, Sequeira AMM, Meekan MG, Duarte CM, Bailey H, et al. Key Questions in Marine Megafauna Movement Ecology The Breadth of Movement Ecology Studies. Trends Ecol Evol. 2016;31: 463–475. 10.1016/j.tree.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 19.Mate B, Mesecar R, Lagerquist B. The evolution of satellite-monitored radio tags for large whales: One laboratory’s experience. Deep Res Part II Top Stud Oceanogr. 2007;54: 224–247. 10.1016/j.dsr2.2006.11.021 [DOI] [Google Scholar]
- 20.Bailey H, Mate BR, Palacios DM, Irvine L, Bograd SJ, Costa DP. Behavioural estimation of blue whale movements in the Northeast Pacific from state-space model analysis of satellite tracks. Endanger Species Res. 2009;10: 93–106. 10.3354/esr00239 [DOI] [Google Scholar]
- 21.Leos-Barajas V, Gangloff EJ, Adam T, Langrock R, van Beest FM, Nabe-Nielsen J, et al. Multi-scale Modeling of Animal Movement and General Behavior Data Using Hidden Markov Models with Hierarchical Structures. J Agric Biol Environ Stat. 2017;22: 232–248. 10.1007/s13253-017-0282-9 [DOI] [Google Scholar]
- 22.Patterson TA, Parton A, Langrock R, Blackwell PG, Thomas L, King R. Statistical modelling of individual animal movement: an overview of key methods and a discussion of practical challenges. AStA Adv Stat Anal. Springer Berlin Heidelberg; 2017;101: 399–438. 10.1007/s10182-017-0302-7 [DOI] [Google Scholar]
- 23.Jonsen ID, Myers RA, Flemming JM. Meta-analysis of animal movement using state-space models. Ecology. 2003;84: 3055–3063. 10.1890/02-0670 [DOI] [Google Scholar]
- 24.Jonsen ID, Flemming JM, Myers RA. Robust state–space modeling of animal movement data. Ecology. 2005;86: 2874–2880. 10.1890/04-1852 [DOI] [Google Scholar]
- 25.Jonsen ID, Myers RA, James MC, Flemming JM, Myers RA. Robust hierarchical state-space models reveal diel variation in travel rates of migrating leatherback turtles. J Anim Ecol. Blackwell Publishing Ltd; 2006;75: 1046–1057. 10.1111/j.1365-2656.2006.01129.x [DOI] [PubMed] [Google Scholar]
- 26.Jonsen ID, Myers RA, James MC. Identifying leatherback turtle foraging behaviour from satellite telemetry using a switching state-space model. Mar Ecol Prog Ser. 2007;337: 255–2007. [Google Scholar]
- 27.Breed GA, Costa DP, Jonsen ID, Robinson PW, Mills-Flemming J. State-space methods for more completely capturing behavioral dynamics from animal tracks. Ecol Modell. Elsevier B.V.; 2012;235–236: 49–58. 10.1016/j.ecolmodel.2012.03.021 [DOI] [Google Scholar]
- 28.Mizroch SA, Rice DW, Zwiefelhofer D, Waite J, Perryman WL. Distribution and movements of fin whales in the North Pacific Ocean. Mamm Rev. 2009;39: 193–227. 10.1111/j.1365-2907.2009.00147.x [DOI] [Google Scholar]
- 29.Reilly SB, Bannister JL, Best PB, Brown M, Brownell Jr. RL, Butterworth DS, et al. Balaenoptera physalus. E.T2478A44210520. TIRL of TS 2013:, editor. IUCN Red List Threat Species 2013 eT2478A44210520. 2013; 20. 10.2305/IUCN.UK.2013-1.RLTS.T2478A44210520.en [DOI]
- 30.Edwards EF, Hall C, Moore TJ, Sheredy C, Redfern J V. Global distribution of fin whales Balaenoptera physalus in the post-whaling era (1980–2012). Mamm Rev. 2015;45: 197–214. 10.1111/mam.12048 [DOI] [Google Scholar]
- 31.Geijer CKA, Notarbartolo di Sciara G, Panigada S. Mysticete migration revisited: are Mediterranean fin whales an anomaly?. Mamm Rev. 2016;46: 284–296. 10.1111/mam.12069 [DOI] [Google Scholar]
- 32.Leatherwood S, Reeves RR, Perrin WFWF, Evans WE, Hobbs L. Whales, Dolphins, and Porpoises of the Eastern North Pacific and adjacent artic waters: a guide to their identification. New York: Dover Publications; 1988. [Google Scholar]
- 33.Urbán R. J, Rojas-Bracho L, Guerrero-Ruiz M, Jaramillo- Legorreta A, Findley L. T. Cetacean diversity and conservation in the Gulf of California En: Cartron J. E, Ceballos G, Felger R. S, editores. Biodiversity, ecosystems, and conservation in northern Mexico Oxford. New York, NY: Oxford University Press; 2005. pp. 276–297. [Google Scholar]
- 34.Thompson PO, Findley LT, Vidal O. 20-Hz pulses and other vocalizations of fin whales, Balaenoptera physalus, in the Gulf of California, Mexico. J Acoust Soc Am. 1992;92: 3051–3057. 10.1121/1.404201 [DOI] [PubMed] [Google Scholar]
- 35.Bérubé M, Urbán J, Dizon AAE, Brownell RL, Palsbøll PJ, Palsboll PJ, et al. Genetic identification of a small and highly isolated population of fin whales (Balaenoptera physalus) in the Sea of Cortez, Mexico. Conserv Genet. 2002;3: 183–190. org/10.1023/A:10152247 [Google Scholar]
- 36.Tershy BR, Urbán-Ramírez J, Breese D, Rojas, Bracho L, Findley LT. Are fin whales resident to the Gulf of California?. Rev Inv Cient. 1993;1: 69–72. [Google Scholar]
- 37.Tershy BR, Breese D, Strong CS, Bresse D, Strong CS. Abundance, Seasonal Distribution and Population Composition of Balanopterid Whales in the Canal de Ballenas, Gulf of California, Mexico. Rep—Int Whal Comm Spec Issue. 1990;12: 369–375. [Google Scholar]
- 38.Širović A, Oleson EM, Buccowich J, Rice A, Bayless AR. Fin whale song variability in southern California and the Gulf of California. Nature. 2017;7: 1–11. 10.1038/s41598-017-09979-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tershy B, Acevedo G, Breese D, Strong C. Diet and feeding behavior of fin and Bryde’s whales in the central Gulf of California, Mexico. Rev Inv Cient. 1993;1: 31–37. [Google Scholar]
- 40.Alvarez-Borrego S, Lara-Lara JR. The Physical Environment and Primary Productivity of the Gulf of California En: American Association of Petroleum Geologist M, editor. The Gulf and Peninsular Province of the Californias. Tulsa; 1991. p. Vol. 47, 555–567. [Google Scholar]
- 41.Santamaría-del-Angel E, Alvarez-Borrego S, Müller-Karger FE. Gulf of California biogeographic regions based on coastal zone color scanner imagery. J Geophys Res. 1994;99: 7411–7421. 10.1029/93JC02154 [DOI] [Google Scholar]
- 42.Álvarez-Salgado XA, Beloso S, Joint I, Nogueira E, Chou L, Pérez FF, et al. New production of the NW Iberian shelf during the upwelling season over the period 1982–1999. Deep Sea Res Part I Oceanogr Res Pap. Elsevier; 2002;49: 1725–1739. 10.1016/S0967-0637(02)00094-8 [DOI] [Google Scholar]
- 43.Hidalgo-González RM, Alvarez-Borrego S. Total and new production in the Gulf of California estimated from ocean color data from the satellite sensor SeaWIFS. Deep Res II. 2004;51: 739–752. 10.1016/j.dsr2.2004.05.006 [DOI] [Google Scholar]
- 44.Lluch-Cota SE, Aragón-Noriega EA, Arreguín-Sánchez F, Aurioles-Gamboa D, Jesús Bautista-Romero J, Brusca RC, et al. The Gulf of California: Review of ecosystem status and sustainability challenges. Prog Oceanogr. 2007;73: 1–26. 10.1016/j.pocean.2007.01.013 [DOI] [Google Scholar]
- 45.Castro-Aguirre JL, Balart EF, Arvizu-Martínez J. Contribución al conocimiento del origen y distribución de la ictiofauna del Golfo de California, México. Hidrobiológica. 1995;5: 57–78. [Google Scholar]
- 46.Morgan L, Maxwell S, Tsao F, Wilkinson TAC, Etnoyer P. Áreas prioritarias marinas para la conservación: Baja California al mar de Béring. Com para la Coop Ambient y Mar Conserv Biol Inst. 2005; 136. [Google Scholar]
- 47.Álvarez-Borrego S, Schwartzlose RA. Masas de agua del Golfo de California. Ciencias Mar. 1979;6: 43–63. [Google Scholar]
- 48.Soto-Mardones L, Marinone SG, Parés-Sierra A. Variabilidad espaciotemporal de la temperatura superficial del mar en el Golfo de California. Ciencias Mar. 1999;25: 1–30. [Google Scholar]
- 49.Heide‐Jørgensen MP, Kleivane L, Ølen N, Laidre KL, Jensen MV. A new technique for deploying satellite transmitters on baleen whales: Tracking a blue whale (Balaenoptera musculus) in the North Atlantic. Mar Mammal Sci. 2001;10: 949–954. 10.1111/j.1748-7692.2001.tb01309.x [DOI] [Google Scholar]
- 50.ARGOS. Argos User’s Manual. 2016. pp. 1–64. [Google Scholar]
- 51.Vincent C, Mcconnell BJ, Ridoux V, Fedak MA. Assessment of Argos Location Accuracy From Satellite Tags Deployed on Captive Gray Seals. Mar Mammal Sci. 2002;18: 156–166. 10.1111/j.1748-7692.2002.tb01025.x Cited by: 172 [DOI] [Google Scholar]
- 52.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. R Foundation for Statistical Computing. 2016. [Google Scholar]
- 53.Spiegelhalter D, Thomas A, Best N, Lunn D. WinBUGS User Manual Permission and Disclaimer. 2003. [Google Scholar]
- 54.Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Ser B (Statistical Methodol. Blackwell Publishers; 2002;64: 583–639. 10.1111/1467-9868.00353 [DOI] [Google Scholar]
- 55.Berg A, Meyer R, Yu J. Deviance Information Criterion for Comparing Stochastic Volatility Models. J Bus Econ Stat. Taylor & Francis; 2004;22: 107–120. 10.1198/073500103288619430 [DOI] [Google Scholar]
- 56.Dempster AP. The direct use of likelihood for significance testing. Stat Comput. 1997;7: 247–252. 10.1023/A:1018598421607 [DOI] [Google Scholar]
- 57.Chan JCC, Grant AL. Fast Computation of the Deviance Information Criterion for Latent Variable Models. Comput Stat Data Anal. 2016;100: 847–859. 10.1016/j.csda.2014.07.018 [DOI] [Google Scholar]
- 58.Python Language Reference. Python Software Foundation. p. v. 2.7.
- 59.Mate BR, Palacios DM, Follett T. Data from: Fin whale movements in the Gulf of California, Mexico, from satellite telemetry. Movebank Data Repository. Newport, OR; 2019. 10.5441/001/1.65h5s5p2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Argote ML, Amador A, Lavín MF. Tidal dissipation and stratification in the Gulf of California. J Geophys Res. 1995;100: 16,103–16,118. [Google Scholar]
- 61.Beier E. A Numerical Investigation of the Annual Variability in the Gulf of California. J Phys Oceanogr. 1997;27: 615–632. 10.1175/1520-0485 [DOI] [Google Scholar]
- 62.Marinone SG. A three-dimensional model of the mean and seasonal circulation of the Gulf of California. J Geophys Res. 2003;108: 1–27. 10.1029/2002JC001720 [DOI] [Google Scholar]
- 63.Navarro-Olache LF, Lavín MF, Alvarez-Sánchez LG, Zirino A. Internal structure of SST features in the central Gulf of California. Deep-Sea Research Part II: Topical Studies in Oceanography. 2004. pp. 673–687. 10.1016/j.dsr2.2004.05.014 [DOI] [Google Scholar]
- 64.ESRI. ESRI ArcGIS Desktop and Spatial Analyst Extension: Release 10.3. Redlands, CA: Environmental Systems Research Institute; 2015. [Google Scholar]
- 65.Hill RD. Theory of geolocation by light levels. Elephant seals Popul Ecol Behav Physiol Univ Calif Press Berkeley. 1994; 227–236. [Google Scholar]
- 66.Kareiva P, Odell G. Swarms of Predators Exhibit “Preytaxis” if Individual Predators Use Area-Restricted Search. Am Nat. 1987;130: 233–270. 10.1086/284707 [DOI] [Google Scholar]
- 67.Rosas Cota A. Corrientes geostróficas en el Golfo de California en la superficie y a 200 metros, durante las estaciones de invierno y verano. Calif Coop Ocean Fish Investig. 1976;19: 89–106. [Google Scholar]
- 68.Antezana J. T. Eufausidos de la costa de Chile. Su rol en la economía del mar. Rev Biol mar, Valparaiso. 1970;14: 19–27. [Google Scholar]
- 69.Gendron D. Population structure of daytime surface swarms of Nyctiphanes simplex (Crustacea: Euphausiacea) in the Gulf of California, Mexico. Mar Ecol Prog Ser. 1992;87: 1–6. [Google Scholar]
- 70.Gómez-Gutiérrez J, Martínez-Gómez S, Robinson CJ. Seasonal growth, molt, and egg production rates of Nyctiphanes simplex (Crustacea: Euphausiacea) juveniles and adults in the Gulf of California. Mar Ecol Prog Ser. 2012;455: 173–194. 10.3354/meps09631 [DOI] [Google Scholar]
- 71.Tershy BR. Body size, diet, habitat use, and social behavior of Balaenoptera whales in the Gulf of California. J Mammal. 1992;73: 477–486. [Google Scholar]
- 72.Croll DA, Tershy BR, Hewitt RP, Demer DA, Fiedler PC, Smith SE, et al. An integrated approch to the foraging ecology of marine birds and mammals. Deep Res II. 1998;45: 1353–1371. 10.1016/S0967-0645(98)00031-9 [DOI] [Google Scholar]
- 73.Breese D, Tershy BR. Relative abundance of cetacea in the Canal de Ballenas, Gulf of California. Mar Mammal Sci. 1993;9: 319–324. 10.1111/j.1748-7692.1993.tb00460.x [DOI] [Google Scholar]
- 74.Silber GK, Newcomer MW, Silber PC, Pérez-Cortés M H, Ellis GM. Cetaceans of the northern Gulf of California: distribution, occurrence, and relative abundance. Mar Mammal Sci. 1994;10: 283–298. [Google Scholar]
- 75.Tershy BR, Breese D, Strong CS. Abundance, seasonal distribution and population composition of balaenopterid whales in the Canal de Ballenas, Gulf of California, Mexico. Rept Int Whal Commn. 199012: 369–375. [Google Scholar]
- 76.Cummings WC. Bryde’s whale Balaenoptera edeni (Anderson, 1878) En: Ridway H, Harrison SR, editores. Handbook of Marine Mammals Vol 3: The Sirenians and Baleen Whales. London, UK: Academic Press; 1985. pp. 137–154. [Google Scholar]
- 77.Watkins W a, Sigurjónsson J, Wartzok D, Maiefski RR, Howey PW, Daher M a. Fin whale tracked by satellite off Iceland. Mar Mammal Sci. 1996;12: 564–569. 10.1111/j.1748-7692.1996.tb00068.x [DOI] [Google Scholar]
- 78.Jahoda M, Lafortuna CL, Almirante C, Biassoni N, Panigada A, Azzellino A, et al. Mediterranean Fin Whale’S (Balaenoptera Physalus) Response To Small Vessels and Biopsy Sampling Assessed Through Passive Tracking. Mar Mammal Sci. 2003;19: 96–110. 10.1111/j.1748-7692.2003.tb01095.x [DOI] [Google Scholar]
- 79.Gendron D. Ecología poblacional de la ballena azul Balaenoptera musculus de la Península de Baja California. PhD. Thesis. Centro de Investigación Científica y de Educación Superior de Ensenada. 2002.
- 80.Brinton E, Townsend AW. Euphausiids in the Gulf of California—the 1957 cruises. Calif Coop Ocean Fish Investig Reports. 1980;21: 211–236. [Google Scholar]
- 81.Brinton E, Fleminger A, Siegel-causey D. The temperate and tropical planktonic biotas of the Gulf of California. CalCOFI Reports. 1986;27: 228–266. [Google Scholar]
- 82.Cisneros-Mata MA, Montemayor-López G, Nevárez-Martínez MO. Modeling deterministc effects of age structure, density dependence, environmental forcing, and fishing on the population dynamics of Sardinops sagax caeruleus in the Gulf of California. CalCOFI Rep. 1996;37: 201–208. [Google Scholar]
- 83.Jaume Schinkel MS. Hábitos alimentarios del rorcual común Balaenoptera physalus en el Golfo de California mediante el uso de isótopos estables de nitrógeno y carbono. MSc. Thesis. Instituto Politécnico Nacional. 2004.
- 84.Gendron D, Aguíñiga S, Carriquiry JD. δ15N and δ13 C in skin biopsy samples: a note on their applicability for examining the relative trophic level in three rorqual species. J Cetacean Res Manag. 2001;3: 41–44. [Google Scholar]
- 85.Tomilin AG. Cetacea Mammals of the USSR and adjacent countries. Izdatel’stvo Akademii Nauk SSSR; 1957. [Google Scholar]
- 86.Rojas B L. Presencia y distribución del rorcual común Balaenoptera physalus (Linnaeus,1758) (Cetacea:Balaenopteridae) en el Golfo de California, México. Universidad Nacional Autónoma de México; 1984. [Google Scholar]
- 87.Aguilar A. Fin whale En: Perrin WF, Würsing B, Thewissen JGM, editores. Encyclopedia of Marine Mammals. San Diego, California: Academic Press; 2002. pp. 435–438. [Google Scholar]
- 88.Mikhalev YA. Humpback whales Megaptera novaeangliae in the Arabian Sea. Mar Ecol Prog Ser. 1997;149: 13–21. [Google Scholar]
- 89.Clapham PJ. Why do baleen whales migrate? A response to Corkeron and Connor. Mar Mamal Sci. 2001;17: 432–436. [Google Scholar]
- 90.Canese S, Cardinali A, Fortuna CM, Giusti M, Lauriano G, Salvati E, et al. The first identified winter feeding ground of fin whales (Balaenoptera physalus) in the Mediterranean Sea. J Mar Biol Assoc United Kingdom. 2006/06/15. Cambridge University Press; 2006;86: 903–907. 10.1017/S0025315406013853 [DOI] [Google Scholar]
- 91.Guerrero Ruiz M, Urbán Ramírez J, Rojas Bracho L. Las ballenas del Golfo de California. Primera ed. Mexico: Secretaría de Medio Ambiente y Recursos Naturales Instituto Nacional de Ecología; 2006. [Google Scholar]
- 92.Urbán J, Cardenas G, Gómez-Gallardo A. Cetáceos de las costas Suroeste del Golfo de California En: Ganster P, Arzipe O, Ivanova A, editores. Los Cabos: Prospectiva de un Paraíso Natural y Turístico. San Diego State: University Pres; 2012. pp. 101–123. [Google Scholar]
- 93.Cubero-Pardo P, Donovan C, Urbán-Ramírez J. A proposal to define vulnerability of cetacean areas to human development: Variables and analysis procedures applied to the gulf of California. Aquat Conserv Mar Freshw Ecosyst. 2011;21 10.1002/aqc.1205 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
The data used in this study are available on Movebank (movebank.org, study name "Fin Whales Gulf of California 2001") and are published in the Movebank Data Repository under DOI: http://doi.org/10.5441/001/1.65h5s5p2.