Abstract
Objectives
This study aims to evaluate the efficacy and safety of mizoribine (MZR) as a steroid-sparing agent compared to methotrexate (MTX) in the treatment of polymyalgia rheumatica in elderly patients.
Patients and methods
Twenty-four patients (9 males, 15 females; mean age 71.7 years; range 50 to 86 years) diagnosed with polymyalgia rheumatica between April 1998 and August 2014, who received prednisone in combination with either MTX or MZR, were included. We collected the data on the cumulative prednisone dose that patients received within 48 weeks after MTX or MZR and its side effect profile.
Results
There were 10 patients in the MTX group and 14 in the MZR group. The cumulative prednisone dose over 0-48 weeks was 2272±396 mg in the MTX group and 1907±241 mg in the MZR group, which was not significantly different (p=0.41). In terms of side effects, in the MTX group, three patients experienced a transient elevation in liver enzymes, and one patient developed gastrointestinal symptoms that led to MTX withdrawal. In the MZR group, one patient was hospitalized due to pneumonia that led to MZR withdrawal.
Conclusion
Mizoribine was non-inferior to MTX in terms of steroid-sparing effects on polymyalgia rheumatica. Also, MZR tended to have fewer side effects than MTX.
Keywords: Methotrexate, mizoribine, polymyalgia rheumatica, prednisone
Introduction
Polymyalgia rheumatica (PMR) is a chronic inflammatory syndrome of unknown cause characterized by pain and morning stiffness in the neck, shoulders, and pelvic girdles in elderly people.(1) Glucocorticoids, starting with 12.5-25 mg daily prednisone, are recommended as the initial treatment of PMR.(1) Patients often require treatment with prednisone for one to two years, and in cases of refractory PMR, for much longer.(2)-(4) Long-term prednisone use frequently causes chronic side effects such as diabetes, hypertension, osteoporosis, infections, and cardiovascular events, which affect nearly 60% of prednisone users.(5,6)
Therefore, 7.5-10 mg weekly methotrexate (MTX) is recommended as a steroid-sparing agent in refractory cases or patients at risk for relapse, side effects, or prolonged therapy.(1) However, it is difficult to use MTX for patients with chronic liver disease, lung disease, or chronic kidney disease, due to its side effect profile.(7) There remains a need for an alternative steroid-sparing agent to MTX, particularly in elderly patients.
Mizoribine (MZR) is an oral immunosuppressive agent, widely used in Japan. MZR is an imidazole nucleoside similar to mycophenolate mofetil (MMF) in terms of possessing an inhibitory effect on inosine monophosphate dehydrogenase (IMPDH).(8) MZR has a less serious side effect profile and contraindications compared to MTX and can be safely used for patients with lung disease or chronic kidney disease, although it sometimes causes side effects including infections, hyperuricemia, anemia, and elevation in liver enzymes.(9) MZR has already been used effectively in clinical practice for the treatment of rheumatoid arthritis (RA,10)-(12) systemic lupus erythematosus (SLE,13)-(15) kidney transplantation,(16) nephrotic syndrome,(9) and immunoglobulin A nephropathy(17) in Japan. However, the role of MZR in the treatment of PMR has not been clarified.
Therefore, in this study, we aimed to evaluate the efficacy and safety of MZR as a steroid- sparing agent compared to MTX in the treatment of PMR in elderly patients.
Patients and Methods
This retrospective single-institution case series included 24 patients (9 males, 15 females; mean age 71.7 years; range 50 to 86 years) diagnosed with PMR at Immuno-Rheumatology Center, St. Luke’s International Hospital (Tokyo, Japan), between April 1998 and August 2014, who received prednisone in combination with either MTX or MZR. All patients fulfilled the 1979 classification criteria for PMR.(18) Exclusion criteria were use of both MTX and MZR, use of other immunosuppressants, giant cell arteritis, and other connective tissue disorders. The choice of MTX or MZR was dependent on the clinical decision in each case. We considered the following factors into decision; patient’s age, comorbidities, and concerns for potential MTX side effects. The study protocol was approved by the St. Luke’s International Hospital Ethics Committee. The study was conducted in accordance with the principles of the Declaration of Helsinki.
The medical charts of patients were further reviewed for 48 weeks after initiation of MTX or MZR. Baseline patient characteristics, including the following risk factors for a relapse or a prolonged therapy for PMR, were analyzed: female sex and high erythrocyte sedimentation rate (ESR) (>40 mm/1 hour) at diagnosis.(19,20) Then, the following data regarding treatment were analyzed: total prednisone dose that patients had received within 48 weeks after starting MTX or MZR, the dose of steroid 48 weeks after starting MTX or MZR, the cumulative prednisone dose between 0-24 weeks and 24-48 weeks, the number of patients who were able to reduce their prednisone dose to less than 5 mg at week 48, the number of flare-ups within 48 weeks, and side effect profile. Flare-up was defined as the development of any signs and symptoms of PMR that needed an increase of the prednisone dose, judged by each attending doctor. Steroid dose reduction was totally dependent on the clinical decision in each case.
Data on the following major side effects of prednisone, MTX, or MZR were collected: infections, new-onset hypertension, new-onset diabetes mellitus, bone fractures, hyperuricemia, anemia, acute kidney injury, elevation in liver enzymes, gastrointestinal complaints, and insomnia.
Statistical analysis
Categorical variables were compared using the Fisher’s exact test, and continuous variables were compared using the unpaired t-test. All statistical analyses were performed using IBM SPSS Version 21.0 (IBM Corp., Armonk, NY, USA) software. P value <0.05 was considered to be statistically significant.
Results
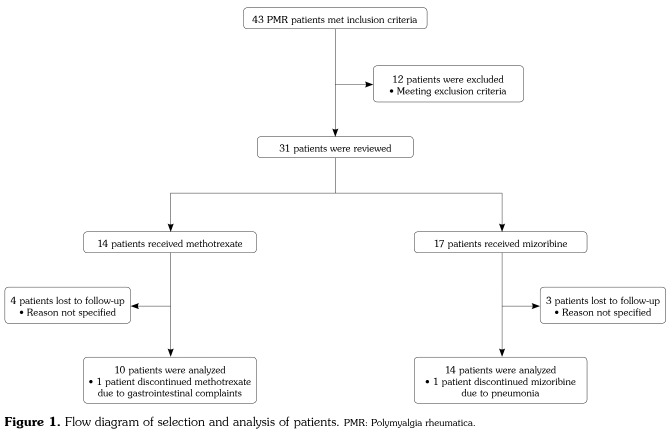
Figure 1 illustrates the process of patient selection and analysis in this study. A total of 43 PMR patients met the initial inclusion criteria and were further reviewed. Twelve patients met the exclusion criteria: five patients had been on other immunosuppressants, three patients had been on both MTX and MZR, and three patients had a diagnosis of PMR plus a diagnosis of another underlying connective tissue disease (two had RA and one had systemic sclerosis). Of seven patients who were lost to follow-up within 48 weeks, all ceased to come to the outpatient clinic without any specific reason.
Figure 1. Flow diagram of selection and analysis of patients. PMR: Polymyalgia rheumatica.
Table 1 shows the baseline characteristics of the 24 patients who met the inclusion criteria. There were 14 patients in the MZR group (5 males, 9 females; mean age 72.9±9.8 years; range 50 to 86 years) and 10 in the MTX group (4 males, 6 females; mean age 70.5±8.7 years; range 52 to 79 years). The maintenance dose of MZR and MTX was 164.3±53.5 mg and 9.4±1.4 mg, respectively. The following baseline characteristics were statistically insignificant between the two groups: age, ethnic origin, ESR at diagnosis, number of patients with high ESR (higher than 40 mm/hour) and C-reactive protein (CRP) at diagnosis, prednisone dose at diagnosis, duration of prednisone monotherapy, prednisone dose at baseline, and comorbidities (hypertension, diabetes mellitus, hyperuricemia, chronic liver disease, and interstitial pneumonia). ESR and CRP at diagnosis were not available for three patients in the MTX group and one patient in the MZR group because the patients were referred from other medical institutions without detailed information.
Table 1. Baseline characteristics of patients.
| Mizoribine (n=14) | Methotrexate (n=10) | ||||
| n | Mean±SD | n | Mean±SD | p | |
| Age (year) | 72.9±9.8 | 70.5±8.7 | 0,52 | ||
| Gender | 0,70 | ||||
| Female | 9 | 6 | |||
| Male | 5 | 4 | |||
| Ethnic origin | 1,00 | ||||
| Asian | 13 | 10 | |||
| European | 1 | 0 | |||
| Erythrocyte sedimentation rate at diagnosis (mm/hr)† | 89.5±25.4 | 81.4±22.7 | 0,49 | ||
| Erythrocyte sedimentation rate >40 mm/hr at diagnosis | 12/13 | 7/7 | 1,00 | ||
| C-reactive protein at diagnosis (mg/dL)† | 5.8±1.8 | 10.0±7.2 | 0,06 | ||
| Prednisone dose at diagnosis (mg/day)† | 20.0±8.8 | 22.1±7.6 | 0,59 | ||
| Duration of prednisone monotherapy (days) | 310±466 | 554±510 | 0,24 | ||
| Prednisone dose at baseline (mg/day) | 12.1±6.7 | 11.4±5.6 | 0,70 | ||
| Comorbidities, no. | |||||
| Hypertension | 6 | 5 | 1,00 | ||
| Diabetes mellitus | 4 | 3 | 1,00 | ||
| Hyperuricemia | 1 | 0 | 1,00 | ||
| SD: Standard deviation; † Data were missing for three patients in methotrexate group and one patient in mizoribine group. | |||||
Table 2 explains the cumulative prednisone dose, prednisone dose reduction from baseline to 48 weeks, and number of disease relapses in the two groups. The cumulative prednisone doses over the 48 weeks of the study were 2272±396 mg in the MTX group and 1907±241 mg in the MZR group, with no significant difference between the groups (p=0.41). The cumulative prednisone dose within 0-24 weeks was similar in the two groups (1288 mg in the MZR group and 1311 mg in the MTX group; p=0.93), but at 24-48 weeks, patients in the MZR group had lower cumulative prednisone dose (619 mg in the MZR group and 962 mg in the MTX group; p=0.14), although the difference was statistically insignificant. The baseline prednisone dose was higher in the MZR group (12.1 mg in the MZR group and 11.4 mg in the MTX group; p=0.70), but the prednisone dose at 48 weeks was lower (2.6 mg in the MZR group and 4.7 mg in the MTX group; p=0.07). In the MZR group, three patients ceased using prednisone without any sign of relapse or re-introduction of prednisone, while only one patient ceased using prednisone in the MTX group. In addition, 93% of patients in the MZR group were able to taper the prednisone dose to less than 5 mg, which was higher than that in the MTX group (60%). The number of flare-ups was insignificantly higher in the MZR group (six times for five patients out of 14; 36%) than in the MTX group (twice for two patients out of 10; 20%).
Table 2. Efficacy of mizoribine and methotrexate.
| Mizoribine (n=14) | Methotrexate (n=10) | ||||
| n | Mean±SE | n | Mean±SE | p | |
| Prednisone dose | |||||
| 0-48 week (mg) | 1907±241 | 2272±396 | 0,41 | ||
| 0-24 week (mg) | 1288±163 | 1311±207 | 0,93 | ||
| 24-48 week (mg) | 619±92 | 962±232 | 0,14 | ||
| At baseline (mg/day) | 12.1±1.8 | 11.4±1.8 | 0,70 | ||
| At 48 week (mg/day) | 2.6±0.5 | 4.7±1.1 | 0,07 | ||
| ≤5 mg at 48 week | 13 | 6 | 0,12 | ||
| Patients who ceased prednisone | 3 | 1 | 0,62 | ||
| Flare-ups | 5 | 2 | 0,65 | ||
| SE: Standard error. | |||||
Table 3 shows adverse effects seen within 48 weeks after initiation of MTX or MZR. The percentage of total adverse events was lower in the MZR group (43% in the MZR group and 60% in the MTX group; p=0.68). In the MTX group, three out of 10 patients experienced a transient elevation in liver enzymes. Of these three patients, one required withdrawal of MTX. In the MZR group, one patient was hospitalized due to pneumonia requiring one week of intravenous administration of antibiotics, which led to MZR withdrawal. Hyperuricemia and anemia were not seen in the MZR group. Although a transient elevation in liver enzymes occurred in one patient in the MZR group, it soon subsided after statin withdrawal. The following steroid-related adverse events did not show a significant difference between the two groups: infections (two patients in the MZR group and one patient in the MTX group), new- onset hypertension (two patients in the MZR group and no patient in the MTX group), new- onset diabetes mellitus (one patient in the MZR group and no patient in the MTX group), and bone fractures (one patient in the MZR group and no patient in the MTX group).
Table 3. Adverse events.
| Mizoribine (n=14) | Methotrexate (n=10) | ||
| n | n | p | |
| Any adverse event | 6 | 6 | 0,68 |
| Opportunistic infection | 2 | 1 | 1,00 |
| New-onset hypertension | 2 | 0 | 0,49 |
| New-onset diabetes mellitus | 1 | 0 | 1,00 |
| Bone fractures | 1 | 1 | 1,00 |
| Anemia | 0 | 2 | 0,16 |
| Elevation in liver enzymes | 1 | 3 | 0,27 |
| Gastrointestinal complaints | 1 | 1 | 1,00 |
| Adverse events requiring drug discontinuation | 1 | 1 | 1,00 |
| Infection | 1 | 0 | 1,00 |
| Gastrointestinal complaints | 0 | 1 | 1,00 |
| Hospitalization | 1 | 0 | 1,00 |
| Infection | 1 | 0 | 1,00 |
Discussion
We showed that MZR was non-inferior to MTX in terms of steroid-sparing effects for 48 weeks after starting each drug. Although not statistically significant, the cumulative prednisone dose in the MZR group was even superior to that of the MTX group during the period of 24-48 weeks. MZR tended to have fewer side effects than MTX. The results of the present study indicate that MZR can be an alternative steroid-sparing agent to MTX in the treatment of PMR. To our knowledge, this study is the first to report on the efficacy and safety of MZR in the treatment of PMR in the elderly.
Mizoribine is an imidazole nucleoside that was originally isolated from the culture medium of Eupenicillium brefeldianum. Similar to MMF, MZR inhibits the enzyme IMPDH,(8) and therefore, MZR is expected to have similar inhibitory effects on lymphocytes as MMF for the treatment of rheumatic diseases. MZR was approved by the Japanese Ministry of Health, Labour and Welfare in 1984. Since then, MZR has been widely used in Japan for renal transplantation and rheumatic diseases, including RA(10)-(12) and SLE.(13)-(15) Nomura et al.(13) reported that 12 patients with SLE were successfully treated with glucocorticoid, MZR and tacrolimus. In the study, no serious side effects were observed and mean prednisone doses were effectively reduced from 11.0 mg to 6.6 mg in 12 months.(13) Furthermore, Ichinose et al.(12) reported that 32 patients with active RA were treated with MZR, and 56.3% of patients responded without serious side effects. These studies and the present study have shown consistent results in terms of efficacy and safety of MZR, and therefore support the rationale of using MZR in the treatment of PMR.
Treatment of patients with PMR still remains challenging, as the standard treatment options are limited to prednisone and MTX, both of which potentially have harmful side effects, particularly in the elderly. Ayoub et al.(2) reported that 22.7% of PMR patients experienced steroid- related adverse effects. Therefore, harmless agents are needed in the treatment of PMR, especially for refractory patients. Infliximab and azathioprine were tested for the treatment of PMR,(21,22) and more recently, tocilizumab monotherapy has been shown to be effective for PMR in a prospective longitudinal study.(23) Compared to these agents, MZR’s strong point is its safety. MZR can be safely used for elderly people,(11,12,15) and therefore, is a promising agent in the treatment of PMR.
This study has some limitations. First, the duration of prednisone monotherapy in the MTX group was longer than that of the MZR group, suggesting that the MTX group might have included more refractory and more prednisone-unresponsive patients with PMR. That is, the study population might have favored MZR. In addition, CRP values in the MZR group tended to be lower than the MTX group at baseline, although not statistically significant (p=0.06). Taking type II error into account, it might reflect the lower disease activity in the MZR group. On the contrary, the mean value of ESR in the MZR group tended to be higher than MTX group at baseline (p=0.49). Altogether, we may assume that the difference of disease activity between these two groups did not differ remarkably. Second, the small study population did not have strong enough power to show statistical significance. Third, the retrospective nature of the study limits the interpretation of the results.
In conclusion, MZR showed non-inferior or even superior steroid-sparing effects than MTX, and adverse events were acceptable in the MZR group. Our results are encouraging for the use of MZR in the treatment of PMR especially for elderly people who cannot tolerate MTX. However, our findings should be validated in larger prospective cohorts in the future.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Dejaco C, Singh YP, Perel P, Hutchings A, Camellino D, Mackie S, et al. 2015 Recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. 2015;74:1799–1807. doi: 10.1136/annrheumdis-2015-207492. [DOI] [PubMed] [Google Scholar]
- 2.Ayoub WT, Franklin CM, Torretti D. Polymyalgia rheumatica. Duration of therapy and long-term outcome. Am J Med. 1985;79:309–315. doi: 10.1016/0002-9343(85)90309-2. [DOI] [PubMed] [Google Scholar]
- 3.Salvarani C, Macchioni PL, Tartoni PL, Rossi F, Baricchi R, Castri C, et al. Polymyalgia rheumatica and giant cell arteritis: a 5-year epidemiologic and clinical study in Reggio Emilia, Italy. Clin Exp Rheumatol. 1987;5:205–215. [PubMed] [Google Scholar]
- 4.Hutchings A, Hollywood J, Lamping DL, Pease CT, Chakravarty K, Silverman B, et al. Clinical outcomes, quality of life, and diagnostic uncertainty in the first year of polymyalgia rheumatica. Arthritis Rheum. 2007;57:803–809. doi: 10.1002/art.22777. [DOI] [PubMed] [Google Scholar]
- 5.Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55:420–426. doi: 10.1002/art.21984. [DOI] [PubMed] [Google Scholar]
- 6.McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008;20:131–137. doi: 10.1097/BOR.0b013e3282f51031. [DOI] [PubMed] [Google Scholar]
- 7.Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372:375–382. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa H. Mizoribine and mycophenolate mofetil. Curr Med Chem. 1999;6:575–597. [PubMed] [Google Scholar]
- 9.Yoshioka K, Ohashi Y, Sakai T, Ito H, Yoshikawa N, Nakamura H, et al. A multicenter trial of mizoribine compared with placebo in children with frequently relapsing nephrotic syndrome. Kidney Int. 2000;58:317–324. doi: 10.1046/j.1523-1755.2000.00168.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka H, Seto Y, Tanaka E, Furuya T, Nakajima A, Ikari K, et al. Management of rheumatoid arthritis: the 2012 perspective. Mod Rheumatol. 2013;23:1–7. doi: 10.1007/s10165-012-0702-1. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura K, Nishino J, Kouchi A, Nakamura N, Nakajima S, Yokoe I, et al. Efficacy and safety of single- dose mizoribine for patients with rheumatoid arthritis: results at 6 months after switching from a multiple-dose regimen without a change in total daily dose. Mod Rheumatol. 2011;21:158–163. doi: 10.1007/s10165-010-0373-8. [DOI] [PubMed] [Google Scholar]
- 12.Ichinose K, Origuchi T, Kawashiri SY, Iwamoto N, Fujikawa K, Aramaki T, et al. Efficacy and safety of mizoribine by one single dose administration for patients with rheumatoid arthritis. Intern Med. 2010;49:2211–2218. doi: 10.2169/internalmedicine.49.3810. [DOI] [PubMed] [Google Scholar]
- 13.Nomura A, Shimizu H, Kishimoto M, Suyama Y, Rokutanda R, Ohara Y, et al. Efficacy and safety of multitarget therapy with mizoribine and tacrolimus for systemic lupus erythematosus with or without active nephritis. Lupus. 2012;21:1444–1449. doi: 10.1177/0961203312458468. [DOI] [PubMed] [Google Scholar]
- 14.Aihara Y, Miyamae T, Ito S, Kobayashi S, Imagawa T, Mori M, et al. Mizoribine as an effective combined maintenance therapy with prednisolone in child- onset systemic lupus erythematosus. Pediatr Int. 2002;44:199–204. doi: 10.1046/j.1328-8067.2001.01534.x. [DOI] [PubMed] [Google Scholar]
- 15.Rokutanda R, Kishimoto M, Ohde S, Shimizu H, Nomura A, Suyama Y, et al. Safety and efficacy of mizoribine in patients with connective tissue diseases other than rheumatoid arthritis. Rheumatol Int. 2014;34:59–62. doi: 10.1007/s00296-012-2633-8. [DOI] [PubMed] [Google Scholar]
- 16.Xing S, Yang J, Zhang X, Zhou P. Comparative efficacy and safety of mizoribine with mycophenolate mofetil for Asian renal transplantation--a meta-analysis. Clin Biochem. 2014;47:663–669. doi: 10.1016/j.clinbiochem.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko K, Nagaoka R, Ohtomo Y, Yamashiro Y. Mizoribine for childhood IgA nephropathy. Nephron. 1999;83:376–377. doi: 10.1159/000045438. [DOI] [PubMed] [Google Scholar]
- 18.Bird HA, Esselinckx W, Dixon AS, Mowat AG, Wood PH. An evaluation of criteria for polymyalgia rheumatica. Ann Rheum Dis. 1979;38:434–439. doi: 10.1136/ard.38.5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cimmino MA, Parodi M, Caporali R, Montecucco C. Is the course of steroid-treated polymyalgia rheumatica more severe in women. Ann N Y Acad Sci. 2006;1069:315–321. doi: 10.1196/annals.1351.030. [DOI] [PubMed] [Google Scholar]
- 20.González-Gay MA, Rodríguez-Valverde V, Blanco R, Fernández-Sueiro JL, Armona J, Figueroa M, et al. Polymyalgia rheumatica without significantly increased erythrocyte sedimentation rate. A more benign syndrome. Arch Intern Med. 1997;157:317–320. doi: 10.1001/archinte.1997.00440240081012. [DOI] [PubMed] [Google Scholar]
- 21.Salvarani C, Macchioni P, Manzini C, Paolazzi G, Trotta A, Manganelli P, et al. Infliximab plus prednisone or placebo plus prednisone for the initial treatment of polymyalgia rheumatica: a randomized trial. Ann Intern Med. 2007;146:631–639. doi: 10.7326/0003-4819-146-9-200705010-00005. [DOI] [PubMed] [Google Scholar]
- 22.De Silva M, Hazleman BL. Azathioprine in giant cell arteritis/polymyalgia rheumatica: a double-blind study. Ann Rheum Dis. 1986;45:136–138. doi: 10.1136/ard.45.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devauchelle-Pensec V, Berthelot JM, Cornec D, Renaudineau Y, Marhadour T, Jousse-Joulin S, et al. Efficacy of first-line tocilizumab therapy in early polymyalgia rheumatica: a prospective longitudinal study. Ann Rheum Dis. 2016;75:1506–1510. doi: 10.1136/annrheumdis-2015-208742. [DOI] [PMC free article] [PubMed] [Google Scholar]