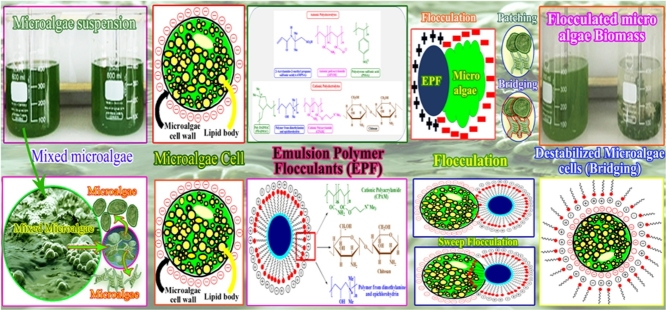
Graphical abstract
Keywords: Microalage, Flocculation, Natural biopolymers, Chemical reactions
Highlights
-
•
Mechanism involved in microalgal flocculation has been reviewed.
-
•
Commercially, bioflocculation is suitable and cost-effective.
-
•
Organic & inorganic flocculants and their features are covered.
-
•
Ideal proportion amongst flocculants and biomass decides their efficiency.
Abstract
Industrially, harvesting of the microalgal biomass is a techno-economic tailback, which essentially meant for the algal biomass industry. It is considered energy as well as cost-intensive in view of the fact that the dewatering process during harvesting. In this review chemical reactions involved in the flocculation of microalage biomass via various certain principal organic polymers are focused. Besides, it focuses on natural biopolymers as flocculants to harvest the cultivated microalgae. Commercially, bio-flocculation is suitable and cost-effective in the midst of a range of adopted harvesting techniques and the selection of an appropriate bioflocculant depends on its efficacy on the several microalgae strains like potential biomass fixation, ecological stride and non-perilous nature. The harvesting of toxin free microalgae biomass in large quantity by such flocculants can be considered to be one of the most cost-effective performances towards sustainable biomass recovery.
1. Introduction
Awareness regarding the energy demand, particularly the development of clean and sustainable fuels to replace fossil fuels in the near future, has grown worldwide [1]. Several types of biomass feedstocks have been studied to produce clean biofuel. However, the choice of food vs fuels is still a crucial concern for the production of biofuel when considering agricultural crops as the source of biofuel [2,3]. The developments of microalgal biomass harvest has been keep growing, since harvesting plays a major role in the economical point of algal biotechnology towards consideration of the mass biomass production, thus, vital for the cost-effective process construction. Many harvesting technologies, such as coagulation, flocculation, and centrifugation, have been invented; however, selecting cost-effective technology is still challenging [4,5]. Some harvesting technologies can be used only for the lab-scale production, not large-scale production, because of the operational cost. Among the harvesting technologies reported, centrifugation can yield high-value biomass without contamination, however, it is not cost-effective [6,7]. Other researchers introduced flotation, which efficiently harvests microalgal biomass by cell flotation [5]. Cell flotation and dispersed ozone flotation techniques hold promises to minimize the cost of operation associated with floatation technology [8]. Another study optimized the flotation technology by combining coagulation and flotation to obtain almost 100% biomass recovery. However, the cost of operation was still not favorable [9].
A recent study on harvesting suggested that using flocculation followed by sedimentation could significantly reduce the cost associated with simple operation [10]. Various flocculation technologies, such as chemical flocculation and bio-flocculation, have been developed and used [10]. Moreover, as the surface charge of the microalgal cells is considered to be negative, a positively charged supplier would be necessary for efficient flocculation and harvesting [11,12]. In previous studies, bio-flocculants have been used to precipitate microalgal biomass, wherein another kind of biomass was used as flocculant, but the requirement of high dosage of the biomass flocculants would limit their use in large-scale production [10]. The polymeric flocculation would be cost-effective, non-toxic and more effective, even in low dosage for harvesting microalgal biomass [13,14]. Flocculation productivity is reliant on a few factors, for example, the type of and charge polymer and in addition on the microalgae species. The microalgae species viz. Chlamydomonas, Chlorella, Scenedesmus, Schizochytrium, Muriellopsis, and Phaeodactylum have been subjected to flocculation examinations with various amounts of flocculants [13,15]. Generally utilized lab strain models for the research in algae field are Chlorella sp., Scenedesmus acuminatus and Chlamydomonas reinhardtii [16]. These methodologies go from customary flocculation strategies that are generally utilized as a part of different fields of industry. The engineered polyacrylamide polymers are normally utilized as concoctions of flocculants in other industries. These are noxious and synthetic polyacrylamide and hence likewise pollute the microalgal biomass [17]. Synthetic chemical flocculation brings about sullying of the biomass, in spite of the fact that the utilization of normal polymers may limit this issue. Flocculants in light of natural biopolymers in this way are of a more secured option, these days.
In this review, we have discussed over the bio-flocculation process along with natural biopolymers as flocculants for the harvesting of microalgae since they can induce efficient flocculation of freshwater microalgae even at low dosages. Furthermore, the chemical reactions involved in this process have been considered.
2. Polymers used in the flocculation
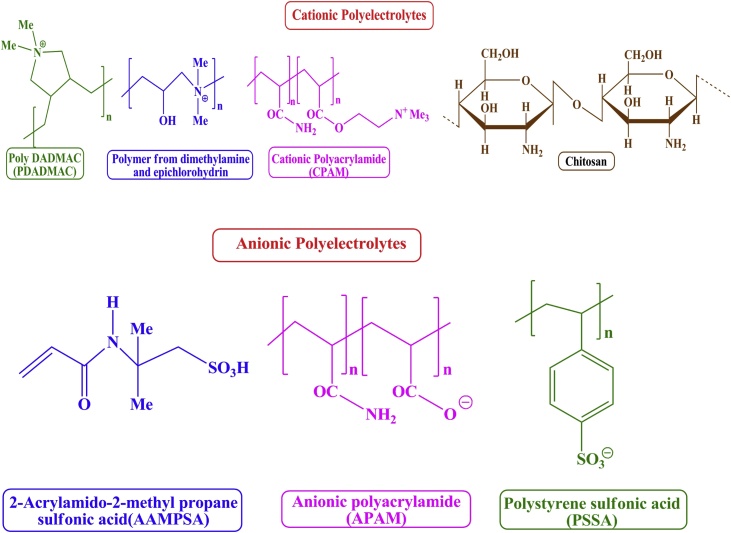
The accumulation of organic polymeric substances called bioflocculants fundamentally improve the effectiveness of the flocculation procedure for microalgal harvesting which is due to the linkages, flanked by various colloidal particles of flocs [18]. Bioflocculants are the polysaccharides which have the best features of both the natural and manufactured polymers and it is the reason behind their edge of the momentum towards the industrial research. In addition, their low carbon content combined with high flocculation have made them not only appropriate flocculants to water treatment but also to microalgal biomass harvesting [19,20]. Moreover, the biopolymers exist in the form of naturally and synthetically structurally modified moieties like carrageenans, alginates, etc., chitosan, starch, etc., and acrylamides, acrylic acids, etc., respectively [20]. These polyelectrolytes are characterized by their functionalities along their main skeleton which are cationic viz. chitosan, inulin, diallyldimethylammonium chloride (DADMAC), ethyleneimine, polyethyleneimine polymers (PED), polyethyleneimine polymers (PEI), vinyleamine, etc., anionic viz. acrylamido 2-methyl propane sulfonic acids (AAMPSA), alginates, pectin, polyacrylamides, polystyrene sulfonic acid (PSSA), etc., non-ionic viz. poly-glutamic acid (PGA) and ampholytes like amylopectin which contains both the carboxylate and amino functionalities [21,22]. Fig. 1 depicts the structure of some important cationic and anionic polymers.
Fig. 1.
The structure of some important cationic and anionic polelectrolytes.
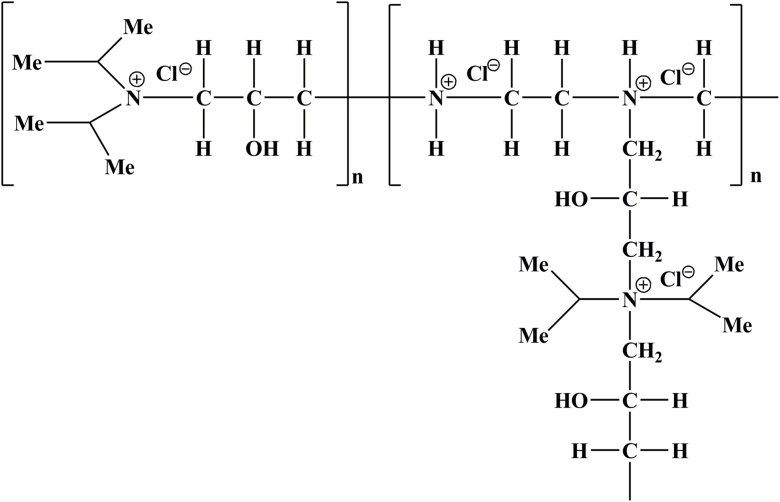
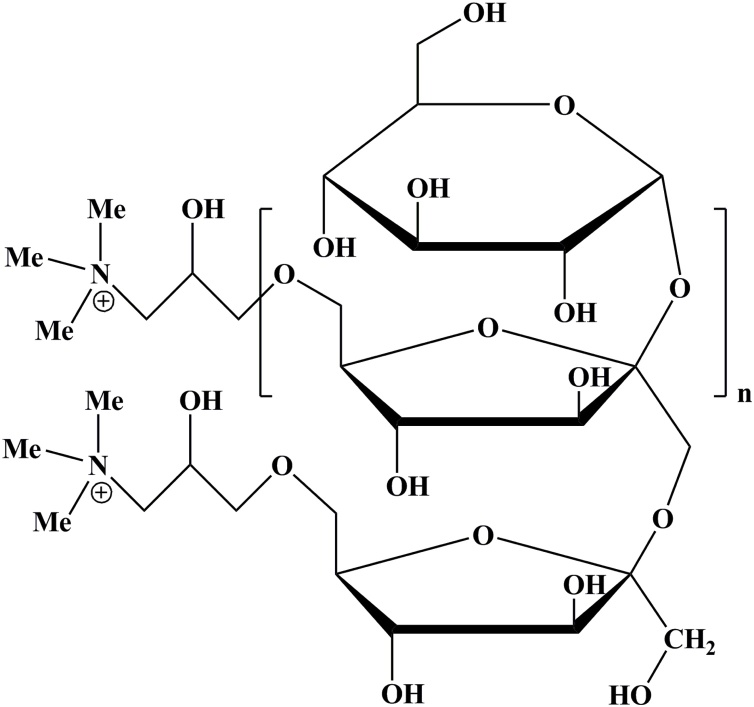
In industrial level, the biopolymer based flocculation is the promising contender. The cationic polymers are other broadly utilized and viable flocculants [[23], [24], [25]], yet are generally much more costly than inorganic flocculants like metal salts, for example, aluminum sulfate etc. Practically, the polyamine cationic bioflocculants (Fig. 2) brings promising outcomes for cost-effective harvesting of microalgal biomass for the energy generation [26]. The growth and development the microalgal species namely Chlorella sp. and Scenedesmus were promising on the reuse of this polymer flocculated wastewater. Table 1 shows applications of commercially available cationic polymers in saline and freshwater microalgae harvesting. Inulin is a natural, renewable, biodegradable and polydispersefructan (Fig. 3) [20]. It covers an extensive variety of applications along with its derivatives. Its basic structure comprises principally of regularβ-d-(2→1)-fructofuranosyl moieties and one α-d-(1→2)-glucopyranosyl unit at the end [27]. A storage polysaccharide exists in the roots and tubers of Asteraceae plants family which mainly consists Inula helenium, [28]. Over the most recent couple of decades, chitosan has risen as a positive flocculating operator in its utilization as a cationic polysaccharide in the harvesting of microalgae [29]. Analysis with other commercially oriented flocculants presents different favorable circumstances, including flocculating particles into bigger flocs [30] bringing about a quicker sedimentation rate (SR), giving a clear residual solution, subsequent to harvesting for higher flocculation productivity and being non-hazardous [29], which makes it conceivable to recycle the residual solution again to the microalgae cultivation [29,30].
Fig. 2.
Structure of polyamine cationic polymer.
Table 1.
Commercially available cationic polymers in saline and freshwater microalgae harvesting.
| Manufacturer | Commercial name |
Price (US$Kg–1) |
Technical specifications Polymer type [P]; Molecular weight [MW]; Charge [C] (Operational pH: 7–8) |
Microalgal Species |
Flocculation condition Cell density [D] g L–1 / cells mL–1 / OD750; Scale [S] mL; Flocculant dose [FD] mg L–1; Settling time [T] min | Medium Freshwater [Fresh]; Marine [Marine] |
Efficiency (%) |
|---|---|---|---|---|---|---|---|
| Allied Chemicals Ltd, UK |
Zetag 63 | NA | P: Polyacrylamide; MW: 1 × 107 |
Chlorella Stimatophora |
D: 106 mL–1; S: 1000; FD: 10; T: 30 | Fresh | 93 |
| Zetag 92 | NA | P: Polyacrylamide; MW: 2 × 107 |
Chlorella Stimatophora |
D: 106 mL–1; S: 100; FD: 10; T: 30 | Fresh | 93 | |
| BASF | Magnafloc LT225 |
NA | P: Polyacrylamide | Chlorella sp. | D: 107 mL–1; S: 100; FD: 35; T: 30 | Fresh | 72 |
|
Chlamydomonas Reinhardtii |
D: 107 mL–1; S: 100; FD: 35; T: 30 | Fresh | 95 | ||||
|
Scenedesmus Acuminatus |
D:107 mL–1; S: 100; FD: 30; T: 30 | Fresh | 95 | ||||
| Zetag 8819 | 8 | P: Polyacrylamide; C: High | Chlorella sp. | S: 1000; FD: 34; T: 60 | Fresh | 98 | |
| Zetag 8185 | 8 | P: Polyacrylamide; MW: High; C: High |
Chlorella Vulgaris |
D: 0.26; S: 3000; FD: 5; T: 30 | Fresh | 100 | |
|
Nannochloropsis Oculata |
D: 0.29; S: 3000; FD: 0.55; T: 30 | Marine | 75 | ||||
| Zetag 7570 | 8 | P: Polyacrylamide |
Nannochloropsis Salina |
S: 2000; FD: 10 | Marine | 10 | |
| Zetag 7557 | 8 | P: Polyacrylamide |
Phaeodactylum Tricornutum |
D: OD750–3.6; S: 5; FD: 0.01; T:120 | Marine | 98 | |
|
Neochloris Oleoabundans |
D: OD750–0.7; S: 5; FD: 0.01; T:120 | Marine | 52 | ||||
| Brenntag Quimica |
EM16 | 1.5–6 | P: Polyelectrolyte; MW: Medium; C: Medium |
Muriellopsis sp. | D: 2.0; S: 250; FD: 10; T: 15 | Fresh | 95 |
| EM22 | 1.5–6 | P: Polyelectrolyte; MW: Large; C: Medium |
Muriellopsis sp. | D: 2.0; S: 250; FD: 10; T: 15 | Fresh | 95 | |
|
Scenedesmus sp. |
D: 2.0; S: 250; FD: 10; T: 15 | Fresh | 95 | ||||
| Brenntag Quimica |
FB1 | 1.5–6 | P: Polyelectrolyte; MW: High; C: Medium |
Muriellopsis sp. | D: 2.0; S: 250; FD: 12; T: 15 | Fresh | 95 |
|
Chlorella Vulgaris |
D: 2.0; S: 250; FD: 3; T: 15 | Fresh | 95 | ||||
|
Scenedesmus Subspicatus |
D: 2.0; S: 250; FD: 2; T: 15 | Fresh | 98 | ||||
| EM1 | 1.5–6 | P: Polyelectrolyte; MW: High; C: High |
Muriellopsis sp. | D: 2.0; S: 250; FD: 15; T: 15 | Fresh | 95 | |
| Chlorella fusca | D: 2.0; S: 250; FD: 8; T: 15 | Fresh | 89 | ||||
| DOW Chemical | C–31 | NA | P: Polyelectrolyte; MW: 5 × 106 |
Mixed Chlorophyta |
D: 0.15; S: 250; FD: 2.5; T: 60 | Fresh | 95 |
| Emsland–Stärk GmbH |
Emfloc KC750 |
1.4 | P: Potato Starch | Chlorella sp. | D: 107 mL–1; S: 100; FD: 70; T: 30 | Fresh | 48 |
|
Chlamydomonas Reinhardtii |
D: 107 mL–1; S: 100; FD: 70; T: 30 | Fresh | 90 | ||||
|
Scenedesmus Acuminatus |
D: 107 mL–1; S: 100; FD: 40; T: 30 | Fresh | 95 | ||||
| Rundo Biotech Japan Co. |
Poly (γ–glutamic acid) |
4.5 | Chitosan |
Chlorella protothecoides |
D: 0.6; S: 8000; FD: 20; T: 120 | Fresh | 98 |
|
Chlorella Vulgaris |
D: 0.6; S: 8000; FD: 20; T: 120 | Marine | 91 | ||||
| Sachtleben Wasserchemie |
Synthofloc 5080H |
NA | P: Polyacrylamide |
Phaeodactylum Tricornutum |
D: OD750–0.7; S: 5; FD: 0.01; T: 120 | Marine | 93 |
|
Neochloris Oleoabundans |
D: OD750–0.8; S: 10; FD: 30 | Marine | 90 | ||||
|
Neochloris Oleoabundans |
D: OD750–0.7; S: 5; FD: 0.01; T: 120 | Marine | 36 | ||||
| Separ Chemi GmbH |
POLY SEPAR® CFL25 |
2.2 | P: Tannin, quaternary ammonia salt; MW: Low; C: High |
Chlorella sp. | D: 107 mL–1; S: 100; FD: 30; T: 30 | Fresh | 95 |
|
Chlamydomonas Reinhardtii |
D: 107 mL–1; S: 100; FD: 20; T: 30 | Fresh | 20 | ||||
|
Scenedesmus Acuminatus |
D: 107 mL–1; S: 100; FD: 70; T: 30 | Fresh | 70 | ||||
| POLY SEPAR® KW100 |
2.7 | P: Quaternary ammonia compound, free of polyacrylamide; C: High |
Chlorella sp. | D: 107 mL–1; S: 100; FD: 30; T: 30 | Fresh | 95 | |
|
Chlamydomonas Reinhardtii |
D: 107 mL–1; S: 100; FD: 50; T: 30 | Fresh | 90 | ||||
|
Scenedesmus Acuminatus |
D: 107 mL–1; S: 100; FD: 60; T: 30 | Fresh | 95 | ||||
| POLY SEPAR® KW45 |
2.7 | P: Quaternary ammonia compound, free of polyacrylamide; C: Low |
Chlorella sp. | D: 107 mL–1; S: 100; FD: 30; T: 30 | Fresh | 65 | |
|
Chlamydomonas Reinhardtii |
D: 107 mL–1; S: 100; FD: 80; T: 30 | Fresh | 80 | ||||
|
Scenedesmus Acuminatus |
D: 107 mL–1; S: 100; FD: 60; T: 30 | Fresh | 35 | ||||
| POLY SEPAR® PK55H |
3.6 | P: Polyacrylamide; MW: High; C: High |
Chlorella sp. | D: 107 mL–1; S: 100; FD: 1.5; T: 30 | Fresh | 95 | |
|
Chlamydomonas Reinhardtii |
D: 107 mL–1; S: 100; FD: 4; T: 30 | Fresh | 99 | ||||
|
Scenedesmus Acuminatus |
D: 107 mL–1; S: 100; FD: 2; T: 30 | Fresh | 98 | ||||
| POLY SEPAR® SK72 |
3.37 | P: Starch | Chlorella sp. | D: 107 mL–1; S: 100; FD: 20; T: 30 | Fresh | 88 | |
|
Chlamydomonas Reinhardtii |
D: 107 mL–1; S: 100; FD: 30; T: 30 | Fresh | 91 | ||||
|
Scenedesmus Acuminatus |
D: 107 mL–1; S: 100; FD: 60; T: 30 | Fresh | 99 | ||||
| POLY SEPAR® SK72 |
3.37 | P: Starch | Chlorella sp. | D: 107 mL–1; S: 100; FD: 20; T: 30 | Fresh | 88 | |
|
Chlamydomonas Reinhardtii |
D: 107 mL–1; S: 100; FD: 30; T: 30 | Fresh | 91 | ||||
|
Scenedesmus Acuminatus |
D: 107 mL–1; S: 100; FD: 60; T: 30 | Fresh | 99 | ||||
| POLY SEPAR® KW745 H |
2.7 | P: Polyacrylamide | Chlorella sp. | D: 107 mL–1; S: 100; FD: 20; T: 30 | Fresh | 90 | |
|
Chlamydomonas Reinhardtii |
D: 107 mL–1; S: 100; FD: 20; T: 30 | Fresh | 90 | ||||
|
Scenedesmus Acuminatus |
D: 107 mL–1; S: 100; FD: 20; T: 30 | Fresh | 90 | ||||
| CFL 217 | 2.5 | P: Poly DADMAC; MW: Low; C: High |
Chlorella sp. | D: 107 mL–1; S: 100; FD: 20; T: 30 | Fresh | 90 | |
|
Chlamydomonas Reinhardtii |
D: 107 mL–1; S: 100; FD: 30; T: 30 | Fresh | 80 | ||||
|
Scenedesmus Acuminatus |
D: 107 mL–1; S: 100; FD: 50; T: 30 | Fresh | 95 | ||||
| CFL 229 | 2.5 | P: Poly DADMAC; MW: Low; C: High |
Chlorella sp. | D: 107 mL–1; S: 100; FD: 40; T: 30 | Fresh | 76 | |
|
Chlamydomonas Reinhardtii |
D: 107 mL–1; S: 100; FD: 40; T: 30 | Fresh | 87 | ||||
|
Scenedesmus Acuminatus |
D: 107 mL–1; S: 100; FD: 50; T: 30 | Fresh | 96 | ||||
| Sigma–Aldrich | Chitosan | 90 | P: Linked d-glucosamine; MW: Medium |
Chlamydomonas Reinhardtii |
D: 0.7; S: 500; FD: 25 | Fresh | 93 |
|
Chlorella Vulgaris |
D: 0.25; S: 100; FD: 8; T: 30 | Fresh | 85 | ||||
|
Chlorella Stimatophora |
D: 106 mL–1; S: 1000; FD: 10; T: 30 | Fresh | 90 | ||||
|
Neochloris Oleoabundans |
D: OD750–0.8; S: 10; FD: 90 | Marine | 66 | ||||
| Sigma–Aldrich | Chitosan | 90 | MW: Low |
Nannochloropsis Salina |
D: 0.7; S: 1500; FD: 3; T: 60 | Marine | 98 |
|
Nannochloropsis sp. |
FD: 100; T: 60 | Marine | 90 | ||||
|
Isochrysis Galbana |
D: 106 mL–1; S: 1000; FD: 10; T: 30 | Marine | 90 | ||||
|
Chaetoceros Calcitrans |
S: 500; FD: 20; T: 240 | Marine | 83 | ||||
| SNF–Floerger | FO4990 | 7.9 | P: Polyacrylamide; MW: 4.5–7.1 × 106; C: Very High |
Chlorella Vulgaris |
D: 0.26; S: 3000; FD: 1.66; T: 30 | Fresh | 99 |
|
Nannochloropsis Salina |
D: 0.7; S: 50; FD: 3; T: 60 | Marine | 94 | ||||
|
Nannochloropsis Oculata |
D: 0.26; S: 3000; FD: 0.55; [T]: 30 | Marine | 90 | ||||
| FO4800 | 3.37 | P: Polyacrylamide; MW: 4.9–7.1 × 106; C: High |
Chlamydomonas Reinhardtii |
D: 0.7; S: 500; FD: 13.5 | Fresh | 97 | |
|
Chlorella Vulgaris |
D: 0.26; S: 3000; FD: 1.66; T: 30 | Fresh | 99 | ||||
|
Nannochloropsis Salina |
D: 0.7; S: 50; FD: 3; T: 60 | Marine | 88 | ||||
| F04650 | 7.9 | P: Polyacrylamide; MW: 4.5–7.1 × 106; C: Medium |
Nannochloropsis Oculata |
D: 0.26; S: 3000; FD: 0.55; T: 30 | Marine | 87 | |
|
Chlorella Vulgaris |
D: 0.26; S: 3000; FD: 1.66; T: 30 | Fresh | 100 | ||||
|
Nannochloropsis Salina |
D: 0.7; S: 50; FD: 3; T: 60 | Marine | 73 | ||||
|
Nannochloropsis Oculata |
D: 0.26; S: 3000; FD: 0.55; T: 30 | Marine | 81 | ||||
| SNF–Floerger | SNF–Floerger FO4550 | 7.9 | P: Polyacrylamide; MW: 4.1–7.1 × 106; C: Low |
Chlorella Vulgaris |
D: 0.26; S: 3000; FD: 1.66; T: 30 | Fresh | 99 |
|
Nannochloropsis Salina |
D: 0.7; S: 50; FD: 3; T: 60 | Marine | 73 | ||||
|
Nannochloropsis Oculata |
D: 0.26; S: 3000; FD: 0.55; T: 30 | Marine | 67 | ||||
| TANAC (Brazil) | Tannin | 1.9 | P: Natural polymer; MW: Low; C: Low–medium |
Microcystis Aeruginosa |
FD: 10; T: 30 | Fresh | 97 |
| Tanfloc SL | 2.25 | P: Natural polymer; MW: Low; C: Low–medium |
Chlorella Vulgaris |
D: 0.26; S: 3000; FD: 5 | Fresh | 100 | |
|
Nannochloropsis Oculata |
D: 0.29; S: 3000; FD: 5; T: 30 | Marine | 97 | ||||
| Nalco (Australia) |
71301 | P: Polyacrylamide; MW: Medium; C: Medium/High |
Chlorococcum sp. |
D: 0.6; S: 1000; FD: 3; T: 30 | Marine | 78 | |
| 71303 | P: Polyacrylamide; MW: Low/Medium; C: Medium |
Chlorococcum sp. |
D: 0.6; S: 1000; FD: 4; T: 30 | Marine | 90 | ||
| 71305 | P: Polyacrylamide; MW: Low; C: Medium/High |
Chlorococcum sp. |
D: 0.6; S: 1000; FD: 3; T: 30 | Marine | 85 |
Fig. 3.
Structure of inulin cationic polymer.
The addition of chitosan to the way of microalgal cultivation at particular concentrations from 10 to 80 ppm caused bio-flocculation took after by perceptible sedimentation of the trailed microalgae. The effectiveness towards the harvesting process of Scenedesmus sp., utilizing chitosan is exceptionally susceptible to the pH of the algal culture medium with pH 7 and 8 [31]. It could be reasoned that, the cultural cell density only decides the required quantity of chitosan for flocculation. The most noteworthy sedimentation rate utilizing 40 ppm chitosan could be accomplished under pH 7 and OD3 (82.1%). Grouping of 80 ppm chitosan accomplished the most noteworthy harvesting rate i.e., 74% at pH 8, while 40 ppm chitosan expected to get 60.1% flocculation for Scenedesmus sp. culture at ahead of schedule logarithmic phase with pH at 7. The cultural cell density impacts the chitosan dosage required for flocculation [31]. An extracellular yield of Bacillus subtilis is the poly (γ-glutamic acid) (γ-PGA) which has been utilized commercially as a microbial flocculant in wastewater treatment also, in large scales [32]. The dosage parameters of γ-PGA to carry out the flocculation process depend on the concentration of biomass and alkalinity for Chlorella protothecoides and Chlorella vulgaris furthermore; γ-PGA has a little impact on microalgal cells [33]. Our outcomes illustrate that γ-PGA is a potential as well as an effective and economical flocculant in the harvesting microalgae for the generation of biodiesel.
Bio-flocculation based method is by all means a definitive assurance for commercial harvesting of microalgae biomass. Anyway inorganic flocculants have some limitations, a perfect approach is the utilization of natural flocculants in light of polymeric materials and structurally modified polymeric materials play a vital role in harvesting process [34]. Artificially, cassia a polysaccharide is made from the linear chain of 1,6 linked α-d-galactopyranose with 1,4-β-d-mannopyranose moieties and are derived basically from the leguminous plant species namely Cassia tora and Cassia obtusifolia. Biopolymers, such as, guar gum and tamarind kernel polysaccharide are being the focus of examination for both the natural and modified of their potential functions [35]. In addition, their formulations which are grafted, polyacrylamide grafted and hydrolyzed tamarind kernel polysaccharide, cationic guar gum and starch grafted polyacrylamide are being intricately contemplated in the flocculation process and are similarly considered to the microalgal flocculation process [34]. Essentially structural modification of tamarind kernel polysaccharide, glycogen and amylopectin [35] has been done and used to flocculate the effluents from textile industries, coal suspension and kaolin, iron mineral individually. Lately, unique methodologies as well as challenges in microalgal flocculation region are well examined [36].
In addition, to have the ability to interface with the negative surface charge on the microalgal cells, these biopolymers should be insistently charged, which is exceptional in nature. An exceptional unequivocally charged biopolymer is chitosan, which is resolved from chitin, a waste thing from the creation of shellfish. Further, chitosan is an incredibly capable flocculant anyway it works exactly at low pH, yet pH of the microalgal cells is modestly high [26,37]. A differentiating choice to chitosan is a cationic starch which is prepared from starch by methods for reaction with quaternary ammonium salt. The charge of that quaternary ammonium group is self-determining towards pH and along these lines cationic starch works over a broader pH than chitosan [38]. The chitosan contains a positive charge inferable from the amino functionalities. Under acidic conditions, these chitosan particles have high positive charge and are dynamic in flocculation by binding to the microorganism which has oppositely charged cell surface. Because of its cationic, biodegradability and low-noxious nature, chitosan has been used in wastewater treatment additionally [39,40]. Distinctive instances of biopolymers that can be used to flocculate microalgae are poly-glutamic acid which is an extracellular polymer made by Bacillus subtilis microscopic organisms [41] or polymers which exist flour of Moringa oleifera seeds [42]. Polymeric flocculants have been used broadly to recover microalgal biomass. Notwithstanding, in examination with inorganic flocculants, for example, aluminum sulfate, a cationic polyelectrolyte might be less viable [43]. Concentrates with Chlorella ellipsoidea at biomass of active mass 0.05–3 kg.m−3 and polymeric concentration of 1 × 10‐5–1 kg.m‐3 have demonstrated a nonattendance of flocculation at polymer fixation up to 0.2 kg.m−3 [22]. Cationic polyethylene imine is a viable flocculant for Chlorella species. In thinks about with them, the quantity of polymers required to start flocculation process diminished as the molar mass of the polymer increased. In any case, additionally increments in molar mass did not enhance the flocculation effectiveness. The adjustments in pH over the scope of 4–7, did not influence the flocculation procedure [22]. Further, the polyvalent natural polymers have been guaranteed as viable flocculants for both the Chlorella and Scenedesmus species [44].
3. Mechanism involved in polymeric flocculation
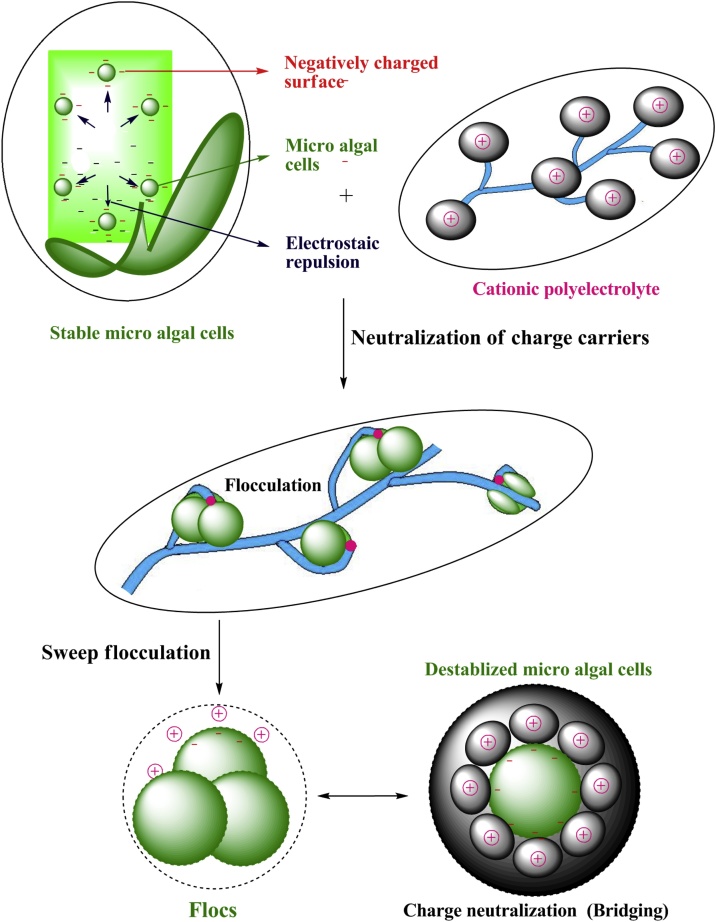
Microalgal cells are smaller in size (3–25 μm), negatively charged cells, which remain in suspension forms in the cultural media as their negative charge balances their amassing. Further, the microalgal cell wall has negative charges which derive principally as a consequence of the carboxylic (−COOH) and amino (–NH2) functional groups. Still, the mechanism of flocculation was not well recognized [[45], [46], [47], [48], [49]]. The polymeric flocculation is an effortless technique for harvesting different types of microalgae [49]. It consistently necessitates the addition of a polyelectrolyte with a strong positive charge; thereby the co-cultivated microalgal biomass harvesting is carried out by certain cationic acrylamide based polyelectrolyte flocculants [21]. Idyllically, these flocculants are cost-effective, non-toxic and more effective than others, even in low concentration [21,50]. Here, the floc forming destabilization mechanisms assume the process of polymeric adsorption and is very pH dependant. There are three floc forming destabilization mechanisms viz, neutralization of charge carriers, sweep flocculation and gravity filtration (Fig. 4).
Fig. 4.
Mechanism involved in polymeric flocculation.
3.1. Neutralization of charge carriers
In inducing the flocs formation for flocculation as the microalgal cells possess a negative charge which prevents the aggregation of cells, the charge neutralization plays a vital role. The stable microalgal cells which carry negative charges are brought closer to the added acrylamide based cationic polyelectrolyte as flocculant to the broth. The surface charge of the negatively charged microalgal cells is neutralized by cationic flocculants via electrolytic repulsion which reduces the zeta-potential and facilitates the process of flocculation. As the gentle mixing accelerates the rate of particle collision, the mixing speed was increased to allow flocculant distribution evenly. Thereby, the positively charged flocculant is attracted towards the negatively charged microalgal colloidal particles by means of some electrostatic forces of attraction via charge, dipole-dipole hydrogen bonding and van der Waals forces of interactions. After addition of the flocculants to the broth, they strongly interact with the single algal cells by adsorption to the surfaces and structuralization of a fibrous network between them has appeared. It was assumed that these flocculants are getting consumed to the cell wall before inciting flocculation. In this way the polymeric adsorption to the surface can be upgraded by charge differences. In the event that the charge differences amongst the polymers and the cell wall are larger, the polymer will be adsorbed rapidly. The algal cell particles are ordinarily negatively charged in arrangement and frame stable colloids so the cationic acrylamide based polymers work viably by means of their inductive and electromeric impacts. The presence of amide functionals (–C O—NH2) and other electronegative molecules in the lattice improves a negative surface charge to the microalgal cells in light of the fact that the lone pair of electrons from —N atom of the amide moved towards more electro negative –O atom.
3.2. Sweep flocculation
The algal cell colloidal particles become destabilized due to the electrostatic attractive forces of the algae cells towards the polyelectrolyte leads to a charge-charge interaction which results in the formation of flocs. Successive collisions and adsorption of the formed microflocs consequently result in the flocs grow which is also due to the flocculants nearby cover almost all the microalgal cell surfaces to permit the cellular interactions. Presently the little stabilized algal cell particles (microflocs) are joined into bigger flocs because of a blend of charge balance and bridging of these particles. It depends upon the charge thickness and length of the polymeric chain of the flocculant. In the last stage, the agitation process is ceased up and the flocs are allowed to settle down. It was observed that the cationic acrylamide based polymer produces large dense flocs. After 10 days, the formed flocs are microscopically visible. Flocculation is affected by quite a few parameters which include the mixing speed, intensity and time of the flocculant [50].
3.3. Gravity filtration
After the flocculation, a low energy centrifugation or gravity filtration of bigger flocs prompts an extra 10-fold higher concentration of the harvested biomass. Following a time of two months, the way of culture media was profitably reused after the flocculation process. It was important to affirm that if flocculant particles left, they don't adversely influence the cell development and the media are driven once more into the way of culture vessel that results to cell partition.
The cost-effective recycling process of the very clear non-toxic growth media after the harvesting of algae cells holds a favorable role for the industrial based applications. Moreover, the main characteristics of the post-treatment process for this kind of microalgae slurry residues involve the neutralization of the flocculated medium, followed by supplementation of nutrients. The microalgal cells can be flocculated by increasing the pH of the recycled culture and it can be observed that the cultivated microalgal biomass in the recycled growth medium is very analogous to that of the fresh medium. Some change in color of the medium after flocculation experiments indicate that the recycled medium after flocculation is not feasible to further cultivation [51]. It is also essential to confirm that the remaining particles of the flocculants do not unconstructively affect the growth, after the separation of algae cells. Moreover, a recycling experiment was conducted over a period of 8 weeks using algae cultures. The biomass can be harvested weekly, using the flocculants and the supernatant can be returned into the bioreactor. The optical density is adjusted to improve the light conditions for the algae growth, after each harvesting.
4. Concluding remarks
Bio-flocculation is thought to be a cost-effective process for the microalgae harvesting. Natural cationic polymers can incite productive bio-flocculation of freshwater microalgae even at low measurements in the vicinity of 1 and 10 mg/L. In any case, the high saltiness of the marine condition was found to repress flocculation process with these polyelectrolytes. Such a phenomenon was examined with three different cationic polymers. The inhibition experienced by flocculation was lessened at elevated salinity levels, and effectual flocculation was accomplished at the salinity levels lower than 5 mg/L. The diminished adequacy of cationic polymers to initiate microalgae bio-flocculation in ocean and salty waters is basically credited to the impact of medium ionic quality on the design and configuration of the polymer, as confirmed by the changes in its intrinsic viscosity. At high ionic potential, the polymer shrinks within its dimensions, and neglects to bind to the microalgal cells.
Currently, researchers have paid immense attention on algae–bacteria [52], algae–fungal [53], algal–algal bioflocculation [54,55] but most approaches are corroborated on lab scale level only [56]. It is due to the requirement of supplementary substrates and energy-intensive sources to sustain growth of the bacterial/fungal cells. There is a possibility of surplus bacterial/fungal contamination for the production plant of microalgae as well as hard to control the cultural set-up. Moreover, there is a basic lack of knowledge on the flocculation mechanisms. However, it is assumed that there are two possible mechanisms for aggregation of microalgae cells by means of bacteria: The first one is based on formation of aggregation via certain polysaccharide and protein compounds produced by bacterial/fungal cell walls [57], and the second one is a direct linking of the bacteria and microalgae, forming aggregated microalgae cells through charge neutralization reactions/electrostatic patching/bridging by extracellular polymeric substances (EPS) [58] to enhance sedimentation process of microalgae [59]. A number of studies propose the possibilities of both bridging and patching mechanisms for aggregation of a large and very close microalgae cells respectively for such bioflocculation processes. In addition, many research deals with genetic engineering, which can induce algae cellular reversible flocculation via dissimilar genetic modifications [60] and such an approach, can be controllable along with high yield [61]. Even though, for increasing the biomass accumulation still require noteworthy advances in the microalgae genomics [62] and the future study should pin points towards the additional screening for self flocculating microalgae to recognize the flocculation genes [63,55].
In light of the results, the usage of anionic and nonionic flocculants can't be recommended for the harvesting of Chlamydomonas, Chlorella and Scenedesmus cells. The flocculation capability of microalgae cells harvesting was entirely affected by the net charge of the examined polymer. Cationic flocculants achieved the most imperative flocculation efficiencies, while anionic, non-ionic and ampholytic flocculants brought about no/inadequate flocculation. The amount of required flocculants differs from 1.5 to 70 mg/L, as indicated by the microalgae species. By virtue of high flocculation efficiencies more than 95% at low quality of 1.5 mg/L, the cationic polyelectrolyte was decided for reusing tests on account of harvesting the species like C. reinhardtii, Chlorella sp., S. acuminatus and so on. It is revealed that the stability for a microalgal suspension of single cells is attributable to the repulsive powers actuated by the charges existing in the cell wall. We recommended that the accomplishment of cationic polymeric flocculants can be credited to the capacity of these flocculants to interface with singular cells and instigate growth of flocs and are framed on the grounds that the cationic functionals of the polymeric flocculant adsorb the negatively charged mass of the stablized cells. Finally, the impact is the destabilization of the cellular suspension consequently; active masses of both the flocculant and the feed must influence the flocculation process.
Amongst, the various flocculants, polymeric organo-flocculants with or without ionic charge along their chain either branched or linear are extensively effective for a choice of industrial functions like treating wastewater, mining process, etc and for effectual microalgae biomass harvesting. Such flocculants can be classified into cationic, anionic, non-ionic or zwitterionic accordingly and the charge carrying flocculants can neutralize microalgae cells which carry opposite surface charges and can join to the particles together via physical/chemical forces of attraction. The effectiveness of such polyelectrolytes are based on their kind, formula weight, charge densities, concentration of the cells, category of algae strain, ionic strength and pH of the cultural medium. This review described that there is an ideal proportion amongst flocculants and biomass that decides the required measure of flocculant at different biomass focuses. Despite the fact that this is like measured dosages announced in other smaller scale algal research, it is roughly 10 times higher than the measurements utilized for the wastewater industry. In addition, increase in the pH prompts the flocculation and was viable (90%) for harvesting of microalgae, primarily in the direct development stage. In any case, the effect of a higher pH on the profitability of polyacrylamide flocculants was negative which may be connected with the hydrolysis of flocculants at higher pH. The successful flocculation of microalgae requires incredibly the charged polymers. High atomic weight polyacrylamide polymer flocculants with higher charge density were profitable for the examined microalgae species. The most confusing efficiencies were enrolled for the flocculants with charge thickness 2–4 meq g–1, which can remove more than 95% of the cells from Chlorella sorokiniana, Scenedesmus obliquus, Scenedesmus subspicatus and Synechoccocus nidulans in the log stage at strengths of 2–5 mg/L. This examination has shown that a little estimation (around 8 mg/L) of polyamine polymer is extremely powerful to harvest of the Scenedesmus sp. The chitosan predominance in flocculation within the sight of polyelectrolytes is credited to its inflexible skeleton that comprises of glycosidic monomers. Moreover, it was found that the bioflocculants of a higher molecular weight and charge density is marginally more prominent for the best flocculation of marine and fresh water microalgae. Harvesting of microalgae utilizing polyacrylamide is prominent amongst the most proficient and costoptimal advances to preconcentrate microalgae for the generation of biofuel.
Conflict of interest
Authors declare no conflict of Interest
Acknowledgements
First author would like to acknowledge the financial Assistance from Ton Duc Thang University. Hochi Minh City, Vietnam.
Contributor Information
Arivalagan Pugazhendhi, Email: arivalagan.pugazhendhi@tdtu.edu.vn.
Gopalakrishnan Kumar, Email: gopalakrishnan.kumar@uis.no.
References
- 1.EIA . US Energy Inf. Adm; 2017. International Energy Outlook 2017 Overview.www.eia.gov/forecasts/ieo/pdf/0484(2016).pdf IEO2017:143. [Google Scholar]
- 2.Kuchler M., Linnér B.O. Challenging the food vs. fuel dilemma: genealogical analysis of the biofuel discourse pursued by international organizations. Food Policy. 2012;37:581–588. doi: 10.1016/j.foodpol.2012.06.005. [DOI] [Google Scholar]
- 3.Filip O., Janda K., Kristoufek L., Zilbermam D. Food versus fuel: an updated and expanded evidence. Energy Econ. 2017 doi: 10.1016/j.eneco.2017.10.033. [DOI] [Google Scholar]
- 4.Behera S., Singh R., Arora R., Sharma N.K., Shukla M., Kumar S. Scope of algae as third generation biofuels. Front. Bioeng. Biotechnol. 2015;2:1–13. doi: 10.3389/fbioe.2014.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laamanen C.A., Ross G.M., Scott J.A. Flotation harvesting of microalgae. Renew. Sustain. Energy Rev. 2016;58:75–86. doi: 10.1016/j.rser.2015.12.293. [DOI] [Google Scholar]
- 6.Dassey A.J., Theegala C.S. Harvesting economics and strategies using centrifugation for cost effective separation of microalgae cells for biodiesel applications. Bioresour. Technol. 2013;128:241–245. doi: 10.1016/j.biortech.2012.10.061. [DOI] [PubMed] [Google Scholar]
- 7.Uduman N., Qi Y., Danquah M.K., Forde G.M., Hoadley A. Dewatering of microalgal cultures: a major bottleneck to algae-based fuels. J. Renew. Sustain. Energy. 2010;2 doi: 10.1063/1.3294480. [DOI] [Google Scholar]
- 8.Ndikubwimana T., Chang J., Xiao Z., Shao W., Zeng X., Ng I.S., Lu Y. Flotation: a promising microalgae harvesting and dewatering technology for biofuels production. Biotechnol. J. 2016;11:315–326. doi: 10.1002/biot.201500175. [DOI] [PubMed] [Google Scholar]
- 9.Xia L., Li Y., Huang R., Song S. Effective harvesting of microalgae by coagulation–flotation. R. Soc. Open Sci. 2017;4 doi: 10.1098/rsos.170867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan C., Alam M.A., Zhao X.Q., Zhang X.Y., Guo S.L., Ho S.H., Chang J.S., Bai F.W. Current progress and future prospect of microalgal biomass harvest using various flocculation technologies. Bioresour. Technol. 2015;184:251–257. doi: 10.1016/j.biortech.2014.11.081. [DOI] [PubMed] [Google Scholar]
- 11.Gultom S.O., Hu B. Review of microalgae harvesting via co-pelletization with filamentous fungus. Energies. 2013;6:5921–5939. doi: 10.3390/en6115921. [DOI] [Google Scholar]
- 12.Shelef G., Sukenik A. Microalgae harvesting and processing: a literature review. Tech. Res. Dev. Found. Ltd. 1984;65 doi: 10.2172/6204677. [DOI] [Google Scholar]
- 13.Bleeke F., Milas M., Winckelmann D., Klock G. Optimization of freshwater microalgal biomass harvest using polymeric flocculants. Int. Aquat. Res. 2015;7:235–244. doi: 10.1007/s40071-015-0108-8. [DOI] [Google Scholar]
- 14.Japar A.S., Takriff M.S., Yasin N.H.M. Harvesting microalgal biomass and lipid extraction for potential biofuel production: a review. J. Environ. Chem. Eng. 2017;5:555–563. doi: 10.1016/j.jece.2016.12.016. [DOI] [Google Scholar]
- 15.Gerde J.A., Yao L.X., Lio J.Y., Wen Z.Y., Wang T. Microalgae flocculation: impact of flocculant type, algae species and cell concentration. Algal Res. Biomass Biofuels Bioprod. 2014;3:30–35. doi: 10.1016/j.algal.2013.11.015. [DOI] [Google Scholar]
- 16.Safi C., Zebib B., Merah O., Pontalier P.Y., Vaca-Garcia C. Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew. Sustain. Energy Rev. 2014;35:265–278. doi: 10.1016/j.rser.2014.04.007. [DOI] [Google Scholar]
- 17.Bratby J. IWA Publishing; 2006. Coagulation and Flocculation in Water and Wastewater Treatment. [Google Scholar]
- 18.Sen G., Ghosh S., Jha U., Pal S. Hydrolyzed polyacrylamide grafted carboxymethylstarch (Hyd. CMS-g-PAM): an efficient flocculant for the treatment of textile industry wastewater. Chem. Eng. J. 2011;171:495–501. doi: 10.1016/j.cej.2011.04.016. [DOI] [Google Scholar]
- 19.C. Banerjee, S. Ghosh, G. Sen, S. Mishra, P. Shukla, R. Bandopadhyay, Study of algal biomass harvesting using cationic guar gum from the natural plant source as flocculant. Carbohydr. Polym. 92, 675–681,doi:10.1016/j.carbpol.2012.09.022. [DOI] [PubMed]
- 20.Rahul R., Sunil K., Usha J., Gautam S. Cationic inulin: a plant based natural biopolymer for algal biomassharvesting. Int. J. Biol. Macromol. 2015;72:868–874. doi: 10.1016/j.ijbiomac.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Lucie V.H., Sasi N. Polyelectrolyte flocculants in harvesting microalgal biomass for food and feed applications. Algal Res. 2017;24:167–180. doi: 10.1016/j.algal.2017.03.022. [DOI] [Google Scholar]
- 22.Tilton R.C., Murphy J., Dixon J.K. The flocculation of algae with synthetic polymeric flocculants. Water Res. 1972;6:155–164. doi: 10.1016/0043-1354(72)90090-5. [DOI] [Google Scholar]
- 23.Chatsungnoen T., Chisti Y. Harvesting microalgae by flocculation–sedimentation. Algal Res. 2016;13:271–283. doi: 10.1016/j.algal.2015.12.009. [DOI] [Google Scholar]
- 24.Chen C.L., Chang J.S., Lee D.J. Dewatering and dryingmethods formicroalgae. Dry Technol. 2015;33:443–454. doi: 10.1080/07373937.2014.997881. [DOI] [Google Scholar]
- 25.Wu J., Liu J., Lin L., Zhang C., Li A., Zhu Y., Zhang Y. Evaluation of several flocculants for flocculating microalgae. Bioresour. Technol. 2015;197:495–501. doi: 10.1016/j.biortech.2015.08.094. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S.K., Kumar M., Guldhe A., Ansari F.A., Rawat I., Kanney K., Bux F. Design and development of polyamine polymer for harvesting microalgae for biofuels production. Energy Convers. Manage. 2014;85:537–544. doi: 10.1016/j.enconman.2014.05.059. [DOI] [Google Scholar]
- 27.de Bruyn A., Alvarez A.P., Sandra P., de Leenheer L. Isolation and identification of β-D-fructofuranosyl-(2,1)-D-fructose, a product of enzymatic hydrolysis of the inulin from Cichorium intybus. Carbohydr. Res. 1992;235:303–308. doi: 10.1016/0008-6215(92)80099-m. [DOI] [PubMed] [Google Scholar]
- 28.Kaur N., Gupta A.K. Applications of inulin and oligofructose in health and nutrition. J. Biosci. 2002;27:703–714. doi: 10.1007/BF02708379. [DOI] [PubMed] [Google Scholar]
- 29.Şirin S., Trobajo R., Ibanez C., Salvadó J. Harvesting the microalgae Phaeodactylum tricornutum with polyaluminum chloride, aluminium sulphate, chitosan and alkalinity-induced flocculation. J. Appl. Phycol. 2012;24:1067–1080. doi: 10.1007/s10811-011-9736-6. [DOI] [Google Scholar]
- 30.Zeng D., Wu J., Kennedy J.F. Application of a chitosan flocculant to water treatment. Carbohy. Polym. 2008;71:135–139. [Google Scholar]
- 31.Xu Y., Purton S., Baganz F. Chitosan flocculation to aid the harvesting of the microalga Chlorella sorokiniana. Bioresour. Technol. 2013;129:296–301. doi: 10.1016/j.biortech.2012.11.068. [DOI] [PubMed] [Google Scholar]
- 32.Yokoi H., Arima T., Hirose J., Hayashi S., Takasaki Y. Flocculation properties of poly (γ-glutamic acid) produced by Bacillus subtilis. J. Ferment. Bioeng. 1996;82:84–87. [Google Scholar]
- 33.Zheng H., Gao Z., Yin J., Tang X., Ji X., Huang H. Harvesting of microalgae by flocculation with poly (γ-glutamic acid) Bioresour. Technol. 2012;112:212–220. doi: 10.1016/j.biortech.2012.02.086. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee C., Ghosh S., Sen G., Mishra S., Shukla P., Bandopadhyay R. Study of algal biomass harvesting through cationic cassia gum, a natural plant based biopolymer. Bioresour. Technol. 2014;151:6–11. doi: 10.1016/j.biortech.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 35.Singh R.P., Pal S., Rana V.K., Ghorai S. Amphoteric amylopectin: a novel polymeric flocculant. Carbohydr. Polym. 2012;91:294–299. doi: 10.1016/j.carbpol.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Vandamme D., Foubert I., Muylaert K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013;31:233–239. doi: 10.1016/j.tibtech.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Choi J.H., Shin W.S., Lee S.H., Joo D.J., Lee J.D., Choi S.J., et al. Application of synthetic polyamine flocculants for dye wastewater treatment. Sep. Sci. Technol. 2001;36:2945–2958. doi: 10.1080/09593332208618213. [DOI] [PubMed] [Google Scholar]
- 38.Yue Q.Y., Gao B.Y., Wang Y., Zhang H., Sun X., Wang S.G., et al. Synthesis of polyamine flocculants and their potential use in treating dye wastewater. J. Hazard. Mater. 2007;152:221–227. doi: 10.1016/j.jhazmat.2007.06.089. [DOI] [PubMed] [Google Scholar]
- 39.Lertsittichai S., Lertsutthiwong P., Phalakornkule C. Improvement of upflow anaerobic sludge bed performance using chitosan. Water Environ. Res. 2007;79:801–807. doi: 10.2175/106143007x175906. [DOI] [PubMed] [Google Scholar]
- 40.Wang J.P., Chen Y.Z., Zhang S.J., Yu H.Q. A chitosan-based flocculant prepared with gamma-irradiation-induced grafting. Bioresour. Technol. 2008;99:3397–3402. doi: 10.1016/j.biortech.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Gao B., Yue Q., Zhan X., Si X., Li C. Flocculation performance of epichlorohydrin-dimethylamine polyamine in treating dyeing wastewater. J. Environ. Manage. 2009;91:423–431. doi: 10.1016/j.jenvman.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Ramanna L., Guldhe A., Rawat I., Bux F. The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour. Technol. 2014;168:127–135. doi: 10.1016/j.biortech.2014.03.064. [DOI] [PubMed] [Google Scholar]
- 43.Pushparaj B., Pelosi E., Torzillo G., Materassi R. Microbial biomass recovery using a synthetic cationic polymer. Bioresour. Technol. 1993;43:59–62. [Google Scholar]
- 44.Golueke C.G., Oswald W.J. Harvesting and processing sewage grown planktonic algae. J. Water Pollut. 1965;37:471–498. [Google Scholar]
- 45.Esser K., Kues U. Flocculation and its implication for biotechnology. Process Biochem. 1983;18:21–23. [Google Scholar]
- 46.Divakaran R., Pillai V.N.S. Flocculation of algae using chitosan. J. Appl. Phycol. 2002;14:419–422. doi: 10.1023/A:1022137023257. [DOI] [Google Scholar]
- 47.Salehizadeh H., Shojaosadati S.A. Extracellular biopolymeric flocculants recent trends and biotechnological importance. Biotechnol. Adv. 2001;19:371–385. doi: 10.1016/S0734-9750(01)00071-4. [DOI] [PubMed] [Google Scholar]
- 48.Strand S.P., Nordengen N., Øtgaard K. Efficiency of chitosans applied for flocculation of different bacteria. Water Res. 2002;36:4745–4752. doi: 10.1016/S0043-1354(02)00173-2. [DOI] [PubMed] [Google Scholar]
- 49.Pushparaj B., Pelosi E., Torzillo G., Materassi R. Microbial biomass recovery using a synthetic cationic polymer. Bioresour. Technol. 1993;43:59–62. doi: 10.1016/0960-8524(93)90083-N. [DOI] [Google Scholar]
- 50.Bleeke F., Milas M., Winckelmann D., Klock G. Optimization of freshwater micro algal biomass harvest using polymeric flocculants. Int. Aquat. Res. 2015;7:235–244. doi: 10.1016/j.rser.2017.10.038. [DOI] [Google Scholar]
- 51.Wu Z., Zhu Y., Huang W., Zhang C., Li T., Zhang Y., Li A. Evaluation of flocculation induced by pH increase for harvesting microalgae and reuse of flocculated medium. Bioresour. Technol. 2012;110:496–502. doi: 10.1016/j.biortech.2012.01.101. [DOI] [PubMed] [Google Scholar]
- 52.Ndikubwimana T., Zeng X., Murwanashyaka T., et al. Harvesting of freshwater microalgae with microbial bioflocculant: a pilot-scale study. Biotechnol. Biofuels. 2016;9:47. doi: 10.1186/s13068-016-0458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C.L., Chang J.S., Lee D.J. Dewatering and drying methods for microalgae. Dry Technol. 2015;33:443–454. doi: 10.1080/07373937.2014.997881. [DOI] [Google Scholar]
- 54.Alam M.A., Wan C., Guo S.L., et al. Characterization of the flocculating agent from the spontaneously flocculating microalga Chlorella vulgaris JSC-7. J. Biosci. Bioeng. 2014;118:29–33. doi: 10.1016/j.jbiosc.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 55.Alam M.A., Vandamme D., Chun W., Zhao X., Foubert I., et al. Bioflocculation as an innovative harvesting strategy for microalgae. Rev. Environ. Sci. Biotechnol. 2016 doi: 10.1007/s11157-016-9408-8. [DOI] [Google Scholar]
- 56.Wan C., Alam M.A., Zhao X.Q., et al. Current progress and future prospect of microalgal biomass harvest using various flocculation technologies. Bioresour. Technol. 2015;184:251–257. doi: 10.1016/j.biortech.2014.11.081. [DOI] [PubMed] [Google Scholar]
- 57.Su Y., Mennerich A., Urban B. Municipal wastewater treatment and biomass accumulation with a wastewaterborn and settleable algal-bacterial culture. Water Res. 2011;45:3351–3358. doi: 10.1016/j.watres.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 58.Ndikubwimana T., Zeng X., He N., et al. Microalgae biomass harvesting by bioflocculation-interpretation by classical DLVO theory. Biochem. Eng. J. 2015;101:160–167. doi: 10.1016/j.bej.2015.05.010. [DOI] [Google Scholar]
- 59.Powell R.J., Hill R.T. Mechanism of algal aggregation by Bacillus sp. Strain RP1137. Appl. Environ. Microbiol. 2014 doi: 10.1128/AEM.00887-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scholz M., Hoshino T., Johnson D., et al. Flocculation of wall-deficient cells of Chlamydomonas reinhardtii mutant cw15 by calcium and methanol. Biomass Bioenergy. 2011;35:4835–4840. doi: 10.1016/j.biombioe.2011.08.020. [DOI] [Google Scholar]
- 61.Lama S., Muylaert K., Karki T.B., et al. Flocculation properties of several microalgae and a Cyanobacterium species during ferric chloride, chitosan and alkaline flocculation. Bioresour. Technol. 2016 doi: 10.1016/j.biortech.2016.08.080. [DOI] [PubMed] [Google Scholar]
- 62.Diaz-Santos E., Vila M., De Vega M., et al. Study of bioflocculation induced bySaccharomyces bayanus var. uvarum and flocculating protein factors in microalgae. Algal Res. 2015;8:23–29. doi: 10.1016/j.algal.2014.12.013. [DOI] [Google Scholar]
- 63.Blatti J.L., Michaud J., Burkart M.D. Engineering fatty acid biosynthesis in microalgae for sustainable biodiesel. Curr. Opin. Chem. Biol. 2013;17:496–505. doi: 10.1016/j.cbpa.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Tenney M.W., Echelberger W.F., Schuessler R.G., Pavoni J.L. Algae flocculation with synthetic organic polyelectrolytes. Appl. Microbiol. 1969;18:965–971. doi: 10.1128/am.18.6.965-971.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]