Abstract
Background
Insomnia has been associated in cross-sectional studies with increased beta (15–35 Hz) EEG power during non-rapid eye movement (NREM) sleep, an index of cortical hyperarousal. However, it is unknown whether this cortical hyperarousal is present before individuals with insomnia develop the disorder. To fill this gap, we examined the association of childhood sleep high-frequency EEG activity with incident insomnia symptoms (i.e., absence of insomnia symptoms in childhood but presence in adolescence).
Methods
We studied a case-control subsample of 45 children (6–11y) from the Penn State Child Cohort, a population-based random sample of 421 children, who were followed-up after 8 years as adolescents (13–20y). We examined low-beta (15–25 Hz) and high-beta (25–35 Hz) relative power at central EEG derivations during NREM sleep and, in secondary analyses, during sleep onset latency, sleep onset, and REM sleep. Incident insomnia symptoms were defined as the absence of parent-reported difficulty falling and/or staying asleep during childhood and a self-report of these insomnia symptoms during adolescence.
Results
Childhood high-beta power during NREM sleep was significantly increased in children who developed insomnia symptoms in adolescence (n=25) as compared to normal sleeping controls (n=20; p=0.03). Multivariable-adjusted logistic regression models showed that increased childhood high-beta EEG power during NREM sleep was associated with a 3-fold odds (95%CI=1.12–7.98) of incident insomnia symptoms in adolescence. No other significant relationships were observed for other sleep/wake states or EEG frequency bands.
Conclusions
Increased childhood high-frequency EEG power during NREM sleep is associated with incident insomnia symptoms in adolescence. This study indicates that cortical hyperarousal during sleep may be a premorbid neurophysiological sign of insomnia, which may mediate the increased risk of psychiatry disorders associated with insomnia.
Keywords: Adolescence, beta, childhood, EEG, hyperarousal, incidence, insomnia symptoms
Introduction
Insomnia symptoms of difficulty falling and/or staying asleep are the most common sleep complaints in childhood and adolescence, with prevalence rates of 17% to 40% (Archbold et al., 2002; Liu and Zhou, 2002; Ohayon et al., 2000; Roberts et al., 2008; Fricke-Oerkermann et al., 2007). Importantly, it has been reported that the incidence of insomnia symptoms in the transition to adolescence is as high as 28% (Roberts et al., 2008; Patten et al., 2000; Zhang et al., 2011; Roberts et al., 1995). Insomnia has been associated with physical, emotional, cognitive and social development, impairing daytime functioning in children and adolescents (Siomos et al., 2010). However, the pathophysiological mechanisms of insomnia symptoms in these early developmental stages remain unclear.
It is possible that a circadian misalignment as a result of the developmental shift towards eveningness in adolescence may play a key role in the incidence of insomnia symptoms in this developmental stage (Fernandez-Mendoza et al., 2010a; Hagenauer et al., 2009). Also, cognitive-emotional and physiological hyperarousal, a known mechanism for the persistence of insomnia in adults, may be a key determinant of the development of insomnia symptoms early on in adolescence (Fernandez-Mendoza et al., 2010b; Bonnet and Arand, 2010). Cortical hyperarousal has specifically been regarded as a mechanistic pathway in the development and maintenance of insomnia and its sleep complaints (Riemann et al., 2010; Perlis et al., 1997). Multiple cross-sectional studies have examined electroencephalogram (EEG) spectral power as an indicator of cortical hyperarousal during sleep in individuals with insomnia and have focused on high-frequency EEG activity in the beta range (15–35 Hz). Most previous cross-sectional studies in young and middle-aged adults have consistently reported that those with insomnia complaints have increased EEG beta power while trying to fall asleep [i.e., during sleep onset latency (SOL)] and while asleep [i.e., during non-rapid eye movement (NREM) sleep] compared to good sleepers (Merica and Gaillard, 1992; Lamarche and Ogilvie, 1997; Cervena et al., 2014; Freedman, 1986; Merica et al., 1998; Perlis et al., 2001a,c; Krystal et al., 2002; Buysse et al., 2008; Spiegelhalder et al., 2010; Corsi-Cabrera et al., 2010; Israel et al., 2012). These previous studies have reported inconsistent associations with other EEG frequency bands (i.e., delta, alpha, sigma and theta) or other sleep states (i.e., REM sleep) (Cervena et al., 2014; Freedman, 1986; Merica et al., 1998; Perlis et al., 2001a,c; Krystal et al., 2002; Spiegelhalder et al., 2012; Israel et al., 2012; Wolynczyk-Gmaj et al., 2011; St-Jean et al., 2013; Wu et al., 2013). The most common interpretation of this cortical hyperarousal during NREM sleep is as an underlying pathophysiologic mechanism of the clinical phenomenology of insomnia (Perlis et al, 2001; Buysse et al, 2011), including the report of being awake, thinking or worrying while asleep, taking longer to fall asleep or sleeping less hours than physiologically obtained (i.e., sleep misperception). However, there is the possibility that cortical hyperarousal during NREM sleep in individuals with insomnia may precede the onset of these clinical phenomena and, thus, may be a potential early sign of the disorder. Given the cross-sectional nature of previous studies, it is unknown whether cortical hyperarousal precedes the onset of insomnia complaints or whether it is the result of the sleep disorder.
No study has examined the longitudinal association between premorbid sleep EEG activity and the development of insomnia. Specifically, no study has taken a developmental approach to study the association of childhood EEG beta activity during NREM sleep with incident insomnia symptoms in adolescence. This developmental approach is important because changes in the sleep EEG occur after the age of 11–12 years (Feinberg and Campbell, 2010; Tarokh and Carskadon, 2010) and identifying early EEG signs that can predict future development of insomnia symptoms in young children will greatly help with understanding the etiology of insomnia, identifying preventive strategies, and developing targeted interventions. We hypothesized that increased beta EEG power, (a neurophysiologic marker of cortical hyperarousal), during NREM sleep in childhood is a significant predictor of incident insomnia symptoms in adolescence. To test this hypothesis, we studied a case-control subsample of 45 children 6–11 years old, drawn from a large, random general population cohort, who were followed-up about 8 years later during adolescence at the age of 13–20 years old.
Methods
Sample
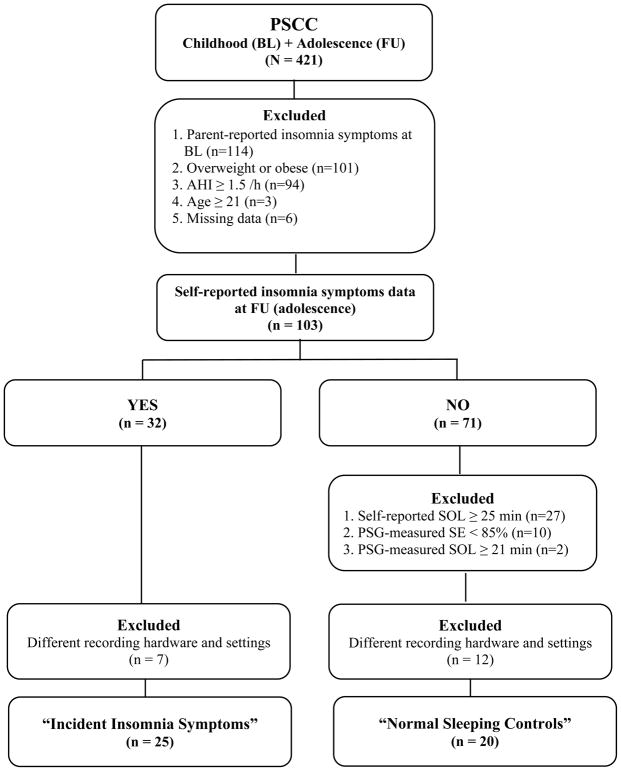
The Penn State Child Cohort (PSCC) is a general population sample of 700 children between ages 5–12 years, of whom 421 were followed up 8.4 years later as adolescents (mean age 17.0 ± 2.2 years, 53.9% male, and 21.9% ethnic minority) (Bixler et al., 2016; Rodríguez-Colón et al., 2015). In order to examine whether increased beta power during NREM sleep is present in individuals with insomnia before they develop the disorder, we studied a case-control subsample of the PSCC to exclude major potential confounders. The incident insomnia symptoms group fulfilled the following criteria: 1) absence of insomnia symptoms at baseline and presence of insomnia symptoms at follow-up, 2) absence of overweight or obesity at follow-up, defined as a body mass index percentile (BMI) < 85% (Kuczmarski et al., 2000), to remove significant confounding by sleep disturbances associated with overweight 3) absence of sleep-disordered breathing (SDB) at follow-up, defined as an apnea-hypopnea index (AHI) < 1.5 events/h of sleep using polysomnography (PSG), given that SDB is associated with changes in EEG activity (i.e., arousals), and 4) age younger than 21 years at follow-up, to include older adolescents (i.e., 18–20 years old) but exclude those few who had reach a psychosocial milestone into young adulthood (e.g., legal drinking age, college graduation). The normal sleeping control group met the following criteria: 1) absence of insomnia symptoms at both baseline and follow-up, 2) absence of overweight or obesity at follow-up, as defined above, 3) absence of SDB at follow-up, as defined above, 4) age younger than 21 years at follow-up, and 5) presence of subjectively reported and objectively measured normal sleep at follow-up, defined as a self-reported SOL below the median of the entire population, i.e., < 25 minutes, and PSG-measured SOL and sleep efficiency (SE) below and above the median, i.e., < 21 minutes and ≥ 85% respectively, of the entire population to ensure that the control group without incident insomnia symptoms were indeed good sleeping adolescents (Fernandez-Mendoza et al., 2016). None of the subjects reported a current use of legal or illegal substances/drugs, except habitual use of morning caffeine. A total of 32 normal sleeping controls and 32 cases of incident insomnia symptoms were selected. However, given that we updated our PSG recording system during the baseline period, we had to exclude 18 subjects (11 normal sleeping controls and 7 incident insomnia symptoms) who were monitored with different PSG system models and recording settings, including sampling rate. Thus, a total of 25 subjects with incident insomnia symptoms and 20 normal sleeping controls were included in this study. Figure 1 depicts the flowchart of subjects’ in the present study. All participants or legal guardians provided informed written consent and the study protocol was approved by Penn State Hershey Institutional Review Board.
Figure 1.
Subjects’ selection and flowchart in this study.
Definition of incident insomnia symptoms
Commensurate with our previous studies (Calhoun et al., 2014; Fernandez-Mendoza et al., 2016), the presence of insomnia symptoms at baseline (childhood) was defined by a parent-report of difficulty falling asleep (DFA) and/or difficulty staying asleep (DSA) on the parent-reported Pediatric Behavior Scale (Calhoun et al., 2014), while the presence of insomnia symptoms at follow-up (adolescence) was defined by a self-report of DFA and/or DSA on the self-reported Pediatric Sleep Questionnaire (Fernandez-Mendoza et al., 2016). Children without parent-reported insomnia symptoms at baseline and without self-reported insomnia symptoms at follow-up were classified as “normal sleeping controls” (n = 20), while children without parent-reported insomnia symptoms at baseline and with self-reported insomnia symptoms at follow-up were classified as “incident insomnia symptoms” (n = 25).
Sleep laboratory
At both baseline and follow-up, all subjects underwent a 9-hour, single-night PSG recording in a sound-attenuated, light- and temperature-controlled room. Each subject was continuously monitored from 22:00 h until 7:00 h using 14-channel recordings of electroencephalogram (EEG), electrooculogram (EOG), and electromyogram (EMG). Respiration was monitored with nasal pressure, thermocouple, and thoracic and abdominal strain gauges and hemoglobin oxygen saturation (SpO2) was obtained from the finger. All data were recorded using Gamma Sleep Recording & Analysis software (Grass-Telefactor; West Warwick, RI). The sleep records were subsequently scored independently according to standardized criteria by a registered polysomnography technician (RPSGT) (Rechtschaffen and Kales, 1968). An apnea was defined as a cessation of airflow with a minimum duration of 5 seconds for age younger than 16 years and 10 seconds for age 16 years or older with an out-of-phase strain gauge movement. A hypopnea was defined as a reduction of airflow of approximately 50% with an associated decrease in SpO2 of at least 3% and/or associated arousal. AHI was calculated as the number of apneas and hypopneas summed per hour of sleep.
Spectral analyses
Commensurate with our previous report (Fernandez-Mendoza et al., 2016), we focused our spectral analyses on central EEG derivations (C3 and C4), beta frequencies (15–35 Hz), and four sleep/wake states [SOL, sleep onset (SO), NREM sleep, and REM sleep]. SOL was defined as time from lights out until the first epoch of NREM sleep stage 2. SO was defined as the first 5 minutes of NREM sleep after SOL (by definition, any segment with wake was not included in the analyses). NREM sleep was defined as any period of at least 15 minutes of NREM sleep. REM sleep was defined as at least 5 minutes of REM sleep. We did not include the time spent awake in the middle of the night [i.e., wake after sleep onset (WASO)] in our primary spectral analyses given that the amount of this wake state was very low in childhood and only 18 subjects had available WASO for spectral analyses; these data are included together with our secondary analyses. All these sleep/wake states are standard in sleep studies and represent measures of sleep continuity, such as time awake before falling asleep (SOL) and in the middle of the night (WASO), and sleep architecture (NREM and REM sleep). We did examine the association of other frequency bands below the beta range as part of our secondary analyses. Overnight EEG was amplified with a band pass between 0.3 to 30 Hz and digitized at 100 Hz. An all-night spectral analysis was performed following Spiegelhalder et al. (2012) procedures with adaptations (Fernandez-Mendoza et al., 2016; Vgontzas et al., 2007). We used the fast Fourier transform (FFT) algorithm to calculate relative spectral power through SleepFFT software (Biosoft Studio, Hershey, PA) (Fernandez-Mendoza et al., 2016; Vgontzas et al., 2007). In each epoch, the spectral resolution was 0.39 Hz. A Hanning window was applied before calculating spectral power within each FFT window (Vgontzas et al., 2007). In order to minimize the effects of artifacts, we visually marked and excluded arousals and periodic leg movements and excluded epochs containing movements or arousals as part of the sleep staging (Spiegelhalder et al., 2012).Furthermore, an epoch was excluded if the deviation was larger than the difference between the median and the first quartile of all median-filtered values across the night. Median-filtered values were defined as the median of values in the 5 minutes preceding and 5 minutes following the epoch (Spiegelhalder et al., 2012). All-night spectral power were obtained across all artifact-free epochs of NREM as well as SOL, SO, REM, and WASO. The averaged C3-A1 and C4-A2 relative power was calculated as [(number of segments at C3 * C3 relative power) + (number of segments at C4 * C4 relative power)] / number of segments at C3 + number of segments at C4). Among the 45 subjects that were studied, 11 were recorded with non-referenced C3 and C4 derivations; however, we linked C3 to A1 as well as C4 to A2 with SleepFFT software (Calhoun et al., 2014; Fernandez-Mendoza et al., 2016) before running the spectral analyses. Relative power was computed by dividing the absolute power of each frequency band by the total power of all frequency bands. The relative power in delta (0.39–3.91 Hz), theta (4.30–7.81 Hz), alpha (8.20–11.72 Hz), sigma (12.11–14.84 Hz), low-beta (15.23–25.00 Hz) and high-beta (25.39–35.16 Hz) and gamma (35.55–49.61 Hz) in the total power (up to 49.6 Hz excluding 0.0 Hz) were calculated for each epoch.
Statistical analyses
Based on the previous cross-sectional studies mentioned above, our primary analyses focused on childhood EEG relative power in the beta range (15–35Hz) during NREM sleep. Mean differences in relative EEG power between normal sleeping controls and those with incident insomnia symptoms in adolescence were analyzed using MANOVA with EEG activity in the beta range analyzed as two levels of low-beta and high-beta. Descriptive presentation of the data includes mean values and standard deviations. Second, we conducted multivariable logistic regression models to examine the independent association between childhood EEG relative beta power during NREM sleep and incident insomnia symptoms in adolescence while adjusting for childhood age, AHI and sex. These logistic regression models included standardized z-scores of EEG relative power in childhood as predictors and incident insomnia symptoms in adolescence as a binary outcome, thus, data resulting from these regression models are reported as odds ratios (OR) and their 95% confidence intervals (95%CI) for one standard deviation increase in childhood EEG relative power. Our secondary analyses examined other EEG frequency bands (i.e., delta, theta, alpha, and sigma) as well as other sleep/wake states (i.e., SOL, SO, REM sleep and WASO). Given that the vast majority of the gamma frequency band was out of range of the recording filter setting (0.3–30 HZ) and WASO was only available in 18 subjects, these data could not be analyzed reliably in the secondary analyses and findings should be interpreted very cautiously. Statistical analyses were performed using SPSS version 21. The level of significance was set at P < 0.05.
Results
Demographic, behavioral and sleep characteristics of the sample
Overall, the incident insomnia symptoms and normal sleeping control groups were not significantly different in terms of several demographic, behavioral or sleep characteristics at either childhood or adolescence (Table 1). We found a significantly lower percentage of stage 2 during childhood in the incident insomnia symptoms group as compared to normal sleeping controls (p=0.041). As expected, the group with incident insomnia symptoms had significantly increased SOL and WASO, shorter total sleep time, and lower sleep efficiency in adolescence as compared to normal sleeping controls.
Table 1.
Demographic, behavioral and sleep characteristics of the study groups
| Normal Sleeping Controls (n=20) | Incident Insomnia Symptoms (n=25) | P | |
|---|---|---|---|
| White race, n (%) | 17 (85.0) | 22 (88.0) | 0.553 |
| Male sex, n (%) | 9 (45.0) | 6 (24.0) | 0.122 |
|
| |||
| Childhood | |||
|
| |||
| Age, years | 8.55 (1.88) | 8.76 (1.79) | 0.703 |
| BMI, percentile | 49.89 (28.23) | 49.63 (19.90) | 0.973 |
| Internalizing, T score | 48.35 (8.50) | 48.00 (8.26) | 0.896 |
| Externalizing, T score | 42.82 (10.09) | 46.30 (10.16) | 0.289 |
| AHI, events/h | 0.60 (0.49) | 0.42 (0.56) | 0.285 |
| Time to bed, h:mm | 22:00 (0:22) | 21:58 (0:31) | 0.374 |
| Time out of bed, h:mm | 6:45 (0:15) | 6:51 (0:25) | 0.592 |
| TIB, minutes | 528.53 (29.13) | 532.56(23.43) | 0.609 |
| SOL, minutes | 22.08 (13.72) | 32.04 (25.59) | 0.124 |
| WASO, minutes | 43.38 (37.93) | 46.23 (37.01) | 0.816 |
| TST, minutes | 462.03 (47.79) | 456.78 (46.81) | 0.713 |
| SE, % | 87.90 (7.85) | 85.89 (9.16) | 0.441 |
| N1, % | 2.95 (2.16) | 3.64 (3.95) | 0.487 |
| N2, % | 52.90 (7.17) | 48.08 (7.98) | 0.041* |
| SWS, % | 24.15 (5.35) | 27.08 (6.18) | 0.101 |
| REM, % | 20.10 (4.79) | 21.20 (5.61) | 0.490 |
|
| |||
| Adolescence | |||
|
| |||
| Age, years | 16.94 (1.95) | 16.57 (1.69) | 0.495 |
| Tanner stage, score | 4.20 (0.83) | 4.00 (1.02) | 0.487 |
| BMI, percentile | 46.21 (23.73) | 47.13 (24.43) | 0.898 |
| MEQ, score | 26.15 (5.64) | 23.76 (4.96) | 0.138 |
| Internalizing, T score | 48.55 (9.25) | 53.36 (10.51) | 0.115 |
| Externalizing, T score | 46.95 (8.88) | 51.40 (12.97) | 0.180 |
| AHI, events/h | 0.80 (0.42) | 0.76 (0.39) | 0.832 |
| Time to bed, h:mm | 21:58 (0:06) | 22:00 (0:19) | 0.617 |
| Time out of bed, h:mm | 6:57 (0:07) | 7:01 (0:19) | 0.370 |
| TIB, minutes | 539.28 (3.94) | 541.12 (4.27) | 0.145 |
| SOL, minutes | 11.75 (4.75) | 32.64 (23.07) | <0.001* |
| WASO, minutes | 38.71 (15.51) | 64.22 (28.19) | <0.001* |
| TST, minutes | 489.95 (15.82) | 445.94 (37.61) | <0.001* |
| SE, % | 90.88 (3.11) | 82.38 (6.70) | <0.001* |
| N1, % | 1.06 (1.96) | 0.74 (0.75) | 0.449 |
| N2, % | 51.50 (7.40) | 50.10 (7.83) | 0.564 |
| SWS, % | 26.86 (8.41) | 28.76 (6.79) | 0.407 |
| REM, % | 20.58 (4.46) | 20.39 (4.99) | 0.896 |
| DFA-only, n (%) | 16 (64) | N/A | |
| DSA-only, n (%) | 2 (8) | ||
| Both, n (%) | 7 (28) | ||
Dara are mean (standard deviation), unless otherwise stated. The higher the T scores, the greater the internalizing symptoms or externalizing behaviors. The lower the MEQ score, the greater the evening circadian preference (i.e., eveningness). AHI = apnea hypopnea index. BMI = body mass index percentile for sex-and-age. DFA= Difficulty falling asleep. DSA= Difficulty staying asleep. MEQ = morningness-eveningness questionnaire. N/A = not applicable. N1 = non-rapid eye movement sleep stage 1. N2 = non-rapid eye movement sleep stage 2. REM = rapid eye movement sleep. SE = sleep efficiency. SOL = sleep onset latency. SWS = slow wave sleep. TIB = time in bed. TST = total sleep time. WASO = wake after sleep onset.
P < 0.05
Childhood high-frequency sleep EEG activity and incident insomnia symptoms in adolescence
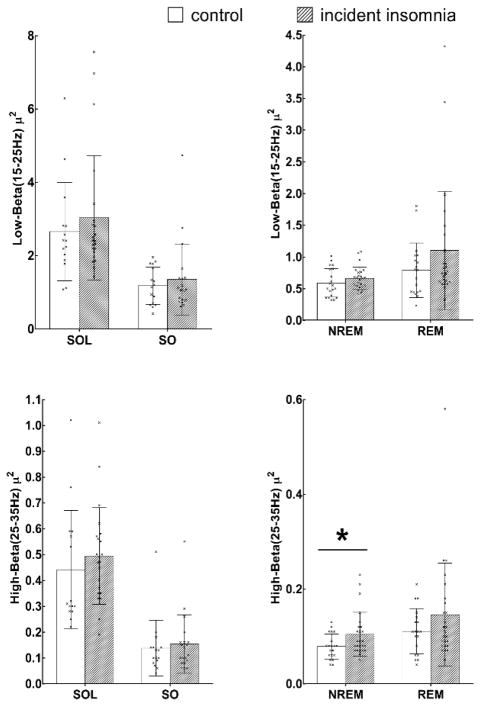
As shown in Table 2, children with incident insomnia symptoms in adolescence had significantly increased high-beta (25–35 Hz) relative power during NREM sleep (p=0.033), while no significant differences during other sleep/wake states (i.e., SO, SOL, and REM) were observed between the incident insomnia symptoms group and normal sleeping controls in terms of childhood EEG low-beta or high-beta relative power. Consistently, the multivariable logistic regression models in Table 3 showed that increased childhood high-beta EEG relative power during NREM sleep was associated with a 3-fold odds of incident insomnia symptoms in adolescence even after adjusting for childhood age, AHI and sex (OR=2.99, 95%CI=1.12–7.98, p=0.029). This association remained similar and in the same direction after further adjusting for childhood percentage of stage 2 (OR=2.88, 95%CI=1.06–7.79, p=0.038); childhood percentage of stage 2 was only marginally associated with incident insomnia symptoms in this multivariable-adjusted regression model (p=0.075). Figure 2 depicts individual data as well as mean values of beta EEG activity in normal sleeping controls and those with incident insomnia symptoms.
Table 2.
Childhood high-frequency EEG relative power during sleep across groups with and without incident insomnia symptoms in adolescence.
| Normal Sleeping Controls | Incident Insomnia Symptoms | P | |||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| SOL | |||||
| Low-β | 15 | 2.66 (1.34) | 21 | 3.02 (1.74) | 0.507 |
| High-β | 15 | 0.44 (0.23) | 21 | 0.47 (0.15) | 0.670 |
| SO | |||||
| Low-β | 16 | 1.18 (0.51) | 19 | 1.38 (0.99) | 0.469 |
| High-β | 16 | 0.14 (0.11) | 19 | 0.16 (0.11) | 0.625 |
| NREM sleep | |||||
| Low-β | 20 | 0.59 (0.23) | 25 | 0.66 (0.18) | 0.231 |
| High-β | 20 | 0.08 (0.03) | 25 | 0.10 (0.05) | 0.033* |
| REM sleep | |||||
| Low-β | 20 | 0.79 (0.43) | 25 | 1.10 (0.93) | 0.181 |
| High-β | 20 | 0.11 (0.05) | 25 | 0.15 (0.11) | 0.205 |
All data are means (standard deviation). Low-β=15–25 Hz, High-β= 25–35 Hz. NREM= non-rapid eye movement (stages 2 and slow wave sleep). REM= rapid eye movement. SO = sleep onset (first 5 minutes of sleep). SOL = sleep onset latency (time between lights off and sleep onset).
P < 0.05
Table 3.
Multivariable-adjusted odds ratios for the association between childhood high-frequency EEG activity during sleep and incident insomnia symptoms in adolescence
| SOL (n=36) | SO (n=35) | NREM (n=45) | REM (n=45) | |
|---|---|---|---|---|
| Low-β | 1.21 (0.57–2.27) | 1.02 (0.46–2.28) | 1.35 (0.69–2.62) | 1.80 (0.56–4.28) |
| High-β | 1.32 (0.62–2.78) | 0.91 (0.42–2.01) | 2.99 (1.12–7.98)* | 1.83 (0.74–4.54) |
Data are odds ratios (95% confidence intervals) adjusted for childhood age, apnea/hypopnea index and sex and indicate the odds of incident insomnia symptoms in adolescence associated with one standard deviation increase in childhood EEG relative power. SOL = sleep onset latency (time between lights off and sleep onset). SO = sleep onset (first 5 minutes of sleep). NREM = non-rapid eye movement sleep (stages 2 and slow wave sleep). REM = rapid eye movement sleep.
P < 0.05
Figure 2.
Individual data and mean values for low-beta and high-beta power during NREM sleep in normal sleeping controls and adolescents with incident insomnia symptoms.
Other childhood sleep EEG frequency bands and sensitivity analyses
In secondary analyses, we examined whether relative power in other frequency bands differed between normal sleeping controls and the incident insomnia symptoms group. As shown in Table 4, relative childhood theta power during SO was marginally higher in the incident insomnia symptoms group as compared to normal sleeping controls (p=0.061); however, this association did not remain significant in multivariable-adjusted logistic regression models (Table S1). No other significant differences were observed in delta, alpha or sigma frequency bands between groups either in terms of mean values (Table 4) or multivariable-adjusted associations (Table S1). Secondary analyses in the small group of subjects who had available data during WASO (8 normal sleeping controls and 10 subjects with incident insomnia symptoms) showed that childhood high-beta power was significantly higher in adolescents with incident insomnia symptoms (Table 4), however, these data should be interpreted cautiously given the small sample size of children with sufficient WASO (n=18) and the filter settings.
Table 4.
Secondary analyses of childhood EEG relative power in other frequency bands across groups with and without incident insomnia symptoms in adolescence.
| Normal Sleeping Controls | Incident Insomnia Symptoms | P | |||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| SOL | |||||
| δ | 15 | 62.28 (10.43) | 21 | 62.44 (10.27) | 0.963 |
| θ | 15 | 21.71 (9.42) | 21 | 23.67 (10.25) | 0.563 |
| α | 15 | 11.64 (7.05) | 21 | 10.02 (4.28) | 0.396 |
| σ | 15 | 1.79 (0.67) | 21 | 1.82 (0.75) | 0.922 |
| γ | 15 | 0.08 (0.05) | 21 | 0.09 (0.04) | 0.670 |
| SO | |||||
| δ | 16 | 75.05 (6.51) | 19 | 72.71 (8.16) | 0.362 |
| θ | 16 | 17.03 (5.26) | 19 | 21.31 (6.38) | 0.061 |
| α | 16 | 3.07 (1.13) | 19 | 3.27 (1.04) | 0.601 |
| σ | 16 | 1.39 (0.69) | 19 | 1.24 (0.48) | 0.477 |
| γ | 16 | 0.02 (0.03) | 19 | 0.03 (0.03) | 0.661 |
| NREM sleep | |||||
| δ | 20 | 79.50 (6.49) | 25 | 80.90 (4.44) | 0.395 |
| θ | 20 | 12.21 (4.91) | 25 | 11.53 (3.03) | 0.573 |
| α | 20 | 4.41 (1.91) | 25 | 3.86 (1.39) | 0.269 |
| σ | 20 | 2.93 (2.06) | 25 | 2.89 (1.75) | 0.948 |
| γ | 20 | 0.01 (0.01) | 25 | 0.02 (0.01) | 0.110 |
| REM sleep | |||||
| δ | 20 | 79.86 (5.22) | 25 | 77.98 (5.26) | 0.246 |
| θ | 20 | 15.41 (4.65) | 25 | 17.06 (4.55) | 0.245 |
| α | 20 | 2.85 (1.44) | 25 | 2.91 (0.96) | 0.858 |
| σ | 20 | 0.78 (0.54) | 25 | 0.91 (0.70) | 0.521 |
| γ | 20 | 0.02 (0.01) | 25 | 0.02 (0.02) | 0.348 |
| WASO | |||||
| δ | 8 | 70.99 (6.67) | 10 | 68.30 (11.22) | 0.559 |
| θ | 8 | 17.41 (4.38) | 10 | 18.03 (7.57) | 0.841 |
| α | 8 | 6.96 (2.97) | 10 | 7.83 (4.19) | 0.627 |
| σ | 8 | 1.52 (0.94) | 10 | 1.80 (0.38) | 0.390 |
| Low-β | 8 | 2.12 (0.92) | 10 | 3.07 (1.07) | 0.065 |
| High-β | 8 | 0.44 (0.21) | 10 | 1.03 (0.74) | 0.046* |
| γ | 8 | 0.13 (0.09) | 10 | 0.59 (0.94) | 0.187 |
All data are means (standard deviation). δ=0.39–3.91 Hz, θ=4.30–7.81 Hz, α=8.20–11.72 Hz, σ=12.11–14.84 Hz, NREM= non-rapid eye movement (stages 2 and slow wave sleep). REM= rapid eye movement. SO = sleep onset (first 5 minutes of sleep). SOL = sleep onset latency (time between lights off and sleep onset).
P < 0.05
In sensitivity analyses, after excluding children in whom non-referenced C3 and C4 EEG recording was used, the results remained similar and in the same direction, i.e., childhood high-beta EEG relative power during NREM sleep was significantly associated with incident insomnia symptoms in adolescence (see Tables S1 and S2).
Discussion
This is the first study to show that children who develop insomnia symptoms in the transition to adolescence already had increased beta EEG power during NREM sleep in childhood despite the absence of parent-reported insomnia symptoms. Our findings indicate that childhood cortical hyperarousal during NREM sleep, as measured by increased EEG power in the 25–35 Hz range is a significant, independent predictor of incident insomnia symptoms in adolescence. These data suggest that cortical hyperarousal may be a premorbid neurophysiologic sign of insomnia. Additional large longitudinal cohorts with a developmental design need to be conducted in order to replicate these findings and confirm that cortical hyperarousal during NREM can serve as a preclinical marker for the detection of those at risk of chronic insomnia phenotypes and associated psychiatric disorders.
Increased beta EEG power during NREM is considered to be a neurophysiologic marker of cortical hyperarousal in chronic insomnia (Perlis et al., 2001b). Previous cross-sectional studies in adults have shown that cortical hyperarousal during NREM sleep is associated with chronic insomnia (Freedman, 1986; Merica et al., 1998; Perlis et al., 2001; Krystal et al., 2002; Buysse et al., 2008; Spiegelhalder et al., 2012), a finding replicated in other cross-sectional studies in adolescents (Fernandez-Mendoza et al.,2016) and young adults (Corsi-Cabrera et al., 2012; Israel et al., 2012). However, given their cross-sectional design, these previous studies could not address the question whether “it is the ongoing cortical hyperarousal leading to complaints of difficulty falling or staying asleep, or vice versa?” Our study shows that childhood cortical hyperarousal during NREM sleep is present in insomnia subjects prior to the development of the sleep complaints in adolescence. This finding gives support to the hypothesis that cortical hyperarousal may be one of the premorbid mechanisms leading to and maintaining insomnia over time.
Our findings of increased childhood beta EEG power during NREM sleep potentially leading to adolescent insomnia complaints suggests a potential dysregulation of the flip-flop switch (i.e., the co-activation of sleep and wake systems) early on and prior to the development of insomnia. However, given that in our study overall PSG differences were minimal between groups during childhood, it is also possible that the flip-flop switch may be preserved and that the cortical hyperarousal observed is related to disrupted cortical networks without any involvement of subcortical structures. In line with this hypothesis, Buysse and colleagues (2011) have proposed that insomnia results from persistent activity in wake-promoting neural structures during NREM sleep and that the simultaneous, regionally-specific “waking” and “sleeping” neural activity helps explain the clinical phenomenology of insomnia, including its core nighttime symptoms. From this perspective, the specific finding of increased childhood beta power during NREM sleep herein could be explained by a dysregulation in “local” processes (limbic and parietal cortices, thalamus, and hypothalamic-brainstem arousal centers) during otherwise “global” (NREM) sleep (Buysse et al, 2011). Another potential explanation for the discrepancy between significant EEG cortical hyperarousal and minimal PSG sleep disturbance in childhood may be that sleep homeostatic mechanisms are stronger at that developmental stage, regardless of the degree of abnormal subcortical or cortical activation taking place. Sleep homeostasis refers to the aspect of sleep regulation that is dependent on the amount of previous wake so that a steady state can be maintained by the organism (Hagenauer et al., 2009). Normally developing children require less amount of wake in order to sleep, thus, their sleep drive is greater than that of adults, which leads to longer sleep duration, less sleep fragmentation and greater sleep depth. This is particularly true before the maturational decline in delta EEG activity occurs at the age of 11–12 years and may well explain the lack of PSG differences at baseline in the young children (6–11 years) in this study. All the hypotheses outlined herein need to be tested in future longitudinal studies combining multiple sources of data from the sleeping brain.
Our findings also have important implications from a transdiagnostic standpoint. While it is well-known that insomnia is highly comorbid with psychiatric disorders (Siomos et al., 2010; Buysse, 2010; Fernandez-Mendoza and Vgontzas, 2013), it is also associated with increased risk for the development of future psychopathologic morbidity, such as depression (Fernandez-Mendoza et al., 2015; Baglioni et al., 2011). Many psychopathologic states have been associated with disrupted cortical networks, including indices such as high-frequency EEG power (Borbély et al., 1984). Our findings suggest that sleep EEG activity may play a role in the increased risk for the development of depression associated with insomnia as early as adolescence or even childhood. Cortical hyperarousal was present in these future insomnia sufferers years before the development of subjective complaints and objective sleep disturbances associated with the disorder. Moreover, this childhood cortical hyperarousal was present in the presence of non-significant marginal differences in internalizing or externalizing behavioral symptoms, such as anxiety or depression, frequently associated with insomnia. These findings suggest that cortical hyperarousal during NREM sleep can serve as an early preclinical marker for the detection of those at risk of insomnia and associated psychiatric disorders. Future studies should replicate these findings in large cohorts with well-defined developmental stages and insomnia phenotypes as well as examine the longitudinal association between sleep EEG activity in childhood and psychopathologic outcomes in adolescence and young adulthood.
There are several strengths and limitations of the current study. Strengths of our study include the longitudinal design between two key developmental periods (i.e., childhood and adolescence), the use of a subsample drawn from a random, representative, general population cohort, and the stringent criteria used to exclude other sleep disorders and potential clinical confounders (e.g., evening circadian preference). However, the presence of some adolescents with a delayed sleep phase cannot be entirely ruled-out. Also, from a conceptual standpoint, this is the first study to examine the association of sleep EEG activity during childhood rather than the overall sleep architecture with incident insomnia symptoms in adolescence. Some limitations should also be noted. First, the relatively small sample size may not have had enough power to test certain associations between childhood sleep EEG activity and incident insomnia symptoms in adolescence; thus, future studies should examine this relationship in the entirety of the PSCC (N=421) as well as the developmental trajectories of sleep EEG activity from childhood to adolescence and their relationship to other significant clinical outcomes, such as incident psychopathology. Second, we analyzed only one night of PSG that might have been affected by the first night effect or may have enhanced the differences between groups. Although recent studies have shown that measures of sleep duration, such as TST and SE, in fixed-time recordings (Gaines et al., 2015 ) and of spectral EEG activity (Israel et al., 2012; Curcio et al., 2004) are relatively stable across consecutive nights, other studies have shown systematic changes in some power spectral data night-to-night (Merica and Gaillard, 1985) and, therefore, future studies should use a multiple-nights design when studying spectral EEG activity in individuals with or at-risk of insomnia. Third, we observed a significant difference in the EEG frequency range from 25 Hz to 35 Hz between the control and incident insomnia symptoms groups. The interpretation of this finding needs to be cautious and take into account the hardware settings of our EEG recording system in childhood, which had a low pass filter at 30 Hz, therefore, the EEG power from the 30Hz to 35Hz is suppressed to some extent in our data. This filter setting, however, should not create any bias towards any particular study group since the EEG signals from all subjects were recorded in the same manner. Finally, we did not have a self-reported measure of habitual total sleep time and concomitant time in bed to calculate self-reported SE, as we do for habitual SOL and, thus, we could not test whether the findings would be similar and in the same direction if self-reported exclusion criteria in the normal sleeping controls would have paralleled the PSG criteria by also including both self-reported SOL and SE.
Conclusion
In summary, increased childhood EEG beta activity during NREM sleep is associated with the incidence of insomnia symptoms later on in adolescence, which suggests that cortical hyperarousal is present prior to the development of insomnia complaints. Our findings give support to the hypothesis that cortical hyperarousal may be an early neurophysiologic sign and potential premorbid mechanism for the development and perpetuation of insomnia over time. Novel treatments for the prevention of insomnia and associated psychiatric morbidity should target cortical hyperarousal in high-risk children.
Supplementary Material
Table S1. Multivariable-adjusted odds ratios for the association between childhood EEG dynamics during sleep and incident insomnia symptoms in adolescence.
Table S2. Childhood EEG relative power during sleep across groups with and without incident insomnia symptoms in adolescence.
Key points.
Cross-sectional studies in adults have shown that insomnia is associated with increased high-frequency cortical activity during sleep; however, it is unknown whether this cortical hyperarousal precedes the onset of insomnia complaints or is the result of the sleep disorder itself, given the lack of studies examining the longitudinal association between premorbid sleep EEG activity and incident insomnia symptoms.
This is the first study to take a developmental approach to study the association of childhood sleep EEG activity with the development of insomnia symptoms in adolescence.
Our novel data showed that increased childhood EEG power in the high-beta range (25–35 Hz) during NREM sleep is associated with incident insomnia symptoms in adolescence.
Our novel finding suggest that cortical hyperarousal is an early neurophysiologic sign of insomnia that precedes the onset of the disorder and may be an early treatment target, particularly among high-risk children.
Acknowledgments
This study was supported by National Institutes of Health R01 HL63772, R01 HL97165, UL1 RR033184, C06 RR16499. JF disclosed ownership of Biosoft Studio (Hershey, PA). The remaining authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: See acknowledgements for disclosures.
Additional Supporting Information may be found in the online version of this article:
References
- Archbold KH, Pituch KJ, Panahi P, Chervin RD. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97–102. doi: 10.1067/mpd.2002.119990. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Fernandez-Mendoza J, Liao D, Calhoun S, Rodriguez-Colon SM, Gaines J, et al. Natural history of sleep disordered breathing in prepubertal children transitioning to adolescence. Eur Respir J. 2016;47:1402–1409. doi: 10.1183/13993003.01771-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Borbély AA, Tobler I, Loepfe M, Kupfer DJ, Ulrich RF, Grochocinski V, et al. All-night spectral analysis of the sleep EEG in untreated depressives and normal controls. Psychiatry Res. 1984;12:27–33. doi: 10.1016/0165-1781(84)90135-5. [DOI] [PubMed] [Google Scholar]
- Buysse DJ. Sleep and Psychiatric Disorders: A Revisit and Reconceptualization. Canadian Journal of Psychiatry. 2010;55:401–402. doi: 10.1177/070674371005500701. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Hall ML, Moul DE, Nofzinger EA, Begley A, et al. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31:1673–1682. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Hall M, Monk TH, Nofzinger EA. A Neurobiological Model of Insomnia. Drug Discov Today Dis Models. 2011;8:129–137. doi: 10.1016/j.ddmod.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun SL, Fernandez-Mendoza J, Vgontzas AN, Liao D, Bixler EO. Prevalence of insomnia symptoms in a general population sample of young children and preadolescents: gender effects. Sleep Med. 2014;15:91–95. doi: 10.1016/j.sleep.2013.08.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervena K, Espa F, Perogamvros L, Perrig S, Merica H, Ibanez V. Spectral analysis of the sleep onset period in primary insomnia. Clin Neurophysiol. 2014;125:979–987. doi: 10.1016/j.clinph.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Corsi-Cabrera M, Figueredo-Rodríguez P, del Río-Portilla Y, Sánchez-Romero J, Galán L, Bosch-Bayard J. Enhanced frontoparietal synchronized activation during the wake-sleep transition in patients with primary insomnia. Sleep. 2012;35:501–511. doi: 10.5665/sleep.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio G, Ferrara M, Piergianni A, Fratello F, De Gennaro L. Paradoxes of the first-night effect: a quantitative analysis of antero-posterior EEG topography. Clin Neurophysiol. 2004;115:1178–1188. doi: 10.1016/j.clinph.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn. 2010;72:56–65. doi: 10.1016/j.bandc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Ilioudi C, Montes MI, Olavarrieta-Bernardino S, Aguirre-Berrocal A, De la Cruz-Troca JJ, Vela-Bueno A. Circadian preference, nighttime sleep and daytime functioning in young adulthood. Sleep and Biological Rhythms. 2010a;8:52–62. [Google Scholar]
- Fernandez-Mendoza J, Li Y, Vgontzas AN, Fang J, Gaines J, Calhoun SL, et al. Insomnia is Associated with Cortical Hyperarousal as Early as Adolescence. Sleep. 2016;39:1029–1036. doi: 10.5665/sleep.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Shea S, Vgontzas AN, Calhoun SL, Liao D, Bixler EO. Insomnia and incident depression: role of objective sleep duration and natural history. J Sleep Res. 2015;24:390–398. doi: 10.1111/jsr.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vela-Bueno A, Vgontzas AN, Ramos-Platón MJ, Olavarrieta-Bernardino S, Bixler EO, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010b;72:397–403. doi: 10.1097/PSY.0b013e3181d75319. [DOI] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN. Insomnia and its impact on physical and mental health. Curr Psychiatry Rep. 2013;15:418. doi: 10.1007/s11920-013-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RR. EEG power spectra in sleep-onset insomnia. Electroencephalogr Clin Neurophysiol. 1986;63:408–413. doi: 10.1016/0013-4694(86)90122-7. [DOI] [PubMed] [Google Scholar]
- Fricke-Oerkermann L, Plück J, Schredl M, Heinz K, Mitschke A, Wiater A, et al. Prevalence and course of sleep problems in childhood. Sleep. 2007;30:1371–1377. doi: 10.1093/sleep/30.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines J, Vgontzas AN, Fernandez-Mendoza J, Basta M, Pejovic S, He F, et al. Short- and Long-Term Sleep Stability in Insomniacs and Healthy Controls. Sleep. 2015 doi: 10.5665/sleep.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31:276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel B, Buysse DJ, Krafty RT, Begley A, Miewald J, Hall M. Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: for some measures, one night is enough. Sleep. 2012;35:1285–1291. doi: 10.5665/sleep.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–640. [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS. CDC growth charts for the United States: Methods and development. National Center for Health Statistics. Vital Health Stat. 2000;11(246) [PubMed] [Google Scholar]
- Lamarche CH, Ogilvie RD. Electrophysiological changes during the sleep onset period of psychophysiological insomniacs, psychiatric insomniacs, and normal sleepers. Sleep. 1997;20:724–733. [PubMed] [Google Scholar]
- Liu X, Zhou H. Sleep duration, insomnia and behavioral problems among Chinese adolescents. Psychiatry Res. 2002;111:75–85. doi: 10.1016/s0165-1781(02)00131-2. [DOI] [PubMed] [Google Scholar]
- Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–1834. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Merica H, Gaillard JM. Statistical description and evaluation of the interrelationships of standard sleep variables for normal subjects. Sleep. 1985;8:261–73. doi: 10.1093/sleep/8.3.261. [DOI] [PubMed] [Google Scholar]
- Merica H, Gaillard JM. The EEG of the sleep onset period in insomnia: a discriminant analysis. Physiol Behav. 1992;52:199–204. doi: 10.1016/0031-9384(92)90258-4. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Roberts RE, Zulley J, Smirne S, Priest RG. Prevalence and patterns of problematic sleep among older adolescents. J Am Acad Child Adolesc Psychiatry. 2000;39:1549–1556. doi: 10.1097/00004583-200012000-00019. [DOI] [PubMed] [Google Scholar]
- Patten CA, Choi WS, Gillin JC, Pierce JP. Depressive symptoms and cigarette smoking predict development and persistence of sleep problems in US adolescents. Pediatrics. 2000;106:E23. doi: 10.1542/peds.106.2.e23. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–188. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Kehr EL, Smith MT, Andrews PJ, Orff H, Giles DE. Temporal and stagewise distribution of high frequency EEG activity in patients with primary and secondary insomnia and in good sleeper controls. J Sleep Res. 2001a;10:93–104. doi: 10.1046/j.1365-2869.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001b;5:363–374. doi: 10.1053/smrv.2001.0151. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001c;24:110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects[J] 1968. [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Lewinsohn PM, Seeley JR. Symptoms of DSM-III-R major depression in adolescence: evidence from an epidemiological survey. J Am Acad Child Adolesc Psychiatry. 1995;34:1608–1617. doi: 10.1097/00004583-199512000-00011. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, Chan W. Persistence and change in symptoms of insomnia among adolescents. Sleep. 2008;31:177–184. doi: 10.1093/sleep/31.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Colón SM, He F, Bixler EO, Fernandez-Mendoza J, Vgontzas AN, Calhoun S, et al. Sleep variability and cardiac autonomic modulation in adolescents - Penn State Child Cohort (PSCC) study. Sleep Med. 2015;16:67–72. doi: 10.1016/j.sleep.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomos KE, Avagianou PA, Floros GD, Skenteris N, Mouzas OD, Theodorou K, et al. Psychosocial correlates of insomnia in an adolescent population. Child Psychiatry Hum Dev. 2010;41:262–273. doi: 10.1007/s10578-009-0166-5. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K, Regen W, Feige B, Holz J, Piosczyk H, Baglioni C, et al. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91:329–333. doi: 10.1016/j.biopsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- St-Jean G, Turcotte I, Pérusse AD, Bastien CH. REM and NREM power spectral analysis on two consecutive nights in psychophysiological and paradoxical insomnia sufferers. Int J Psychophysiol. 2013;89:181–194. doi: 10.1016/j.ijpsycho.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33:801–809. doi: 10.1093/sleep/33.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bixler EO, Basta M, et al. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007;292:E253–261. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- Wolynczyk-Gmaj D, Szelenberger W. Waking EEG in primary insomnia. Acta Neurobiol Exp (Wars) 2011;71:387–392. doi: 10.55782/ane-2011-1860. [DOI] [PubMed] [Google Scholar]
- Wu YM, Pietrone R, Cashmere JD, Begley A, Miewald JM, Germain A, et al. EEG power during waking and NREM sleep in primary insomnia. J Clin Sleep Med. 2013;9:1031–1037. doi: 10.5664/jcsm.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lam SP, Li SX, Li AM, Lai KY, Wing YK. Longitudinal course and outcome of chronic insomnia in Hong Kong Chinese children: a 5-year follow-up study of a community-based cohort. Sleep. 2011;34:1395–1402. doi: 10.5665/SLEEP.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Multivariable-adjusted odds ratios for the association between childhood EEG dynamics during sleep and incident insomnia symptoms in adolescence.
Table S2. Childhood EEG relative power during sleep across groups with and without incident insomnia symptoms in adolescence.