Abstract
There is increasing use of head-to-head clinical trials in dermatology when establishing the efficacy of a new treatment. Active comparator trials (ACTs) can be classified into three distinct study trial designs: non-inferiority, equivalence, and superiority. A better understanding of the statistical parameters, such as acceptable treatment differences (also known as the margin or delta), is necessary to properly design and interpret findings of active comparator trials (ACTs) in the field of dermatology. Therefore, the objective of this study is to summarize the maximum acceptable treatment differences in clinical trials that examine the efficacy of an oral or biologic psoriasis therapy with an active comparator. We conducted a systematic search using MEDLINE, Scopus, EMBASE, Cochrane Central Register of Controlled Trials, LILACS, Web of Science, and ClinicalTrials.gov from inception to August 31, 2017. All ACTs with adult participants that had a primary outcome of the Psoriasis Area and Severity Index score were included. Bibliographies of articles were further reviewed. Two investigators independently assessed for article inclusion and separately completed data extraction of pre-defined data points. When there was a disagreement, a third investigator was consulted. Of the 49 ACTs included, there were 13 superiority, 8 non-inferiority, and 7 equivalence trials. Another 21 studies had inadequate information for classification. All of the non-inferiority trials reported the margin, 1 of the superiority, and 6 of the equivalence trials stated the treatment difference explicitly. For superiority trials, acceptable treatment differences ranged from 14% to 20%. The non-inferiority studies reported lower bound margins ranging from −20% to −10%. The equivalence trials reported upper and lower bound margins ranging from ±12.5% to ±18%. The results demonstrate the need for harmonization in the conduct of dermatological clinical trials and in the approaches of reporting research parameters.
Introduction
Clinical trials may include the use of placebo (PBO), active treatment (an approved therapy, standard care, or the best available intervention), or no control group.1 Active-controlled clinical trials (ACTs) are increasingly the benchmark in many fields of medicine when establishing the efficacy of a new treatment.2,3 Even if ACTs do not achieve the desired primary outcomes or prove the hypothesis, negative results still provide highly valuable information4 by identifying sub-populations who may stand to gain the most benefits and ascertain those in whom the drug should be avoided. Prior to the 2000’s, ACTs were quite uncommon for systemic agents in moderate to severe psoriasis. For example, a systematic review of dermatological studies from 1977–2000 found only 31 (12.5%) randomized controlled trials (RCTs) comparing treatment modalities in different therapeutic classes.5 Although placebo-controlled trials often precede active-controlled trials in psoriasis research, head-to-head comparisons for new therapies are becoming the rule rather than the exception.6 Preference for ACTs may be attributed to the pragmatic nature of such trials as they assess the comparative values of different treatments as opposed to choosing to treat or not (i.e., PBO). However, head-to-head clinical trials present unique challenges in the design and the interpretation of results.
ACT methodology can be classified into different types of comparisons: non-inferiority trials (which aims to demonstrate a minimum level of efficacy in the investigational intervention vs. the comparator by a pre-specified amount); equivalence trials (often employed in biosimilar studies to evaluate whether the investigational intervention is comparable enough, or not “too different,” to the originator drug); and superiority trials7 (the most convincing way to determine whether the investigational intervention is more efficacious than the comparator) (Fig. 1).8,9 It is critical to understand the type of comparison as the design directly impacts the conclusions that can be drawn from the results. Particularly, it is crucial that the margins or delta of comparison, also known as the clinically acceptable maximum difference between two treatments, be well-defined and justified as this difference directly influences sample size estimates as well as study conclusions.
Figure 1.
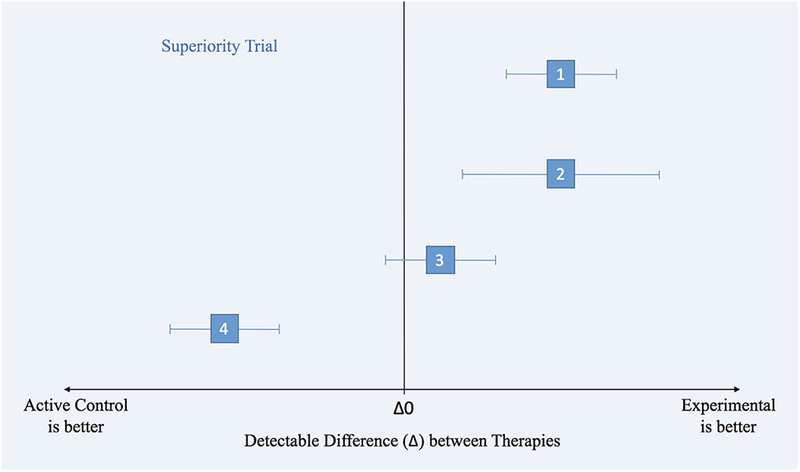
Statistical differences between superiority, equivalence, and non-inferiority clinical trials.
Figure 1a. Superiority Clinical Trial Principles illustrated by different scenarios (1–4), where the effects of an active control and experimental treatment are within the confidence intervals (along the x-axis).
1) Experimental is superior to active control. The CI does not include zero treatment difference.
2) Experimental is superior to active control but it has a wider CI than in (1), meaning that the estimate of the difference between treatments is less precise (due to more variance or a smaller sample size).
3) There is no difference between treatments.
4) The entire CI lies below the line of Δ0, favoring active control.
We recently conducted a systematic review of active-controlled trials of biosimilars and found that 5 out of the 9 studies did not report adequate information to ascertain key aspects of study design, which led us to conduct a larger systematic review inclusive of all systemic and biologic ACTs in psoriasis.10 Given the increasing use of active controls, development of new investigative therapies, and implications for evidence-based practice, we sought to determine the range of statistical parameters for active-controlled systemic and biologic therapeutic trials in psoriasis. The objective of this study is to summarize the different types of ACTs (superiority, NI, or equivalence trials) and to synthesize current evidence to inform future comparative trials in psoriasis.
Methods
We conducted a systematic search based on the Cochrane Handbook for Systematic Reviews of Interventions guidelines using MEDLINE, Scopus, EMBASE, Cochrane Central Register of Controlled Trials, LILACS, Web of Science, and ClinicalTrials.gov from inception to August 31, 201711 to identify eligible articles using relevant keywords and Medical Subject Headings with the assistance of a professional librarian (Supplementary Table 1). To avoid possible publication bias and identify ongoing or unpublished trials, we also searched clinicaltrialresults.org, World Health Organization International Clinical Trials Registry Platform and Food and Drug Administration websites on September 7, 2017. To obtain data from unpublished studies and abstracts, we contacted corresponding authors or industry sponsors. Supplementary data were retrieved if the full-text publications did not contain data points of interest. Our inclusion criteria (Supplementary Table 2) included adult patients (≥18 years old) with plaque psoriasis (PsO) randomized to receive monotherapy of an experimental intervention or active control. Our outcome of interest was the Psoriasis Area and Severity Index (PASI) score, as this is the measure typically used in clinical trials to define successful treatment,12 measured at week 12 or later.
Study Selection
Study selection was carried out according to the PRISMA statement (Fig.2).13 Two reviewers (MTW and JA) independently reviewed the titles and abstracts of all studies for inclusion and exclusion criteria which had been downloaded from the search into EndNote X7.7.1 (Thompson Reuters). EndNote X7.7.1 and STATA 13.1 (College Station, Texas, USA) were used to identify duplicates. Titles and abstracts were screened for inclusions, followed by full-text retrieval if abstracts were insufficient to determine whether studies met inclusion and exclusion criteria. In cases of disagreements, the two reviewers (MTW and JA) compared findings and reached consensus about eligibility and on classification. If consensus was not reached, a third reviewer (EDD) was consulted. Single-center studies were excluded as small sample sizes typically yield studies that are inadequately powered. Although our search criteria included phototherapy, we excluded phototherapies after the database search in addition to topical treatments as PsO RCTs generally examine oral and biologic therapies.
Figure 1b.
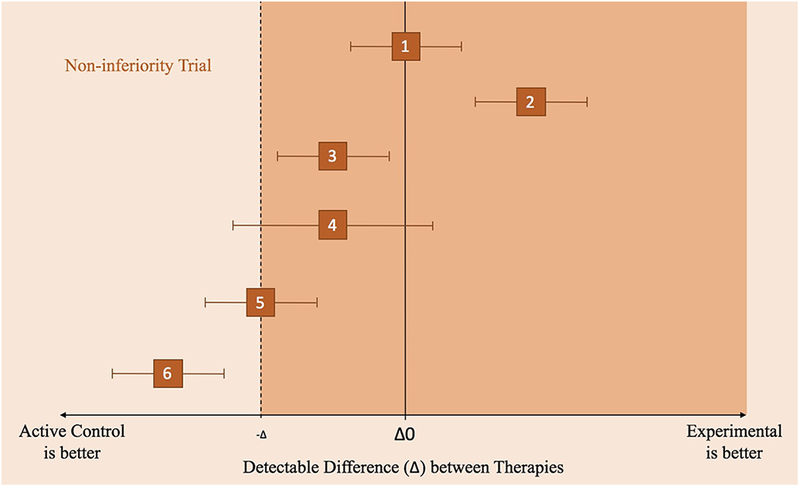
Non-inferiority Clinical Trial Principles illustrated by different scenarios (1–6), where the effects of an active control and experimental treatment are within the confidence intervals (along the x-axis).
1) Experimental non-inferior to control but not significantly different from active control.
2) Experimental is non-inferior to control but is significantly different from control.
3) Experimental is somewhat inferior to control. Experimental is not inferior because the CI does not include the non-inferior margin or the line of Δ0.
4) Inconclusive because the CI includes values on both sides of the non-inferiority margin and the line of no treatment difference (Δ0).
5) Experimental is inferior to control because it includes the non-inferiority margin.
6) Experimental is inferior to control and is more certain of its inferiority as compared to scenario (5) because it does not include the non-inferiority margin.
Data Extraction and Management
Two authors (MTW and JA) independently extracted pre-determined data from all 49 RCTs that met inclusion criteria. RCTs were classified into superiority, NI, or equivalent trial design based on the language used in the publications and the reported statistical parameters between the experimental and active control groups. ACTs were deemed as “other head-to-head comparisons” if the authors could not retrieve adequate information to classify the RCTs as one of the three aforementioned study designs. For the purpose of this manuscript, ACTs were classified according to the type of comparison of the investigative drug versus the active control (i.e., a study was considered as a NI ACT if any of the hypotheses tested non-inferiority of an investigative intervention versus active control, even if it also explored superiority to placebo). For studies with multiple hypotheses tested, ACTs were classified according to whichever hypothesis was tested first sequentially. We abstracted the following data: study design, sample size, power, confidence intervals, margin or delta, primary outcome, and response rates (Tables 1–4). From the extracted data, we summarized ranges for continuous data and calculated proportions and percentages for categorical data. Studies with missing values were excluded from specific analyses. Statistical analyses were performed using STATA 13.1.
Table 1.
Characteristics of the Superiority Randomized, Controlled Trials with an Active Comparator.
| Source | Sponsor (Completion Year) | Title | Experimental | Active Comparator | PBO | Sample Size (Experiment/Comparator/PBO) A priori/Observed | Primary Outcome | Week Measured | Response Rate A priori/Observed |
Delta (%)a | CI (%) | Power | Study Qualityb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Table1A. Superiority Trials with complete power analyses data. | |||||||||||||
| NCT0067973132 | AbbVie (2009) | A 52-week Trial Comparing Briakinumab with Methotrexate in Patients with Psoriasis | Briakinumab | MTX | - | 250 (1:1) 317 (154/163) |
PASI75 | 24 | 70% (Briakinumab) vs. 50% (MTX) | 20 | 95 | 0.9 | 1 |
| NCT0045458433 | Centocor (2009) | ACCEPT | UST – 45mg & 90mg | ETA | - | 850 (3:5:5)c 903 (209/347/347) |
PASI75 | 12 | 65% (UST 90mg) vs. 50% (ETA) | 15 | 95 | >0.97 | 1 |
| 64% (UST 45mg) vs. 50% (ETA) | 14 | 95 | 0.87 | 1 | |||||||||
| NCT0071058034 | AbbVie (2013) | Study Comparing the efficacy and safety of ABT-874 to Etanercept and Placebo in subjects with Moderate to Severe Chronic Plaque Psoriasis | Briakinumab | ETA | PBO | 350 (2:2:1) 350 (139/139/72) |
PASI75 | 12 | 70% (Briakinumab) vs. 50% (ETA) vs. 4% (PBO)d | 20 | 95 | 0.9 | 1 |
| NCT0069196435 | AbbVie (2013) | Study Comparing the efficacy and safety of ABT-874 to Etanercept and Placebo in subjects with Moderate to Severe Chronic Plaque Psoriasis | Briakinumab | ETA | PBO | 350 (2:2:1) 347 (138/141/68) |
PASI75 | 12 | 70% (Briakinumab) vs. 50% (ETA) vs. 4% (PBO)d | 20 | 95 | 0.9 | 1 |
| NCT0205448116 | Boehringer Ingelheim (2016) | BI 655066 Dose Ranging in Psoriasis, Active Comparator Ustekinumab | BI655066 (RIS) – 18mg & 90mg & 180mg | UST | - | 160 (2 pooled:1) 166 (43/41/42/40) |
PASI90 | 12 | 70% (pooled 90mg and 180mg RIS) vs. 45% (UST) | 25 | 90 | >0.8 | 1 |
| NCT0172975429 | Merck (2019) | reSURFACE2 | Tildrakizumab – 200mg & 100mg | ETA | PBO | 1050 (2:2:1:2) | PASI75 | 12 | 73% (TIL) vs. 56% (ETA) | 17 | 95 | >0.98 | 1 |
| Table1B. Superiority Trials with incomplete power analyses data. | |||||||||||||
| NCT0170860336 | Amgen (2014) |
AMAGINE-2 | BRO - 140mg & 210mg | UST | PBO | 1800 (2:2:1:1) 1831 (612/610/300/309) |
PASI100 | 12 |
26% BRO 140mg (p=.08)
& 44% BRO 210mg (p<.001) vs. 22% UST |
NR | 95 | ≥0.9 | 1 |
| NCT0170862936 | AMAGINE-3 | 1800 (2:2:1:1) 1881 (624/629/313/315) |
PASI100 | 12 |
27% BRO 140mg (p=.007)
& 37% BRO 210mg (p<.001) vs. 19% UST |
NR | 95 | ≥0.9 | 1 | ||||
| NCT0207498237 | Novartis (2017) |
CLEAR | AIN457 (SEC) | UST | - | 640 (1:1) 676 (338/338) |
PASI90 | 16 | 71% (SEC) vs. 51% (UST) |
20 | NR | >0.99 | 1 |
| NCT0220723138 | Janssen (2020) |
VOYAGE 1 | Guselkumab 100mg | ADA | PBO | 750
(2:2:1) 837 |
PASI90 | 24 | 73.3% (Guselkumab) vs. 49.7% (ADA) vs. 2.9% (PBO)e | NR | 95 | NR | 1 |
| NCT0220724439 | Janssen (2020) |
VOYAGE 2 | Guselkumab 100mg | ADA | PBO | 992 (2:1:1) | PASI90 | 24 | 70.0% (Guselkumab) vs. 46.8% (ADA) vs. 2.4% (PBO)e | NR | 95 | NR | UA |
| Table1C. Unpublished Superiority Trials | |||||||||||||
| NCT0282660340 | Novartis (2018) |
CLARITY | SEC | UST | - | 1109 | PASI90 | 12 | UA | UA | UA | UA | UA |
| NCT0316825641 | Can-Fite BioPharma (2019) | CF101 Therapy in Patients With Moderate-to-severe Plaque Psoriasis | CF101 – 2mg & 3mg | Apremilast | PBO | 407 (3:3:3:2) | PASI75 | 16 | UA | UA | UA | UA | UA |
The italicized numbers are the observed data, instead of the expected response rates and sample size required for power analyses decided a priori.
Abbreviations: Bi-weekly (BIW); Briakinumab (BRI) brodalumab (BRO); every 2 weeks (Q2W); every 4 weeks (Q4W); every 8 weeks (Q8W); every 12 weeks (Q12W); Adalimumab (ADA); Confidence Interval (CI); Etanercept (ETA); Infliximab (INF); methotrexate (MTX); not reported (NR); placebo (PBO); Risankizumab (RIS); Secukinumab (SEC); subcutaneous (SC); unavailable data point (UA); ustekinumab (UST).
The hypothesized true magnitude of difference between the arms (or effect size) was calculated according to reported statistical parameters from the manuscript that were decided a priori. The reported delta is based on the experimental vs. active comparator.
Using a modified rating of individual studies from the Oxford Centre for Evidence-based medicine,42 1 indicates a properly powered and conducted randomized clinical trial.
Power to detect treatment difference at an alpha level of 0.05 between 90mg UST and ETA was 0.97 and between 45mg and ETA was 0.87.
These reported power calculations were based on PGA 0/1 (co-primary endpoint), not PASI75.
Power calculations based on experimental vs. placebo had >0.99 power. Secondary statistical analyses included the experimental vs. active control.
Table 4.
Head-to-head research trials that could not be classified as any of the aforementioned three statistical designs.
| Source | Sponsor/Author (Completion Year) | Title | Study Arms | Sample Size (Experimental/Active Comparator/PBO) A Priori/Observed |
Primary Outcome | Week Measured | Response Rate of Primary Outcome | CI (%) | Delta / Margin | Study Power | Study Qualitya |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Table 4A. Published | |||||||||||
| N/A | Flystrom et al17 (2005) |
Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial | MTX vs. Ciclosporin | 70 (35/35) | mean change in PASI | 12 | 58% (MTX) vs. 72% (ciclosporin) | 95 | NR | 0.9 | 1 |
| NCT0025164158 | Merck (2008) |
RESTORE1 | INF vs. MTX |
868 (653/215) 800 (3:1) |
PASI75 | 16 | 78% (INF) vs. 42% (MTX) | 95 | NR | NR | 1 |
| N/A | Noor et al18 (2011) |
Efficacy and safety of MTX vs. acitretin in chronic plaque psoriasis | MTX vs. Acetretin |
142 (71/71) | PASI75 | 24 | 53.5% (MTX) vs 25.3% (Acetretin) | NR | NR | NR | 1 |
| NTR155919 | De Vries et al (2012) |
PIECE | INF vs. ETA |
48
(25/23) 120 |
PASI75 | 12 | 75% (INF) vs 50% (ETA) | 95 | NR | 0.8 | 1 |
| Table 4B. Unpublished | |||||||||||
| NCT0169029959 | Celgene (Apr 2016) |
LIBERATEb | Apremilast (30mg BID) vs. ETA (50mg SC weekly) vs. PBO | 250 (1:1:1) | PASI75 & PASI 50 | 16 | UA | UA | UA | UA | UA |
| NCT0247408260 | Novartis (June 2016) |
PRIME | SEC vs. Fumaric Acid | 200 | PASI75 | 24 | UA | UA | UA | UA | UA |
| NCT0248922761 | Coherus Biosciences (Mar 2017) |
Comparison of CHS-1420 Versus Humira in Subjects With Chronic Plaque Psoriasis | CHS-1420 (ADA biosimilar) vs. ADA (HUMIRA®) | 545 | PASI75 | 12 | UA | UA | UA | UA | UA |
| NCT0258134562 | Momenta (Apr 2017) |
Phase 3 Study of M923 and Humira® in Subjects With Chronic Plaque-type Psoriasis | M923 vs. ADA (HUMIRA®) vs. M923 & ADA | 827 | PASI75 | 16 | UA | UA | UA | UA | UA |
| NCT0268437063 | AbbVie (Aug 2017) |
BI 655066 (RIS) Compared to Placebo and Active Comparator (UST) in Patients With Moderate to Severe Chronic Plaque Psoriasis | ABBV-066/BI 655066 (RIS) vs. UST vs. PBO | 500 | PASI90 | 16 | UA | UA | UA | UA | UA |
| NCT0269452364 | AbbVie (Aug 2017) |
BI 655066/ABBV-066 (RIS) Compared to Active Comparator (ADA) in Patients With Moderate to Severe Chronic Plaque Psoriasis | ABBV-066/BI 655066 (RIS) vs. ADA vs. PBO | 605 | PASI90 | 16 | UA | UA | UA | UA | UA |
| NCT0268435765 | AbbVie (Sept 2017) |
BI 655066 Compared to PBO & Active Comparator (UST) in Patients With Moderate to Severe Chronic Plaque Psoriasis | ABBV-066/BI 655066 (RIS) vs. UST vs. PBO | 500 | PASI90 | 16 | UA | UA | UA | UA | UA |
| NCT0263480166 | Eli Lilly (Nov 2017) | A Study of Ixekizumab (LY2439821) in Participants With Moderate-to-Severe Plaque Psoriasis Naive to Systemic Treatment | Izekizumab vs. Fumaric Acid vs. MTX | 162 | PASI75 | 24 | UA | UA | UA | UA | UA |
| NCT0285096567 | Boehringer Ingelheim (Dec 2017) |
Efficacy, Safety and Immunogenicity of BI 695501 Versus Humira® in Patients With Moderate to Severe Chronic Plaque Psoriasis | BI 695501 (ADA Biosimilar) vs. ADA (HUMIRA®) | 318 | PASI75 | 16 | UA | UA | UA | UA | UA |
| NCT0295153368 | Janssen-Cilag (June 2018) |
POLARIS | Guselkumab vs. Fumaric Acid Esters | 119 | PASI90 | 24 | UA | UA | UA | UA | UA |
| NCT0325538269 | AbbVie (Jul 2018) |
A Study to Assess the Efficacy of RIS Compared to FUMADERM® in Subjects With Moderate to Severe Plaque Psoriasis Who Are Naïve to and Candidates for Systemic Therapy | RIS vs. Fumaderm | 110 | PASI90 | 24 | UA | UA | UA | UA | UA |
| NCT0234624070 | UCB Biopharma (Dec 2018) |
CIMPACT | Certolizumab Pegol 200mg vs. Certolizumab Pegol 400mg vs. ETA vs. PBO | 559 | PASI75 | 12 | UA | UA | UA | UA | UA |
| NCT0266058071 | EMD Serono (Aug 2020) |
MSB11022 in Moderate to Severe Chronic Plaque Psoriasis | MSB11022 (ADA Biosimilar) vs. ADA (HUMIRA®) | 458 | PASI75 | 16 | UA | UA | UA | UA | UA |
| NCT0321943772 | AbbVie (Sept 2021) |
A Study Comparing the Safety and Efficacy of RIS to Methotrexate in Subjects With Moderate to Severe Plaque Psoriasis | RIS vs. MTX | 100 | PASI90 | 28 | UA | UA | UA | UA | UA |
| Table 4C. Unpublished: Study Terminated | |||||||||||
| NCT0190078273 | Novartis (2013) | Efficacy and Safety Study of Subcutaneous SEC in Treatment of Subjects With Moderate to Severe Chronic Plaque-type Psoriasis as Compared to ETA and PBO | SEC vs. ETA vs. PBO | 0 | PASI | 12 | UA | UA | UA | UA | UA |
| NCT0278673274 | MedDerm Associates (2017) |
Phase 3 Study to Evaluate the Efficacy and Safety of Induction and Maintenance Regimens of Brodalumab Compared With PBO and UST in Subjects With Moderate to Severe Plaque Psoriasis | Brodalumab 210mg vs. Brodalumab 140mg vs. PBO vs. UST | 15 | PASI | 12 | UA | UA | UA | UA | UA |
| NCT0193668875 | Merck (2018) |
A Study to Evaluate the Efficacy and Safety/Tolerability of Subcutaneous MK-3222 in Participants With Moderate-to-Severe Chronic Plaque Psoriasis (MK-3222–012) | MK-3222 200mg vs. MK-3222 100mg vs. ETA 50mg vs. PBO | 0 | PASI75 | 12 | UA | UA | UA | UA | UA |
The italicized numbers are the observed data, instead of the expected response rates and sample size required for power analyses decided a priori.
Abbreviations: Bi-weekly (BIW); every 2 weeks (Q2W); every 4 weeks (Q4W); every 6 weeks (Q6W); every 8 weeks (Q8W); every 12 weeks (Q12W); Once daily (OD); Adalimumab (ADA); Etanercept (ETA); Infliximab (INF); methotrexate (MTX); Not reported (NR); placebo (PBO); Risankizumab (RIS); Secukinumab (SEC); subcutaneous (SC); unavailable data point (UA); ustekinumab (UST).
Using a modified rating of individual studies from the Oxford Centre for Evidence-based medicine,42 1 indicates a properly powered and conducted randomized clinical trial.
Study was not powered to compare experimental vs. active comparator. Power analyses were reported for the experimental treatment vs. placebo only
Risk of bias
Often, systematic reviews include an assessment of the risk of bias which involves rating each RCT on several domains such as randomization, blinding, and missing data.14,15 However, a formal risk of bias assessment was not applicable to our study outcome as we focused on specific details in statistical analyses and publication.
Results
We identified 49 psoriasis RCTs for oral and biologic therapies with active comparators. Most were phase 3 trials; however, there was one phase 2 trial16 and four RCTs17–20 that did not specify the trial phase. Of the 49 ACTs, there were 13 superiority studies (Table 1), 8 non-inferiority studies (Table 2), 7 equivalence trials (Table 3), and 21 were “other head-to-head” comparisons (Table 4) that could not be classified into one of these 3 categories based on our methodology. The “other head-to-head” studies did not use appropriate wording, or statistical parameters necessary to ascertain the a priori design of the trial. We were also unable to obtain this information after requests to the study sponsors and corresponding authors.
Table 2.
Characteristics of the Non-Inferiority Randomized, Controlled Trials with an Active Comparator.
| Source | Sponsor (Completion Year) | Title | Experimental | Active Comparator | PBO | Sample Size (Experiment/Comparator/PBO) A priori/Observed | Primary Outcome | Week Measured | Response Rate A priori/Observed |
CI (%)a | Margin (%) | Power | Reported Analyses | Margin Justified | Study Qualityb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Table2A. Non-inferiority Trials with complete power analyses data. | |||||||||||||||
| NCT00235820 43,44 | Abbott (2008) |
CHAMPION | ADA | MTX | PBO | 250 (2:2:1) 271 (108/110/53) |
PASI75 | 16 | 62% ADA vs. 60% MTX | 95 | −20 | 0.80 | ITT, PP | No | 1 |
| NCT0135857845 | Novartis (2013) |
FIXTUREc | SEC (AIN457) – 150mg & 300mg | ETA | PBO | 1264 (1:1:1:1) 1305 (327/323/323/324) |
PASI75 | 12 | 55% SEC vs. 50% ETA | 98.75 | −10 | 0.90 | ITT | Yes, prior studies | 1 |
| NCT0124159146 | Pfizer (2015) |
Tofacitinib vs. ETA or PBO | Tofacitinib – 5mg & 10mg | ETA | PBO | 1101 (329/330/335/107) | PASI75 | 12 | 39.5% 5mg vs. 63.6% 10mg vs. 58.8% ETA vs. 5.6% PBO | 95 | −15 | 0.95 | ITT | No | 1 |
| NCT0172693347 | Almirall (2015) |
BRIDGE | LAS41008 (DMF) | Fumaderm® (LASW1835) | PBO | 690 (2:2:1) 671 (267/273/131) |
PASI75 | 16 | 50% DMF vs. 35% Fumaderm® | 95 | −15 | 0.9 | ITT, PP | No | 1 |
| NCT0256180648 | Eli Lilly and Co. (2017) |
IXORA-Sd | Izekizumab | UST | - | 300 (1:1) 302 (166/136) |
PASI90 | 12 | 70% IXE vs. 43% UST | 95 | −12.6 | >0.95 | ITT | Yes, “in-house data” | 1 |
| Table2B. Non-inferiority Trials with incomplete power analyses data. | |||||||||||||||
| NCT0159724549 | Eli Lilly and Co. (2019) |
UNCOVER-2e | Ixekizumab – Q2W & Q4W | ETA | PBO | 1225 (2:2:2:1) 1224 (351/347/358/168) |
PASI75 | 12 | 89.7% IXE Q2W vs. 77.5% IXE Q4W vs. 41.6% ETA vs. 2.4% PBO | 97.5 | −12 | NR | ITT | No | UA |
| NCT0164617749 | Eli Lilly and Co. (2019) |
UNCOVER-3e | Ixekizumab | ETA | PBO | 1225 (2:2:2:1) 1346 (385/386/382/193) |
PASI75 | 12 | 87.3% IXE Q2W vs. 84.2% IXE Q4W vs. 53.4% ETA vs. 7.3% PBO | 97.5 | −12 | NR | ITT | No | UA |
| Table2C. Unpublished Non-Inferiority Trial | |||||||||||||||
| NCT0309010050 | Janssen (2018) |
ECLIPSE | Guselkumab & matched-PBO | SEC | - | 1048 | PASI90 | 48 | UA | UA | −10 | UA | UA | No | UA |
The italicized numbers are the observed data, instead of the expected response rates and sample size required for power analyses decided a priori.
Abbreviations: Bi-daily (BID); Bi-weekly (BIW); every 2 weeks (Q2W); every 4 weeks (Q4W); every 6 weeks (Q6W); Once daily (OD); Adalimumab (ADA); Confidence Interval (CI); Etanercept (ETA); Intention-To-Treat (ITT); Ixekizumab (IXE); Methotrexate (MTX); not reported (NR); Per-Protocol (PP); placebo (PBO); Secukinumab (SEC); subcutaneous (SC); unavailable data point (UA); ustekinumab (UST).
Although in non-inferiority trials, only one boundary (one-sided CI) of the classical two-sided CI is used for analysis.
Using a modified rating of individual studies from the Oxford Centre for Evidence-based medicine,42 1 indicates a properly powered and conducted randomized clinical trial.
A closed-testing procedure used to evaluate different hypotheses. The non-inferiority testing was followed by superiority testing between experimental and placebo.
Firstly, evaluated non-inferiority and secondly evaluated superiority of ixekizumab to ustekinumab, as measured by the proportion of patients achieving a PASI 90 response at week 12
Study power was not identified for non-inferiority, only for superiority as >0.93 and therefore, may result in an underpowered study for testing non-inferiority. This study was classified as non-inferiority because the non-inferiority hypothesis was required to be satisfied prior to superiority testing.
Table 3.
Characteristics of the Equivalence Randomized, Controlled Trials with an Active Comparator (none had placebo control).
| Source | Title | Author/Sponsor (Completion Year) | Experimental | Active Comparator | Sample Size (Experiment/Comparator/PBO) A priori/Observed | Primary Outcome | Week Measured | Response Rate A Priori/Observed |
CI (%) | Margin | Power | Reported Analyses | Margin Justified | Study Qualitya |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Table 3A. Equivalence Trials with complete power analyses data. | ||||||||||||||
| NTR74320 | Fumarates vs. MTX in moderate to severe chronic plaque psoriasis | Fallah Arani et al (2009) |
MTX | Fumarate | 50 (1:1) 60 (30/30) |
Mean change from baseline PASI | 12 |
7.8 decrease
(MTX) vs. 7.6 decrease (Fumarate) |
95 | ±5 points in mean PASI | 0.90 | ITT | No | 1 |
| NCT0189186451 | EGALITY – Study 302 | Sandoz (2015) |
GP2015 | ETA (ENBREL®) | 546 (1:1) 531 (264/267) |
PASI75 | 12 | 73.4% (GP2015) vs. 75.7% (ETA) | 95 | ±18% | 0.90 | ITT, PP | Yes, prior studies | 1 |
| NCT0197048852 | Study to Compare Efficacy and Safety of ABP 501 and ADA (HUMIRA®) | Amgen (2016) |
ADA-atto (Amjevita) | ADA (HUMIRA®) | 340 (1:1) 350 (175/175) |
% Improvement in PASI from Baseline | 16 | 80.9% (ABP501) vs. 83.1% (ADA) | 95 | ±15% | 0.90 | ITT | No | 1 |
| NCT0276295553 | CALYPSO | Biocad (2016) |
BCD-057 | ADA (HUMIRA®) | 344 (1:1) | PASI75 | 16 | 71% (BCD-057) vs. 71% (ADA) | 95 | ±14% | 0.80 | NR | NR | 1 |
| Table 3B. Equivalence Trials with incomplete power analyses data. | ||||||||||||||
| NCT0213421054,55 | Comparison of CHS-0214 to Enbrel (ETA) in Patients With Chronic Plaque Psoriasis (PsO) | Coherus
Biosciences (2016) |
CHS-0214 | ETA (ENBREL®) | 521 (1:1) 456 (228/228) |
PASI75b | 12 | 64.5% (CHS-0214) vs. 62.3% (ETA) | 95 | ±18% | NR | NR | NR | UA |
| Mean % change in PASIb | 12 | 76.7% (CHS-0214) vs. 73.4% (ETA) | 95 | ±12.5% | NR | NR | NR | UA | ||||||
| NCT0201610556 | ADACCESS | Sandoz (2016) |
GP2017 | ADA (HUMIRA®) | 421 (231/190) | PASI75b | 16 | 67.0% (GP2017) vs 62.4% (ADA) | 90 | ±18% | NR | NR | NR | UA |
| Table3C. Unpublished Equivalence Trial | ||||||||||||||
| NCT0271432257 | MYL-1401A Efficacy and Safety Comparability Study to Humira® | Mylan (2017) | MYL-1401A | ADA (HUMIRA®) | 294 (2:1) | PASI % change from Baselinec | 12 | UA | UA | UA | UA | UA | UA | UA |
The italicized numbers are the observed data, instead of the expected response rates and sample size required for power analyses decided a priori.
Abbreviations: Bi-weekly (BIW); every 2 weeks (Q2W); every 4 weeks (Q4W); every 6 weeks (Q6W); Once daily (OD); Adalimumab (ADA); Confidence Interval (CI); Etanercept (ETA); methotrexate (MTX); not reported (NR); placebo (PBO); subcutaneous (SC); unavailable data point (UA).
Using a modified rating of individual studies from the Oxford Centre for Evidence-based medicine,42 1 indicates a properly powered and conducted randomized clinical trial.
The secondary endpoint was percent change in PASI from baseline at week 16, with 90% CI and ±15% margins.
Primary outcome previously PASI75 at Week 16
Of particular interest is the ambiguous reporting of margins or delta, as this directly impacts the interpretation of results. Of the 49 studies, 1 of 13 (0.08%), 8 of 8 (100%), and 6 of 7 (85.17%) of the superiority, NI, and equivalence trials, respectively, reported the margin explicitly (i.e., the reader did not have to perform power analyses or calculations to derive missing information). However, in 5 of the superiority trials, we were able to manually calculate the delta (otherwise known as the effect size or acceptable treatment difference).
Our findings indicate that the acceptable difference between treatment arms range from 14% to 20% for superiority trials for PASI75, PASI90, or PASI100 endpoints. The NI studies reported lower bound margins ranging from −20% to −10% for PASI75 or PASI90 endpoints. The equivalence trials reported upper and lower margins ranging from ±12.5% to ±18% for PASI75 or PASI change (mean or percent) from baseline endpoints. Of which, one equivalence RCT reported ±5 points in the mean PASI score. None of the “other head-to-head” trials reported margins or acceptable differences between treatment arms.
Discussion
This systematic review demonstrated considerable heterogeneity in the conduct of head-to-head dermatology trials, reflective of underlying variations in protocol parameters. Although several regulatory bodies have provided lengthy guidance documents for head-to-head trial design21–23 and reporting,24,25 our results revealed discrepancies in the conduct and reporting of ACTs in dermatology. Regarding study conduct, there was a diverse range of acceptable treatment differences and inconsistency in the choice of statistical analyses. Inconsistency in the choice of statistical analyses is not problematic per se, as this should be dictated by the primary study question (i.e., establishing non-inferiority, equivalence, or superiority). More problematic, however, is variation in clinical trials reporting. For example, there was a high proportion of studies that omitted data, had ambiguity in power analyses, and violated guidelines for good reporting.24 Indeed, only one of the 8 NI trials successfully indicated non-inferiority study design in the title of the manuscript – the first component of the CONSORT guidelines. Our findings indicate a lack of standardization in study design and reporting, which is critical to allow for appropriate comparison and interpretation of results. Clinical Trials study design and reporting are fundamental because even the slightest change in any study parameters could result in entirely different study conclusions and determination of treatment efficacy.
Studies used language implying either superiority (such as “superior to”), non-inferiority or equivalence RCT design but had much variation in the margins and choice of analyses. Designing ACTs requires a delicate balance of utilizing parameters from current literature and expertise from clinical and research specialists to determine clinically acceptable measures, otherwise known as the margin of error or delta in clinical trials. Our review found a wide range of acceptable treatment differences in superiority, non-inferiority, and equivalence trials (range: 14 to 20%, −20% to −10%, and ±12.5% to ±18%, respectively). Interpretation and application of the margin or delta influences clinical decision making as healthcare providers and patients may differ in their opinions of the maximum acceptable difference between interventions. For example, if the true PASI90 response rates were 60% for an active control and 50% for an investigative treatment, this would be considered as sufficiently similar responses in an equivalence study with ±10% margins. Although one healthcare professional may consider this to be sufficiently “equal” and comfortably prescribe the investigative drug as a replacement, another physician might require more conservative margins.
Readers should also be made aware that the choice of statistical analyses can bias study conclusions. For superiority trials, the intention-to-treat analyses imply a conservative effect on the outcome of the trial. However, for non-inferiority and equivalence trials, ITT analyses do not have the same conservative effect, and it is unclear if per protocol analyses have a conservative effect. Therefore, it is recommended to conduct both intention-to-treat (full analysis set) and “as treated” (per protocol) analyses to support research findings.26 Without both analyses supporting study conclusions in NI and equivalence trials, readers should be cautious of the comparative efficacy conclusions. Only 28.57% (2/7) of the completed NI dermatology trials performed both intention-to-treat and per protocol analyses, which is comparatively worse than another systematic review inclusive of other fields of medicine which found 44% of NI trials conducting both analyses.27 Of the three full-text manuscripts for equivalence trials, only 1 performed both ITT and per protocol analyses. This demonstrates a lack of handling of missing data using more modern and statistically valid approaches.
In addition to the above, details of power analyses and margin rationale were omitted and inconsistent,25 further complicating interpretation of study findings. The International Committee of Medical Journal Editors states that statistical methods should be described with “enough detail to enable a knowledgeable leader with access to the original data to verify the reported results.”28 However, only 7 of the 13 superiority trials reported all of the necessary information to calculate the hypothesized difference between treatments. Of these, only one trial explicitly stated the difference of treatment effect29 whereas we, the authors, used the reported statistical parameters to find this “acceptable difference” for the other trials. In our review, the non-inferiority margin was explicitly stated in all of the trials (8/8, 100%) which is similar to previous findings from Rehal et al.30 reporting 98% (164/168) margin specification. 6 of 7 equivalent studies (85.71%) reported the margins, which is comparable to another study quoting 36 of 42 (86.00%).27 Critically, there was a lack of transparency as we could not make definitive conclusions about 21 trials (classified as “other head-to-head comparisons”) due to missing data. Although a high proportion of these is ongoing or unpublished, the lack of transparency should not be minimized as these parameters are typically decided a priori.
Margin rationale can involve utilizing historical data and clinical judgment as recommended by the International Conference on Harmonisation.23 However, our findings for psoriasis ACT margin justification are comparatively worse than other fields of medicine. For example, a recent systematic review of NI trials indicated that less than half of the publications reported clinical or statistical justification for the margin of error.30 In our review, 2 of the 8 (25.0%) NI trials and only one of the equivalence trials (1/7, 14.29%) presented reason for margin selection as opposed to 48% (80/162) and 50% (21/42), respectively.27 Our findings are similar to other studies that reviewed margin specification but in other aspects lacked compliance with study conduct and methodology reporting.
As with all studies, our systematic review has limitations. Our results should be approached with caution as there were relatively fewer studies to derive conclusions from (as compared to other fields of medicine). Furthermore, restricting medications to only oral and biologic therapies may have limited our study findings. Lastly, we did not conduct a formal meta-estimate. Typically, meta-analyses utilize observed response rates to estimate the effect of treatment size. However, in this case, it would be inappropriate to conduct a formal meta-analysis based on arbitrarily determined numbers (by clinical trialists) and pooling such findings may not lead to more certain outcomes.31 Finally, we were unable to obtain information as described above despite repeated efforts to contact sponsors.
Our findings show incongruity in ACT conduct and reporting which is required for proper evaluation of research findings. Clinicians should be made aware of the different types of study design and of varying acceptable treatment differences, from which conclusions are derived. Readers should be wary of studies that do not report necessary data to reproduce information that is required to determine acceptable differences. Though treatment differences utilized in clinical trials depends upon clinical expertise, there is currently no clear consensus of acceptable treatment differences for ACTs in psoriasis. The lack of agreement makes it difficult to interpret and apply ACT results. We recommend publishing an international consensus statement for various dermatological comparative study designs that include multiple-arm clinical trials (i.e., experimental, active control, and placebo) and suggestions of clinically acceptable differences between treatment options. In the meantime, we strongly urge authors to explicitly state study parameters and communicate consistently per the CONSORT statement for RCT reporting. Lastly, we suggest that sponsors may want to participate by increasing transparency and openly reporting study parameters that are decided a priori.
Conclusion
Considering the impact of these trials on evidence-based medicine, it is imperative for clinicians to understand the variety of statistical measures to interpret research findings appropriately. Treatment differences that appear to be acceptable to clinical trials range from 14% to 20% for superiority, −20% to −12% for non-inferiority and ±12.5% to ±18% for equivalence active comparator trials. The results demonstrate the need for standardized ACT design and clarity in reporting clinical research findings. A better understanding of identifying acceptable margins in the field of dermatology may help future comparison study design.
Supplementary Material
Figure 1c.
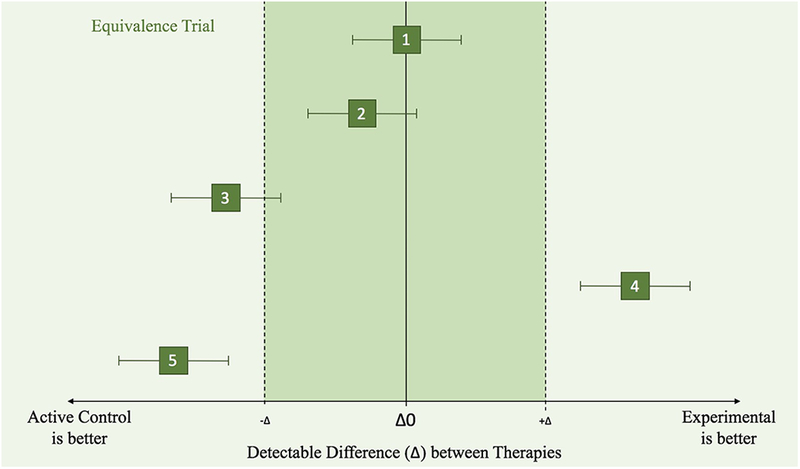
Equivalence Clinical Trial Principles illustrated by different scenarios (1–5), where the effects of an active control and experimental treatment are within the confidence intervals (along the x-axis).
1) Experimental is equivalent to active control because it is within both the upper and lower margins.
2) Experimental is equivalent to active control because it is within both the upper and lower margins.
3) Inconclusive because the CI has values inside and outside the equivalence range.
4) Experimental is not equivalent to active control. It is superior to active control.
5) Experimental is not equivalent to the new treatment and is worse than the active control.
Figure 2.
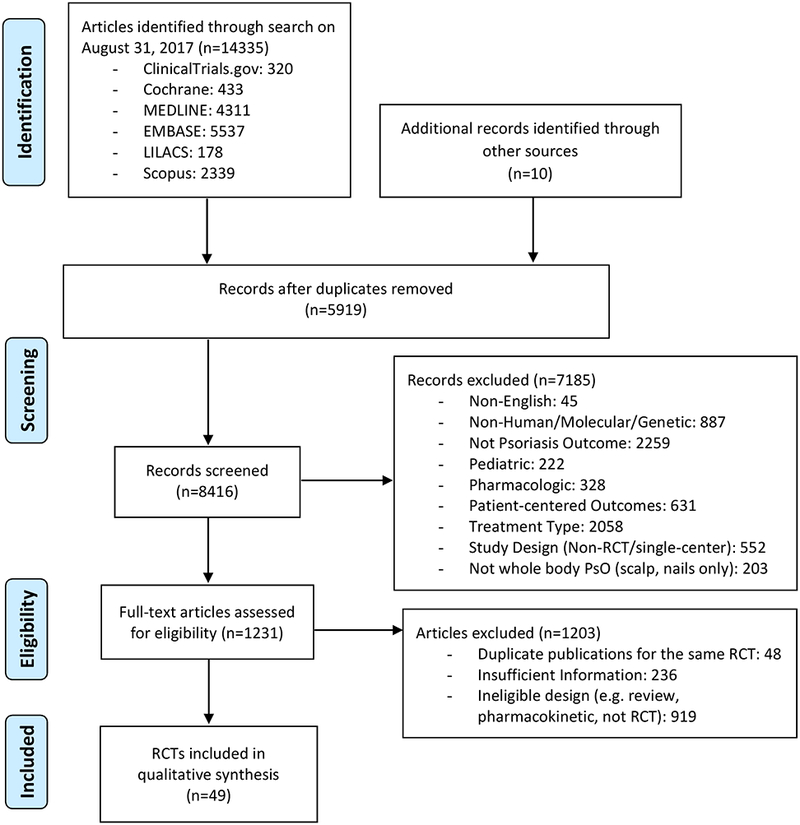
Evidence Search and Selection.
Acknowledgement
We are indebted to Maylene Qiu from the Biomedical Sciences Library at the University of Pennsylvania for her expert advice in systematic review methodology.
Funding/Support: This study was supported in part by a grant (K24 AR064310) from NIH/NIAMS (Gelfand) and a medical dermatology fellowship from the National Psoriasis Foundation (Wan).
Financial Disclosure:
Dr. Wu is an investigator for AbbVie, Amgen, Eli Lilly, Janssen, Novartis, and Regeneron.
Dr. Gelfand served as a consultant for BMS, Boehringer Ingelheim, GSK, Janssen Biologics, Menlo Therapeutics, Novartis Corp, Regeneron, Dr Reddy’s labs, Sanofi and Pfizer Inc., receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Janssen, Novartis Corp, Sanofi, Celgene, Ortho Dermatologics, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Lilly and Ortho Dermatologics. Dr. Gelfand is a co-patent holder of resiquimod for treatment of cutaneous T cell lymphoma. Dr Gelfand is a Deputy Editor for the Journal of Investigative Dermatology receiving honoraria from the Society for Investigative Dermatology.
We confirm that this manuscript has not been published elsewhere and is not under consideration by another journal. This study does not require IRB review.
References
- 1.Castro M. Placebo versus best-available-therapy control group in clinical trials for pharmacologic therapies: which is better? Proc Am Thorac Soc. 2007;4(7):570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halpern SD, Ubel PA, Berlin JA, Townsend RR, Asch DA. Physicians’ preferences for active-controlled versus placebo-controlled trials of new antihypertensive drugs. J Gen Intern Med. 2002;17(9):689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff (Millwood). 2013;32(6):1116–1125. [DOI] [PubMed] [Google Scholar]
- 4.Matosin N, Frank E, Engel M, Lum JS, Newell KA. Negativity towards negative results: a discussion of the disconnect between scientific worth and scientific culture. Dis Model Mech. 2014;7(2):171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naldi L, Svensson A, Diepgen T, et al. Randomized clinical trials for psoriasis 1977–2000: the EDEN survey. J Invest Dermatol. 2003;120(5):738–741. [DOI] [PubMed] [Google Scholar]
- 6.Chalmers RJ, Griffiths CE. Resetting the research agenda for psoriasis. J Invest Dermatol. 2003;120(5):ix–x. [DOI] [PubMed] [Google Scholar]
- 7.Lesaffre E. Use and misuse of the p-value. Bull NYU Hosp Jt Dis. 2008;66(2):146–149. [PubMed] [Google Scholar]
- 8.Christensen E. Methodology of superiority vs. equivalence trials and non-inferiority trials. J Hepatol. 2007;46(5):947–954. [DOI] [PubMed] [Google Scholar]
- 9.Mansley EC, Elbasha EH, Teutsch SM, Berger ML. The decision to conduct a head-to-head comparative trial: a game-theoretic analysis. Med Decis Making. 2007;27(4):364–379. [DOI] [PubMed] [Google Scholar]
- 10.Wan MT, Strober BE, Wu JJ, Shin DB, Gelfand JM. How similar are the treatment responses to biosimilars in patients with psoriasis? A systematic review of statistical margins in comparative clinical trials. J Am Acad Dermatol. 2017;77(3):569–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S, Wiley InterScience (Online service). Cochrane handbook for systematic reviews of interventions Cochrane book series. Chichester, England; Hoboken, NJ: Wiley-Blackwell,; 2008: http://hdl.library.upenn.edu/1017.12/1339079 Connect to full text. [Google Scholar]
- 12.American Academy of Dermatology Work Group, Menter A, Korman NJ, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65(1):137–174. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 15.Methods CB. Assessing Risk of Bias in Included Studies. http://methods.cochrane.org/bias/assessing-risk-bias-included-studies. Accessed January 24, 2018.
- 16.Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis. N Engl J Med. 2017;376(16):1551–1560. [DOI] [PubMed] [Google Scholar]
- 17.Flytstrom I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. Br J Dermatol. 2008;158(1):116–121. [DOI] [PubMed] [Google Scholar]
- 18.Noor SM, Ayub N, Paracha MM. Efficacy and safety of methotrexate versus acitretin in chronic plaque psoriasis. Journal of Postgraduate Medical Institute. 2017;31(1):4–7. [Google Scholar]
- 19.de Vries AC, Thio HB, de Kort WJ, et al. A prospective randomized controlled trial comparing infliximab and etanercept in patients with moderate-to-severe chronic plaque-type psoriasis: the Psoriasis Infliximab vs. Etanercept Comparison Evaluation (PIECE) study. Br J Dermatol. 2017;176(3):624–633. [DOI] [PubMed] [Google Scholar]
- 20.Fallah Arani S, Neumann H, Hop WC, Thio HB. Fumarates vs. methotrexate in moderate to severe chronic plaque psoriasis: a multicentre prospective randomized controlled clinical trial. Br J Dermatol. 2011;164(4):855–861. [DOI] [PubMed] [Google Scholar]
- 21.Gomberg-Maitland M, Frison L, Halperin JL. Active-control clinical trials to establish equivalence or noninferiority: methodological and statistical concepts linked to quality. Am Heart J. 2003;146(3):398–403. [DOI] [PubMed] [Google Scholar]
- 22.Draft Guidance for Industry: Non-Inferiority Clinical Trials. 2010; https://www.fda.gov/downloads/Drugs/Guidances/UCM202140.pdf. Accessed September 7, 2017.
- 23.ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials. International Conference on Harmonisation E9 Expert Working Group. Stat Med. 1999;18(15):1905–1942. [PubMed] [Google Scholar]
- 24.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG, Group C. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594–2604. [DOI] [PubMed] [Google Scholar]
- 26.Lesaffre E. Superiority, equivalence, and non-inferiority trials. Bull NYU Hosp Jt Dis. 2008;66(2):150–154. [PubMed] [Google Scholar]
- 27.Schiller P, Burchardi N, Niestroj M, Kieser M. Quality of reporting of clinical non-inferiority and equivalence randomised trials--update and extension. Trials. 2012;13:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Editors. ICoMJ. Uniform requirements for manuscripts submitted to biomedical journals: writing and editing for biomedical publication. 2018; www.icmje.org. Accessed January 25, 2018.
- 29.Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288. [DOI] [PubMed] [Google Scholar]
- 30.Rehal S, Morris TP, Fielding K, Carpenter JR, Phillips PP. Non-inferiority trials: are they inferior? A systematic review of reporting in major medical journals. BMJ Open. 2016;6(10):e012594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glass GV. Primary, Secondary, and Meta-Analysis of Research. Educational Researcher. 1976;5(10):3–8. [Google Scholar]
- 32.Reich K, Langley RG, Papp KA, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365(17):1586–1596. [DOI] [PubMed] [Google Scholar]
- 33.Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118–128. [DOI] [PubMed] [Google Scholar]
- 34.Strober BE, Crowley JJ, Yamauchi PS, Olds M, Williams DA. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165(3):661–668. [DOI] [PubMed] [Google Scholar]
- 35.Gottlieb AB, Leonardi C, Kerdel F, Mehlis S, Olds M, Williams DA. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165(3):652–660. [DOI] [PubMed] [Google Scholar]
- 36.Lebwohl M, Strober B, Menter A, et al. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N Engl J Med. 2015;373(14):1318–1328. [DOI] [PubMed] [Google Scholar]
- 37.Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409. [DOI] [PubMed] [Google Scholar]
- 38.Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparatore-controlled VOYAGE 1 trial. Journal of the American Academy of Dermatology. 2017;76(3):405–417. [DOI] [PubMed] [Google Scholar]
- 39.Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. Journal of the American Academy of Dermatology. 2017;76(3):418–431. [DOI] [PubMed] [Google Scholar]
- 40.Study of Secukinumab Compared to Ustekinumab in Subjects With Plaque Psoriasis. https://ClinicalTrials.gov/show/NCT02826603. Accessed December 1, 2017.
- 41.CF101 Therapy in Patients With Moderate-to-severe Plaque Psoriasis. https://ClinicalTrials.gov/show/NCT03168256. Accessed December 1, 2017.
- 42.Group OLoEW. The Oxford Levels of Evidence 2. https://www.cebm.net/index.aspx?o=5653. Accessed December 1, 2017.
- 43.Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158(3):558–566. [DOI] [PubMed] [Google Scholar]
- 44.Saurat JH, Langley RG, Reich K, Unnebrink K, Sasso EH, Kampman W. Relationship between methotrexate dosing and clinical response in patients with moderate to severe psoriasis: subanalysis of the CHAMPION study. Br J Dermatol. 2011;165(2):399–406. [DOI] [PubMed] [Google Scholar]
- 45.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. [DOI] [PubMed] [Google Scholar]
- 46.Bachelez H, van de Kerkhof PC, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):552–561. [DOI] [PubMed] [Google Scholar]
- 47.Mrowietz U, Szepietowski JC, Loewe R, et al. Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate-to-severe chronic plaque psoriasis: a randomized, double-blind, Fumaderm(R) - and placebo-controlled trial (BRIDGE). Br J Dermatol. 2017;176(3):615–623. [DOI] [PubMed] [Google Scholar]
- 48.Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177(4):1014–1023. [DOI] [PubMed] [Google Scholar]
- 49.Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551. [DOI] [PubMed] [Google Scholar]
- 50.A Study to Evaluate the Comparative Efficacy of CNTO 1959 (Guselkumab) and Secukinumab for the Treatment of Moderate to Severe Plaque-type Psoriasis. https://ClinicalTrials.gov/show/NCT03090100. Accessed December 1, 2017.
- 51.Griffiths CEM, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2017;176(4):928–938. [DOI] [PubMed] [Google Scholar]
- 52.Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: A randomized, double-blind, multicenter, phase III study. J Am Acad Dermatol. 2017;76(6):1093–1102. [DOI] [PubMed] [Google Scholar]
- 53.Biocad. Comparative Clinical Trial of Efficacy and Safety of BCD-057 and Humira® in Patients With Moderate to Severe Plaque Psoriasis. https://ClinicalTrials.gov/show/NCT02762955. Accessed December 1, 2017.
- 54.Leonardi C, Tang H, Kelleher C, Finck B. Evaluation of CHS-0214 as a proposed biosimilar to etanercept for the treatment of chronic plaque psoriasis: One-year results from a randomized, double-blind global trial. Journal of the American Academy of Dermatology. 2017;76(6):AB128. [Google Scholar]
- 55.Kivitz AJ, Papp K, Devani A, et al. Randomized, double-blind study comparing CHS-0214 with etanercept (ENBREL) in patients with psoriasis and psoriatic arthritis. Arthritis and Rheumatology. 2016;68:2142–2143. [Google Scholar]
- 56.Blauvelt A, Fowler J, Schuck E, Jauch J, Woehling H, Leonardi C. A randomized, double-blind, multicenter study to compare the efficacy, safety, and immunogenicity of a proposed adalimumab biosimilar (GP2017) with originator adalimumab in patients with moderate-to-severe chronic plaque-type psoriasis. Journal of the American Academy of Dermatology. 2017;76(6):AB22. [Google Scholar]
- 57.MYL-1401A Efficacy and Safety Comparability Study to Humira®. https://ClinicalTrials.gov/show/NCT02714322. Accessed December 1, 2017.
- 58.Barker J, Hoffmann M, Wozel G, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol. 2011;165(5):1109–1117. [DOI] [PubMed] [Google Scholar]
- 59.Reich K, Gooderham M, Green L, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31(3):507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Study of Secukinumab Compared to Fumaderm® in Adults With Moderate to Severe Psoriasis. https://ClinicalTrials.gov/show/NCT02474082. Accessed December 1, 2017.
- 61.Comparison of CHS-1420 Versus Humira in Subjects With Chronic Plaque Psoriasis. https://ClinicalTrials.gov/show/NCT02489227. Accessed December 1, 2017.
- 62.Phase 3 Study of M923 and Humira® in Subjects With Chronic Plaque-type Psoriasis. https://ClinicalTrials.gov/show/NCT02581345. Accessed December 1, 2017.
- 63.BI 655066 (Risankizumab) Compared to Placebo and Active Comparator (Ustekinumab) in Patients With Moderate to Severe Chronic Plaque Psoriasis. https://ClinicalTrials.gov/show/NCT02684370. Accessed December 1, 2017.
- 64.BI 655066/ABBV-066 (Risankizumab) Compared to Active Comparator (Adalimumab) in Patients With Moderate to Severe Chronic Plaque Psoriasis. https://ClinicalTrials.gov/show/NCT02694523. Accessed December 1, 2017.
- 65.BI 655066 Compared to Placebo & Active Comparator (Ustekinumab) in Patients With Moderate to Severe Chronic Plaque Psoriasis. https://ClinicalTrials.gov/show/NCT02684357. Accessed December 1, 2017.
- 66.A Study of Ixekizumab (LY2439821) in Participants With Moderate-to-Severe Plaque Psoriasis Naive to Systemic Treatment. https://ClinicalTrials.gov/show/NCT02634801. Accessed December 1, 2017.
- 67.Efficacy, Safety and Immunogenicity of BI 695501 Versus Humira® in Patients With Moderate to Severe Chronic Plaque Psoriasis. https://ClinicalTrials.gov/show/NCT02850965. Accessed December 1, 2017.
- 68.A Study to Compare the Efficacy of Guselkumab to Fumaric Acid Esters for the Treatment of Participants With Moderate to Severe Plaque Psoriasis. https://ClinicalTrials.gov/show/NCT02951533. Accessed December 1, 2017.
- 69.A Study to Assess the Efficacy of Risankizumab Compared to FUMADERM® in Subjects With Moderate to Severe Plaque Psoriasis Who Are Naïve to and Candidates for Systemic Therapy. https://ClinicalTrials.gov/show/NCT03255382. Accessed December 1, 2017.
- 70.Efficacy and Safety Study of Certolizumab Pegol (CZP) Versus Active Comparator and Placebo in Subjects With Plaque Psoriasis (PSO). https://ClinicalTrials.gov/show/NCT02346240. Accessed December 1, 2017.
- 71.MSB11022 in Moderate to Severe Chronic Plaque Psoriasis. https://ClinicalTrials.gov/show/NCT02660580. Accessed December 1, 2017.
- 72.A Study Comparing the Safety and Efficacy of Risankizumab to Methotrexate in Subjects With Moderate to Severe Plaque Psoriasis. https://ClinicalTrials.gov/show/NCT03219437. Accessed December 1, 2017.
- 73.Efficacy and Safety Study of Subcutaneous Secukinumab in Treatment of Subjects With Moderate to Severe Chronic Plaque-type Psoriasis as Compared to Etanercept and Placebo. Accessed December 1, 2017.
- 74.Study to Evaluate Broadlumab vs Placebo and Ustekinumab. https://ClinicalTrials.gov/show/NCT02786732. Accessed December 1, 2017.
- 75.A Study to Evaluate the Efficacy and Safety/Tolerability of Subcutaneous MK-3222 in Participants With Moderate-to-Severe Chronic Plaque Psoriasis (MK-3222–012). https://ClinicalTrials.gov/show/NCT01936688. Accessed December 1, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


