Abstract
Objective
The standard treatment for patients with advanced/metastatic soft tissue sarcomas (ASTS) is systemic chemotherapy with doxorubicin. A previous meta-analysis of 8 randomized controlled trials (RCTs) demonstrated the superiority of single-agent doxorubicin over doxorubicin-based combination chemotherapy for ASTS. However, meta-analyses of all RCTs that compare doxorubicin to other single-agent or combination regimens as first-line treatments for ASTS are lacking. We conducted a systematic review and meta-analysis to evaluate the efficacy and toxicity of current primary treatments for ASTS.
Methods
Eligible studies were RCTs of first-line chemotherapies for ASTS comparing doxorubicin alone to other single agents or to combination therapies (experimental arm). Data from studies reporting hazard ratios (HR) and 95% confidence intervals (CI) for overall survival (OS) and progression-free survival (PFS) were pooled. Other time-to-event endpoints were extracted from the studies based on Kaplan-Meier estimates, and pooled odds ratios (OR) and 95% CI were calculated.
Results
Twenty-seven eligible RCTs comprising 6156 patients were identified. Overall, the 1-year OS (OR 0.88, 95% CI 0.79–0.99, P = 0.03) was significantly improved in the experimental arm over the doxorubicin-only arm; however, there was no significant difference in 2-year OS (OR 0.87, 95% CI 0.73–1.03, P = 0.11) or OS (HR 0.97, 95% CI 0.91–1.03, P = 0.28) between the two groups. PFS and other time-to-event endpoints were not significantly different between the two treatment arms. While incidences of overall severe adverse events were not significantly different (OR 1.20, 95% CI 0.88–1.65, P = 0.26), severe nausea/vomiting was significantly more frequent in the experimental arm (OR 1.90, 95% CI 1.27–2.83, P = 0.002).
Conclusion
The efficacies of doxorubicin-only and experimental arm regimens were similar, although toxicities were more frequent in the experimental arms. Hence, doxorubicin monotherapy remains suitable as a standard first-line regimen for ASTS.
Introduction
Soft tissue sarcomas (STS) are rare malignant tumors that comprise approximately 1% of all malignant tumors [1]. The Soft Tissue Tumor Registry of the Japanese Orthopaedic Association had 1529 STS patients in Japan registered in 2015 [2]. The standard treatment for all localized STS is surgical resection, whereas systemic chemotherapy is the preferred treatment for patients with advanced and metastatic STS (ASTS).
The standard first-line regimen for ASTS as recommended by worldwide guidelines is doxorubicin (DOX) alone [3–5]. The efficacy of DOX against ASTS has been demonstrated by previous randomized controlled trials (RCTs), and the superiority of DOX monotherapy over combination chemotherapy was shown in a meta-analysis of 8 RCTs of first-line treatment for ASTS by Bramwell et al. in 2003 [6]. The concomitant agents used in the experimental groups of these RCTs were streptozotocin, vincristine, cyclophosphamide, dacarbazine, vindesine, ifosfamide, cisplatin, and mitomycin.
Pazopanib, the first molecular-targeted therapeutic agent for ASTS, was approved in the United States, Europe, and Japan in 2012 [7]. More recently, trabectedin, eribulin, and olaratumab were also approved for ASTS [8–10]. Therefore, several more recent RCTs comparing DOX alone with combination chemotherapy or other regimens were performed. These included RCTs comparing DOX alone to trabectedin [11–13], while another comparing DOX to pazopanib is currently ongoing [14]. Notably, the combination of olaratumab and DOX as a first-line treatment for ASTS has shown superior overall survival (OS) over DOX alone [10]. These results suggested it would be valuable to perform an updated meta-analysis of RCTs for ASTS, including the modern trials of new agents.
In this meta-analysis of 27 RCTs, we compared the efficacy of DOX monotherapy with that of other single-agent and combination chemotherapy regimens for the first-line treatment of ASTS.
Methods
Study selection
PubMed, Scopus, EBSCOhost MEDLINE, and the Cochrane Central Register of Controlled Trials were searched in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15]. The search algorithm followed the method previously described [16], except for the inclusion of the keywords ‘doxorubicin or adriamycin or anthracycline’ and ‘first line or first-line’. We included phase II and III RCTs of first-line systemic therapies for ASTS that compared single-agent DOX with other chemotherapy regimens and were published in English between January 1974 and September 2018. RCTs investigating bone sarcoma, rhabdomyosarcoma, other pediatric sarcomas, Kaposi sarcoma, and gastrointestinal stromal tumors were excluded owing to the distinct biological characteristics and treatment strategies for these tumors. Reviews, meta-analyses, and non-randomized clinical trials were also excluded. All studies retrieved by the search were independently screened and crosschecked according to the above eligibility criteria by 2 authors (KT and MK). In case of discrepancy, a third author (TI or II) was consulted.
Data extraction
Data extracted from eligible RCTs included publication date; study phase; primary and secondary endpoints; dose of standard-arm DOX; regimen and dose of the experimental arm; presence of intention-to-treat (ITT) analysis; sample size; and patient age, sex, and performance status. The following were also recorded: sarcoma subtypes, histologic grades, number of patients with advanced or metastatic disease, number of patients with prior radiotherapy, response rates (RRs), PFS (or time-to-progression [TTP]), OS, severe (grade 3 or higher) adverse events (AEs), and descriptions of post-protocol treatment. For survival data, medians, hazard ratios (HRs), confidence intervals (CIs), and P-values were extracted. The RR was defined as the proportion of patients assessed as having achieved complete or partial response based on the criteria described in each study. Three-month (or 12-week) PFS, 6-month (or 24-week) PFS, 1-year PFS, 1-year OS, and 2-year OS based on Kaplan-Meier (KM) estimates were extracted from the studies. When these data were not described in the articles, PFS or OS KM curves were used to calculate estimations as binary proportions.
Statistical analysis
In the meta-analyses, pooled odds ratios (ORs) and corresponding 95% CIs were calculated for RR; 3-month, 6-month, and 1-year PFS; 1- and 2-year OS; and AEs. Additionally, pooled HRs and 95% CIs were calculated for PFS and OS using the Mantel-Haenszel and inverse variance random or fixed effects model. A random effects model was applied if the P-value for the heterogeneity test was less than 0.1. Heterogeneity among study results was quantified using Cochrane’s Q-test and I2 statistics. Primary and major secondary endpoints of the present study were OS and PFS, respectively, based on the previous surrogacy analysis of endpoint [16]. The risk of bias in the included studies was assessed using the Cochrane Risk of Bias Assessment Tool, and publication bias was evaluated using a funnel plot. Meta-analyses were performed using Review Manager (RevMan), version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark). Other statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA). All statistical tests were 2-sided, and P-values ≤0.05 were considered statistically significant.
Results
Characteristics of eligible studies
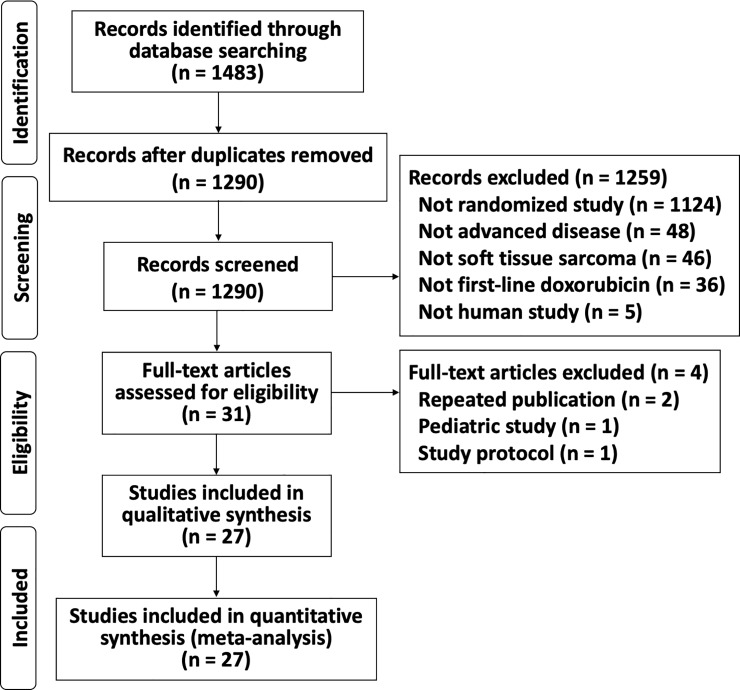
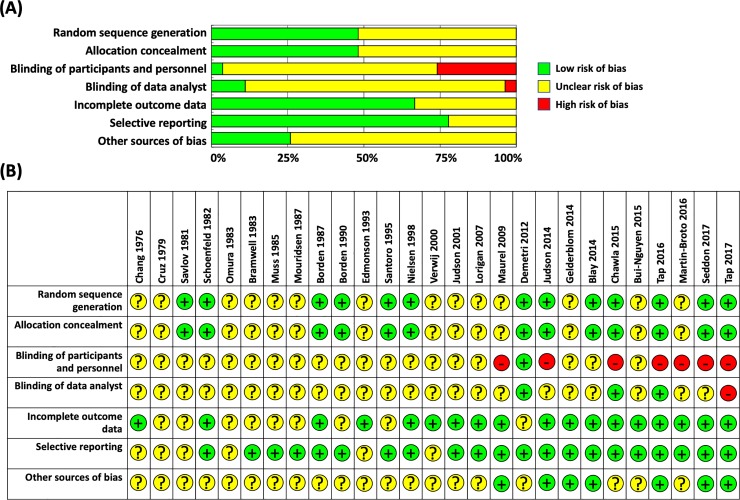
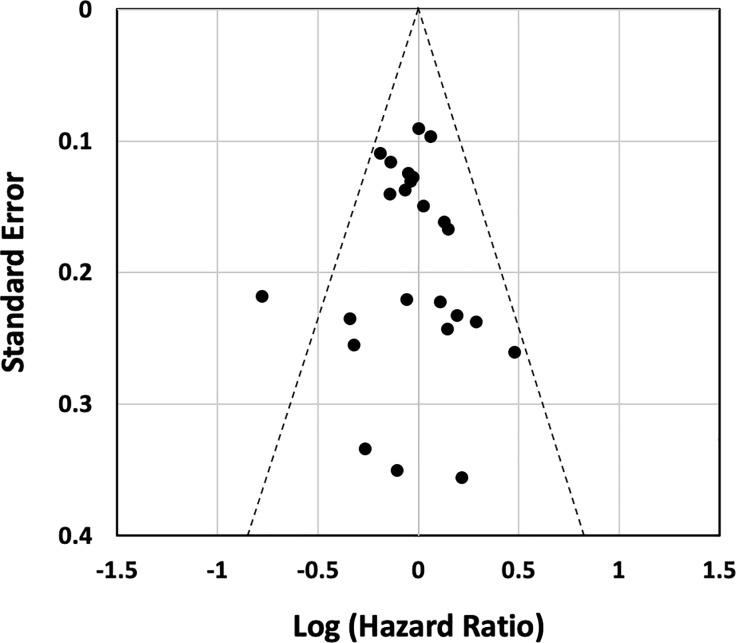
The search initially unearthed 1483 articles. After eliminating duplicates, 1290 abstracts were further screened and 1259 studies were excluded because they were not RCTs, described cancers other than sarcomas or STS, did not describe advanced/metastatic diseases, were non-human studies, or did not use DOX alone as the first-line standard treatment. The full texts of the remaining 31 articles were further evaluated, and 2 duplicate publications, 1 study protocol-only paper, and 1 pediatric population study were also excluded. Ultimately, 27 RCTs were included in the final analysis (Fig 1) [10–13,17–39]. The characteristics of the 27 eligible RCTs are summarized in Tables 1 and 2. Due to the difficulty of masking of the treatment by intra-venous infusion of chemotherapeutic drugs, risk of bias for blinding of participants and personnel and outcome assessment were found across studies. Moreover, many studies did not described detail about random sequence generation and allocation concealment (Fig 2). Although there was some asymmetry of a small study with outlier, there was no strong evidence of publication bias for RCTs of first-line DOX for ASTS based on the funnel plot (Fig 3).
Fig 1. PRISMA flow diagram.
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1. Characteristics of RCTs.
| RCTs, overall | Treatment in experimental arm | ||||||
|---|---|---|---|---|---|---|---|
| Combination chemotherapy with DOX | Other regimens without DOX | ||||||
| No. of studies (%) | No. of patients (%) | Median no. of patients | No. of studies (%) | No. of patients (%) | No. of studies (%) | No. of patients (%) | |
| 27 (100) | 6156 (100) | 133 | 14 (100) | 3954 (100) | 13 (100) | 2202 (100) | |
| Trial phase | |||||||
| II | 10 (37.0) | 1137 (18.5) | 122 | 4 (28.6) | 508 (12.8) | 6 (46.2) | 629 (28.6) |
| III | 11 (40.7) | 3534 (57.4) | 279 | 5 (35.8) | 2169 (54.9) | 6 (46.2) | 1365 (62.0) |
| Not specified | 6 (22.2) | 1485 (24.1) | 268 | 5 (35.8) | 1277 (32.3) | 1 (7.6) | 208 (9.4) |
| Primary endpoint | |||||||
| OS | 2 (7.4) | 1095 (17.8) | NA | 2 (14.3) | 1095(27.7 | 0 | 0 |
| Other time-to-event (PFS, 3m-PFS, etc) | 10 (37.0) | 1589 (25.8) | 130 | 4 (28.6) | 508 (12.8) | 6 (46.2) | 1081 (49.1) |
| RR | 1 (3.7) | 95 (1.5) | N | 0 | 0 | 1 (7.6) | 95 (4.3) |
| Not specified | 14 (51.9) | 3377 (54.9) | 215.5 | 8 (57.1) | 2351 (59.5) | 6 (46.2) | 1026 (46.6) |
| ITT analysis included | |||||||
| Yes | 9 (33.3) | 2075 (33.7) | 133 | 4 (28.6) | 1343 (34.0) | 5 (38.5) | 732 (33.2) |
| No | 18 (66.7) | 4081 (66.3) | 209 | 10 (71.4) | 2611 (66.0) | 8 (61.5) | 1470 (66.8) |
| Post-protocol treatment described | |||||||
| Yes | 14 (51.9) | 2897 (47.1) | 132.5 | 7 (50.0) | 1824 (46.1) | 8 (61.5) | 1188 (54.0) |
| No | 13 (48.1) | 3259 (52.9) | 279 | 7 (50.0) | 2130 (53.9) | 5 (38.5) | 1014 (46.0) |
A phase II/III study was counted as phase III study. Abbreviations: RCT, randomized controlled trial; DOX, doxorubicin; OS, overall survival; PFS, progression-free survival; 3m-PFS, 3 month-PFS; RR, response rate; ITT, intention-to-treat.
Table 2. Description of RCTs.
| Study | No. of patients | Experimental regimen | Study phase | Primary Endpoint | ITT analysis | Post-protocol treatment |
|---|---|---|---|---|---|---|
| RCTs comparing DOX and DOX-based combination chemotherapy | ||||||
| Chang 1976 [17] | 33 | DOX+Streptozotocin | not specified | not specified | not specified | not specified |
| Schoenfeld 1982 [18] | 221 | 1) VCR+DOX+CPA 2) VCR+Act-D+CPA |
not specified | not specified | not specified | Crossover |
| Omura 1983 [19] | 315 | DOX+DTIC | not specified | not specified | not specified | not specified |
| Muss 1985 [20] | 132 | DOX+CPA | III | not specified | not specified | not specified |
| Borden 1987 [21] | 361 | DOX+DTIC | not specified | not specified | not specified | not specified |
| Borden 1990 [22] | 347 | DOX+Vindesine | not specified | not specified | not specified | not specified |
| Edmonson 1993 [23] | 279 | 1) DOX+IFM 2) DOX+MMC+CDDP |
III | not specified | not specified | not specified |
| Santoro 1995 [24] | 663 | 1) DOX+IFM 2) CYVADIC |
III | not specified | not specified | not specified |
| Maurel 2009 [25] | 132 | DOX+IFM | II | PFS | not specified | IFM, DTIC, GEM+DTIC |
| Demetri 2012 [26] | 128 | DOX+Conatumumab | II | PFS | not specified | Roll over |
| Judson 2014 [27] | 455 | DOX+IFM | III | OS | + | DOX, EPI, IFM, TRAB, PAZ, ERIB, DTIC, GEM+DOC, etc |
| Tap 2016 [10] | 133 | DOX+Olaratumab | II | PFS | + | DOX, GEM+DOC, TRAB, PAZ, ERIB, GEM, DTIC, DOC, etc |
| Martin-Broto 2016 [13]) | 115 | DOX+TRAB | II | PFS | + | not specified |
| Tap 2017 [28] | 640 | DOX+Evofosfamide | III | OS | + | DOX, IFM, TRAB, GEM+DOC, PAZ, ERIB, GEM, DTIC, etc |
| RCTs comparing DOX and other chemotherapy without DOX | ||||||
| Cruz1979 [29] | 117 | 1) Act-D+LPAM 2) Act-D+LPAM+VCR 3) Act-D+LPAM+NSC1026 |
III | not specified | not specified | Crossover |
| Savlov 1981 [30] | 208 | Cycloleucine | not specified | not specified | not specified | Crossover |
| Bramwell 1983 [31] | 71 | Carminomycin | II | not specified | not specified | Crossover |
| Mouridsen 1987 [32] | 210 | EPI | II/III | not specified | not specified | Crossover |
| Nielsen 1998 [33] | 334 | EPI | III | not specified | not specified | not specified |
| Verweij 2000 [34] | 86 | DOC | II | not specified | not specified | Crossover |
| Judson 2001 [35] | 95 | Liposomal doxorubicin | II | RR | + | not specified |
| Lorigan 2007 [36] | 326 | IFM | III | PFS | not specified | not specified |
| Gelderblom 2014 [37] | 118 | Brostallicin | II | 26-week PFR | not specified | DOX-based, IFM, etc |
| Blay 2014 [11] | 121 | TRAB | III | PFS | + | TRAB, etc |
| Bui-Nguyen 2015 [12] | 133 | TRAB | II | PFS | + | not specified |
| Chawla 2015 [38] | 123 | Aldoxorubicin | II | PFS | + | not specified |
| Seddon 2017 [39] | 257 | GEM+DOC | III | 24-week PFR | + | DOX, IFM, TRAB, PAZ, GEM+DOC, GEM, etc |
Abbreviations: RCT, randomized controlled trial; OS, overall survival; PFS, progression-free survival; PFR, progression-free rate; RR, response rate; ITT, intention-to-treat; DOX, doxorubicin; VCR, vincristine; CPA, cyclophosphamide; Act-D, actinomycin D; DTIC, dacarbazine; IFM, ifosfamide; MMC, mitomycin C; CDDP, cisplatin; CYVADIC, CPA+VCR+DOX+DTIC; TRAB, trabectedin; LPAM, melphalan; EPI, epirubicin; DOC, docetaxel; GEM, gemcitabine; PAZ, pazopanib; ERIB, eribulin.
Fig 2. Assessment of the risk of bias of the included studies.
(A) Risk of bias graph. (B) Risk of bias summary.
Fig 3. Funnel plot of the including studies evaluating the presence of publication bias.
Altogether, 6156 patients were randomly assigned to experimental or DOX-only arms, which included 3371 and 2785 patients, respectively. The median number of patients per RCT was 133. All 27 RCTs had single-agent DOX as the control arm. After excluding 1 older study with a DOX dose of 1.2 mg/kg [29], the median DOX dose in the control arms of the remaining studies was 75 mg/m2 (range 60–80 mg/m2). Among 32 experimental arms in 27 RCTs, 30 consisted of cytotoxic drugs (either single-agent or combination) and 2 included molecular-targeted drugs. Ten RCTs were phase II and 11 were phase III. Six RCTs did not specify their study phases. Primary endpoint and ITT analyses were defined in 13 (48.1%) and 9 (33.3%) RCTs, while post protocol treatments were described in 14 (51.9%). For OS, HR was described in 11RCTs (40.7%) and estimated using KM curve in 13 RCTs (48.1%), while there was no OS data in the remaining 3 RCTs. On the other hand, HR for PFS was described in 11 RCTs (40.7%) and estimated using KM curve in 12 RCTs (44.4%). PFS data was not shown in the remaining 4 RCTs.
In the 14 RCTs investigating combination chemotherapy with DOX, a total of 3954 patients were randomly assigned (Table 1); there were 4 and 5 phase II and III studies, respectively. Primary endpoint and ITT analyses were described in 6 and 4 of the 14 RCTs, respectively.
Meta-analysis of efficacy
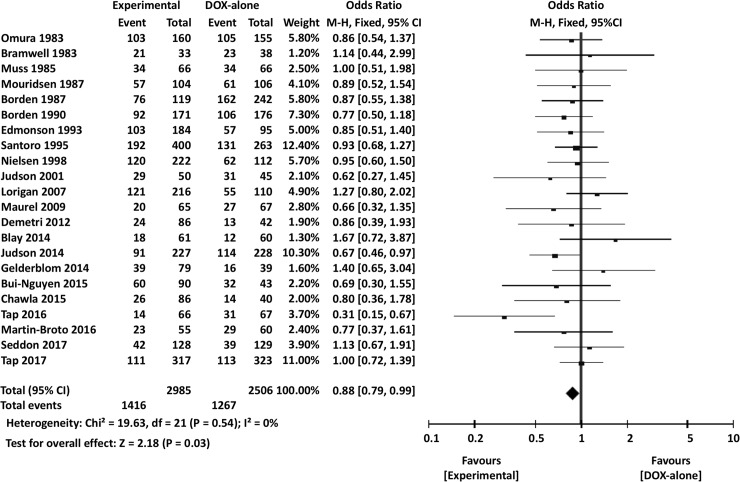
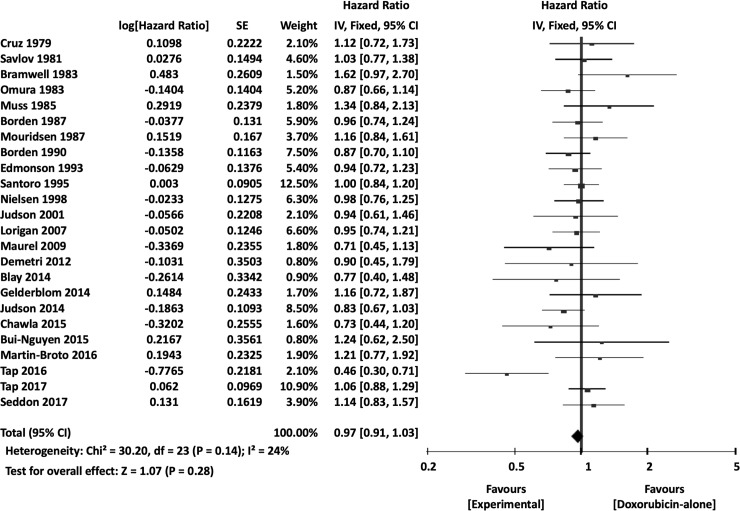
The meta-analysis results are summarized in Table 3. Overall, the experimental arm demonstrated significantly better 1-year OS (OR 0.88, 95% CI 0.79–0.99, P = 0.03) (Fig 4). However, there were no significant differences between DOX single-agent and experimental arms in terms of 2-year OS (OR 0.87, 95% CI 0.73–1.03, P = 0.11) or overall OS (HR 0.97, 95% CI 0.91–1.03, P = 0.28) (Fig 5).
Table 3. Summary of the meta-analysis.
| Endpoint | All RCTs | RCTs comparing DOX vs DOX-based combination therapy | ||
|---|---|---|---|---|
| HR/OR (95% CI) | P | HR/OR (95% CI) | P | |
| OS | 0.97 (0.91–1.03) | 0.28 | 0.92 (0.82–1.03) | 0.13 |
| 1-year OS | 0.88 (0.79–0.99) | 0.03 | 0.82 (0.72–0.94) | 0.004 |
| 2-year OS | 0.87 (0.73–1.03) | 0.11 | 0.84 (0.67–1.05) | 0.14 |
| PFS | 1.02 (0.91–1.13) | 0.74 | 0.91 (0.85–0.99) | 0.02 |
| 3-month PFS | 1.11 (0.85–1.46) | 0.43 | 0.77(0.58–1.01) | 0.06 |
| 6-month PFS | 0.91 (0.73–1.15) | 0.44 | 0.81 (0.61–1.06) | 0.13 |
| 1-year PFS | 0.88 (0.69–1.13) | 0.33 | 0.77 (0.64–0.91) | 0.003 |
| 2-year PFS | 0.88 (0.70–1.09) | 0.23 | 1.04 (0.81–1.33) | 0.78 |
| RR | 1.11 (0.85–1.46) | 0.45 | 0.76 (0.60–0.97) | 0.03 |
| AEs, overall | 1.20 (0.88–1.65) | 0.26 | 1.81 (1.35–2.43) | <0.0001 |
| Nausea/vomiting | 1.90 (1.27–2.83) | 0.002 | 2.52 (1.47–4.33) | 0.0008 |
| Leukopenia | 1.17 (0.72–1.89) | 0.52 | 2.51 (2.00–3.16) | <0.00001 |
| Neutropenia | 0.79 (0.52–1.21) | 0.28 | 1.08 (0.61–1.93) | 0.79 |
Abbreviations: RCT, randomized controlled trial; DOX, doxorubicin; HR, hazard ratio; OR, odds ratio; CI, confidence interval; OS, overall survival; PFS, progression-free survival; RR, response rate; AEs, adverse events.
Fig 4. Comparisons of doxorubicin alone vs experimental chemotherapy: Forest plots of 1-year overall survival.
M-H, Mantel-Haenszel; CI, confidence interval.
Fig 5. Comparisons of doxorubicin alone vs experimental chemotherapy: Forest plots of overall survival.
M-H, Mantel-Haenszel; CI, confidence interval.
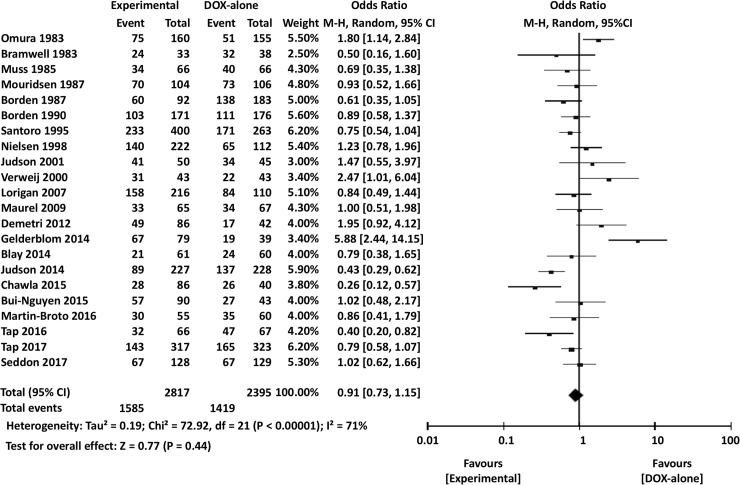
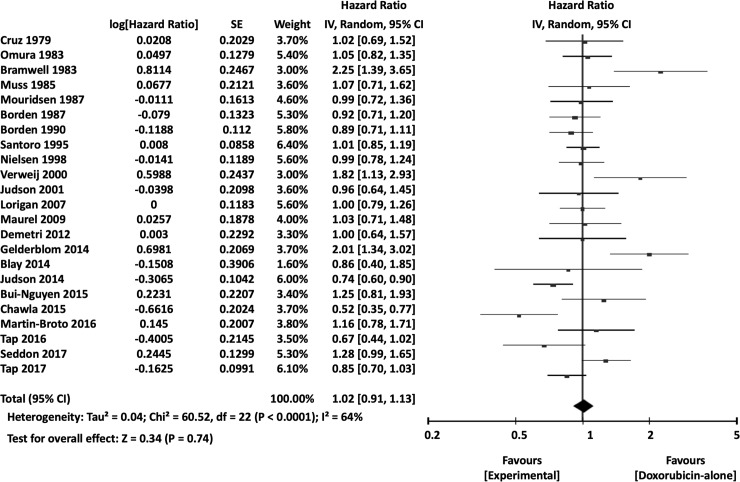
Our analyses revealed no significant differences between control and experimental arms in terms of 3-month PFS (OR 1.11, 95% CI 0.85–1.46, P = 0.43), 6-month PFS (OR 0.91, 95% CI 0.73–1.15, P = 0.44) (Fig 6), 1-year PFS (OR 0.88, 95% CI 0.69–1.13, P = 0.33), 2-year PFS (OR 0.88, 95% CI 0.70–1.09, P = 0.23), overall PFS (HR 1.02, 95% CI 0.91–1.13, P = 0.74) (Fig 7), or RR (OR 1.11, 95% CI 0.85–1.46, P = 0.45).
Fig 6. Comparisons of doxorubicin alone vs experimental chemotherapy: Forest plots of 6-month progression-free survival.
CI, confidence interval; M-H, Mantel-Haenszel.
Fig 7. Comparisons of doxorubicin alone vs experimental chemotherapy: Forest plots of progression-free survival.
CI, confidence interval; M-H, Mantel-Haenszel.
Meta-analysis of adverse events
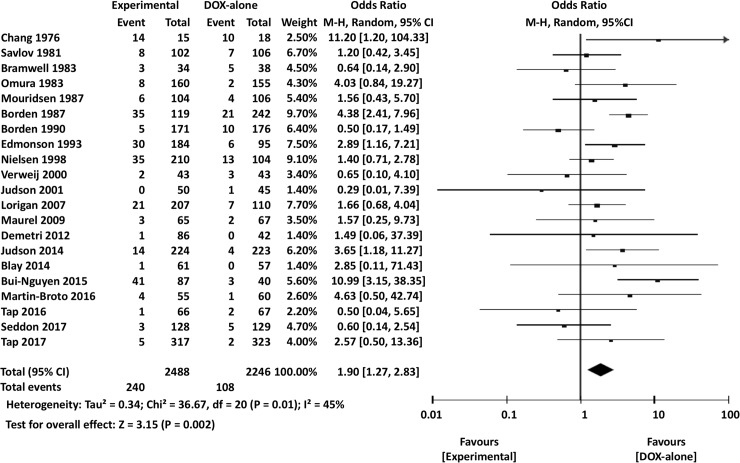
The incidences of overall severe AEs (grades 3 or higher) were not significantly different between experimental and DOX-only arms (OR 1.20, 95% CI 0.88–1.65, P = 0.26). There was also no significant difference in the occurrence of severe leukopenia (OR 1.17, 95% CI 0.72–1.89, P = 0.52) or neutropenia (OR 0.79, 95% CI 0.52–1.21, P = 0.28) between DOX-only and experimental arms. However, severe nausea or vomiting was significantly less frequent in DOX-only arms than in experimental arms (OR 1.90, 95% CI 1.27–2.83, P = 0.002) (Fig 8).
Fig 8. Comparisons of doxorubicin alone vs experimental chemotherapy: Forest plots of the incidence of severe nausea/vomiting adverse events.
CI, confidence interval; M-H, Mantel-Haenszel.
Meta-analysis of RCTs comparing DOX alone and DOX-based combination chemotherapy
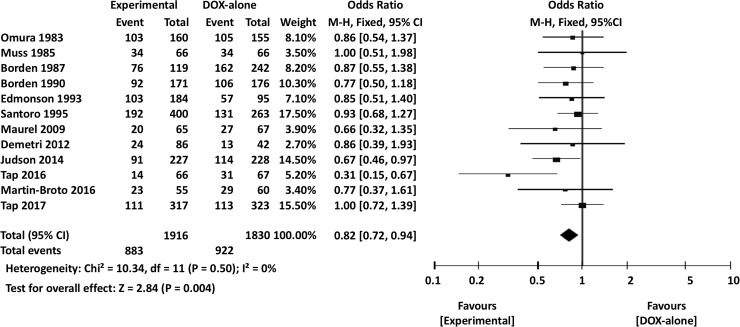
Next, subgroup meta-analyses of 14 RCTs comparing DOX alone to DOX-based combination regimens were performed. As in the overall analysis, the 1-year OS was significantly longer in combination chemotherapy arms than in DOX-only arms (OR 0.82, 95% CI 0.77–0.94, P = 0.004) (Fig 9). On the other hand, DOX and experimental arms did not have significantly different 2-year OS (OR 0.84, 95% CI 0.67–1.05, P = 0.14) or overall OS (HR 0.92, 95% CI 0.82–1.03, P = 0.13).
Fig 9. Comparisons of doxorubicin alone vs doxorubicin-based combination chemotherapy: Forest plots of 1-overall survival.
M-H, Mantel-Haenszel; CI, confidence interval.
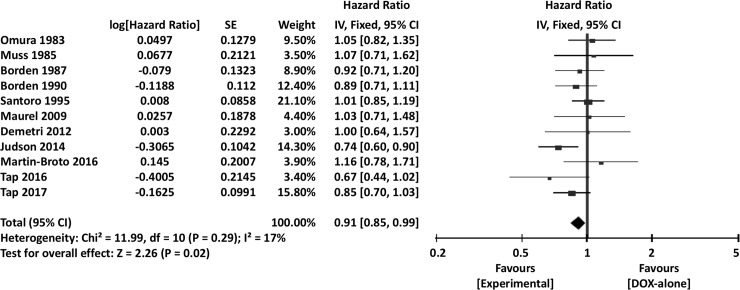
When the surrogate endpoints were analyzed, 1-year PFS (OR 0.77, 95% CI 0.64–0.91, P = 0.003), overall PFS (HR 0.91, 95% CI 0.85–0.99, P = 0.02) (Fig 10), and RR (OR 0.76, 95% CI 0.60–0.97, P = 0.03) were significantly more favorable in the combination chemotherapy groups. Additional meta-analyses of the 3-month PFS (OR 0.77, 95% CI 0.58–1.01, P = 0.06), 6-month PFS (OR 0.81, 95% CI 0.61–1.06, P = 0.13), and 2-year PFS (OR 1.04, 95% CI 0.81–1.33, P = 0.78) showed no significant differences between DOX-only and combination therapy arms.
Fig 10. Comparisons of doxorubicin alone vs doxorubicin-based combination chemotherapy: Forest plots of progression-free survival.
SE, standard error; IV, inverse variance; CI, confidence interval.
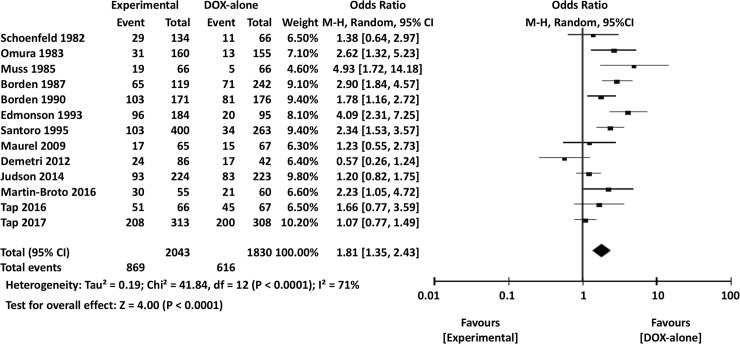
Overall severe AEs (OR 1.81, 95% CI 1.35–2.43, P<0.0001) (Fig 11), leukopenia (OR 2.51, 95% CI 2.00–3.16, P<0.00001), and nausea or vomiting (OR 2.52, 95% CI 1.47–4.33, P = 0.0008) were significantly less frequent in DOX-only arms than in combination therapy arms. There were no significant differences in the incidences of severe neutropenia between DOX-only and experimental arms (OR 1.08, 95% CI 0.61–1.93, P = 0.79).
Fig 11. Comparisons of doxorubicin alone vs doxorubicin-based combination chemotherapy: Forest plots of the overall incidence of severe adverse events.
M-H, Mantel-Haenszel; CI, confidence interval.
Discussion
Bramwell et al.’s meta-analysis collected 8 RCTs that compared DOX alone to DOX-based combination chemotherapy for treating ASTS [6]. Subsequently, 6 similar RCTs have been conducted, as well as 13 additional RCTs of primary therapy for ASTS that compared DOX alone to other single agents or combination regimens without DOX. Ours is the first meta-analysis of the abovementioned 27 RCTs of first-line chemotherapy with standard DOX for ASTS.
Bramwell et al.’s meta-analysis included 10 DOX-based combination chemotherapy regimens administered in 8 studies, as well as 9 single-agent DOX standard arms in 8 RCTs. Two of the 8 RCTs demonstrated significantly better RRs in the combination arm than in the DOX-only arm. None of the RCTs exhibited significant differences in 1-year and 2-year mortality rates between the 2 treatment groups. Bramwell et al.’s meta-analysis revealed no significant differences in RR (OR 1.26, 95% CI 0.96–1.67, P = 0.10), death at 1 year (OR 0.87, 95% CI 0.73–1.05, P = 0.14), and death at 2 years (OR 0.84, 95% CI 0.67–1.05, P = 0.13) between DOX-only and DOX-based combination regimens [6]. However, other time-to-event endpoints such as overall and 3-month PFS were not analyzed in their study. On the other hand, AEs including nausea/vomiting and hematologic toxicities tended to be frequent for combination regimens, although the differences in overall AE rates among the 8 RCTs were not statistically analyzed. Therefore, their meta-analysis concluded that single-agent DOX was a suitable standard treatment for chemotherapy-naive patients with ASTS; this has remained the case in worldwide guidelines [3–5].
Conatumumab, ifosfamide, trabectedin, evofosfamide, and olaratumab were used in combination with DOX in 6 similar RCTs performed after Bramwell et al.’s meta-analysis. The combination of DOX and olaratumab significantly prolonged OS over DOX alone. In a randomized phase II study, OS was significantly better with the combination therapy (HR 0.46, 95% CI 0.30–0.71, P = 0.0003), although the number of patients was small (67 in the DOX arm and 66 in the DOX plus olaratumab arm) [10].
The present meta-analysis demonstrated that RR and PFS were significantly improved with the combination therapy compared to DOX alone, suggesting that RR and PFS have improved since the RCTs investigated in Bramwell et al.’s study. However, there was no significant difference in OS between the 2 groups. On the other hand, severe overall AEs, leukopenia, and nausea/vomiting rates were significantly higher in patients receiving the combination regimens. Therefore, in agreement with Bramwell et al.’s conclusion, our meta-analysis of 14 RCTs comparing DOX to combination therapy revealed that DOX alone ought to remain the recommended first-line regimen for patients with ASTS.
Recently, a meta-analysis of 22 RCTs of single agents and combination therapies for ASTS found that OS (HR 0.79, 95% CI 0.65–0.97, P = 0.02) and PFS (OR 0.86, 95% CI 0.73–1.00, P = 0.05) were significantly improved in patients receiving the combination regimens [40]. However, the actual numbers of the RCTs analyzed for OS and PFS were only 7 and 11, respectively. No RCT published before 2008 was involved in the analysis. Their study further included study abstracts, although the results were often different from those in the fully published articles, and also included studies using cytostatic/biological agents only. Moreover, the lines of treatment and patient backgrounds in each RCT were different, while the control regimens in each also varied. These caveats suggest that the results of their study ought to be interpreted with greater caution.
The limitations of our study are as follows: 1) The present meta-analysis was based only on published data, as we were unable to access individual data of the patients included in each RCT; 2) several older studies included certain subjects, such as those with mesothelioma and bone tumors, who were excluded from more recent trials; 3) several studies did not define their time-to-event endpoints; 4) the patient characteristics among the RCTs, such as histologic grade, subtypes, and proportions of metastatic and unresectable tumors, varied; and 5) some studies included a small number of patients who had received prior chemotherapy (175 out of 6156 patients: 2.8%). These limitations should be noted for interpretation of the results of the study.
Currently, a phase III trial of DOX plus olaratumab is being conducted; if the results of this trial will be in agreement with the randomized phase II trial by Tap et al. [10], there is a possibility that the standard therapy might be changed from DOX alone to a combination of DOX plus olaratumab. Presently, however, DOX single agent ought to remain the optimal standard therapy for primary ASTS treatment based on our meta-analysis that included the abovementioned randomized phase II trial.
Supporting information
(DOC)
Acknowledgments
We thank the statisticians of the Kureha Special Laboratory for their kind assistance in statistical analyses. We would like to thank Editage for English language editing.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was supported in part by the National Cancer Center Research and Development Fund (29-A-3), by Japan Agency for Medical Research and Development under Grant Number JP17ck0106336, and by Japan Society for the Promotion of Science, grant number 18 K09110. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Cancer Society: Cancer facts and figures 2017: American Cancer Society, Atlanta, 2017. [Google Scholar]
- 2.Japanese Orthopaedic Association Musculoskeletal Tumor Committee: Soft tissue tumor registry in Japan 2015: National Cancer Center, Tokyo, 2015. [Google Scholar]
- 3.Casali PG, Abecassis N, Bauer S, Biagini R, Bielack S, Bonvalot S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 10.1093/annonc/mdy096 [DOI] [PubMed] [Google Scholar]
- 4.Dangoor A, Seddon B, Gerrand C Dangoor A, Seddon B, Gerrand C, et al. UK guidelines for the management of soft tissue sarcomas. Clin Sarcoma Res 2016;6: 20 10.1186/s13569-016-0060-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López-Pousa A, Martin Broto J, Martinez Trufero J, Sevilla I, Valverde C, Alvarez R, et al. : SEOM Clinical Guideline of management of soft-tissue sarcoma (2016). Clin Transl Oncol 2016;18: 1213–1220. 10.1007/s12094-016-1574-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramwell VH, Anderson D, Charette ML. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev 2003;CD003293 10.1002/14651858.CD003293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379: 1879–1886. 10.1016/S0140-6736(12)60651-5 [DOI] [PubMed] [Google Scholar]
- 8.Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016;34: 786–793. 10.1200/JCO.2015.62.4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schöffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 2016;387: 1629–1637. 10.1016/S0140-6736(15)01283-0 [DOI] [PubMed] [Google Scholar]
- 10.Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388: 488–497. 10.1016/S0140-6736(16)30587-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blay JY, Leahy MG, Nguyen BB, Patel SR, Hohenberger P, Santoro A, et al. Randomised phase III trial of trabectedin versus doxorubicin-based chemotherapy as first-line therapy in translocation-related sarcomas. Eur J Cancer 2014;50: 1137–1147. 10.1016/j.ejca.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 12.Bui-Nguyen B, Butrynski JE, Penel N, Blay JY, Isambert N, Milhem M, et al. A phase IIb multicentre study comparing the efficacy of trabectedin to doxorubicin in patients with advanced or metastatic untreated soft tissue sarcoma: the TRUSTS trial. Eur J Cancer 2015;51: 1312–1320. 10.1016/j.ejca.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 13.Martin-Broto J, Pousa AL, de Las Peñas R, García Del Muro X, Gutierrez A, Martinez-Trufero J, et al. Randomized phase II study of trabectedin and doxorubicin compared with doxorubicin alone as first-line treatment in patients with advanced soft tissue sarcomas: A Spanish Group for Research on Sarcoma study. J Clin Oncol 2016;34: 2294–2302. 10.1200/JCO.2015.65.3329 [DOI] [PubMed] [Google Scholar]
- 14.Karch A, Koch A, Grünwald V. A phase II trial comparing pazopanib with doxorubicin as first-line treatment in elderly patients with metastatic or advanced soft tissue sarcoma (EPAZ): study protocol for a randomized controlled trial. Trials 2016;17:312 10.1186/s13063-016-1434-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339: b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zer A, Prince RM, Amir E, Abdul Razak A. Evolution of randomized trials in advanced/metastatic soft tissue sarcoma: End point selection, surrogacy, and quality of reporting. J Clin Oncol 2016;34: 1469–1475. 10.1200/JCO.2015.64.3437 [DOI] [PubMed] [Google Scholar]
- 17.Chang P, Wiernik PH. Combination chemotherapy with adriamycin and streptozotocin. I. Clinical results in patients with advanced sarcoma. Clin Pharmacol Ther 1976;20:605–610. [DOI] [PubMed] [Google Scholar]
- 18.Schoenfeld DA, Rosenbaum C, Horton J, Wolter JM, Falkson G, DeConti RC. A comparison of adriamycin versus vincristine and adriamycin, and cyclophosphamide versus vincristine, actinomycin-D, and cyclophosphamide for advanced sarcoma. Cancer 1982;50:2757–2562. [DOI] [PubMed] [Google Scholar]
- 19.Omura GA, Major FJ, Blessing JA, Sedlacek TV, Thigpen JT, Creasman WT, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983;52:626–632. [DOI] [PubMed] [Google Scholar]
- 20.Muss HB, Bundy B, DiSaia PJ, Homesley HD, Fowler WC Jr, Creasman W, et al. Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group). Cancer 1985;55:1648–1653. [DOI] [PubMed] [Google Scholar]
- 21.Borden EC, Amato DA, Rosenbaum C, Enterline HT, Shiraki MJ, Creech RH, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987;5:840–850. 10.1200/JCO.1987.5.6.840 [DOI] [PubMed] [Google Scholar]
- 22.Borden EC, Amato DA, Edmonson JH, Ritch PS, Shiraki M. Randomized comparison of doxorubicin and vindesine to doxorubicin for patients with metastatic soft-tissue sarcomas. Cancer 1990;66:862–867. [DOI] [PubMed] [Google Scholar]
- 23.Edmonson JH, Ryan LM, Blum RH, Brooks JS, Shiraki M, Frytak S, et al. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993;11:1269–1275. 10.1200/JCO.1993.11.7.1269 [DOI] [PubMed] [Google Scholar]
- 24.Santoro A, Tursz T, Mouridsen H, Verweij J, Steward W, Somers R, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–1545. 10.1200/JCO.1995.13.7.1537 [DOI] [PubMed] [Google Scholar]
- 25.Maurel J, López-Pousa A, de Las Peñas R, Fra J, Martín J, Cruz J, et al. Efficacy of sequential high-dose doxorubicin and ifosfamide compared with standard-dose doxorubicin in patients with advanced soft tissue sarcoma: an open-label randomized phase II study of the Spanish group for research on sarcomas. J Clin Oncol 2009;27:1893–1898. 10.1200/JCO.2008.19.2930 [DOI] [PubMed] [Google Scholar]
- 26.Demetri GD, Le Cesne A, Chawla SP, Brodowicz T, Maki RG, Bach BA, et al. First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: a phase I/II open-label and double-blind study. Eur J Cancer 2012;48:547–563. 10.1016/j.ejca.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 27.Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014;15:415–423. 10.1016/S1470-2045(14)70063-4 [DOI] [PubMed] [Google Scholar]
- 28.Tap WD, Papai Z, Van Tine BA, Attia S, Ganjoo KN, Jones RL, et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): an international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2017;18:1089–1103. 10.1016/S1470-2045(17)30381-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz AB Jr, Thames EA Jr, Aust JB, Metter G, Ramirez G, Fletcher WS, et al. Combination chemotherapy for soft-tissue sarcomas: a phase III study. J Surg Oncol. 1979;11:313–323. [DOI] [PubMed] [Google Scholar]
- 30.Savlov ED, MacIntyre JM, Knight E, Wolter J. Comparison of doxorubicin with cycloleucine in the treatment of sarcomas. Cancer Treat Rep 1981;65:21–27. [PubMed] [Google Scholar]
- 31.Bramwell VH, Mouridsen HT, Mulder JH, Somers R, Van Oosterom AT, Santoro A, et al. Carminomycin vs adriamycin in advanced soft tissue sarcomas: an EORTC randomised phase II study. Eur J Cancer Clin Oncol 1983;19:1097–1104. [DOI] [PubMed] [Google Scholar]
- 32.Mouridsen HT, Bastholt L, Somers R, Santoro A, Bramwell V, Mulder JH, et al. Adriamycin versus epirubicin in advanced soft tissue sarcomas. A randomized phase II/phase III study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer Clin Oncol 1987;23:1477–1483. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen OS, Dombernowsky P, Mouridsen H, Crowther D, Verweij J, Buesa J, et al. High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer 1998;78:1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verweij J, Lee SM, Ruka W, Buesa J, Coleman R, van Hoessel R, et al. Randomized phase II study of docetaxel versus doxorubicin in first- and second-line chemotherapyfor locally advanced or metastatic soft tissue sarcomas in adults: a study of the european organization for research and treatment of cancer soft tissue and bone sarcoma group. J Clin Oncol 2000;18:2081–2086. 10.1200/JCO.2000.18.10.2081 [DOI] [PubMed] [Google Scholar]
- 35.Judson I, Radford JA, Harris M, Blay JY, van Hoesel Q, le Cesne A, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicinin the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissueand Bone Sarcoma Group. Eur J Cancer 2001;37:870–877. [DOI] [PubMed] [Google Scholar]
- 36.Lorigan P, Verweij J, Papai Z, Rodenhuis S, Le Cesne A, Leahy MG, et al. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 2007;25:3144–3150. 10.1200/JCO.2006.09.7717 [DOI] [PubMed] [Google Scholar]
- 37.Gelderblom H, Blay JY, Seddon BM, Leahy M, Ray-Coquard I, Sleijfer S, et al. Brostallicin versus doxorubicin as first-line chemotherapy in patients with advanced or metastatic soft tissue sarcoma: an European Organisation for Research and Treatment of Cancer Soft Tissueand Bone Sarcoma Group randomised phase II and pharmacogenetic study. Eur J Cancer 2014;50:388–396. 10.1016/j.ejca.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 38.Chawla SP, Papai Z, Mukhametshina G, Sankhala K, Vasylyev L, Fedenko A, et al. First-Line Aldoxorubicin vs Doxorubicin in Metastatic or Locally Advanced Unresectable Soft-Tissue Sarcoma: A Phase 2b Randomized Clinical Trial. JAMA Oncol 2015;1:1272–1280. 10.1001/jamaoncol.2015.3101 [DOI] [PubMed] [Google Scholar]
- 39.Seddon B, Strauss SJ, Whelan J, Leahy M, Woll PJ, Cowie F, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol 2017;18:1397–1410. 10.1016/S1470-2045(17)30622-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zer A, Prince RM, Amir E, Abdul Razak AR. Multi-agent chemotherapy in advanced soft tissue sarcoma—A systematic review and meta-analysis. Cancer Treat Rev 2018;63: 71–78. 10.1016/j.ctrv.2017.12.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.