Abstract
Background
Long non-coding RNA of myocardial infarction associated transcript (lncRNA-MIAT) has a reported role in microvascular dysfunction. This study aimed to investigate the role of lncRNA-MIAT and its effects on transforming growth factor-β1 (TGF-β1) signaling in patients with diabetic retinopathy and in ARPE-19 adult retinal pigment epithelial cells in vitro.
Material/Methods
Study participants provided plasma samples and included patients with non-proliferative diabetic retinopathy (n=52), patients with diabetes without diabetic retinopathy (n=63), and healthy controls (n=56). Plasma levels of lncRNA-MIAT and TGF-β1 were detected by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA), respectively. Pearson correlation analysis was performed on the plasma data, and the diagnostic relevance of plasma levels of lncRNA-MIAT for diabetic retinopathy was evaluated by receiver operating characteristic (ROC) curve analysis. Cells of the human retinal pigment epithelial cell line, ARPE-19, were cultured in high glucose with construction and transfection of a MIAT expression plasmid vector. Viability of ARPE-19 cells was detected by the MTT assay and Western blot measured the expression levels of TGF-β1.
Results
Plasma levels of lncRNA-MIAT were significantly increased in patients with diabetic retinopathy compared with patients with diabetes without diabetic retinopathy and with healthy controls. ARPE-19 cells cultured in a high glucose environment showed reduced cell viability and upregulation of lncRNA-MIAT expression.
Conclusions
Increased plasma levels of lncRNA-MIAT were significantly associated with the presence of diabetic retinopathy, and increased expression of lncRNA-MIAT reduced the viability of ARPE-19 cells in vitro by upregulating TGF-β1 signaling.
MeSH Keywords: Diabetic Retinopathy; RNA, Long Noncoding; Transforming Growth Factor beta1
Background
Worldwide, diabetic retinopathy is a common complication of diabetes mellitus and is a leading cause of loss of impaired vision and blindness in middle-aged and elderly people [1]. With the increasing prevalence of diabetes, by 2030, the incidence of diabetic retinopathy has been predicted to increase by 69% in developing countries and by 20% in developed countries, when compared with epidemiologic data from 2010 [2]. More than 80% of patients who have had diabetes for more than 20 years will develop diabetic retinopathy [3]. With adequate glycemic control, the severity of diabetic retinopathy in 90% of patients with diabetic retinopathy will significantly improve, but blindness occurs in the remaining 10% of patients even with good glycemic control, for reasons that remain unknown [4]. Early detection and treatment are important for the prevention of diabetes-related visual impairment, and diagnostic biomarkers that may be used to detect the early onset or risk for diabetic retinopathy continue to be investigated.
Increased levels of transforming growth factor-β (TGF-β) has been shown in the aqueous humor of the eyes of patients with diabetes compared with normal eyes [5]. Activation of TGF-β signaling has been shown to affect the development of diabetic retinopathy [6]. However, while increased TGF-β signaling may be protective and prevent the rapid progression of retinopathy, TGF-β signaling also promotes thickening of the basal lamina of retinal capillaries [6]. There have been recently reported studies that have shown that the effects of TGF-β signaling may affect the microvasculature through interactions with long non-coding RNAs (lncRNAs) [7,8], The lncRNAs are a subgroup of non-coding RNAs composed of more than 200 nucleotides that have been shown to have important functions in several human diseases [9].
It has previously been reported that lncRNA of myocardial infarction associated transcript (MIAT) regulates microvascular dysfunction, which is the major pathological change in diabetic retinopathy [10,11]. Therefore, this study aimed to investigate the role of lncRNA-MIAT and its effects on transforming growth factor-β1 (TGF-β1) signaling in patients with diabetic retinopathy and in ARPE-19 adult retinal pigment epithelial cells in vitro.
Material and Methods
Patients and controls subjects
The study included 52 patients with diabetes mellitus and non-proliferative diabetic retinopathy, and 63 patients with diabetes mellitus without diabetic retinopathy, who were diagnosed and treated in the Second Hospital of Jilin University from March 2015 to October 2017. A control group consisted of 56 healthy individuals. The diagnostic criteria for diabetes and diabetic retinopathy were in accordance with the Chinese Medical Association recommendations, 2014. All patients with diabetes included in the study were diagnosed and treated for the first time. Patients who had diabetes mellitus combined with other diseases were excluded from the study. The local Ethics Committee of the Second Hospital of Jilin University approved this study. All participants signed informed consents to participate in the study.
Preparation of plasma
About 10 ml of whole blood was obtained from the elbow vein of each participant on the day of clinic admission. Blood was collected in a Vacutainer® PPT™ plasma preparation tube (BD Biosciences, Franklin Lakes, NJ, USA) at room temperature, followed by centrifugation at 1600×g for 15 min to collect the plasma. Plasma samples were stored at −80ºC before use.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used for total RNA extraction. RNA concentrations were measured using the NanoDrop™ 2000 spectrophotometer (Thermo Fisher Scientific., lnc., Waltham, MA, USA). The RNA samples were transcribed into cDNA using the SuperScript III reverse transcriptase kit (Thermo Fisher Scientific., lnc., Waltham, MA, USA) using the following reaction conditions: 55ºC for 25 min, and 80ºC for 15 min.
The following primers were used in the polymerase chain reaction (PCR) reactions:
lncRNA-MIAT: 5′-TTTACTTTAACAGACCAGAA-3′ (forward), and
5′-CTCCTTTGTTGAATCCAT-3′ (reverse);
β-actin: GACCTCTATGCCAACACAGT (forward), and
AGTACTTGCGCTCAGGAGGA (reverse).
The PCR reaction parameters were: 45 s at 95°C, followed by 40 cycles of 25 s at 95°C and 35 s at 56°C. Data were normalized using 2−ΔΔCT method.
Enzyme-linked immunosorbent assay (ELISA)
Serum levels of TGF-β1 were measured using an ELISA kit (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer’s instructions.
Cell culture and transfection of ARPE-19 adult retinal pigment epithelial cells in vitro
The human retinal pigment epithelial cell line, ARPE-19, was purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Cell culture and subculture were performed in accordance with manufacturer’s instructions. Full-length myocardial infarction associated transcript (MIAT) cDNA was obtained from PCR amplification and inserted into the linearized pEGFP-C3 plasmid vector (Clontech, Palo Alto, CA, USA) to create the MIAT expression vector. Vector DNA was mixed with Lipofectamine 2000 reagent (11668-019) (Invitrogen, Carlsbad, CA, USA) and incubated at room temperature for 30 min to allow the formation of vector-reagent complexes. The vector-reagent complexes were then incubated with ARPE-19 cells at 37ºC for 6 h to achieve transfection. Overexpression of MIAT was confirmed by qRT-PCR before subsequent experiments.
MTT assay
After transfection and confirmation of overexpression, ARPE-19 cells were harvested and used to make a cell suspension at a cell density of 4×104 cells/ml. Then, 4×103 cells in 100 μl cell suspension were added into each well of 96-well plate and 30 mM D-glucose (dextrose) was added to each well. Cells were cultured at 37°C for 6h. Then, 10 μl of MTT was added, and cell culture was performed for another 4 h. Absorbance at 570 nm was then measured using a Fisherbrand™ AccuSkan™ GO UV/Vis microplate spectrophotometer (Fisher Scientific., lnc., Waltham, MA, USA).
Western blot
Radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific., lnc., Waltham, MA, USA) was used for total protein extraction. Protein content was measured using the bicinchoninic acid (BCA) protein assay. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10%) was then performed using 20 μg of denatured protein in each well. Blocking was performed by incubating the membranes with 5% dried skimmed milk powder at room temperature for 1 h. After washing three times with Tris-buffered saline with Tween® 20 (TBST) (0.3% Tween), the membranes were incubated with rabbit anti-human primary antibodies to transforming growth factor-β1 (TGF-β1) (1: 2000) (ab92486) (Abcam, Cambridge MA, USA) and anti-GAPDH antibody (1: 1000) (ab9485) (Abcam, Cambridge MA, USA) overnight at 4°C. After washing three times with TBST (0.3% Tween), the membranes were further incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG secondary antibody (1: 1000) (MBS435036) (MyBioSource, San Diego, CA, USA) at room temperature for 1 h. After washing three times with TBST (0.3% Tween), enhanced chemiluminescence (ECL) reagent (Sigma-Aldrich, St Louis, MO, USA) was added to develop the signals. ImageJ software was used to normalize the relative expression level of TGF-β1 to GAPDH.
Statistical analysis
GraphPad Prism 6 was used to analyze the data. All numerical data were expressed as the mean ± standard deviation (SD). Comparisons between two groups and among multiple groups were performed by the Student’s t-test and one-way analysis of variance (ANOVA), followed by Tukey’s test. A p-value <0.05 represented a statistically significant difference. Receiver operating characteristic (ROC) curve analysis and the evaluation of area under the curve (AUC) were performed.
Results
Study groups
Three study groups underwent analysis of plasma samples. The 52 patients with diabetic retinopathy included 30 men and 22 women, with an age range from 23–69 years (mean, 45.5±6.2 years). The 63 patients with diabetes without diabetic retinopathy included 36 men and 27 women, with an age range from 25–68 years (mean 46.1±6.5 years). The 56 healthy people in the control group included 32 men and 24 women, with an age range from 22–67 years (mean, 45.3±7.2 years).
Plasma levels of long non-coding RNA of myocardial infarction associated transcript (lncRNA-MIAT) were upregulated in patients with diabetic retinopathy
As shown in Figure 1, significant differences were found between the three groups in the plasma levels of lncRNA-MIAT. Specifically, plasma levels of lncRNA-MIAT were significantly increased in patients with diabetic retinopathy compared with patients with diabetes without diabetic retinopathy, and healthy controls (p<0.05). Although increased plasma levels of lncRNA-MIAT were found in patients with diabetes without diabetic retinopathy compared with healthy controls, this difference did not reach statistical significance (p>0.05).
Figure 1.
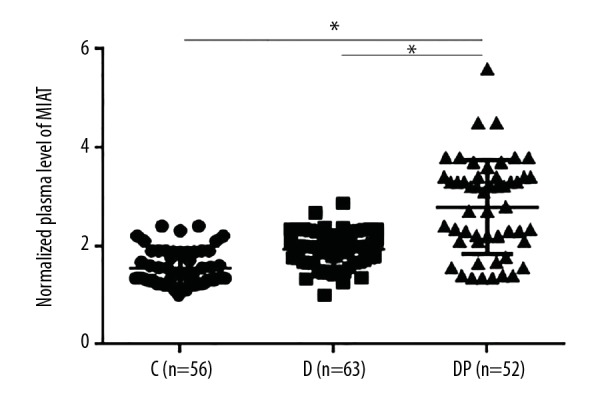
Plasma levels of long non-coding RNA of myocardial infarction associated transcript (lncRNA-MIAT) were upregulated in patients with diabetic retinopathy. * p<0.05; C – control; D – diabetes, DR – diabetic retinopathy.
Analysis of plasma lncRNA-MIAT levels as a diagnostic biomarker for diabetic retinopathy in diabetes mellitus
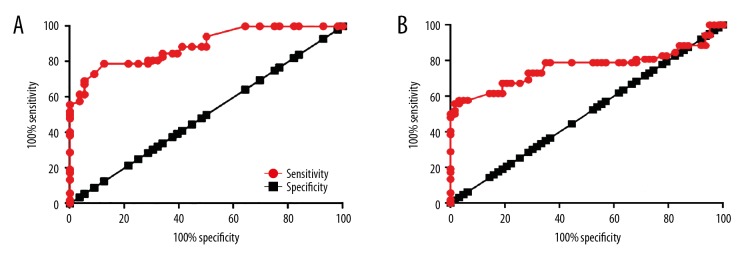
Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic value of plasma lncRNA-MIAT for diabetic retinopathy. As shown in Figure 2, with healthy controls as the reference, the area under the curve (AUC) was 0.8896, the standard error was 0.3055, and the 95% confidence interval (CI) was 0.8297–0.9495 (Figure 2A). In patients with diabetes without diabetic retinopathy as the reference, the AUC was 0.7633, the standard error was 0.05021, and the 95% CI was 0.6648–0.8617 (Figure 2A). Therefore, increased plasma levels of lncRNA-MIAT distinguished between patients with diabetes mellitus and diabetic retinopathy, and patients with diabetes mellitus without diabetic retinopathy, and from both healthy controls.
Figure 2.
Diagnostic receiver-operating characteristic (ROC) curve of plasma long non-coding RNA of myocardial infarction associated transcript (lncRNA-MIAT) in diabetic retinopathy. This figure shows the diagnostic value of measuring plasma long non-coding RNA of myocardial infarction associated transcript (lncRNA-MIAT) for diabetic retinopathy. (A) Plasma lncRNA-MIAT measurements in healthy controls. (B) Plasma lncRNA-MIAT measurements in patients with diabetes without the complication of diabetic retinopathy.
Plasma levels of lncRNA-MIAT and transforming growth factor-β1 (TGF-β1) were significantly correlated with the presence of diabetic retinopathy
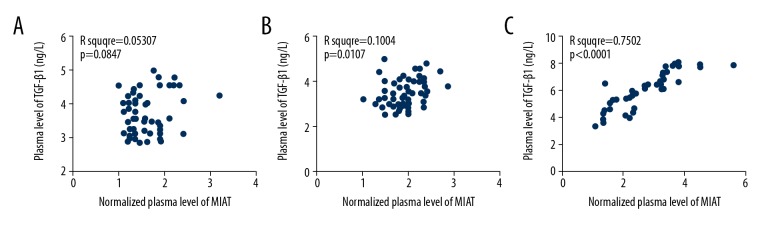
The relationship between plasma levels of lncRNA-MIAT and transforming growth factor-β1 (TGF-β1) were studied and showed that increased plasma levels of lncRNA-MIAT and TGF-β1 were significantly correlated with the presence of diabetic retinopathy in patients with diabetes (Figure 3A), when compared with patients with diabetes without diabetic retinopathy (Figure 3B) and healthy controls (Figure 3C).
Figure 3.
Plasma levels of long non-coding RNA of myocardial infarction associated transcript (lncRNA-MIAT) and transforming growth factor-β1 (TGF-β1) were positively correlated in patients with diabetic retinopathy. This figure shows the Pearson correlation analysis of plasma levels of long non-coding RNA of myocardial infarction associated transcript (lncRNA-MIAT) and transforming growth factor-β1 (TGF-β1). (A) Pearson correlation analysis in healthy controls. (B) Pearson correlation analysis in patients with diabetes without the complication of diabetic retinopathy. (C) Pearson correlation analysis in patients with diabetes with diabetic retinopathy.
A high glucose culture environment upregulated MIAT lncRNA expression in ARPE-19 adult retinal pigment epithelial cells in vitro
ARPE-19 adult retinal pigment epithelial cells were cultured in high glucose conditions. Clinically, the normal range for fasting blood glucose in our unit is 3.9–6.1 mM. Therefore, D-glucose (dextrose) at a concentration of 5 mM was used as a control concentration, and D-glucose at concentrations of 10, 20, and 30 mM were used in the culture media of ARPE-19 cells to provide a high glucose environment. As shown in Figure 4, significant differences in MIAT lncRNA expression were found in ARPE-19 cells for different concentrations of D-glucose. Compared with the control group, a high glucose environment significantly upregulated the expression level of MIAT lncRNA in ARPE-19 cells, in a dose-dependent manner.
Figure 4.
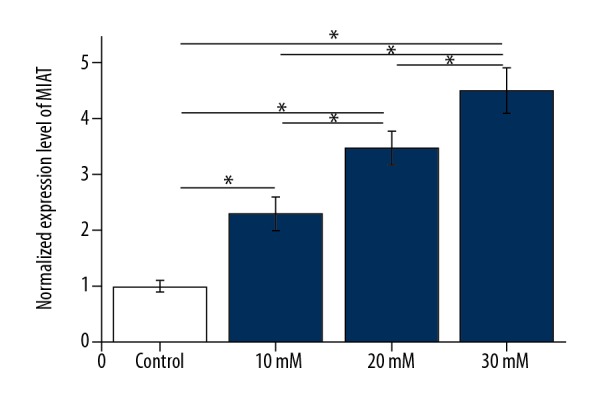
A high glucose environment upregulated long non-coding RNA of myocardial infarction associated transcript (lncRNA-MIAT) expression in adult retinal pigment epithelial ARPE-19 cells in vitro.
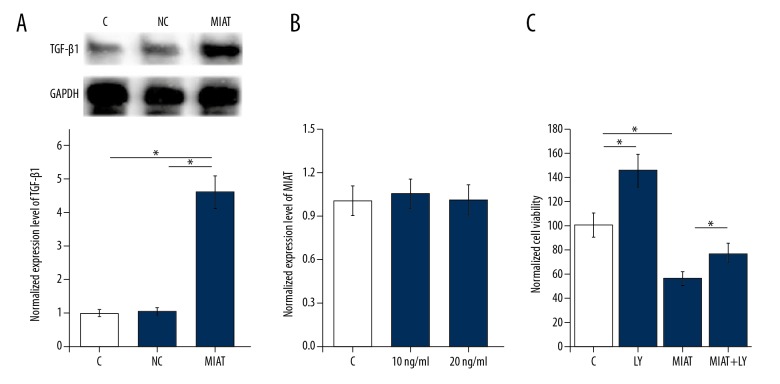
MIAT lncRNA overexpression upregulated TGF-β1 expression and promoted apoptosis of ARPE-19 cells in vitro
To further investigate the potential interactions between MIAT lncRNA and TGF-β1, the MIAT expression vector plasmid was transfected into ARPE-19 cells, and TGF-β1 expression was measured by Western blot. As shown in Figure 5A, significant differences were found in expression levels of TGF-β1 between the groups. MIAT lncRNA overexpression led to significantly upregulated expression of TGF-β1 (p<0.05). However, treatment with TGF-β1 (Sigma-Aldrich, St Louis, MO, USA) at doses of 10 and 20 ng/ml showed no significant effects on MIAT lncRNA expression (Figure 5B) and MIAT lncRNA expression was significantly reduced. However, treatment with TGF-β inhibitor, LY2109761 (LY) (Sigma-Aldrich, St Louis, MO, USA) at a dose of 100 nM increased the viability of ARPE-19 cells under cell culture conditions with 30 nM D-glucose (p<0.05) (Figure 5C). Also, ARPE-19 cells that underwent both MIAT lncRNA overexpression and TGF-β1 inhibition showed significantly increased cell viability compared with cells treated by MIAT lncRNA overexpression alone (p<0.05) (Figure 5C).
Figure 5.
Overexpression of the myocardial infarction associated transcript (MIAT) gene upregulated transforming growth factor-β1 (TGF-β1) expression and promoted apoptosis of ARPE-19 adult retinal pigment epithelial cells in vitro. This figure shows the effects of lncRNA-MIAT overexpression on TGF-β1 expression. (A) The effects of TGF-β1 treatment on expression of MIAT lncRNA. (B) The effects of TGF-β1 treatment on apoptosis of mouse ARPE-19 cells with overexpression of MIAT lncRNA. (C) The effects of TGF-β1 inhibitor treatment. * p<0.05; C – control; NC – negative control cells transfected with empty vector LY, TGF-β inhibitor LY2109761.
Discussion
The key findings of this study were that plasma levels of long non-coding RNA of myocardial infarction associated transcript (lncRNA-MIAT) were significantly increased in patients with diabetic retinopathy, and that in ARPE-19 human retinal pigment epithelial cells cultured in a high glues environment in vitro, increased expression of lncRNA-MIAT reduced cell viability by the activation of transforming growth factor-β1 (TGF-β1) signaling.
Recent studies in animal models have shown that the onset and development of diabetes are accompanied by changes in expression patterns of lncRNAs, which may be upregulated or down-regulated in diabetes to promote or inhibit the pathological changes caused by hyperglycemia [12]. Upregulation of the expression of lncRNA-MIAT has been shown in retinal endothelial cells in a high glucose environment [9]. Therefore, in the present study, an in vitro cell experiment was conducted using ARPE-19 cells, which also showed that a high glucose environment significantly upregulated the expression level of MIAT lncRNA in a dose-dependent manner. However, when comparing the plasma findings in the three patient groups, that although there were significant differences in plasma levels of lncRNA-MIAT between patients with diabetes with diabetic retinopathy and patients with diabetes without diabetic retinopathy, there was no significant difference between patients with diabetes without diabetic retinopathy and healthy controls. Therefore, it is possible that a high glucose environment may alter lncRNA-MIAT expression in certain types of cells, while significant changes in levels of circulating lncRNA-MIAT are only observed under more severe disease conditions, including diabetic retinopathy. Another explanation might be that patients with diabetes without retinopathy may still have the ability to antagonize or reduce the upregulation of MIAT lncRNA, while patients with retinopathy may have lost this ability.
Diabetic retinopathy usually develops as a complication of chronic or uncontrolled diabetes mellitus and is currently evaluated by regular ophthalmological examination. Based on the stage of the disease, the current treatment is laser therapy, which is used to prevent of loss of vision [13]. However, currently available treatment strategies for diabetic retinopathy are applicable only for patients at advanced stages and are reported to be associated with significant adverse effects [14]. Therefore, early diagnosis of diabetic retinopathy and the identification of methods for the prediction for patients who are most at risk remain important. Because the development of human disease can be accompanied by either new biomarkers or increased expression of markers in the blood, the identification and detection of diagnostic biomarkers may identify disease at an early stage and provide guidance regarding response to treatment [15]. Currently, circulating diagnostic biomarkers that have been proposed for diabetic retinopathy include endothelial cell and inflammatory biomarkers, which can be affected by high blood glucose levels and have low diagnostic specificity [16].
All patients included in this study who had diabetes mellitus with diabetic retinopathy had non-proliferative stage diabetic retinopathy, which is considered to be an early stage of this disease. Increased levels of lncRNA-MIAT were detected in the plasma of all patients in this group. Although high glucose environment significantly upregulated the expression level of lncRNA-MIAT in ARPE-19 cells, increased plasma levels of lncRNA-MIAT only appeared in patients with diabetes combined with retinopathy but not in patients with diabetes without retinopathy. Receiver operating characteristic (ROC) curve analysis also showed that increased plasma levels of lncRNA-MIAT significantly distinguished between patients with and without diabetic retinopathy. Therefore, measurement of plasma levels of lncRNA-MIAT may be a specific biomarker for diabetic retinopathy. Further controlled studies are required to support these findings.
The activation of TGF-β signaling has previously been reported to affect the development of diabetic retinopathy [6]. TGF-β signaling mediates inflammatory responses that may also promote the progression of diabetic retinopathy [17,18], which can be regarded as an inflammatory disease [19]. The findings of the present study showed a significant correlation between plasma TGF-β1 levels and lncRNA-MIAT levels in patients with diabetic retinopathy. Also, increased expression of lncRNA-MIAT significantly promoted the expression of TGF-β1 in ARPE-19 cells and reduced cell viability under high glucose culture conditions, while treatment with a TGF-β inhibitor increased cell viability and reduced the inhibitory effects of lncRNA-MIAT overexpression on cell viability. Therefore, it is possible that lncRNA-MIAT inhibited the viability of ARPE-19 cells by activating TGF-β1 signaling. Importantly, the significant correlation between of plasma levels TGF-β1 and lncRNA-MIAT in patients with diabetes and diabetic retinopathy was not observed in patients with diabetes without diabetic retinopathy, or and in healthy controls. Therefore, the regulatory effects of lncRNA-MIAT on TGF-β signaling may serve as a target for the treatment of diabetic retinopathy. Also, TGF-β1 inhibition may not completely prevent endothelial cell damage associated with lncRNA-MIAT overexpression, suggesting the possible involvement of other pathways involved in the lncRNA-MIAT-mediated loss of endothelial cell viability.
Conclusions
Long non-coding RNA of myocardial infarction associated transcript (lncRNA-MIAT) was significantly upregulated in patients with diabetes and diabetic retinopathy when compared with patients with diabetes without diabetic retinopathy, and levels could be detected in the plasma. The lncRNA-MIAT may promote the development of diabetic retinopathy by reducing the viability of adult retinal pigment epithelial cells by activating transforming growth factor-β1 (TGF-β1) signaling, as demonstrated by the findings from a preliminary in vitro study using ARPE-19 cells. The findings of this study provided new insights into the pathogenesis of diabetic retinopathy. Further controlled clinical studies are required to further evaluate the potential diagnostic role of plasma lncRNA-MIAT measurement and diabetic retinopathy, with the potential for developing a clinical diagnostic method and possibly a therapeutic target for diabetic retinopathy.
Footnotes
Source of support: Departmental sources
References
- 1.Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin Exp Ophthalmol. 2016;44(4):260–77. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Kertes PJ, Johnson TM, editors. Evidence-based eye care. 2nd edition. Wolters Kluwer Lippincott Williams & Wilkins Health; Philadelphia: 2014. [Google Scholar]
- 4.Tapp RJ, Shaw JE, Harper CA, et al. The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care. 2003;26(6):1731–37. doi: 10.2337/diacare.26.6.1731. [DOI] [PubMed] [Google Scholar]
- 5.Ochiai Y, Ochiai H. Higher concentration of transforming growth factor-β in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn J Ophthalmol. 2002;46(3):249–25. doi: 10.1016/s0021-5155(01)00523-8. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler SE, Lee NY. Emerging roles of transforming growth factor β signaling in diabetic retinopathy. J Cell Physiol. 2017;232(3):486–89. doi: 10.1002/jcp.25506. [DOI] [PubMed] [Google Scholar]
- 7.Yuan J, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25(5):666–81. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Kang Y. A new Lnc in metastasis: long noncoding RNA mediates the prometastatic functions of TGF-β. Cancer Cell. 2014;25(5):557–59. doi: 10.1016/j.ccr.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi X, Sun M, Liu H, et al. Long non-coding RNAs: A new frontier in the study of human diseases. Cancer Lett. 2013;339(2):159–66. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Yan B, Liu J, Yao J, et al. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015;16(7):1143–56. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 11.Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: Clues towards understanding pathogenesis? Eye. 2009;23(7):1496–506. doi: 10.1038/eye.2009.108. [DOI] [PubMed] [Google Scholar]
- 12.Jiang GJ, Zhang T, An T, et al. Differential expression of long noncoding RNAs between sperm samples from diabetic and non-diabetic mice. PLoS One. 2016;11(4):e0154028. doi: 10.1371/journal.pone.0154028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kollias AN, Ulbig MW. Diabetic retinopathy: Early diagnosis and effective treatment. Dtsch Arztebl Int. 2010;107(5):75–83. doi: 10.3238/arztebl.2010.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simó-Servat O, Simó R, Hernández C. Circulating biomarkers of diabetic retinopathy: An overview based on physiopathology. J Diabetes Res. 2016;2016 doi: 10.1155/2016/5263798. 5263798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joglekar MV, Januszewski AS, Jenkins AJ, et al. Circulating microRNA biomarkers of diabetic retinopathy. Diabetes. 2016;65(1):22–24. doi: 10.2337/dbi15-0028. [DOI] [PubMed] [Google Scholar]
- 16.Sasongko MB, Wong TY, Jenkins AJ, et al. Circulating markers of inflammation and endothelial function, and their relationship to diabetic retinopathy. Diabet Med. 2015;32(5):686–91. doi: 10.1111/dme.12640. [DOI] [PubMed] [Google Scholar]
- 17.Worthington JJ, Kelly A, Smedley C, et al. Integrin αvβ8-mediated TGF-β activation by effector regulatory T cells is essential for suppression of T-cell-mediated inflammation. Immunity. 2015;42(5):903–15. doi: 10.1016/j.immuni.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez VL, Caspi RR. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015;36(6):354–63. doi: 10.1016/j.it.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semeraro F, Cancarini A, Rezzola S, et al. Diabetic retinopathy: Vascular and inflammatory disease. J Diabetes Res. 2015;2015 doi: 10.1155/2015/582060. 582060. [DOI] [PMC free article] [PubMed] [Google Scholar]