Aminoglycosides have been used since the 1940’s to treat bacterial infections1. At present, tobramycin, gentamicin, amikacin, streptomycin, neomycin, and paromomycin are approved for use in patients. These antibiotics bind to prokaryotic ribosomal RNA to inhibit protein synthesis. In addition to their antibacterial properties, aminoglycosides have numerous other activities2. Some aminoglycosides bind to human ribosomal mRNA which can result in read-through of premature stop codons and these drugs have been used in clinical trials for treatment of genetic disorders. Aminoglycosides also bind to other RNAs including ribozymes, resulting in inhibition of the latter’s activity, and to the TAR element in HIV, resulting in dissociation of the HIV Tat protein from the viral genome. Gopinath et al.3 report in this issue of Nature Microbiology that certain aminoglycosides can inhibit virus replication in cell culture, and that prophylactic application of these antibiotics to mucosal surfaces in mice can reduce virus replication. They show that the effect of aminoglycosides is independent of the microbiome of the animal, since similar effects are seen in germ-free mice. Aminoglycosides induced expression of interferon (IFN)-stimulated genes (ISGs) in a TLR3 dependent manner, which likely accounts for their antiviral activity. Recruitment of dendritic cells to the mucosa was required for ISG expression. Certain aminoglycosides including neomycin and kanamycin, induced ISGs, while others such as streptomycin and amikacin did not. Importantly, only aminoglycosides that that induced ISGs protected mice from challenge with herpes simplex virus (HSV) infection.
IFNs are critical components of the host response to virus infection (Fig. 1). IFNs induce expression of hundreds of ISGs that inhibit virus replication4. These ISGs have sequences in their promoters termed IFN-stimulated response elements (ISREs). Using a high-throughput screen of over 2,000 drugs, Patel et al. identified 64 compounds that activate ISREs5. These included 4 antibiotics, 11 cardiovascular drugs, and 6 anti-neoplastic drugs. Several anthracyclines approved for cancer therapy, including daunorubicin, doxorubicin, and idarubicin, activated ISREs; idarubicin was found to increase expression of several ISGs and inhibit replication of encephalomyocarditis virus in vitro without inducing cytotoxicity. A subsequent study by these authors showed that several statins activated ISREs, increased expression of ISGs when expressed with IFN-β, and inhibited replication of human rhinovirus and respiratory syncytial virus in vitro6. Thus, many drugs can activate ISREs and inhibit virus replication, including certain aminoglycosides.
Fig. 1. Activation of interferon (IFN) response genes (ISGs) by aminoglycosides, poly I:C, or double-stranded (ds) RNA after virus infection.
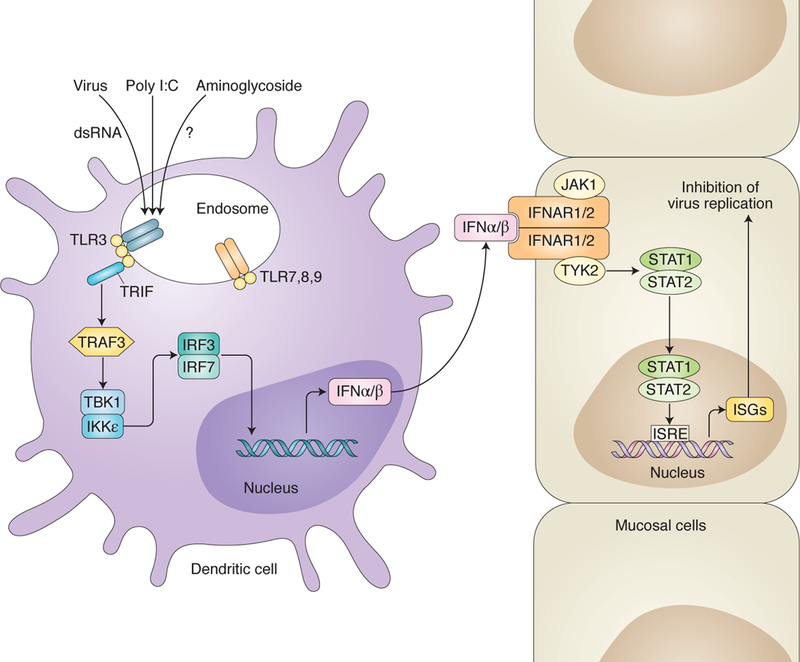
Endosomal toll-like receptor 3 (TLR3) is activated by dsRNA or poly I:C triggering a signaling cascade that results in production of IFN-α and -β. These latter two cytokines bind to IFN-α/β receptor subunits 1 and 2 (IFNAR1/2) on other cells which ultimately results in production of ISGs to inhibit virus infection. Aminoglycoside activation of ISGs is dependent on activation of TLR3, TRIF, and IRF3/7. Aminoglycosides are known to bind to host cell RNA which might trigger TLR3 activation, but this has not been established.
Gopinath et al.3 found that induction of ISGs by neomycin was due, at least in part, to activation of the toll-like receptor (TLR)-3 pathway and dependent on signaling by TRIF and ILR3/7. TLR3, along TLR7, 8, and 9 are located in endosomes of cells (Fig. 1). Activation of TLR3 by doubled-stranded RNA induces a signaling pathway that leads to synthesis of IFN and production of ISGs in neighboring cells to inhibit virus infection. TLR3 and TLR9 agonists are the basis of several vaccine adjuvants and they enhance immune responses, including production of IFN and ISGs7. CpG, is a TLR9 agonist and was recently approved as an adjuvant for a hepatitis B vaccine. Poly I:C is a TLR3 agonist and an investigational adjuvant for vaccines, particularly for cancer. Like aminoglycosides, poly I:C has been reported to inhibit replication of several viruses including HSV and influenza. Thus, aminoglycosides are TLR3-dependent activators of ISGs and may have potential as adjuvants.
Gopinath et al.3 report that prophylactic application of aminoglycosides to the nasal or vaginal mucosa of mice protected the animals from infection with certain DNA or RNA viruses, but the effects were transient. Prophylactic treatment of mice with intravaginal neomycin prior to genital challenge with HSV reduced disease; however vaginal shedding, a key endpoint in the mouse HSV infection model, was only inhibited during the first three days of infection and then reached levels similar to those in untreated mice. Pretreatment of mice deficient in Mx1, an ISG that is critical for protection of animals from influenza, with intranasal neomycin significantly improved survival; however, about 50% of the mice died. Pretreatment of mice with intravaginal neomycin or kasugamycin reduced levels of Zika virus RNA in vaginal mucosa for the first 2 days after challenge; however, after the third day the effect was no longer significant in the neomycin treated animals. Thus, while prophylactic use of certain aminoglycosides significantly improved outcomes in animals, the effects were modest.
Might aminoglycosides, or their derivatives, be useful for inhibiting virus infections at mucosal surfaces in humans? As noted above, aminoglycosides were found to induce ISGs resulting in an antiviral state, but only when used for several days before infection with virus. When initiated 24 hours after infection of mice with HSV, at a time when animals were still asymptomatic, aminoglycosides had a variable effect on disease. Imiquimod, a TLR-7 agonist, induces production of IFN-α and TNF-α and is approved for topical treatment of preexisting human papillomavirus infections and is used off label as topical therapy for acyclovir-resistant HSV8. Thus, it is likely that topical aminoglycosides, which also work through the IFN pathway would be less effective than topical imiquimod. However, while imiquimod may induce an inflammatory response that may limit its use, topical aminoglycosides might be better tolerated. As the authors note, topical administration of aminoglycosides would alter the microbiome and this may increase the risk of outgrowth of pathogenic bacteria. Thus, while current aminoglycosides seem unlikely to be clinically useful to prevent virus infections, derivatives of these drugs might be more effective with less effect on the microbiome. The work of Gopinath et al.3 provide yet another activity for an old class of drugs, and suggest that in addition to altering the bacterial microbiome, treatment of patients with aminoglycosides might also alter their virome by inducing ISGs.
Footnotes
Competing interests
The author declares no competing interests.
References
- 1.Krause KM, Serio AW, Kane TR, & Connolly LE Cold Spring Harb Perspect Med 6:a027029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermann T Cell Mol Life Sci 64:1841–52 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gopinath S et al. Nat. Microbiol xx:xx-xx (2018). [Google Scholar]
- 4.Schlee M & Hartmann G Nat Rev Immunol 16:566–80 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel DA, Patel AC, Nolan WC, Zhang Y, & Holtzman MJ PLoS One 7:e36594 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel DA, et al. J Biomol Screen 19:119–30 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alving CR, Peachman KK, Rao M, Reed SG Curr Opin Immunol 24:310–5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanley MA Clin Exp Dermatol 27:571–7 (2002). [DOI] [PubMed] [Google Scholar]


