Calcific Aortic Valve Disease is the most common indication for surgical valve replacement in the world[1]. For years this disease was thought to be a passive degenerative phenomenon. Understanding of the cellular mechanisms of this valve lesion will present new cellular therapeutic options to slow disease progression. In this study by Egan et al[2], identifies for the first time in human calcifying aortic valves a population of circulating osteogenic precursor cells(COP) in calcified human aortic valves. Their finding of these CD45+ OCN+ COP cells in areas of calcification and not in the unaffected calcified tissues provides another level of evidence that mesenchymal derived cell populations are responsible for the development of osteogensis in the calcified aortic valve. Specifically, the study demonstrated that these cells were localized to areas of confirmed endochondral ossification and bone formation. Within the regions of interest there were areas of mature bone with the characteristic architecture including osteocytes and bone lining cells. However, within the limits of the study there was no consistent involvement of the valve leaflet layers as the areas of endochondral ossification was found to extend to variable depths. The conclusions from this study provides the first evidence in human calcifying aortic valve tissue that a novel cellular origin is found on the calcific aortic valve and that these COP cells play a role in the cellular mechanisms of osteogensis.
Previously, we and Mohler et al, have demonstrated that aortic valve calcification is associated with endochondral bone formation and an osteoblast bone-like phenotype[3, 4]. This bone phenotype is regulated by canonical Wnt pathway in experimental cardiovascular calcification[5, 6]. We have also shown that the canonical Wnt/Lrp5 pathway is upregulated in diseased human valves from patients with valvular heart disease[7]. Bone and cartilage are major tissues in the vertebrate skeletal system, which is primarily composed of three cell types: osteoblasts, chrondrocytes, and osteoclasts. In the developing embryo, osteoblast and chrondrocytes, both differentiate from common mesenchymal progenitors in situ, whereas osteoclasts are of hematopoietic origin and brought in later by invading blood vessels. Osteoblast differentiation and maturation lead to bone formation controlled by two distinct mechanisms: intramembranous and endochondral ossification, both starting from mesenchymal condensations.
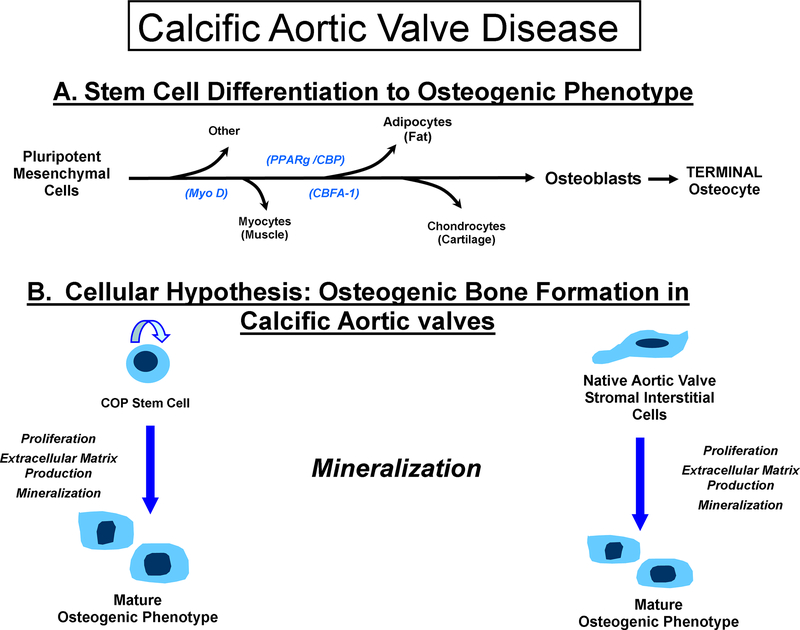
Two osteoblast-specific transcripts have been identified: 1) Cbfa1 and 2) osteocalcin (OC). The transcription factor Cbfa1[8] has all the attributes of a ‘master gene’ differentiation factor for the osteoblast lineage and bone matrix gene expression. During embryonic development, Cbfa1 expression precedes osteoblast differentiation and is restricted to mesenchymal cells destined to become osteoblast. In addition to its critical role in osteoblast commitment and differentiation, Cbfa1 appears to control osteoblast activity, i.e., the rate of bone formation by differentiated osteoblasts The regulatory mechanism of osteoblast differentiation from osteoblast progenitor cells as shown in Figure 1, Panel A into terminally differentiated cells is via a well orchestrated and well studied pathway which involves initial cellular proliferation events and then synthesis of bone matrix proteins, which requires the actions of specific paracrine/hormonal factors/BMP and the activation of the canonical Wnt pathway[9]. In a previous study by Suda et al, they have shown that these isolated COP cells can express BMP and can form bone in vivo[10]. Confirming the hypothesis that these COP cells are capable of homing to sites of valve calcification and neovascularization and form bone. The studies to date indicate that the cellular origins of bone forming cells in the calcifying aortic valve have two distinct pathways as shown in Figure 1, Panel B. The cells can either be the COP cell capable of differentiating to bone at the site of calcification and disease. The other cell type is the interstitial aortic valve cell that is capable of differentiating to bone in vivo, as described in the most recent NHLBI working group paper on calcific aortic valve disease[11]. Further evidence for the circulating stem cell was published in a study by Tanaka et al, which demonstrated using transplanted bone marrow cells composed 17% of the population of calcifying cells in the native atherosclerotic valve[12]. The presence of variable depths of the COP cell in the calcific valve is consistent with the hypothesis that these cells can home to the diseased valve but are not responsible for the entire bone formation process. The native interstitial cells also have the potential to differentiate to bone in situ and contribute to the calcifying cells in the native valve. The contribution of these two cell types toward the development of calcification in the aortic valve requires further ongoing investigation. In summary, this study provides incremental understanding into the role of calcific aortic valve disease. In the future, these studies will provide the roadmap for targeted cellular therapies for the treatment of this disease process to slow the progression of this disease and delay surgery in this patient population.
Figure 1.
Demonstrates the Calcific Aortic Valve Disease: Cellular Origins of Valves Calcification and the mechanisms of osteogensis in these cell types. Panel A: The potential cellular phenotypes for mesenchymal derived cells, Panel B: The two different cell origins for valve calcification: the COP cell and the native interstitial cell both contributing to the hypothesis of osteogenesis in calcific aortic valve disease.
Acknowledgement:
Dr. Rajamannan is the inventor on a patent for methods to slow progression of valvular heart disease. This patent is owned by the Mayo Clinic and the author does not receive any royalties from this patent. This work is supported by NIH grant funding: 5R01HL085591 and 3R01HL085591S1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- a1.Roberts WC, Ko JM, Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation, 2005. 111: p. 920–5. [DOI] [PubMed] [Google Scholar]
- 2.Egan KP, Kim J, Mohler EM, Pignolo RJ, Role for Circulating Oteogenic Precursor (COP) cells in Aortic Valvular Disease, ATVB, In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T, Human aortic valve calcification is associated with an osteoblast phenotype. Circulation, 2003. 107: p. 2181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohler ER 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS, Bone formation and inflammation in cardiac valves. Circulation, 2001. 103: p. 1522–8. [DOI] [PubMed] [Google Scholar]
- 5.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA, Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest, 2005. 115: p. 1210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC, Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation, 2005. 112(9 Suppl): p. I229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caira FC, et al. , Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol, 2006. 47: p. 1707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation.[see comment]. Cell 1997;89:747–54. [DOI] [PubMed] [Google Scholar]
- 9.Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone 1995;17(2 Suppl):77S–83S [DOI] [PubMed] [Google Scholar]
- 10.Suda RK, Bilings PC, Egan KP, Kim JH, McCarrick-Walmsley R, Glaser DL, Shore EM, Pignolo RJ, Circulating osteogenic precursor cells in heterotopic bone formation, Stem Cells 2009; 27:2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen JK, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM, Calcific Aortic Valve Disease: Not Simply a Degenerative Process A Review and Agenda for Research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group, Circulation in Press October 19,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka K, Sata M, Fukuda D, Suematsu Y, Motomura N, Takamoto S, Hirata Y, Nagai R, Age- Associated aortic stenosis in apoliprotein E- Deficient mice, J Am Coll Cardiol 2005; 46: 134–141. [DOI] [PubMed] [Google Scholar]