Abstract
Mesenchymoangioblast (MB) is the earliest precursor for endothelial and mesenchymal cells originating from APLNR+PDGFRα+KDR+ mesoderm in human pluripotent stem cell cultures. MBs are identified based on their capacity to form FGF2-dependent compact spheroid colonies in a serum-free semisolid medium. MBs colonies are composed of PDGFRβ+CD271+EMCN+DLK1+CD73− primitive mesenchymal cells which are generated through endothelial/angioblastic intermediates (cores) formed during first 3–4 days of clonogenic cultures. MB-derived primitive mesenchymal cells have potential to differentiate into mesenchymal stromal/stem cells (MSCs), pericytes, and smooth muscle cells. In this review, we summarize the specification and developmental potential of MBs, emphasize features that distinguish MBs from other mesenchymal progenitors described in the literature and discuss the value of these findings for identifying molecular pathways leading to MSC and vasculogenic cell specification, and developing cellular therapies using MB-derived progeny.
Keywords: Mesenchymoangioblasts, Human pluripotent stem cells, Embryonic stem cells, Induced pluripotent stem cells, Mesoderm development, Mesenchymal stem cells, Mesoangioblast, Mesospheres, Hemangioblasts, Cardiovascular progenitors, Pericytes, Smooth muscles, Embryonic mesenchyme
Introduction
Mesenchymal tissues are critical components of any organ in the human body, including vasculature. Although mesenchymal cells within many organs share a common phenotype and capacity to grow in adherent cultures, they are comprised of functionally and developmentally diverse cell populations. Recent advances in human pluripotent stem cell (hPSC) technologies have demonstrated the feasibility of generating different types of mesenchymal cell populations, including mesenchymal stroma/stem cells (MSCs), pericytes (PCs), and smooth muscle cells (SMCs) de novo. However, applying hPSC-derived mesenchymal cells for studying mesenchymal cell development, cellular therapies, and tissue engineering is hampered by the lack of knowledge regarding a mesenchymal progenitor hierarchy and markers that allow to discriminate different mesenchymal cell populations. Identification of a common mesodermal progenitor for mesenchymal and endothelial cells, mesenchymoangioblast (MB), was an important milestone toward solving these problems [1]. The purpose of this review is to summarize our current knowledge of MBs and their differentiation potential, and to discuss the implications of these findings for studies mesenchymal cells and development of novel cellular therapies.
Mesenchyme formation during embryonic development
During embryonic development, the connective tissues, including bone, cartilage, adipose, blood cells, and vasculature are derived from mesenchyme. The formation of embryonic mesenchyme is one of the most critical events during embryogenesis that leads to the establishment of cardiovascular, hematopoietic, skeletal, and soft tissues. During gastrulation, the first mesenchymal cells forming primitive streak arise from epiblast through epithelial-to-mesenchymal transition. Following differentiation and migration, primitive mesenchymal cells partition into three major components forming lateral plate, intermediate, and paraxial mesoderm [2–5]. In addition, the earliest mesenchymal cells transgressing through the primitive streak give rise to extraembryonic mesoderm that forms yolk sac and allantois tissues. Another, relatively smaller portion of mesenchyme is produced from neural crest that originates from ectoderm at the margins of neural tube. Mesenchymal cells derived from neural crest produce craniofacial connective tissues, while connective tissues in the body are predominantly of mesodermal origin [6–8]. Although in vivo lineage mapping experiments in avian and mouse have extensively characterized germ layer contributions to mesenchymal derivatives, including bone marrow MSCs, smooth muscle cells (SMC), and pericytes (PCs), and have demonstrated the mosaic origin of mural cells and MSCs within vasculature and bone marrow as related to the site of origin [9–13], the hierarchy of mesenchymal progenitors formed during early stages of embryogenesis remains poorly understood. Moreover, due to fundamental developmental differences between mouse and human [14], it is critical to assess how mesenchymal cells develop in human ontogeny.
Development of MB defines the onset of mesenchymo- and endotheliogenesis in human pluripotent stem cell cultures
Discovery of hPSCs, including embryonic stem cells (hESCs) [15] and induced pluripotent stem cells (hiPSCs) [16, 17], opens opportunities to produce de novo SMCs and PCs [18–21], obtains the earliest mesodermal/mesenchymal populations, otherwise inaccessible in humans, and assesses the most primitive stages of embryonic development in vitro. Using hPSCs directed toward mesendodermal differentiation, Vodyanik et al. [1] revealed a novel clonal progenitor for endothelial and mesenchymal cells, designated as MB by analogy with hemangioblast (HB), a common progenitor for endothelial and hematopoietic cells. MBs were identified by their capacity to form compact spheroid colonies in FGF2-supplemented semisolid medium, which are capable of differentiating into endothelial and mesenchymal cells [1]. Formation of MB colonies solely depends on FGF2, but not other factors (VEGF, SCF, IGF1, EGF, and HGF), and requires serum-free medium [1]. Addition of PDGF-BB to FGF2-supplemented clonogenic medium enhances the frequency and size of MB colonies. However, PDGF-BB alone was not sufficient to support MB colony formation. In contrast, TGFβ1 or activin A completely abrogates MB colony formation.
MBs appear very early and exist only transiently during differentiation. In coculture with OP9, or in chemically defined conditions, MBs emerge on day 2 of differentiation from a mesodermal population expressing apelin receptor (APLNR), PDGFRα and KDR [1, 22]. MB colonies are dramatically reduced on day 3 of differentiation and entirely disappear on day 4 of differentiation. MBs precede development of HBs [1, 22], thereby suggesting that MB is the earliest clonogenic mesodermal progenitors to mark the onset of endothelio- and mesenchymogenesis in hPSC cultures.
MB generates mesenchymal cells through endothelial intermediates
Using time-lapse imaging, single cell deposition assay, and chimeric hESC lines containing equal proportions of EGFP and mOrange-marked H1 hESCs, we demonstrated a single cell origin of MB colonies. In addition, time-lapse microscopy revealed unique morphogenic events involved in MB colony formation [1]. When placed in clonogenic medium with FGF2, mesodermal cells from day 2 hESC differentiation cultures exhibit high motility. Following several divisions, a single mesodermal cell forms an immotile structure (core) composed of approximately 30 cells expressing KDR and typical endothelial genes, including PECAM and CDH5 (day 3 of clonogenic culture). Subsequently, cells at the core periphery undergo endothelial-to-mesenchymal transition (EndMT) and form a shell of tightly packed mesenchymal cells that continue to expand for up to 12–14 days of clonogenic culture (Fig. 1). Interestingly, the aggregation of migrating gastrulating cells and KDR upregulation in response to FGF produced by endoderm was also observed in chicken embryo [23, 24], thereby suggesting that morphogenic events observed during early stages of MB colony formation in FGF2 supplemented clonogenic culture from day 2 differentiated hPSCs resemble angioblast formation in vivo.
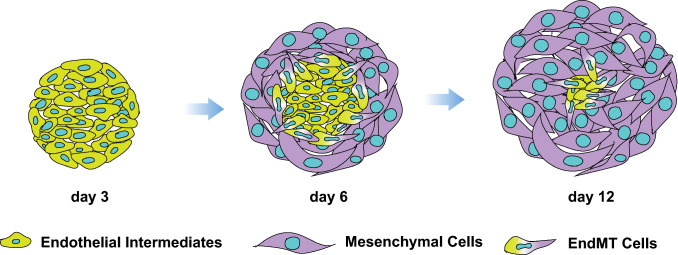
Fig. 1.
Schematic diagram demonstrating mesenchymal colony formation from MBs. During the first 3 days in serum-free clonogenic cultures with FGF2, mesodermal cells forms tight aggregates of endothelial/angioblastic cells (cores). Subsequently cores undergo EndMT giving rise to primitive mesenchymal cells
To analyze the differentiation potential of MB colonies at different stages of development, we isolated individual colonies on days 3, 6, and 12 of clonogenic culture and assessed endothelial and mesenchymal cell generation in coculture with OP9 [1]. These studies revealed that 3-day-old colonies (core stage) formed primarily endothelial clusters, while day 12 colonies formed predominantly mesenchymal clusters. In contrast, day 6 colonies primarily formed mesenchymoendothelial clusters, i.e., clusters composed of both endothelial and mesenchymal cells. Overall, these observations demonstrate that MB generates mesenchymal cells through endothelial/angioblastic intermediates and provides the direct link between endothelio- and mesenchymogenic populations.
MBs give rise to different mesenchymal cell lineages
Detailed phenotypic analysis of fully formed individual MB colonies (day 12 of clonogenic cultures in semisolid medium) revealed that they consist of a uniform population of cells with PDGFRβ+CD146+CD90+CD56+CD166+CD31−CD43−CD73− mesenchymal cell phenotype and gene expression profile of embryonic mesenchyme originating from primitive posterior mesoderm [1]. Mesenchymal cell forming MB colonies have broad differentiation potential and can generate different types of mesenchymal lineages, including MSCs, SMCs, and PCs (Figs. 2, 3). In medium supplemented with FGF2, MB colonies generate fibroblast-like cells with PDGFRβ+CD146+CD90+CD73+CD56+CD34−CD31−CD43−CD45−NG2low/−Calponinlow/− typical MSC phenotype and the capacity to differentiate into chondro-, osteo-, and adipogenic cells [1, 25], including brown adipocytes following exposure to TGFβ pathway inhibitor SB431542 together with ascorbic acid and EGF [26]. MB-derived MSCs have very strong expansion potential and can be maintained in culture on fibronectin- and collagen-coated plates with FGF2 for up to 25 passages. Cell cultures derived from a single MB colony accumulate up to 1022 MSCs [1].
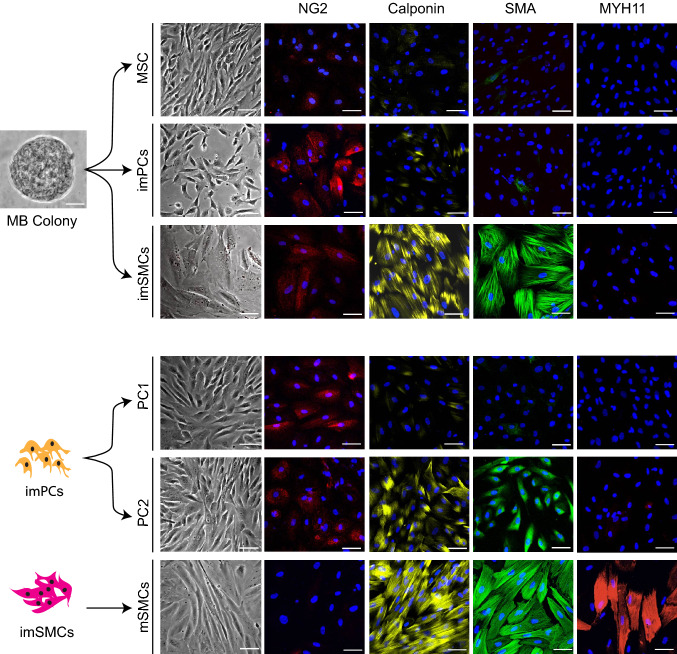
Fig. 2.
Generation of distinct mesenchymal populations form MB colonies. Morphology and expression of typical PC and SMC markers by different PC and SMC populations generated from MBs are shown. MB colony scale bar is 25 μm. Immunofluorescent images scale bars are 50 μm. Presented data is published in [25] but showing a different set of experiments
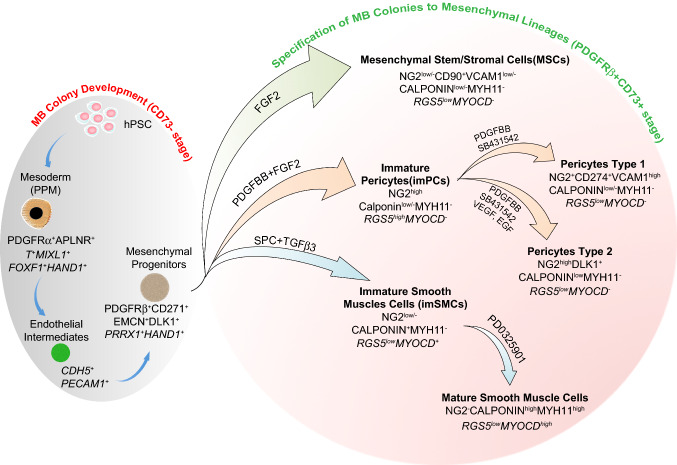
Fig. 3.
Model of mesoderm-derived mesenchymal cell development through MB pathway. Primitive posterior mesoderm (PPM) induced from hPSCs possess a potential to form FGF2-dependent compact spheroid colonies in semisolid medium with mesenchymal and endothelial cell potentials which define MBs. Development of MB colonies proceeds through endothelial/angioblastic cell intermediates which subsequently undergo EndMT giving rise to primitive PDGFRβ+CD271+DLK1+EMCN+CD73− multipotential mesenchymal progenitors. When MB colonies are collected and cultured in adherent conditions in the presence of the listed factors, they give rise to MSCs, imPCs, and imSMCs. The emerging imPCs could be further specified into CD274+ capillary-like PC1 and DLK1+ arteriolar-like PC2 with proinflammatory and contractile phenotype, respectively. Treatment of imSMCs with MEK inhibitor induces their maturation. Distinctive phenotypic and gene (in italic) expression features of corresponding cell populations are shown
Culture of MB colonies in the presence of PDGF-BB and FGF2 induces formation of NG2highSMAlowCalponinlow/−MYH11− proliferative immature PCs (imPCs), that can be expanded for up to 12 passages and further specified into capillary-like NG2+SMAlow/−Desminlow/−Calponinlow/−MYH11− PC1 in presence of PDGF-BB and TGFβ inhibitor SB431542, or arteriolar-like NG2highSMA+Desmin+Calponinlow/−MYH11− PC2 in presence of PDGF-BB, SB431542, VEGF and EGF [25]. In vivo and in vitro functional analysis of MB-derived PCs revealed that all different PC populations align with endothelial cells and strongly support vessel formation. Interestingly, PC1 and especially PC2, support endothelial tube formation in vitro for up to 7 days.
In contrast, cultures of MB colonies in the presence of SMC inducers, transforming growth factor β3 (TGFβ3) and sphingosylphosphorylcholine (SPC), produce NG2low/−SMA+Calponin+MYH11− proliferative/synthetic/immature SMCs that can be expanded up to seven passages and differentiated into mature NG2−CalponinhighMYH11+ SMCs (mSMCs) following treatment with MEK inhibitor PD0325901 [25]. SMC maturation is associated with transition from a rhomboid morphology, typical of synthetic SMCs, to an elongated morphology with well-organized contractile proteins, and marked upregulation of TAGLN, SYNPO2, PLN, MYOCD, MLK, ACTA2, CNN1, and MYH11 typical SMC gene expression. imSMCs, and especially mSMCs, exhibit a basal contractile tone in gel lattice assay and strongly contract in response to carbachol. However, SMCs failed to support tube formation in vivo and in vitro [25].
Identification of lineage tree and lineage-specific markers of MB-derived mesenchymal cells in hPSC cultures
Study of mesenchymal cells and molecular pathways regulating their development and applying this knowledge to tissue engineering is hampered by the lack of knowledge regarding a mesenchymal progenitor hierarchy and difficulties in distinguishing different types of mesenchymal cells ex vivo, since the most reliable criterion for identification of MSCs and mural cells, the anatomical location [27, 28], cannot be applied to hPSC cultures. Discovery of a well-defined clonogenic mesodermal progenitor for mesenchymal cells, MB, and demonstrating discrete mesenchymal cell differentiation pathways from MBs, has allowed us to characterized different stages of mesenchyme specification and define markers specific for these stages in hPSC cultures (Fig. 3) [25].
We have found that MB appears in hPSC cultures committed to mesendoderm differentiation on day 2 of culture along with upregulation of APLNR [1], which is typically expressed in primitive streak and adjacent embryonic and extraembryonic mesoderm, and later in lateral plate mesoderm [29–31]. Sorting experiments revealed that MB colony-forming cells almost entirely segregate to the APLNR+ fraction [1]. Phenotypic analysis of APLNR+ cells on day 2 of differentiation demonstrated that they express other primitive streak and early mesodermal markers PDGFRα and KDR [32–34], but no typical endothelial, MSCs, and hematopoietic markers (CD73, VE-cadherin, CD31, CD43 and CD45). Thus, we designated this stage of mesodermal development as EMHlin−APLNR+PDGFRα+KDR+ phenotype, where EMHlin− denotes the lack of expression of endothelial, MSCs, and hematopoietic markers [35, 36]. Molecular profiling of APLNR+ cells revealed expression of MIXL1, T, and EOMES primitive streak genes and FOXF1, HAND1, HAND2, IRX3, BMP4, and WNT5A genes typically found in lateral plate/extraembryonic mesoderm, but not note genes associated with neural crest, endoderm, paraxial, and intermediate mesoderm, thereby indicating that MB arises from a cell population that resembles very early primitive posterior mesoderm in the gastrulating embryo [1].
MB differentiation and MB colony development in clonogenic cultures proceed through two discrete stages: (1) formation of a core composed of tightly adherent endothelial-like angiogenic cells and (2) development of mesenchymal progenitors from endothelial cores through EndMT (Figs. 1, 3). Endothelial intermediates forming cores express KDR and typical endothelial genes including CDH5, PECAM, FLT1, TEK, SELE, and ICAM2. Most likely these cells resemble early aggregates of angioblastic cells, or vascular primordia that express KDR and certain markers of endothelial cells, but have yet to form lumen [24, 37]. Following EndMT, MB cores give rise to PDGFRβ+ primitive mesenchymal cells that form a shell of tightly packed cells around the angiogenic core. Mesenchymal progenitors forming MB colonies can be discriminated by surface expression of primitive mesenchymal markers CD271, Endomucin and delta-like non-canonical Notch ligand 1 (DLK1), and lack of the typical MSC marker CD73 [1, 25]. In addition, our molecular profiling studies of mesenchymal cells throughout all stages of specification and diversification revealed that MB colonies uniquely express HAND1, TBR1, and LHX genes [25] that are involved in tissue morphogenesis and PRRX1 gene known to be present in the primitive bone marrow mesenchymal progenitors [38].
PDGFRβ+CD271+EMCN+DLK1+CD73− primitive mesenchymal cells from MB colonies can be differentiated into MSCs and juvenile proliferative imPCs and imSMCs following treatment with molecules involved in specification of these mesenchymal lineages (Figs. 2, 3). This transition is associated with acquisition of mesenchymal marker CD73, which is expressed by all types of differentiated mesenchymal cells, including MSCs, PCs, and SMCs [25]. imPCs generated from MBs can be further specified into NG2+SMA− capillary (PC1) and NG2+SMA+ arteriolar (PC2) PCs, while immature/synthetic SMCs can be induced to differentiated into MYH11+ contractile SMCs (Figs. 2, 3). Distinguishing between PCs and SMCs could be made based on the expression of NG2 and calponin. All PC populations express NG2 and low levels of calponin, while lacking NG2 and high calponin expression could be used to define SMCs. MSCs typically do not express NG2 and calponin. In addition, expression of MYOCD gene is very specific for imSMCs and helps to discriminate these cells from imPCs and MSCs. When MB-derived imSMCs transition into contractile mSMCs, they upregulate expression of MYH11 protein, a very specific marker of mature SMCs.
Molecular profiling studies MB-derived PCs revealed that capillary PC1 generated from MBs has a “proinflammatory” gene expression profile as signified by high levels of chemoattractants, inflammatory, VCAM1, and programmed cell death ligand PDL1 (CD274), gene expression. In contrast, arteriolar PC2 could be distinguished by high expression of DLK1, which is found to be expressed by fetal arteriolar PCs [39]. Based on these findings, the utility of flow cytometric analysis for phenotypic discrimination between CD274+VCAMhigh capillary PC1 and DLK1+ arteriolar PC2 was demonstrated [25].
Interestingly, CD146 or MCAM, which is typically used to isolate PCs from somatic tissues [40], is expressed at all stages of mesenchymal development, including MB colonies and all differentiated mesenchymal cells [25], thereby suggesting the limited utility of this marker for identifying mural cell populations in hPSC cultures.
Overall, these studies have defined the hierarchy of mesoderm-derived mesenchymal cells in hPSC cultures and established a platform for studying the molecular pathways guiding mesenchymal cell specification. In addition, mesenchymal cell differentiation through a well-defined clonogenic progenitor stage, followed by directed specification of primitive mesenchymal intermediates to a particular mesenchymal lineage, makes it possible to generate pure populations of MSCs, PCs, and SMCs for tissue engineering and cellular therapies.
Distinguishing MBs from other types of embryonic mesenchymal progenitors
Mesoangioblast
Mesoangioblasts were initially described as expandable cell lines generated from dispersed E9.5 embryonic mouse aorta cells by limiting dilution [41]. Mesoangioblast cell lines express endothelial Cd34, Flk1, and Cdh5 genes with some cells within cell lines showing SMA protein expression. Embryonic mesoangioblasts have capacity to differentiate into osteoblasts, adipocytes, cartilage, cardiomyocytes, skeletal and smooth muscle, osteoclasts, and macrophages [41]. Later mesoangioblast terminology was also applied to mesenchymal or pericyte-like alkaline phosphatase positive cells isolated following 7 day culture of minced skeletal muscle cells on collagen type I-coated plates [42]. It has been shown that these cells have a capacity to differentiate into skeletal muscle in vitro and incorporate into skeletal muscles following in vivo transplantation [43–45]. In addition, the term “mesoangioblast” is used to describe hPSC-derived fibroblastoid cells that were reprogrammed into skeletal muscle cells with MYOD1 [46], a transcription factor that directly coverts fibroblasts and PSCs into skeletal muscle cells [47–50]. Since no single marker was identified that denotes mesoangioblasts across different stages of development and species, it was concluded that mesoangioblasts are primarily defined by their isolation method and functional properties [51].
In contrast to embryonic mesoangioblasts, MBs arise at primitive streak stage of development from APLNR+PDGFRα+KDR+ mesodermal cells, before endothelial markers are expressed and vascular cells are formed. MB is a transient cell population, which is defined by the capacity to form compact spheroid colonies in semisolid medium in response to FGF2. MBs have potential to differentiate into endothelial and mural cell populations through distinct KDR+CDH5+PECAM+ angioblastic/endothelial and PDGFRβ+CD271+EMCN+DLK1+CD73− primitive mesenchymal intermediates [1, 25]. Although MBs generate MSC lines with robust expansion potential, these cell lines do not retain MB properties, i.e., capacity to differentiate into endothelial cells [1]. In addition, MBs have a more restricted differentiation potential as compare to embryonic mesoangioblast, and do not differentiate into hematopoietic cells and cardiomyocytes.
Hemangioblast and cardiovascular clonogenic mesodermal progenitors
Flk1+ (KDR+) mesodermal cells emerging within primitive streak possess a broad differentiation potential. In addition to MBs, Flk1+ cells contain clonogenic progenitors with hematopoietic (HB) and cardiac potentials [52–54]. HB, a mesodermal progenitors with capacity to form colonies composed of immature (blast) hematopoietic cells in semisolid medium supplemented with VEGF, SCF, and conditioned medium from the endothelial cell line D4T, was initially identified in mouse embryonic stem cell (ESC) differentiation cultures [55]. Later studies revealed that blast colony-forming cells (BL-CFCs) also possess capacity to generate endothelial cells [56], leading to the conclusion that BL-CFCs represent a common precursor for endothelial and hematopoietic cells, HB. In addition, in vivo studies revealed BL-CFCs in the Flk1+ posterior primitive streak of E7.5 mouse embryo [52], thereby providing evidence that HB exists in vivo. Interestingly, formation of HB colonies in clonogenic medium similar to MBs proceeds through endothelial intermediates [1, 57]. However, in contrast to MBs, endothelial intermediates in HB colonies undergo endothelial-to-hematopoietic transition and form blood. It has been shown that cells collected from clonogenic HB cultures from hESCs generate MSCs [58]. However, it remains unclear whether MSCs generated by this method originate from HBs, MBs or other types of mesenchymal progenitors which are commonly formed along with HBs in semisolid colony-forming cultures [1, 59–61]. Thus, a conclusion regarding MSC potential of HBs can be made only by analyzing individual HB colonies. When we collected individual HB colonies and cultured them on fibronectin/collagen-coated plates in presence of FGF2, we failed to generate MSCs (data not published), thereby suggesting that HB is an unlikely source for MSCs.
Flk1+ cells with cardiac CFC potential in posterior primitive streak and ESC cultures, were detected in semisolid medium supplemented with cytokines known to function in mesoderm and early heart formation, including VEGF, FGF2, BMP4, and the Wnt inhibitor, DKK1 [53, 62]. In these conditions, mesodermal cells form tightly packed colonies expressing an array of cardiac markers and capable of spontaneous contraction when replated on gelatin substrate in serum-free medium. Gene expression analysis of expanded cardiac colonies revealed that they express endothelial PECAM and CDH5 and SMC SMA and CNN1 genes, suggesting that cardiac colonies may have endothelial and SMC potential [53]. However, in contrast to MB colonies, cardiac colonies and cells expanded from these colonies express TBX5, TNX20, and MYL2 cardiac genes [53, 54]. HBs and cardiovascular colony-forming progenitors are transient progenitors similarly to MBs. In PSC cultures, clonogenic mesodermal progenitors emerge in well-defined temporary order: MB colonies appears first, typically on day 2 differentiation [1, 22], HBs emerges next, typically on day 3 of differentiation [1, 35, 63], and cardiac colonies are formed a day later following HBs [53, 62]. Thus, mesodermal clonogenic progenitors within the Flk1+ population can be distinguished not only by functional properties, but distinct temporal kinetics during differentiation.
Mesospheres and other types of mesenchymal progenitors
Studies by Elefanty’s group [59] revealed that addition of WNT3A to HB clonogenic cultures inhibited formation of HB colonies and promoted development of mesodermal colonies termed, mesospheres. Mesospheres display a morphology and gene expression profile similar to MB colonies and could differentiate into adipocytes, osteocytes and SMCs. However, in contrast to MBs, mesospheres develop in presence of VEGF [59], which blocks formation of MB colonies at angiogenic core stage [1, 64]. Currently, it remains unclear whether mesospheres represent MB or a distinct mesenchymal progenitor. One can argue that differences in mesenchymal colony-forming potential in presence of VEGF indicate that mesospheres and MBs are different progenitors. On the other hand, it is possible that addition of WNT3A to clonogenic cultures may overcome VEGF-mediated inhibition of endothelial-to-mesenchymal transition and allow for MB colony formation in presence of VEGF.
Vasculogenic potential of Flk1+ cells was initially described by Yamashita et al., [65] who revealed that mouse ESC-derived Flk1+ mesodermal cells contribute to endothelial and mural cells following intracardiac injection in chicken embryo and that single Flk1+ cells can produce mixed colonies composed of SMA and CD31 expressing cells. These studies demonstrated for the first time the existence of common progenitors for endothelial cells and SMCs. However, later studies described above revealed the heterogeneity of mesenchymogenic progenitors within Flk1+/KDR+ population and demonstrated that SMCs and endothelial cells can be produced by at least two different types of progenitors, including at MBs and cardiovascular colony-forming cells.
Evseenko et al. [66] have found that the earliest mesodermal progenitors generated from hESCs in presence of activin A, BMP4, VEGF, and FGF2 can be identified by a CD326−CD56+KDRdim phenotype. These progenitors are capable to generate all mesodermal lineages, including blood, endothelial cells, bone, cartilage, and adipocytes. Lentiviral tagging experiments suggest that CD326−CD56+KDRdim population can be specified into bipotential mesenchymal/endothelial and hematopoietic/endothelial progenitors [67]. Thus, CD326−CD56+KDRdim cells described by Evseenko et al. [66] likely represent a more immature mesodermal population which emerges before MB and HB specification occurs.
MB potential for cellular therapies
Mesenchymal cells for tissue repair and regeneration
MSCs and perivascular cells isolated from adult tissues are recognized as a promising cell source for tissue regeneration. MSCs have capacity to differentiate into bone and cartilage and regenerate corresponding tissues directly. In addition, MSCs and perivascular cells can regenerate tissues indirectly by suppressing inflammation, stimulating angiogenesis, and recruiting tissue-specific progenitors to the site of injury (reviewed in Refs. [68–70]). MSCs also exhibit potent immunosuppressive activities, which are at least partially mediated by signaling activated by MSC apoptosis following infusion [71]. MSC treatments for a variety conditions have been pursued in numerous clinical trials, including bone and cartilage healing, cardiovascular regeneration, and graft-versus-host disease (GVHD), a major complication following allogeneic hematopoietic stem cell transplantation [70, 72, 73]. However, therapeutic application of MSCs faces several important challenges. This includes significant inconsistency among MSC preparations due to donor-to-donor variability, differences in tissue source, isolation and cell culture techniques, and potential contaminations of mesenchymal cell preparations with non-mesenchymal cells (macrophages, endothelial cells, and others). In addition, limited expansion potential of MSCs from adult tissues and lack of markers to define composition and biological function of MSC preparations hamper production of cellular preparations with reproducible pharmacological effects [72, 74–77].
Advantages and challenges in therapeutic application of mesenchymal cells from hPSCs
Human pluripotent stem cells can be expanded indefinitely and could potentially provide an unlimited number of mesenchymal cells for regenerative therapies from a single donor, thereby eliminating a variability in MSC preparations related to donor source. However, multiple origins of mesenchymal cells, which determine their functional heterogeneity, create a major challenge for standardizing mesenchymal cell production from hPSCs. During development, mesenchymal cells, including mural cells, arise from a number of embryonic sites, including neural crest, lateral plate mesoderm, paraxial, and intermediate mesoderm [9–13, 28]. Embryonic mesenchymal progenitors convey their origin-specific heterogeneity to their mesenchymal derivatives, which display unique functional properties related to their lineage origin [28]. In seminal studies, Cheung et al. [21] generated SMCs from hPSCs through well-defined neural crest, lateral plate and paraxial mesoderm intermediates, and demonstrated that these cells recapitulate origin-specific proliferative and secretory responses to growth factors, cytokines, and vasoactive agonists. Other studies by Chin et al. [78] revealed phenotypical and functional heterogeneity within hPSC-derived mesenchymal cells defined by typical PDGFRβ+CD90+CD105+CD44+CD73+ MSC phenotype. CD146highCD73highPDGFRα− subset within phenotypical MSC population expressed genes associated with hematopoietic stem cell (HSC) niche and supported HSC expansion in vitro. In contrast, CD146lowCD73lowPDGFRα+ mesenchymal cell population supported HSC differentiation.
Although multiple protocols for generating mesenchymal cells from hPSCs have been described (reviewed in Refs. [79–81]), origin and heterogeneity of mesenchymal cell populations obtained in these studies has not been addressed. Typically, hPSC-MSC protocols rely on selective expansion of fibroblastoid cells, which were spontaneously differentiated from hPSCs in presence of feeders, serum, or platelet lysate, or following treatment with TGFβ inhibitor. In some studies, fibroblastoid cells were isolated from differentiation cultures using CD105, CD73, or CD146 markers that are expressed by all types of mesenchymal lineages, including MSCs, SMCs and PCs. It is highly likely that the spontaneous differentiation process generates a mixture of functionally diverse mesenchymal cells of unknown embryonic origin and at different stages of development, which limits its utility for reproducible generation of pure mesenchymal cell populations for clinical use. Thus, developing well-defined progenitor-based protocols and identifying markers allowing to discriminate functionally distinct mesenchymal populations would be very critical to achieve standardization of hPSC-derived mesenchymal cell products.
The safety issues related to hPSC-derived MSCs are of paramount significance. Since MSCs are administered in relatively high numbers, the presence of even small numbers of undifferentiated hPSCs raises concern for potential teratoma formation (reviewed in Refs. [82–84]). It is also important to ensure the genomic integrity and epigenetic stability of iPSC lines used for mesenchymal cell manufacturing. Initially, iPSCs were generated using retroviral vectors in which transgenes are permanently integrated into the genome. However, within a short period of time, transgene-free reprogramming technologies have been developed, including episomal plasmids, modified RNA, Sendai virus, and protein-based methods (reviewed in Ref. [85]). It has been demonstrated that most genomic variations observed in iPSCs are inherited from the cells of origin and are not related to reprogramming [86–88]. Nevertheless, the possibility of de novo generation of genomic aberrations during reprogramming process cannot be entirely excluded [89]. Genomic instability can also occur following long-term expansion and differentiation of hiPSCs [90–92]. Therefore, careful monitoring genome integrity of hiPSCs and their derivatives is required to ensure hiPSC-MSC safety in clinical applications.
Benefits of using MB-based protocol for clinical grade MSC and perivascular cell generation
Mesenchymal cells generation through MB progenitor pathway using defined steps directing MBs to MSCs, PCs, or SMCs allows for the production of pure well-defined mesenchymal cell populations and standardization of the cell manufacturing process. Since semisolid medium used for generating MB colonies does not support the growth of singularized undifferentiated hPSCs, this step permits not only selection of primitive mesenchymal progenitors, but also effective elimination of undifferentiated hPSCs that pose a tumorigenicity risk. MSC products obtained from MBs are naturally free of immune cells, including macrophages, because MBs emerge in cultures before HBs and blood cells are formed. This prevents any possibility of adverse immunologic reaction to MSC products due to presence of contaminating immune cells.
MSCs generated from MBs have a robust expansion potential. Up to 1022 MSCs can be generated from a single MB colony, following continuous expansion for 120 days in culture [1]. Given that hPSC lines are capable of growing indefinitely in culture and can be expanded up to 1072 fold [93], essentially unlimited number of MSCs can be generated from a single hPSC line obtained from a single donor. It has been shown that MSCs isolated from adult tissues lose their therapeutic properties following prolonged in vitro culture making scaling of adult MSC products difficult [94, 95]. In contrast, MB hPSC-based protocol eliminates the need for excessive MSC expansion and allows for scalable manufacturing of low passage MSCs to meet the clinical needs for young cell products.
MBs can be efficiently differentiated in chemically defined conditions from hPSCs, which were also expanded in chemically defined conditions [22, 96]. Protocols for generating MB colonies and MSCs require serum- and xenogen-free conditions [1]. Therefore, all MSC differentiation steps through the MB pathway can be easily adopted for good manufacturing practice (GMP). In addition, the MB platform can be adopted for cell-mediated drug delivery. hPSCs can be genetically modified using CRISPR/Cas9 to express therapeutic molecules and clonally selected, thereby ensuring homogeneity of genomic editing and eliminating clones with deleterious off-target effects. Subsequently engineered hPSC lines can be used to produce an unlimited number of drug-loaded MSCs.
Overall, the MB-based platform for MSC production eliminates donor-to-donor variability, establishes the consistency in a product and its manufacturing process, and makes feasible a low cost/high volume manufacturing of an unlimited off-the-shelf supply of well-defined drug-like cellular products.
Experimental therapies with MB-derived MSCs
Within the last decade, several animal studies have demonstrated the therapeutic efficacy of hESC and hiPSC-derived MSCs in animal models of bone and cartilage injury, autoimmune and inflammatory diseases, myocardial infarction, and pulmonary injury (reviewed in Ref. [79]). Interestingly, hESC-derived MSCs performed more effectively compared to bone marrow MSCs in reducing experimental autoimmune encephalitis [97], pulmonary hypertension [98], and acute pulmonary injury [99], and hiPSC-derived MSCs outperformed bone marrow MSCs in a mouse model of anthracycline-induced cardiomyopathy [100]. MB-derived MSCs have already been tested mouse models of hindlimb ischemia and chronic airway disease. In hindlimb ischemia, intramuscular injection of MB-derived MSCs inhibited tissue damage, improved peripheral blood flow and significantly reduced toe necrosis, suggesting that these cells have a significant protective effect against ischemic insult [101]. Royce et al. [102], induced chronic allergic airways disease (AAD)/asthma by administering aerosolized ovalbumin to ovalbumin-sensitized mice. After establishing chronic AAD, mice were treated with MSCs administered intravenously or intranasally. Both intravenous and intranasal MB-derived MSC administration protected from airway inflammation, airway remodeling including goblet cell metaplasia, excessive fibroblast proliferation and collagen deposition, and airway hyperresponsiveness. It was also noted that intranasal MSC administration directly to the lungs offered greater protection against ovalbumin-induced airway remodeling and hyperresponsiveness. The immunomodulatory effect of MB-MSCs was greater than bone marrow or adipose MSCs.
Concluding remarks
During development, mesenchymal cells originate from multiple mesodermal and neural crest sources, which dictates their functional heterogeneity and complicates the process of generating well-defined pure mesenchymal cell populations from hPSCs. Discovery of MBs as the earliest clonogenic mesodermal progenitor for mesenchymal cells was a critical first step that permitted selection and interrogation of mesenchymal lineages originating from a single mesodermal cell with distinct clonogenic properties. Moreover, demonstration that MBs generate mesenchymal cells through endothelial/angioblastic intermediates, provided clear evidence of a direct developmental link between endothelial and mesenchymal progenitor populations. The recent identification of sequential stages of MB differentiation toward MSCs, PCs, and SMCs, in addition to novel stage- and lineage-specific markers, created a platform for exploring the molecular mechanisms guiding specification and diversification of mesenchymal lineages of mesodermal origin and modeling genetic diseases associated with vascular and skeletal abnormalities using patient-specific iPSCs. The MB-based protocol for SMC differentiation provided access to well-defined synthetic and contractile SMC populations, thus offering an in vitro system to study mechanisms of neointimal hyperplasia and drug testing for conditions associated with dysregulation of vascular SMC proliferation.
Despite progress with understanding mesenchymal cell specification through the MB pathway, the complexity mesenchymal cell development in hPSC cultures remains poorly understood. Mesenchymal cell generation from neural crest and different mesodermal compartments [10, 21] and cardiovascular clonogenic progenitors [53, 54] has been demonstrated. However, the distinct developmental stages and progenitors formed in these conditions remain uncharacterized. Thus, further studies are needed to decipher mesenchymal cell genesis of various origins at the progenitor level. Comparing the phenotype and function of developmentally diverse mesenchymal cell populations will be essential for better understanding the vascular and skeletal lineages in development and diseases states. It may also aid in designing cellular therapies that better meet specific clinical needs.
hPSCs are a logical alternative source of mesenchymal cells for cellular therapies and tissue engineering. MB-based mesenchymal cell generation protocols, in contrast to protocols based on spontaneous hPSC differentiation, allow for standardized manufacturing of well-defined pure MSC, PC, and SMC cell populations for clinical applications. The therapeutic efficacy of MB-derived MSCs has been demonstrated in animal models of hindlimb ischemia and chronic allergic airways disease [101, 102]. Further animal and in vitro studies are needed to compare the therapeutic potential of MB-derived MSCs and various PC subsets, to explore whether more specific and efficient targeting of inflammation versus tissue degeneration could be achieved with these populations. imPCs and capillary PC1 demonstrated superior vessel-stabilizing potential in vitro and in vivo [25], suggesting that these PCs could be more efficient in treating tissue damage associated with vascular diseases. Comparative analysis of the immunosuppressive properties of distinct MB-derived mesenchymal populations may help to design better cellular therapies for GVHD and autoimmune diseases. Finally, advances in gene editing technologies have created opportunities for using MB-derived MSCs as a vehicle for drug delivery, which can be manufactured in unlimited quantities. Further exploration of these technologies in preclinical studies will be essential for advancing the field.
iPSC-derived MSCs already reached clinical translation. Recently, Australian stem cell and regenerative medicine company, Cynata Therapeutics has conducted the first phase I clinical trial to evaluate safety and efficacy of allogeneic MB-derived MSCs for treating steroid resistant GVHD (NCT02923375). Interim data from 8 patients in this trial revealed the overall response rate 100% and complete response rate 50% without treatment-related serious events during a primary evaluation period (100 days post-treatment). This milestone already made important inroads to moving iPSC-based MSC technologies into clinic.
Acknowledgements
We thank Matthew Raymond for editorial assistance. I.I.S. and A.K are supported by funds from the National Institute of Health (U01HL134655, U01HL099773 and P51 RR000167). I.I.S. is a founding shareholder and consultant for Cynata Therapeutics.
Abbreviations
- MB
Mesenchymoangioblast
- HB
Hemangioblast
- PC
Pericytes
- SMC
Smooth muscle cells
- MSC
Mesenchymal stem/stromal cells
- hPSC
Human pluripotent stem cells
- hESC
Human embryonic stem cells
- hiPSCs
Human-induced pluripotent stem cells
References
- 1.Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, Slukvin II. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7(6):718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tam PP, Behringer RR. Mouse gastrulation: the formation of a mammalian body plan. Mech Dev. 1997;68(1–2):3–25. doi: 10.1016/S0925-4773(97)00123-8. [DOI] [PubMed] [Google Scholar]
- 3.Lawson A, Schoenwolf GC. Cell populations and morphogenetic movements underlying formation of the avian primitive streak and organizer. Genesis. 2001;29(4):188–195. doi: 10.1002/gene.1023. [DOI] [PubMed] [Google Scholar]
- 4.Sadler TW. Langman’s medical embryology. Philadelphia: Lippincott Williams and Wilkins; 2006. [Google Scholar]
- 5.Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233(3):706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- 6.Noden DM. The control of avian cephalic neural crest cytodifferentiation. I. Skeletal and connective tissues. Dev Biol. 1978;67(2):296–312. doi: 10.1016/0012-1606(78)90201-4. [DOI] [PubMed] [Google Scholar]
- 7.Noden DM. Origins and patterning of craniofacial mesenchymal tissues. J Craniofac Genet Dev Biol Suppl. 1986;2:15–31. [PubMed] [Google Scholar]
- 8.Mayor R, Theveneau E. The neural crest. Development. 2013;140(11):2247–2251. doi: 10.1242/dev.091751. [DOI] [PubMed] [Google Scholar]
- 9.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27(6):1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 10.Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa S. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129(7):1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Isern J, Garcia-Garcia A, Martin AM, Arranz L, Martin-Perez D, Torroja C, Sanchez-Cabo F, Mendez-Ferrer S. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife. 2014;3:e03696. doi: 10.7554/eLife.03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller FD. Riding the waves: neural and nonneural origins for mesenchymal stem cells. Cell Stem Cell. 2007;1(2):129–130. doi: 10.1016/j.stem.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Morikawa S, Mabuchi Y, Niibe K, Suzuki S, Nagoshi N, Sunabori T, Shimmura S, Nagai Y, Nakagawa T, Okano H, Matsuzaki Y. Development of mesenchymal stem cells partially originate from the neural crest. Biochem Biophys Res Commun. 2009;379(4):1114–1119. doi: 10.1016/j.bbrc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Rossant J, Tam PPL. New insights into early human development: lessons for stem cell derivation and differentiation. Cell Stem Cell. 2017;20(1):18–28. doi: 10.1016/j.stem.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 18.Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF, Grainger SJ, Kapp FG, Sun L, Christensen K, Xia Y, Florido MH, He W, Pan W, Prummer M, Warren CR, Jakob-Roetne R, Certa U, Jagasia R, Freskgard PO, Adatto I, Kling D, Huang P, Zon LI, Chaikof EL, Gerszten RE, Graf M, Iacone R, Cowan CA. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol. 2015;17(8):994–1003. doi: 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlova VV, Drabsch Y, Freund C, Petrus-Reurer S, van den Hil FE, Muenthaisong S, Dijke PT, Mummery CL. Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts. Arterioscler Thromb Vasc Biol. 2014;34(1):177–186. doi: 10.1161/ATVBAHA.113.302598. [DOI] [PubMed] [Google Scholar]
- 20.Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, Germanguz I, Amit M, Itskovitz-Eldor J. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125(1):87–99. doi: 10.1161/CIRCULATIONAHA.111.048264. [DOI] [PubMed] [Google Scholar]
- 21.Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30(2):165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uenishi G, Theisen D, Lee JH, Kumar A, Raymond M, Vodyanik M, Swanson S, Stewart R, Thomson J, Slukvin I. Tenascin C promotes hematoendothelial development and T lymphoid commitment from human pluripotent stem cells in chemically defined conditions. Stem Cell Rep. 2014;3(6):1073–1084. doi: 10.1016/j.stemcr.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flamme I, Breier G, Risau W. Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol. 1995;169(2):699–712. doi: 10.1006/dbio.1995.1180. [DOI] [PubMed] [Google Scholar]
- 24.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, D’Souza SS, Moskvin OV, Toh H, Wang B, Zhang J, Swanson S, Guo LW, Thomson JA, Slukvin II. Specification and diversification of pericytes and smooth muscle cells from mesenchymoangioblasts. Cell Rep. 2017;19(9):1902–1916. doi: 10.1016/j.celrep.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hafner AL, Contet J, Ravaud C, Yao X, Villageois P, Suknuntha K, Annab K, Peraldi P, Binetruy B, Slukvin II, Ladoux A, Dani C. Brown-like adipose progenitors derived from human induced pluripotent stem cells: Identification of critical pathways governing their adipogenic capacity. Sci Rep. 2016;6:32490. doi: 10.1038/srep32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Shen EM, McCloskey KE. Development of mural cells: from in vivo understanding to in vitro recapitulation. Stem Cells Dev. 2017;26(14):1020–1041. doi: 10.1089/scd.2017.0020. [DOI] [PubMed] [Google Scholar]
- 29.D’Aniello C, Lonardo E, Iaconis S, Guardiola O, Liguoro AM, Liguori GL, Autiero M, Carmeliet P, Minchiotti G. G protein-coupled receptor APJ and its ligand apelin act downstream of Cripto to specify embryonic stem cells toward the cardiac lineage through extracellular signal-regulated kinase/p70S6 kinase signaling pathway. Circ Res. 2009;105(3):231–238. doi: 10.1161/CIRCRESAHA.109.201186. [DOI] [PubMed] [Google Scholar]
- 30.Devic E, Paquereau L, Vernier P, Knibiehler B, Audigier Y. Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech Dev. 1996;59(2):129–140. doi: 10.1016/0925-4773(96)00585-0. [DOI] [PubMed] [Google Scholar]
- 31.Zeng XX, Wilm TP, Sepich DS, Solnica-Krezel L. Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev Cell. 2007;12(3):391–402. doi: 10.1016/j.devcel.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Orr-Urtreger A, Bedford MT, Do MS, Eisenbach L, Lonai P. Developmental expression of the alpha receptor for platelet-derived growth factor, which is deleted in the embryonic lethal Patch mutation. Development. 1992;115(1):289–303. doi: 10.1242/dev.115.1.289. [DOI] [PubMed] [Google Scholar]
- 33.Sakurai H, Era T, Jakt LM, Okada M, Nakai S, Nishikawa S. In vitro modeling of paraxial and lateral mesoderm differentiation reveals early reversibility. Stem Cells. 2006;24(3):575–586. doi: 10.1634/stemcells.2005-0256. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118(2):489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 35.Choi KD, Vodyanik MA, Togarrati PP, Suknuntha K, Kumar A, Samarjeet F, Probasco MD, Tian S, Stewart R, Thomson JA, Slukvin II. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2(3):553–567. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slukvin II. Deciphering the hierarchy of angiohematopoietic progenitors from human pluripotent stem cells. Cell Cycle. 2013;12(5):720–727. doi: 10.4161/cc.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flamme I, Frolich T, Risau W (1997) Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J Cell Physiol 173(2):206–210. https://doi.org/10.1002/(SICI)1097-4652(199711)173:2<206:AID-JCP22>3.0.CO;2-C [DOI] [PubMed]
- 38.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, Pinho S, Ciero P, Nakahara F, Ma’ayan A, Bergman A, Merad M, Frenette PS. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science. 2015 doi: 10.1126/science.aad0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129(11):2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 42.Tonlorenzi R, Dellavalle A, Schnapp E, Cossu G, Sampaolesi M. Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr Protoc Stem Cell Biol. 2007;Chapter 2:Unit 2B 1. doi: 10.1002/9780470151808.sc02b01s3. [DOI] [PubMed] [Google Scholar]
- 43.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 44.Loperfido M, Jarmin S, Dastidar S, Di Matteo M, Perini I, Moore M, Nair N, Samara-Kuko E, Athanasopoulos T, Tedesco FS, Dickson G, Sampaolesi M, VandenDriessche T, Chuah MK. piggyBac transposons expressing full-length human dystrophin enable genetic correction of dystrophic mesoangioblasts. Nucleic Acids Res. 2016;44(2):744–760. doi: 10.1093/nar/gkv1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iyer PS, Mavoungou LO, Ronzoni F, Zemla J, Schmid-Siegert E, Antonini S, Neff LA, Dorchies OM, Jaconi M, Lekka M, Messina G, Mermod N. Autologous cell therapy approach for duchenne muscular dystrophy using piggybac transposons and mesoangioblasts. Mol Ther. 2018;26(4):1093–1108. doi: 10.1016/j.ymthe.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tedesco FS, Gerli MF, Perani L, Benedetti S, Ungaro F, Cassano M, Antonini S, Tagliafico E, Artusi V, Longa E, Tonlorenzi R, Ragazzi M, Calderazzi G, Hoshiya H, Cappellari O, Mora M, Schoser B, Schneiderat P, Oshimura M, Bottinelli R, Sampaolesi M, Torrente Y, Broccoli V, Cossu G. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci Transl Med. 2012;4(140):140ra189. doi: 10.1126/scitranslmed.3003541. [DOI] [PubMed] [Google Scholar]
- 47.Dekel I, Magal Y, Pearson-White S, Emerson CP, Shani M. Conditional conversion of ES cells to skeletal muscle by an exogenous MyoD1 gene. New Biol. 1992;4(3):217–224. [PubMed] [Google Scholar]
- 48.Rao L, Tang W, Wei Y, Bao L, Chen J, Chen H, He L, Lu P, Ren J, Wu L, Luan Z, Cui C, Xiao L. Highly efficient derivation of skeletal myotubes from human embryonic stem cells. Stem Cell Rev. 2012;8(4):1109–1119. doi: 10.1007/s12015-012-9413-4. [DOI] [PubMed] [Google Scholar]
- 49.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-X. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka A, Woltjen K, Miyake K, Hotta A, Ikeya M, Yamamoto T, Nishino T, Shoji E, Sehara-Fujisawa A, Manabe Y, Fujii N, Hanaoka K, Era T, Yamashita S, Isobe K, Kimura E, Sakurai H. Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi Myopathy in vitro. PLoS One. 2013;8(4):e61540. doi: 10.1371/journal.pone.0061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonfanti C, Rossi G, Tedesco FS, Giannotta M, Benedetti S, Tonlorenzi R, Antonini S, Marazzi G, Dejana E, Sassoon D, Cossu G, Messina G. PW1/Peg3 expression regulates key properties that determine mesoangioblast stem cell competence. Nat Commun. 2015;6:6364. doi: 10.1038/ncomms7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432(7017):625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 53.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11(5):723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 55.Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller G. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386(6624):488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 56.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125(4):725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 57.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457(7231):892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimbrel EA, Kouris NA, Yavanian GJ, Chu J, Qin Y, Chan A, Singh RP, McCurdy D, Gordon L, Levinson RD, Lanza R. Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cells Dev. 2014;23(14):1611–1624. doi: 10.1089/scd.2013.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gertow K, Hirst CE, Yu QC, Ng ES, Pereira LA, Davis RP, Stanley EG, Elefanty AG. WNT3A promotes hematopoietic or mesenchymal differentiation from hESCs depending on the time of exposure. Stem Cell Rep. 2013;1(1):53–65. doi: 10.1016/j.stemcr.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faloon P, Arentson E, Kazarov A, Deng CX, Porcher C, Orkin S, Choi K. Basic fibroblast growth factor positively regulates hematopoietic development. Development. 2000;127(9):1931–1941. doi: 10.1242/dev.127.9.1931. [DOI] [PubMed] [Google Scholar]
- 61.Robertson SM, Kennedy M, Shannon JM, Keller G. A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-1. Development. 2000;127(11):2447–2459. doi: 10.1242/dev.127.11.2447. [DOI] [PubMed] [Google Scholar]
- 62.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8(2):228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109(7):2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slukvin II, Vodyanik M. Endothelial origin of mesenchymal stem cells. Cell Cycle. 2011;10(9):1370–1373. doi: 10.4161/cc.10.9.15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408(6808):92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 66.Evseenko D, Zhu Y, Schenke-Layland K, Kuo J, Latour B, Ge S, Scholes J, Dravid G, Li X, MacLellan WR, Crooks GM. Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2010;107(31):13742–13747. doi: 10.1073/pnas.1002077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chin CJ, Cooper AR, Lill GR, Evseenko D, Zhu Y, He CB, Casero D, Pellegrini M, Kohn DB, Crooks GM. Genetic tagging during human mesoderm differentiation reveals tripotent lateral plate mesodermal progenitors. Stem Cells. 2016;34(5):1239–1250. doi: 10.1002/stem.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, Tawonsawatruk T, Lazzari L, Soo C, Peault B. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2014;71(8):1353–1374. doi: 10.1007/s00018-013-1462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014;54(5):1418–1437. doi: 10.1111/trf.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, von Bonin M, Barbieri L, Halai K, Ward S, Weng L, Chakraverty R, Lombardi G, Watt FM, Orchard K, Marks DI, Apperley J, Bornhauser M, Walczak H, Bennett C, Dazzi F. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9(416):eaam7828. doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 72.Majka M, Sulkowski M, Badyra B, Musialek P. Concise review: mesenchymal stem cells in cardiovascular regeneration: emerging research directions and clinical applications. Stem Cells Transl Med. 2017;6(10):1859–1867. doi: 10.1002/sctm.16-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munneke JM, Spruit MJA, Cornelissen AS, van Hoeven V, Voermans C, Hazenberg MD. The potential of mesenchymal stromal cells as treatment for severe steroid-refractory acute graft-versus-host disease: a critical review of the literature. Transplantation. 2016;100(11):2309–2314. doi: 10.1097/TP.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 74.Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14(2):141–145. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Viswanathan S, Keating A, Deans R, Hematti P, Prockop D, Stroncek DF, Stacey G, Weiss DJ, Mason C, Rao MS. Soliciting strategies for developing cell-based reference materials to advance mesenchymal stromal cell research and clinical translation. Stem Cells Dev. 2014;23(11):1157–1167. doi: 10.1089/scd.2013.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin I, De Boer J, Sensebe L, Therapy MSCCotISfC A relativity concept in mesenchymal stromal cell manufacturing. Cytotherapy. 2016;18(5):613–620. doi: 10.1016/j.jcyt.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Liu ST, de Castro LF, Jin P, Civini S, Ren JQ, Reems JA, Cancelas J, Nayak R, Shaw G, O’Brien T, McKenna DH, Armant M, Silberstein L, Gee AP, Hei DJ, Hematti P, Kuznetsov SA, Robey PG, Stroncek DF. Manufacturing differences affect human bone marrow stromal cell characteristics and function: comparison of production methods and products from multiple centers. Sci Rep. 2017;7:46731. doi: 10.1038/srep46731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chin CJ, Li S, Corselli M, Casero D, Zhu Y, He CB, Hardy R, Peault B, Crooks GM. Transcriptionally and functionally distinct mesenchymal subpopulations are generated from human pluripotent stem cells. Stem Cell Rep. 2018 doi: 10.1016/j.stemcr.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luzzani CD, Miriuka SG. Pluripotent stem cells as a robust source of mesenchymal stem cells. Stem Cell Rev. 2017;13(1):68–78. doi: 10.1007/s12015-016-9695-z. [DOI] [PubMed] [Google Scholar]
- 80.Xu J, Gong T, Heng BC, Zhang CF. A systematic review: differentiation of stem cells into functional pericytes. FASEB J. 2017;31(5):1775–1786. doi: 10.1096/fj.201600951RRR. [DOI] [PubMed] [Google Scholar]
- 81.Maguire EM, Xiao Q, Xu Q. Differentiation and application of induced pluripotent stem cell-derived vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2017;37(11):2026–2037. doi: 10.1161/ATVBAHA.117.309196. [DOI] [PubMed] [Google Scholar]
- 82.Neofytou E, O’Brien CG, Couture LA, Wu JC. Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Investig. 2015;125(7):2551–2557. doi: 10.1172/JCI80575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol. 2016;17(3):194–200. doi: 10.1038/nrm.2016.10. [DOI] [PubMed] [Google Scholar]
- 84.Tapia A, Salgado MS, Martin MP, Lapuerta M, Rodriguez-Fernandez J, Rossi MJ, Cabanas B. Molecular characterization of the gas-particle interface of soot sampled from a diesel engine using a titration method. Environ Sci Technol. 2016;50(6):2946–2955. doi: 10.1021/acs.est.5b05531. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi K, Yamanaka S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat Rev Mol Cell Biol. 2016;17(3):183–193. doi: 10.1038/nrm.2016.8. [DOI] [PubMed] [Google Scholar]
- 86.Abyzov A, Mariani J, Palejev D, Zhang Y, Haney MS, Tomasini L, Ferrandino AF, Rosenberg Belmaker LA, Szekely A, Wilson M, Kocabas A, Calixto NE, Grigorenko EL, Huttner A, Chawarska K, Weissman S, Urban AE, Gerstein M, Vaccarino FM. Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature. 2012;492(7429):438–442. doi: 10.1038/nature11629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng L, Hansen NF, Zhao L, Du Y, Zou C, Donovan FX, Chou BK, Zhou G, Li S, Dowey SN, Ye Z, Program NCS, Chandrasekharappa SC, Yang H, Mullikin JC, Liu PP. Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression. Cell Stem Cell. 2012;10(3):337–344. doi: 10.1016/j.stem.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Young MA, Larson DE, Sun CW, George DR, Ding L, Miller CA, Lin L, Pawlik KM, Chen K, Fan X, Schmidt H, Kalicki-Veizer J, Cook LL, Swift GW, Demeter RT, Wendl MC, Sands MS, Mardis ER, Wilson RK, Townes TM, Ley TJ. Background mutations in parental cells account for most of the genetic heterogeneity of induced pluripotent stem cells. Cell Stem Cell. 2012;10(5):570–582. doi: 10.1016/j.stem.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Narva E, Ng S, Sourour M, Hamalainen R, Olsson C, Lundin K, Mikkola M, Trokovic R, Peitz M, Brustle O, Bazett-Jones DP, Alitalo K, Lahesmaa R, Nagy A, Otonkoski T. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471(7336):58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 90.Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, Lee JH, Loh YH, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert ML, Yu J, Kirkness EF, Izpisua Belmonte JC, Rossi DJ, Thomson JA, Eggan K, Daley GQ, Goldstein LS, Zhang K. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471(7336):63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R, Lynch C, Harness JV, Lee S, Barrero MJ, Ku S, Martynova M, Semechkin R, Galat V, Gottesfeld J, Izpisua Belmonte JC, Murry C, Keirstead HS, Park HS, Schmidt U, Laslett AL, Muller FJ, Nievergelt CM, Shamir R, Loring JF. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8(1):106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Varela C, Denis JA, Polentes J, Feyeux M, Aubert S, Champon B, Pietu G, Peschanski M, Lefort N. Recurrent genomic instability of chromosome 1q in neural derivatives of human embryonic stem cells. J Clin Investig. 2012;122(2):569–574. doi: 10.1172/JCI46268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lei Y, Schaffer DV. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc Natl Acad Sci USA. 2013;110(52):E5039–E5048. doi: 10.1073/pnas.1309408110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22(5):675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 95.Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8(5):424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang X, Kimbrel EA, Ijichi K, Paul D, Lazorchak AS, Chu J, Kouris NA, Yavanian GJ, Lu SJ, Pachter JS, Crocker SJ, Lanza R, Xu RH. Human ESC-derived MSCs outperform bone marrow MSCs in the treatment of an EAE model of multiple sclerosis. Stem Cell Rep. 2014;3(1):115–130. doi: 10.1016/j.stemcr.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y, Liao S, Yang M, Liang X, Poon MW, Wong CY, Wang J, Zhou Z, Cheong SK, Lee CN, Tse HF, Lian Q. Improved cell survival and paracrine capacity of human embryonic stem cell-derived mesenchymal stem cells promote therapeutic potential for pulmonary arterial hypertension. Cell Transpl. 2012;21(10):2225–2239. doi: 10.3727/096368912X653020. [DOI] [PubMed] [Google Scholar]
- 99.Hao Q, Zhu YG, Monsel A, Gennai S, Lee T, Xu F, Lee JW. Study of bone marrow and embryonic stem cell-derived human mesenchymal stem cells for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells Transl Med. 2015;4(7):832–840. doi: 10.5966/sctm.2015-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y, Liang X, Liao S, Wang W, Wang J, Li X, Ding Y, Liang Y, Gao F, Yang M, Fu Q, Xu A, Chai YH, He J, Tse HF, Lian Q. Potent paracrine effects of human induced pluripotent stem cell-derived mesenchymal stem cells attenuate doxorubicin-induced cardiomyopathy. Sci Rep. 2015;5:11235. doi: 10.1038/srep11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koch JM, D’Souza SS, Schwahn DJ, Dixon I, Hacker TA. Mesenchymoangioblast-derived mesenchymal stromal cells inhibit cell damage, tissue damage and improve peripheral blood flow following hindlimb ischemic injury in mice. Cytotherapy. 2016;18(2):219–228. doi: 10.1016/j.jcyt.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 102.Royce SG, Rele S, Broughton BRS, Kelly K, Samuel CS. Intranasal administration of mesenchymoangioblast-derived mesenchymal stem cells abrogates airway fibrosis and airway hyperresponsiveness associated with chronic allergic airways disease. FASEB J. 2017;31(9):4168–4178. doi: 10.1096/fj.201700178R. [DOI] [PubMed] [Google Scholar]