Abstract
XMEN disease (X-linked immunodeficiency with Magnesium defect, Epstein-Barr virus infection and Neoplasia) is a novel primary immune deficiency caused by mutations in MAGT1 and characterised by chronic infection with Epstein-Barr virus (EBV), EBV-driven lymphoma, CD4 T-cell lymphopenia, and dysgammaglobulinemia(1). Functional studies have demonstrated roles for magnesium as a second messenger T-cell receptor signalling(1), and for NKG2D expression and consequently NK- and CD8 T-cell cytotoxicity(2). 7 patients have been described in the literature; the oldest died at 45 years and was diagnosed posthumously(1–3). We present the case of a 58-year-old Caucasian gentleman with a novel mutation in MAGT1 with the aim of adding to the phenotype of this newly described disease by detailing his clinical course over more than 20 years.
Case Report
The patient was originally referred to immunology at the age of 36 years, with a long history of sinopulmonary infections and left-sided bronchiectasis. In childhood he had 3 episodes of pneumonia requiring hospital admission, and family history was remarkable for unexplained neonatal death of a male sibling. Initial investigations revealed normal immunoglobulins, serum protein electrophoresis, and absolute counts of peripheral T-, B- and NK-cells, although his CD4:CD8 ratio was reversed at 0.76. He was able to mount responses to protein vaccines; however, failed to respond to pneumococcal polysaccharide. He was therefore diagnosed with specific polysaccharide antibody deficiency and, in view of co-existing bronchiectasis, was commenced on immunoglobulin infusions with a good response over the next 16 years. Although his platelet count was normal at presentation, a mild intermittent thrombocytopenia was noted during follow-up with counts ranging from 104–256×109/L.
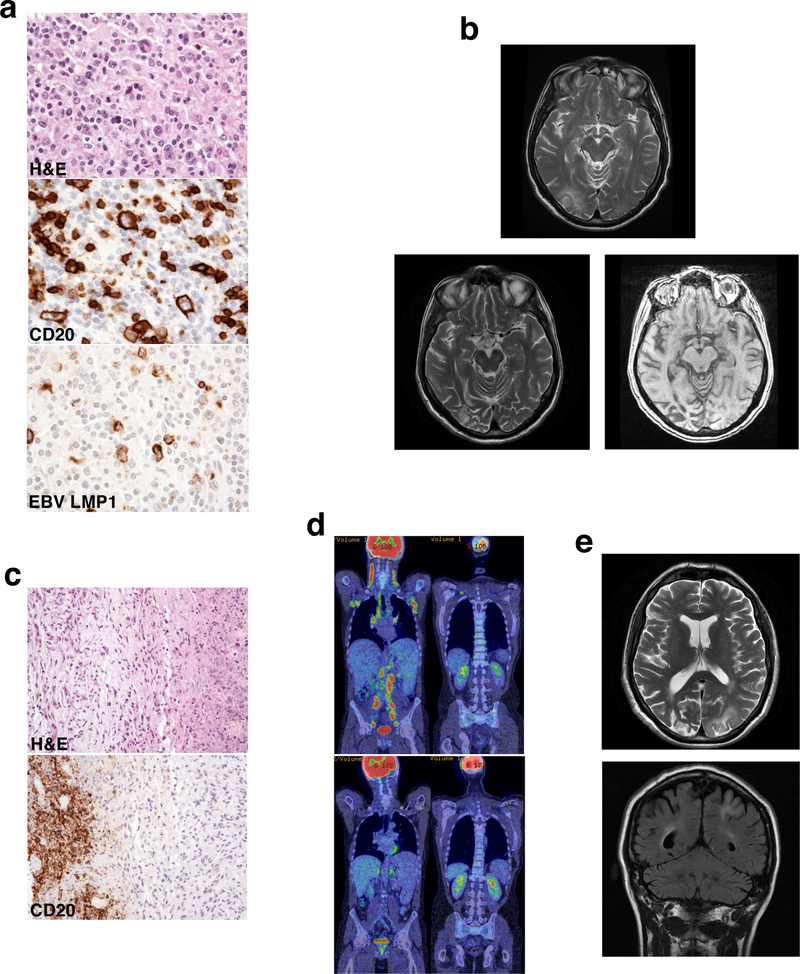
At the age of 52 he began to suffer from increased respiratory tract infections, associated with waxing and waning lymphadenopathy, hepatosplenomegaly and B-symptoms. Investigations demonstrated fluctuating EBV viremia up to 18,000 copies/ml and a decline of his previously normal serum IgA and IgM to undetectable levels. PET-CT showed widespread adenopathy with avid fluorodeoxyglucose (FDG) uptake and lymph node histology showed an EBV-driven lymphoproliferative disorder (Figure 1a). He was initially treated with 4 cycles of single agent Rituximab, however full R-CHOP chemotherapy was later instituted as his B-symptoms persisted. Treatment was curtailed after 5 out of 6 planned cycles due to Pneumocystis pneumonia (PCP). Despite this, he made a good clinical and radiological response, his EBV viral load became undetectable and serum IgA and M recovered to normal levels. Shortly afterwards he developed left-sided homonymous hemianopia and pupillary mydriasis; MRI brain showed features thought to be suggestive of posterior reversible encephalopathic syndrome (PRES) secondary to chemotherapy (Figure 1b); lumbar puncture was not performed. Although his neurological symptoms resolved over 4–6 months, 4 years later the MRI changes were persistent and associated with underlying atrophy (Figure 1b).
Fig. 1. Lymph node histology and imaging.
a Lymph node histology showing EBV-driven lymphoproliferative disease with atypical Reed-Sternberg-like cells (arrow, top image), staining for CD20 (middle image) and EBV LMP1 (bottom image). b MRI brain showing high T-2 signal in right occipital lobe (top image), which persisted and was associated with underlying atrophy 4 years later (bottom images). c Lymph node histology showing diffuse large B-cell lymphoma with extensive necrosis and fibrosis (top image) and ghostly outlines of very large CD20+ cells (bottom image). d PET-CT images showing widespread adenopathy with avid FDG uptake, hepatosplenomegaly and bony uptake in the pelvis and spine (top images) with regression of nodal and bony disease post-chemotherapy (bottom images). e MRI brain showing multifocal white matter changes in the occipital and parietal lobes
At the age of 57 symptoms suggestive of lymphoproliferative disease recurred, this time associated with worsening of his intermittent thrombocytopenia, with platelet counts ranging from 33–237 ×109/L, intermittent neutropenia, severe CD4 and B-cell lymphopenia (CD4 count 0.09×109/L; B cell count 0.02 ×109/L) and an EBV viral load of a million copies/ml. PET-CT revealed enlarged FDG-avid nodes on both sides of the diaphragm with bone involvement (Figure 1d); diffuse large B-cell lymphoma was diagnosed on lymph node histology (Figure 1c). In situ hybridization for EBV (EBER) was negative, however the sample was necrotic and it was therefore not clear whether this was an accurate result. Treatment with R-CHOP chemotherapy was limited to 2 cycles due to cumulative doxorubicin dose. He subsequently received 4 cycles of R-GCVP chemotherapy followed by 2 cycles of Rituximab and made a good response with striking regression of his nodal and bony disease (Figure 1d). Coincident with the end of chemotherapy he developed rapid visual deterioration without any associated field defects, extra-ocular muscle weakness or retinal pathology. MRI brain showed multifocal white matter changes affecting the occipital and parietal lobes (Figure 1e). Analysis of cerebrospinal fluid revealed raised protein (639mg/L), and although polymerase chain reaction was negative for EBV, cytomegalovirus (CMV), herpes simplex, varicella zoster (VZV) and enterovirus, JC virus was detected at 1.72 copies/ml, rising to 77.1 copies/ml a month later. Progressive neurological decline ensued with seizures, expressive dysphasia, perseveration of speech, and right-sided neglect and weakness in keeping with progressive multifocal leucoencephalopathy (PML), and the patient died a few months later.
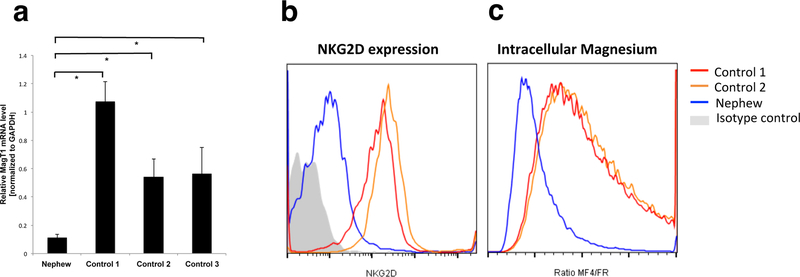
During his follow-up it became apparent that one of his nephews developed an EBV lymphoproliferative disease at the age of 13 years. He was treated with chemotherapy, followed by brachytherapy for recurrence. Investigations for known X-linked defects (SAP, XIAP, CD40L, and WASp), including those involving EBV susceptibility, were normal. Recently a novel mutation in exon 5 of MAGT1 (c.712C>T, p.R238X) was identified in the nephew. MAGT1 mRNA in peripheral blood mononuclear cells (PBMCs) was reduced 5–10 fold compared to healthy controls (Figure 2a and supplementary methods section). In addition, NKG2D expression on CD8+ T-cells (Figure 2b) and basal intracellular free magnesium in PBMCs (Figure 2c), measured as previously described(1, 2), were both reduced consistent with a diagnosis of XMEN disease. The same mutation has subsequently been found in our patient (Table 1, and supplementary family pedigree).
Fig. 2. Validation of novel mutation in MAGT1.
a RT-PCR showing the level of MagT1 mRNA normalized to GAPDH in PBMCs from the nephew compared to three controls. All samples were assayed on at least two different days and each time in duplicate or triplicate.Asterisks indicate P<0.02. b Flow cytometry of NKG2D surface expression on CD8 cells from the nephew and normal controls. c Intracellular magnesium level from then nephew and normal controls. Flow cytometry shows the ratio of the mean fluorescent intensity (MFI) of a Mg2+-specific fluorescent probe (MagFluo4) to the MFI of a Ca2+-sensitive probe (FuraRed) as (MF4/FR) in PBMCs. The ratio is set at 1 for the normal controls. Isotype control antibody staining indicates background fluorescence in both panels. All plots are representative of at least 2 independent experiments.
Table 1.
Clinical and immunological features of the patient and his nephew compared with other cases of XMEN disease reported in the literature
| Patient | Nephew | Other Reported Cases (n=7)(1–3) | ||||
|---|---|---|---|---|---|---|
| Clinical Phenotype | ||||||
| Age at presentation | 36 years | 13 years | 3–45 years | |||
| Age & cause of death | 58 years DLBCL, PML | _ | 23, 45 years (2/7) Post-HSCT for lymphoma | |||
| Presenting diagnosis | • Recurrent respiratory tract infections with bronchiectasis • Specific polysaccharide antibody deficiency |
• EBV-driven LPD | • Recurrent infections • Persistent EBV viremia • Lymphoma • Chronic active EBV (1/7) |
|||
| Lymphoproliferative disease (LPD) (Age; Treatment) | • EBV-driven B-cell LPD (52 years; RCHOP) • DLBCL (58 ears; RCHOP, RGCVP) |
•EBV-driven B-cell LPD (13 years; chemotherapy) • Recurrent B-cell LPD disease (14 years; brachytherapy) |
• B-cell LPD, Burkitt’s, Hodgkin’s, non-Hodgkin’s lymphoma • Age 7–45 years • 2/7 had 2 episodes of lymphoma • 2/2 died post HSCT • 3/7 (3–7 years old) with no LPD |
|||
| Recurrent infections | • EBV Viral load range: undetectable to 1.26×106 copies/ml • Sinopulmonary bacterial infections with bronchiectasis |
• EBV Viral load range: 9×103 to 1.11×105 copies/ml |
• EBV (7/7) • Other viral infections e.g. herpes simplex, recurrent VZV, molluscum, pneumonia (4/7) • Bacterial respiratory tract infections (6/7) |
|||
| Immunological Phenotype | ||||||
| Time Point | At Presentation | During Follow-up | At Presentation | During Follow-up | ||
|
Immunoglobulins g/L (normal range) |
IgG | 9 (6–16) | 4.7–14.9 (trough levels on replacement) | 4.62 (5.2–14.8) | 6.27 (6.4–17.3) | Dysgammaglobuinemia with variable serum immunoglobulins and vaccine responses |
| IgA | 1.21 (0.8–3) | <0.06–1.2 (0.8–3) | 0.33 (0.5–3.8) | 0.28 (0.9–4.9) | ||
| IgM | 1.26 (0.4–2.5) | <0.04–1.24 (0.4–2.5) | 0.44 (0.4–2) | 5.7 (0.3–3.4) | ||
| Comment | • Vaccine responses normal to protein-conjugates, absent to polysaccharide • Started on immunoglobulin replacement |
• Undetectable IgA and IgM during LPD only | • Immunoglobulins measured post-chemotherapy • Vaccine responses not measured |
• Values above represent immunoglobulins measured prior to IgG infusions for thrombocytopenia | ||
|
Lymphocyte Subpopulations x109/L (normal range) |
Total | 3.78 (1–2.8) | 0.51–2.01 (1–2.8) | 2.94 (1.3–3.6) | 2.49 (1.3–3.6) | - |
| CD3 | 2.27 (0.7–2.1) | 0.22–1.16 (0.7–2.1) | 1.55 (0.6–2.3) | 1.38 (0.6–2.3) | % low in 1/6, normal in 5/6 | |
| CD4 | 0.95 (0.3–1.4) | 0.09–1.26 (0.3–1.4) | 0.62 (0.3–1.6) | 0.53 (0.3–1.6) | • Absolute counts often above 0.3×109/L • % low in 4/6, normal in 1/6, high in1/6 |
|
| CD8 | 1.25 (0.2–0.9) | 0.13–1.10 (0.2–0.9) | 0.71 (0.15–0.79) | 0.71 (0.15–0.79) | % low in1/6, normal in 4/6, high in 1/6 | |
| CD19 | 0.98 (0.1–0.5) | <0.001–1.06 (0.1–0.5) | 0.64 (0.08–0.5) | 0.51 (0.08–0.5) | % low in1/6 on Rituximab, normal in 4/6, high in 1/6 | |
| NK | Not Done | 0.10–0.36 (0.09–0.6) | 0.24 (0.11–0.61) | 0.13 (0.11–0.61) | % low in1/6, normal in 5/6 | |
| Comment | - | • B-cells 0.01 in the context of LPD (before treatment) & undetectable after Rituximab | - | - | • Expressed at % of total lymphocytes • Only measured in 6/7 |
|
| CD4:CD8 Ratio | Inverted | Inverted | Inverted in 6/7 | |||
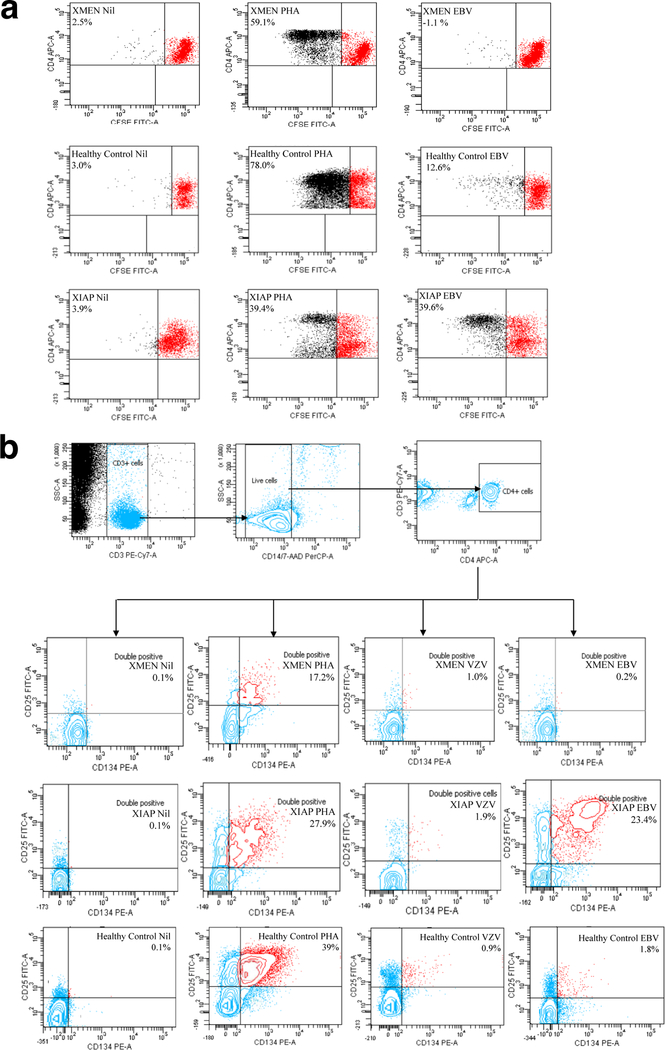
Given the predominant susceptibility of patients with MAGT1 deficiency to EBV, we decided to look at EBV-specific T-cell responses in our patient (supplementary methods section). Antigen-specific CFSE (Figure 3a) showed an absent proliferative response to EBV, despite a viral load of a million copies/ml and a preserved response to phytohemagglutinin (PHA). A similar pattern was seen when looking for upregulation of CD25 and OX-40 on antigen-specific CD4 T-cells (4), which demonstrated a defective response to EBV, although responses to PHA, and other common viruses such as varicella zoster (VZV) (Figure 3b) and cytomegalovirus (not shown) were normal. These results were in contrast to those from a patient with a hypomorphic mutation in XIAP (c .396G>T; p.G466X) and no current EBV viremia (Figure 3a and b), which consistently showed a stronger proliferative and OX-40 response to EBV compared to healthy controls, suggesting that these assays could potentially provide a simple strategy for differentiating XMEN disease from other immunodeficiencies with EBV susceptibility, although further work is needed.
Fig. 3. EBV-specific T-cell responses in XMEN patient compared with a healthy control and a patient with a hypomorphic mutation in XIAP resulting in the expression of a truncated protein.
a CFSE proliferation assay of PBMCs using medium (nil), PHA, and EBV lysate. b OX-40 assay using medium (nil), PHA, VZV and EBV lysates. Cells are gated to acquire 30, 000 CD3+ events, a negative gate is applied to exclude monocytes and apoptotic cells that may aberrantly express activation markers, then dual expression of activation markers CD25 and CD134 (OX-40) is measured amongst the CD4+ population; the gate coordinates are fixed to give 0.1% double positive events in the nil tube (supplementary methods section)
Discussion
Mutations in MAGT1, which encodes a selective magnesium transporter, have been described in 7 male patients ranging from 3–45 years with chronic EBV, EBV-driven lymphoproliferative disorders, inverted CD4:CD8 ratios, and dysgammaglobulinemia(1–3). Loss-of-function mutations in MAGT1 have been shown to abrogate the transient influx of free magnesium resulting from T-cell receptor signaling leading to delayed PLCγ1 activation(1). Although EBV specific CD8+ memory T-cells have been demonstrated via tetramer staining, there is defective cytotoxic T-cell and NK cell cytotoxicity due to reduced NKG2D expression, resulting from reduced intracellular basal free magnesium(2).
Optimal treatment has not yet been established. Oral magnesium restored NKG2D expression and diminished the percentage of EBV-infected B-cells in 2 patients(2); however, long-term clinical correlates are lacking and the patients may only come to medical attention following the development of an EBV-driven lymphoproliferative disease, which can occur as late as the 4th decade, precluding early institution of treatment(3). Given that the main cause of morbidity and mortality in XMEN disease is EBV-driven lymphoproliferative disease, perhaps there is a role for B-cell ablative therapies, particularly in the context of late diagnosis, although this will not be curative and caution should be exercised in light of the development of PML in the patient presented. Patients presenting with lymphoma should be treated with chemotherapy, although recurrence is likely. Standard of care for relapsed aggressive lymphoma usually involves high dose chemotherapy with autologous stem cell transplantation; although allogeneic transplantation might be curative, 2 patients with XMEN disease and lymphoma underwent transplant and died of transplant related complications(2, 3).
Although PML has been described in the context of other primary immune deficiencies associated with idiopathic CD4 T-cell lymphopaenia(5–9), this is the first description in a patient with XMEN disease. The patient had several known immunological risk factors for the condition including profound CD4 lymphopaenia, B-cell malignancy and treatment with chemotherapy and Rituximab(10, 11); it is also possible that the T-cell activation defect demonstrated in MAGT1 deficiency(1) may have played a role. Evidence suggests that the JC virus establishes latent infection in epithelial cells of the kidney and haematopoietic cells in the bone marrow, including B-cells(12–14). In the case presented, treatment with chemotherapy and Rituximab could have increased the mobilisation of latently infected B-cells from the bone marrow in order to restore peripheral B-cell numbers(11). This, in combination with his cellular immune defect, could have led to viral reactivation, oligodendrocyte infection and the development of PML.
In summary, we have described the case of a 58 year-old-man with a novel nonsense mutation in MAGT1. We hope to have added to the phenotype of this newly described disease by describing his clinical course over more than 20 years and characterising his EBV-specific T-cell responses. This case demonstrates that lymphomas can fail to show staining for EBV and identifies PCP and PML as possible complications, particularly in the context of treatment with chemotherapeutic agents and Rituximab. The difference in the age of presentation with EBV-driven lymphoproliferative disease between the patient and his nephew (52 Vs 13 years) suggests additional roles for environmental factors (such as age at first EBV encounter) and/or genetic modifiers. This, together with the treatment difficulties highlighted, emphasises the importance of early diagnosis, in order to ensure that the patient is in the optimal clinical state for treatments directed against the molecular defect, such as oral magnesium, allogeneic hematopoietic stem cell transplantation, or even, in the future, gene therapy.
Supplementary Material
Acknowledgements
This work was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases. We thank Tammy Krogmann for preparing RNA and cDNA from the nephew.
Footnotes
Work originated from the Department of Clinical Immunology and the Nuffield Department of Medicine, John Radcliffe Hospital, Oxford.
References
- 1.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011;475(7357):471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaigne-Delalande B, Li FY, O’Connor GM, Lukacs MJ, Jiang P, Zheng L, et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science 2013;341(6142):186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li FY, Chaigne-Delalande B, Su H, Uzel G, Matthews H, Lenardo MJ. XMEN disease: a new primary immunodeficiency affecting Mg2+ regulation of immunity against Epstein-Barr virus. Blood 2014;123(14):1248–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadler R, Bateman EA, Heath V, Patel SY, Schwingshackl PP, Cullinane AC, et al. Establishment of a healthy human range for the whole blood ‘OX40’ assay for the detection of antigen-specific CD4+ T cells by flow cytometry. Cytometry B Clin Cytom 2014. [DOI] [PubMed]
- 5.Misbah SA, Spickett GP, Zeman A, Esiri MM, Wallington TB, Kurtz JB, et al. Progressive multifocal leucoencephalopathy, sclerosing cholangitis, bronchiectasis and disseminated warts in a patient with primary combined immune deficiency. J Clin Pathol 1992;45(7):624–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chikezie PU, Greenberg AL. Idiopathic CD4+ T lymphocytopenia presenting as progressive multifocal leukoencephalopathy: case report. Clin Infect Dis 1997;24(3):526–7. [DOI] [PubMed] [Google Scholar]
- 7.Haider S, Nafziger D, Gutierrez JA, Brar I, Mateo N, Fogle J. Progressive multifocal leukoencephalopathy and idiopathic CD4+lymphocytopenia: a case report and review of reported cases. Clin Infect Dis 2000;31(4):E20–2. [DOI] [PubMed] [Google Scholar]
- 8.Inhoff O, Doerries K, Doerries R, Scharf J, Groden C, Goerdt S, et al. Disseminated cutaneous Kaposi sarcoma and progressive multifocal leukoencephalopathy in a patient with idiopathic CD4+ T lymphocytopenia. Arch Dermatol 2007;143(5):673–5. [DOI] [PubMed] [Google Scholar]
- 9.Delgado-Alvarado M, Sedano MJ, González-Quintanilla V, de Lucas EM, Polo JM, Berciano J. Progressive multifocal leukoencephalopathy and idiopathic CD4 lymphocytopenia. J Neurol Sci 2013;327(1–2):75–9. [DOI] [PubMed] [Google Scholar]
- 10.Carson KR, Focosi D, Major EO, Petrini M, Richey EA, West DP, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol 2009;10(8):816–24. [DOI] [PubMed] [Google Scholar]
- 11.Ferenczy MW, Marshall LJ, Nelson CD, Atwood WJ, Nath A, Khalili K, et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 2012;25(3):471–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houff SA, Major EO, Katz DA, Kufta CV, Sever JL, Pittaluga S, et al. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N Engl J Med 1988;318(5):301–5. [DOI] [PubMed] [Google Scholar]
- 13.Monaco MC, Atwood WJ, Gravell M, Tornatore CS, Major EO. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J Virol 1996;70(10):7004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houff SA, Berger JR. The bone marrow, B cells, and JC virus. J Neurovirol 2008;14(5):341–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.