Abstract
Background and Objectives:
We compared the outcome of robotic hysterectomy (RH) with laparoscopic hysterectomy (LH) for large uteri (≥16 weeks).
Methods:
This was a retrospective review over 5 years of 165 women (RH, 46; LH, 119). Demographic data, conversion, hemoglobin drop, indication, operating time, postoperative stay, and intra-operative strategies (adhesiolysis, myomectomy) were recorded.
Results:
Mean age was 45.7 ± 6.4 years and 44.5 ± 5.4 years (no diff) and body mass index was 30.2 ± 6.3 kg/m2 and 27.8 ± 4.8 kg/m2 (P = .009) in the RH and LH groups. There was no difference in percentage of women with previous laparotomy (RH, 15.2% vs LH, 13.4%) and mean number of lower-segment caesarean section (RH, 1.0 vs LH, 0.8). Mean size of uterus was similar (RH, 20.0 weeks vs LH, 17.4 weeks). The mean number of ports was higher in the RH group (RH, 4.2 vs LH, 3.4; P < .001) as was needed for adhesiolysis (RH, 71.7% vs LH, 35.3%; P < .001). Difficult bladder dissection was more in the RH group (56.5% vs 26.1%; P < .001). Vaginal morcellation was similar in both groups (RH, 89.1%; LH, 83.2%). RH took longer operating time (131.0 vs 110.6 minutes; P = .006). RH had less drop in Hb (1.0 vs 1.8 g/dL; P < .001) and remained the same after multiple regression analysis. Postoperative stay was similar in both groups (1.4 days). Requirement of intravenous analgesia was significantly lower in the RH group (12.5 vs 30.9 hours; P < .001). Open conversion rate was 4.3% (RH) and 10.9% (LH) but not significant.
Conclusion:
A higher body mass index, more adhesiolysis, and difficult bladder dissection imply a more challenging nature of women who underwent RH. Despite this, RH was shown to be feasible and safe with a lower blood loss.
Keywords: Hysterectomy, Fibroids, Robotic, Laparoscopy, Conversion rate, Large uterus
INTRODUCTION
Hysterectomy is the most common operation that women undergo after caesarean section. Fibroid uterus is common indication for hysterectomy for large uterus. The presence of fibroids raises technical difficulties in performing hysterectomy by minimal access approach, although there is no denying its advantages in the overall recovery in women. Even today, the world over doctors approach hysterectomy for large uterus abdominally. In a nationwide survey in the United States, when asked for preferred mode of hysterectomy for themselves or their spouse, only 8% chose abdominal hysterectomy, 55.5% chose vaginal hysterectomy, and 40.6% chose laparoscopic hysterectomy.1 We assessed outcome of minimally invasive hysterectomy for large uteri, clinically ≥16 weeks by robotic hysterectomy (RH) and compared it with the outcome of laparoscopic hysterectomy (LH).
METHODS
This was a retrospective review of all women over a 5-year period 2013–2018 who underwent a minimally invasive hysterectomy and had a large uterus (≥16-week size). We chose to do a clinical assessment of the size for comparison rather than post-surgery weight as this is useful in selecting the patients preoperatively. This preoperative assessment and correct selection of case is the most important factor in successfully completing hysterectomy. All cases were preoperatively evaluated for size by a single surgeon who also performed all cases in both groups of patients. One-hundred sixty-five women (RH, 46; LH, 119) were included in this study. The decision to offer LH or RH was based on primarily two factors. The first was surgical difficulty. In general, patients were considered for RH only if surgery was expected to be technically challenging. For those meeting this criterion, the second critical factor was economical. RH was more expensive than LH and hence many patients were not in a position to afford it. Both modalities were on offer during the entire study time period. A single surgeon (RS) performed all surgeries. This surgeon has been performing LH and RH for 20 years and 6 years, respectively.
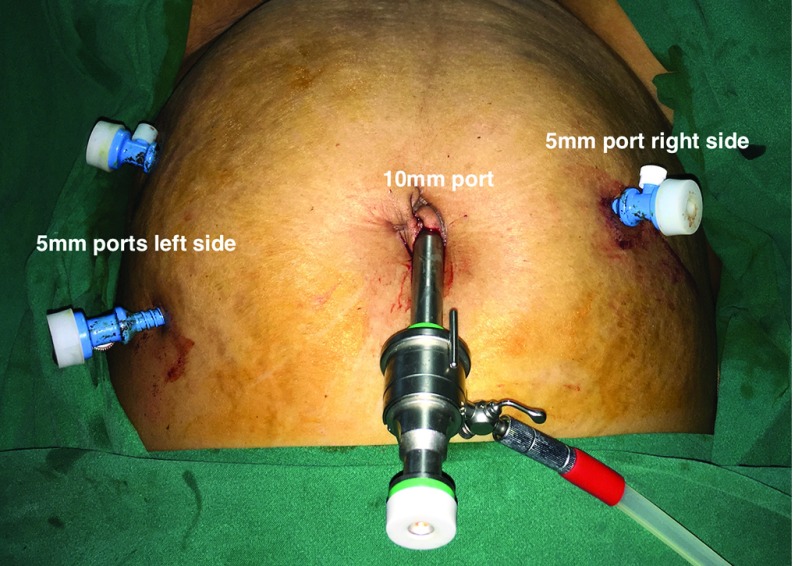
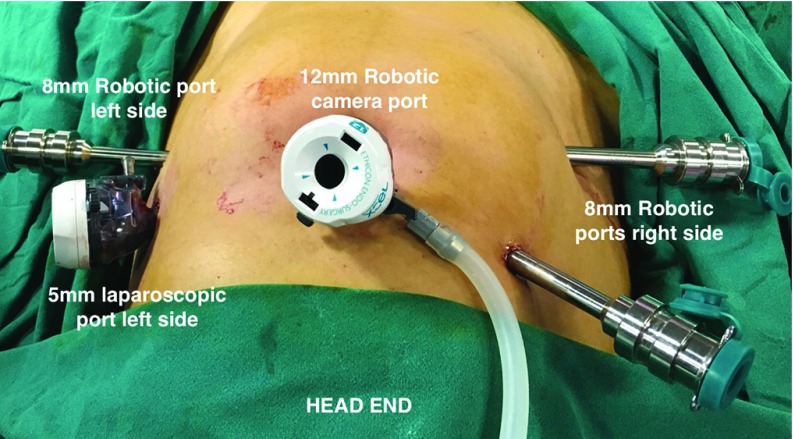
Eleven women who underwent an open hysterectomy during this period were excluded from analysis. Demographic data, age, and BMI were recorded for all. The presentations were classified as heavy menstrual bleeding, heavy menstrual bleeding associated with dysmenorrhea, mass felt per abdomen, and post-menopausal bleeding. The diagnosis for which hysterectomy was performed was also noted. Any history of previous abdominal surgery that included caesarean section was noted. Data from operative notes such as number of ports, adhesions, and need for adhesiolysis. For the laparoscopic hysterectomy, the primary port (10 mm) was either placed at the umbilicus or above the umbilicus at the Lee Huang point. Two secondary ports (5 mm) were placed laterally on the left side of the abdomen and one 5-mm port on the right side of the abdomen (Figure 1). For robotic hysterectomy, the primary port (12 mm) was either placed at the umbilicus or at the Lee Huang point. Two robotic secondary ports (8 mm) were placed laterally on the right side of the abdomen and one 8-mm robotic port on the left side of the abdomen and one 5-mm assistant port on the right side above the 8-mm port (Figure 2). Difficult bladder dissection or any organ injury such as bladder or ureter was recorded. Intra-operative strategies to complete the hysterectomy—use of uterine elevator or myoma screw for manipulation, intra-operative myomectomy—were noted. We noted the need to put a suprapubic incision, which could be for completing the hysterectomy or for specimen retrieval. The operative time (OT) was recorded as total time from incision to port closure (OT-1), time taken for placement of ports, setting up laparoscopic equipment in the LH group or docking time in RH group, morcellation, and vault closure vaginally (OT-2), time needed to perform the laparoscopic part of surgery (LH) or the console time in (RH) group (OT-3). Strategy for specimen retrieval (vaginal, suprapubic incision, or by enlarging the primary port) was also noted. The drop in hemoglobin was recorded as the difference between the preoperative hemoglobin level minus the preoperative hemoglobin level the morning after the surgery. Postoperative stay and the need for intravenous analgesia was recorded as a surrogate marker for immediate postoperative recovery parameters. The statistical analysis was done by using the t-test for the continuous variables and normal test for proportion for categorical variables.
Figure 1.
Port placement for laparoscopic hysterectomy.
Figure 2.
Port placement for robot assisted laparoscopic hysterectomy.
RESULTS
The mean age (±SD) was similar in both groups, 45.7 ± 6.4 years in the RH group and 44.5 ± 5.4 years in LH group. BMI (±SD) was 30.2 ± 6.3 kg/m2 and 27.8 ± 4.8 kg/m2 (P = .009) in the RH and LH groups, respectively. The women who underwent RH were significantly heavier than those who underwent LH. Heavy menstrual bleeding, heavy menstrual bleeding with dysmenorrhea, abdominal mass, and postmenopausal bleeding were the presenting symptoms in 30.4%, 58.7%, 2.2%, and 8.7% in the RH group and 39.5%, 56.3, 4.2%, and 0% in the LH group, respectively (P = .009). The most common indication in both groups was fibroid (RH, 65.2%; LH, 85.7%). There was no difference in the percentage of women with previous laparotomy (RH, 15.2% vs LH, 13.4%) as well as mean number of previous lower segment cesaerean section (LSCS) (RH, 1.0 vs LH, 0.8). The demographic data were tabulated in Table 1. The mean size of uterus was similar (RH, 20.0 weeks vs LH, 17.4 weeks). The mean number of ports was higher in the RH group (RH, 4.2 vs LH, 3.4; P < .001). The need for adhesiolysis was significantly higher in the RH group (RH, 71.7% vs LH, 35.3%; P < .001). The intra-operative strategy of doing myomectomy to complete the surgery was used only in the LH group in 40.3% of cases. Difficult bladder dissection was encountered significantly more often in the RH group (56.5 vs 26.1%; P < .001). Intra-operative organ injury occurred only in the LH group (3 bladder and 1 ureteric injury). Vaginal morcellation was carried out in a similar number in both groups (RH, 89.1%; LH, 83.2%). Three cases in the RH group and two cases in the LH group required primary port (umbilical) enlargement for specimen retrieval, all other specimens were removed via vaginal morcellation. A power-morcellation device was not used in any case, and all specimens were removed as cold-knife morcellation by scalpel. One case in the RH group needed suprapubic incision to remove an intact uterus for suspicion of endometrial carcinoma. The results are tabulated in Table 2. RH took longer OT when compared with the LH group (OT1, 131.0 vs 110.6 minutes, P = .006; OT2, 27.1 vs 22.4 minutes, P = .04; OT3, 104.46 vs 87.31 minutes, P = .006). There was less of a drop in hemoglobin levels in the RH group as compared to the LH group (1.0 vs 1.8 g/dL; P < .001), which was statistically significant. This result of OT is tabulated in Table 3. On multiple regression analysis, the difference in drop in hemoglobin was noted to be lower with the RH group even after controlling for multiple risk factors such as uterine size, BMI, organ injury (bladder, ureter), and previous laparotomy. Postoperative stay was similar (1.4 days in both). The mean hours of requirement of intravenous analgesia was significantly lower in the RH group: 12.5 vs 30.9 hours in the LH group (P < .001). Conversion to open surgery was necessary in 4.3% and 10.9% in the RH and LH groups, respectively, which was not statistically significant.
Table 1.
Demographic Data
| Robotic Hysterectomy (46) | Laparoscopic Hysterectomy (119) | P Value | |
|---|---|---|---|
| Age (years) | 45.7 ± 6.4 | 44.5 ± 5.4 | .165 |
| BMI | 30.2 ± 6.3 | 27.8 ± 4.8 | .009 |
| Women with previous laparotomy | 7 (15.2) | 16 (13.4) | .97 |
| Mean number of previous LSCS | 0.80 ± 1.02 | 0.56 ± 0.85 | .125 |
| Average size of uterus (weeks) | 19.96 ± 14.80 | 17.43 ± 2.12 | .07 |
LSCS, lower segment cesarean section. Patients who underwent robotic hysterectomy had a significantly higher body mass index.
Table 2.
Results
| Robotic Hysterectomy (46) | Laparoscopic Hysterectomy (119) | P Value | |
|---|---|---|---|
| Mean number of ports | 4.17 ± 0.38 | 3.53 ± 0.55 | <.001 |
| Need for adhesiolysis | 33 (71.7) | 42 (35.3) | <.001 |
| Vaginal morcellation | 41 (89.1) | 99 (83.2) | .48 |
| Drop in Hb g/dL | 1.03 ± 0.76 | 1.76 ± 0.9 | <.001 |
| Mean hours of IV analgesia | 12.52 ± 14.96 | 30.86 ± 13.31 | <.001 |
| Conversion to open surgery | 4.3 | 10.9 | 1 |
Hb, Hemoglobin; IV, intravenous.
Table 3.
Operative Time
| Robotic Hysterectomy | Laparoscopic Hysterectomy | P Value | |
|---|---|---|---|
| OT-1 | 130.98 ± 54.92 | 110.59 ± 35.72 | .006 |
| OT-2 | 27.07 ± 22.67 | 22.39 ± 6.76 | .044 |
| OT-3 | 104.46 ± 48.02 | 87.31 ± 29.74 | .006 |
OT, operative time.
Results are presented either as n (%) for categorical variables or Mean ± SD for continuous variables.
DISCUSSION
The aim of doing this comparison was to evaluate whether robotic assistance improved clinical outcomes in women who underwent hysterectomy for large uterus. The biggest challenge here is to avoid conversion to laparotomy as this increases the morbidity in the patient. We had conversion to open surgery in 4.3% and 10.9% in the RH and LH groups, respectively, which was not statistically significant. However, if we analyze individual cases, only 3 patients needed suprapubic incision to complete the procedure in the RH group, and all had BMI > 44 kg/m2. One of these had a large (10 × 10 cm) cervical fibroid, which made dissection at the vault level difficult. The second case was a large adenomyotic uterus, performed early in our learning curve. The third suprapubic incision in the RH group was made for specimen retrieval to avoid morcellation (endometrial carcinoma). In the LH group, 13 patients needed conversion to complete the hysterectomy due to hemorrhage or organ damage. The technical difficulties that made us convert in the LH group were overcome in the RH group. In a systematic review of RH in obese women, the conversion rate reported was 4.1% (92 of 2226 patients).2 Analysis by Uccella and group3 identified 6 studies of LH for uteri weighing ≥1 kg for a total of 62 patients; conversion to open surgery was necessary in 6 (9.7%) patients, and an additional 13 (21%) received a minilaparotomic incision to extract the uterus. Successful laparoscopic-assisted vaginal hysterectomy is also reported with success in 14 of 15 women in women with extremely large uterus.4 The number of ports used was significantly higher in the robotic surgery group. This is due to the technique advocated by the Da Vinci operative manual. However, with increased experience and the learning curve in our unit, we now perform robotic hysterectomy with only 4 ports (12 mm for telescope, 2 ports of 8 mm for robotic instruments, and 1 5-mm port for assistance), which is similar to a 10-mm port for telescope and 3 5-mm ports during laparoscopic hysterectomy. The assistant ports in both the groups are used for suction and manipulation of uterus by myoma screw. In their study, James Fanning et al5 use a 5-port technique during laparoscopic hysterectomy. They used a 5-mm trocar in the supraumbilical area or left upper quadrant (depending on uterus size, previous abdominal/pelvic incisions, and BMI). Two additional 5-mm ports were placed in the right and left side. Single-port RHs have been described but require more critical evaluation before they can be recommended for routine use.7 However, Dällenbach8 describes a 3-port technique in 53 cases of RH as feasible and safe for simple hysterectomy.
One of the strategies used by our group to improve visualization of pedicles during LH was to do intraoperative myomectomy. This was done after injecting diluted vasopressin and the myoma was left in the right paracolic gutter for vaginal retrieval later. The intra-operative strategy of doing myomectomy to complete the surgery was used 40.3% of the time in the LH group and none in RH group. This technique is particularly useful if there is a large myoma in the lower part of the uterus either in the anterior or posterior wall. This improves the vision for bladder dissection anteriorly or uterosacral dissection posteriorly. Large lateral fibroid extending into lateral pelvic wall, when removed, restores the anatomy of pedicles and surrounding organs and one can proceed with hysterectomy. We have described this technique in our previously published paper.9 However, we did not feel the need to adopt this strategy in our RH cases as the control and movement of camera facilitated reaching odd locations in limited pelvic spaces to complete the hysterectomy. The average mean size of uterus was marginally larger in the RH group when compared with the LH group (20 weeks vs 17.4 weeks), although not statistically different. In their paper, Taniguchi et al10 describe a similar technique of intra-operative myomectomy in 52% of their laparoscopic hysterectomies and completed surgery without conversion. However, when not done swiftly, this technique can increase the risk for intra-operative blood loss.
Surrounding organ damage increases the morbidity, especially if not recognized intra-operatively. A systematic review by Wong et al11 reported an incidence of urinary-tract injuries as 0.33% (95% CI, 0.30–0.36). Bladder injury (0.24%; 95% CI, 0.22–0.27) was overall 3 times more frequent than ureteral injury (0.08%; 95% CI, 0.07–0.10). Most ureteral injuries resulted from electrosurgery (33.3%; 95% CI, 24.3–45.8), whereas most bladder injuries resulted from lysis of adhesions (23.3%; 95% CI, 18.7–29.0). We encountered 3 bladder and 1 ureteric injury in the LH group. The bladder injuries were detected and managed intra-operatively. However, the ureteric injury presented late on seventh post-operative day and was managed by ureteric stenting. Difficult bladder dissection was encountered significantly more often in the RH group (56.5% vs 26.1%; P < .001) when compared to the LH group. Absence of bladder injury in the RH group can be accounted for improved vision and dexterity with robotic platform. Nezhat and team12 describe a reverse vesicouterine fold dissection as a useful alternative technique for laparoscopic hysterectomy in women with a history of prior cesarean deliveries. Risk factors such as high BMI and presence of endometriosis increases the likelihood of urinary-tract damage. In multivariate logistic analysis, a BMI of 26 to 30 kg/m2 was associated with an increased risk of ureteral injury as compared to a BMI ≤ 25 kg/m2 and the presence of endometriosis were associated with an increased risk of bladder injury.13 Removing the specimen at the end of the hysterectomy for large uterus is of special importance as power morcellation has fallen into disrepute since the black box Food and Drug Administration, USA (FDA) warning in 2014, although fragmented extraction of uterine and leiomyoma tissue in gynecologic surgery has been performed for decades and can be done through enlarging a laparoscopic port, a minilaparotomy incision, or through a colpotomy. In our study, we did not use a mechanical power morcellation device and all specimens were extracted by cutting them into small pieces by scalpel (cold-knife morcellation). Vaginal morcellation was carried out in a similar number in both groups (RH, 89.1%; LH, 83.2%) in this study. Experienced vaginal surgeons can adapt to remove specimen vaginally and continue to provide minimally invasive hysterectomies without compromising patient outcomes and safety.14 Among patients undergoing vaginal hysterectomy with morcellation, the incidence of occult uterine carcinoma is 0.82%. Uncontained vaginal morcellation when used concomitantly with vaginal hysterectomy does not appear to negatively impact patient prognosis or outcomes.15
In our series, 3 cases in the RH group and two cases in the LH group required primary-port (umbilical) enlargement for specimen retrieval. One case in the RH group had a suprapubic incision to remove an intact uterus for suspicion of endometrial carcinoma. In-bag morcellation is also a viable option when surgeons choose to use mechanical morcellation devices during hystyerectomy of large uterus. Peritoneal washings after contained morcellation were all negative for malignant or smooth-muscle cells.16,17 When the 3 methods of morcellation were compared, there was no significant difference between the 3 morcellation techniques in peri-operative outcomes, hence all 3 can be viable options for tissue retrieval. However, the longest operative times were for the minilaparotomy approach. The various techniques used by this group was electronic power morcellation, manual vaginal morcellation via the vagina, or manual morcellation via minilaparotomy.18 Robot-assisted hysterectomy took significantly longer time in this study. Docking and equipment setup time is often blamed for longer operating time in RH cases. If we analyze the time individually in our study, OT2 time is the reflection of the setting up equipment, docking, and morcellation time. Although significantly different, the actual time difference is an average 5 minutes between the 2 groups. This extra 5 minutes includes docking and morcellation time in the RH group. Thus, with improved learning curve of the whole team, the equipment setup time can be reduced to a minimum and does not influence the overall time. The longer OT3 time in the RH group is again a reflection of the fact that the average size of uteus was larger in the RH group, as well as the need for adhesiolysis, and difficult bladder dissection was encountered more in this group. Nezhat and group,19 when compared with traditional robotic assisted laparoscopic hysterectomy (RALH) and total laparoscopic hysterectomy (TLH), found that the mean operative time was 276 minutes and 206 minutes, respectively. Learning curve matters in reducing time in any procedure. In a recent study published by Woelk et al,20 recorded operative times for robotic hysterectomy as 210 minutes when surgeons first started robotic surgery, which decreased to 160 minutes after 3 years. Chiu et al,21 in their comparison of hysterectomy for large uterus with adhesions reported shortened operation time in the LH group as compared with the RH group (113.9 ± 38.4 minutes vs 164.3 ± 81.4 minutes; P = .007). In a different study, when a subgroup analysis of obese patients was done, procedure time was longer in RALH and LH (P < .001).22
Our comparative study shows statistically less blood loss in the RH group. When multiple regression analysis was performed, the difference in drop in Hb was lower with RH even after controlling for multiple risk factors like uterine size, BMI, organ injury (bladder, ureter), and previous laparotomy. Nezhat and group19 reported a mean EBL Estimated blood loss (EBL) in the patients who had RALH and TLH as 250 mL and 300 mL, respectively. When mean blood loss in RH was compared with minilaparotomy hysterectomy by Smorgick et al,23 after adjusting for differences in uterine weight using a multivariate linear regression analysis, they concluded that the mean blood loss and the rate of hemorrhage were no longer significantly different between the 2 groups. Reduced blood loss in the RH group when compared with LH was also reoprted by Chiu et al21 (187.5 ± 148.7 mL vs 385.7 ± 482.6; P = .044). The mean hours of requirement of intravenous analgesia was significantly lower in the RH group in this study. The RH group needed for 12.5 hours when compared with 30.9 hours in the LH group (P < .001). More precise dissection and reduced tissue damage can be a reason for less post-operative pain. The patients in the RH group reported less pain at port site despite the port size in the RH group being 8 mm as compared to 5 mm in laparoscopic surgery. This reduced pain is due to the fulcrum effect. The robotic instruments move with their wrist inside the patient's abdomen whereas the laparoscopic instruments use the abdominal wall as leverage for movement, causing more tissue damage at the abdominal wall. El Hachem et al24 did not find any significant differences in postoperative pain scores and narcotic compared to laparoscopcy in benign cases. A significant lower opiod or fentanyl dose requirement was reported in the robotic group in cervical and endometrial cancer cases.25,26 Retrospective analysis of the data is the main weakness of the study. With nonrandom allocation of cases, there is the likelihood that the more difficult cases were selected and counseled for robotic assisted surgery. The RH group included in this study was also the part of the learning curve of this surgical group.
This study has important limitations. The data are retrospective and the case allocation nonrandom. While departmental policy is clear, the exact decision-making process in case of allocation is not known for individual patients. The groups were not exactly comparable. However, the bias was in the direction of more challenging cases being offered RH. All cases were operated by a single surgeon, hence the data might not be applicable to all institutions. The main elements of the surgical team remained constant through the study period.
CONCLUSION
Robotic hysterectomy is associated with a lower blood loss and post-operative requirement of analgesia. A higher BMI, more need for adhesiolysis, and greater number of women with difficult bladder dissection possibly imply the more challenging nature of women who underwent RH in the presence of large uterus. Despite this, RH was shown to be feasible and safe with a lower blood loss, albeit at the cost of a greater number of ports and longer operative time. RH is as good if not a better approach for difficult hysterectomy with large uteri.
Contributor Information
Rooma Sinha, Department of Gynecology, Apollo Hospitals, Jubilee Hills, Hyderabad, India..
Rupa Bana, Department of Gynecology, Apollo Hospitals, Jubilee Hills, Hyderabad, India..
Madhumathi Sanjay, Department of Gynecology, Apollo Hospitals, Jubilee Hills, Hyderabad, India..
References:
- 1. Einarsson JI, Matteson KA, Schulkin J, Chavan NR, Sangi-Haghpeykar H. Minimally invasive hysterectomies—A survey on attitudes and barriers among practicing gynecologists. J Minim Invasive Gynecol. 2010;17(2):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iavazzo C, Gkegkes ID. Robotic assisted hysterectomy in obese patients: A systematic review. Arch Gynecol Obstet. 2016;293(6):1169–1183. [DOI] [PubMed] [Google Scholar]
- 3. Uccella S, Cromi A, Serati M, Casarin J, Sturla D, Ghezzi F. Laparoscopic hysterectomy in case of uteri weighing ≥1 kilogram: A series of 71 cases and review of the literature. J Minim Invasive Gynecol. 2014;21(3):460–465. [DOI] [PubMed] [Google Scholar]
- 4. Park JY, Kim TJ, Kang HJ, et al. Laparoendoscopic single site (LESS) surgery in benign gynecology: Perioperative and late complications of 515 cases. Eur J Obstet Gynecol Reprod Biol. 2013;167(2):215–218. [DOI] [PubMed] [Google Scholar]
- 5. Fanning J, Fenton B, Switzer M, Johnson J, Clemons J. Laparoscopic-assisted vaginal hysterectomy for uteri weighing 1000 grams or more. JSLS. 2008;12(4):376–379. [PMC free article] [PubMed] [Google Scholar]
- 6. Iavazzo C, Gkegkes ID. Single-site port robotic-assisted hysterectomy: A systematic review. Arch Gynecol Obstet. 2014;289(4):725–731. [DOI] [PubMed] [Google Scholar]
- 7. Fanfani F, Monterossi G, Fagotti A, Scambia G. Laparoendoscopic single-site hysterectomy: Is it safe and feasible? Curr Opin Obstet Gynecol. 2014;26(4):275–280. [DOI] [PubMed] [Google Scholar]
- 8. Dällenbach P, Petignat P. Perioperative outcomes of three-port robotically assisted hysterectomy: A continuous series of 53 cases. J Robot Surg. 2014;8(3):221–226. [DOI] [PubMed] [Google Scholar]
- 9. Sinha R, Swarnasree G, Rupa B, Madhumathi S. Laparoscopic hysterectomy for large uteri: Outcomes & techniques. J Minim Access Surg. 2018. March 23 Available at 10.4103/jmas.JMAS_205_17 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taniguchi F, Koike N, Kikukawa T, et al. An evaluation of laparoscopic hysterectomy alone versus in combination with laparoscopic myomectomy for patients with uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2017;210:132–138. [DOI] [PubMed] [Google Scholar]
- 11. Wong JMK, Bortoletto P, Tolentino J, Jung MJ, Milad MP. Urinary tract injury in gynecologic laparoscopy for benign indication: A systematic review. Obstet Gynecol. 2018;131(1):100–108. [DOI] [PubMed] [Google Scholar]
- 12. Nezhat C, Grace LA, Razavi GM, Mihailide C, Bamford H. Reverse vesicouterine fold dissection for laparoscopic hysterectomy after prior cesarean deliveries. Obstet Gynecol. 2016;128(3):629–633. [DOI] [PubMed] [Google Scholar]
- 13. Tan-Kim J, Menefee SA, Reinsch CS, et al. Laparoscopic hysterectomy and urinary tract injury: Experience in a health maintenance organization. J Minim Invasive Gynecol. 2015;22(7):1278–1286. [DOI] [PubMed] [Google Scholar]
- 14. Wesol A, Woolley S. Impact of power morcellator removal on hysterectomy practice patterns. Eur J Obstet Gynecol Reprod Biol. 2017;215:41–44. [DOI] [PubMed] [Google Scholar]
- 15. Wasson M, Magtibay P, 2nd, Magtibay P, Magrina J. Incidence of occult uterine malignancy following vaginal hysterectomy with morcellation. J Minim Invasive Gynecol. 2017;24(4):665–669. [DOI] [PubMed] [Google Scholar]
- 16. Rimbach S, Schempershofe M. In-bag morcellation as a routine for laparoscopic hysterectomy. Biomed Res Int. 2017;2017:6701916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krentel H, Wilde RL. Laparoscopic supracervical hysterectomy with in-bag morcellation in very large uterus. Case Rep Med. 2017;2017:9410571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meurs EAIM, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24(5):843–849. [DOI] [PubMed] [Google Scholar]
- 19. Nezhat C, Lavie O, Lemyre M, Gemer O, Bhagan L, Nezhat C. Laparoscopic hysterectomy with and without a robot: Stanford experience. JSLS. 2009;13(2):125–128. [PMC free article] [PubMed] [Google Scholar]
- 20. Woelk JL, Casiano ER, Weaver AL, Gostout BS, Trabuco EC, Gebhart JB. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121(1):87–95. [DOI] [PubMed] [Google Scholar]
- 21. Chiu LH, Chen CH, Tu PC, Chang CW, Yen YK, Liu WM. Comparison of robotic surgery and laparoscopy to perform total hysterectomy with pelvic adhesions or large uterus. J Minim Access Surg. 2015;11(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borahay MA, Tapısız ÖL, Alanbay İ Kılıç GS. Outcomes of robotic, laparoscopic and open hysterectomy for benign conditions in obese patients. J Turk Ger Gynecol Assoc. 2018;19(2):72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smorgick N, Dalton VK, Patzkowsky KE, Hoffman MR, Advincula AP, As-Sanie S. Comparison of 2 minimally invasive routes for hysterectomy of large uteri. Int J Gynaecol Obstet. 2013;122(2):128–131. [DOI] [PubMed] [Google Scholar]
- 24. El Hachem L, Acholonu UC, Jr, Nezhat FR. Postoperative pain and recovery after conventional laparoscopy compared with robotically assisted laparoscopy. Obstet Gynecol. 2013;121(3):547–553. [DOI] [PubMed] [Google Scholar]
- 25. Soliman PT, Langley G, Munsell MF, Vaniya HA, Frumovitz M, Ramirez PT. Analgesic and antiemetic requirements after minimally invasive surgery for early cervical cancer: A comparison between laparoscopy and robotic surgery. Ann Surg Oncol. 20(4)13;20:1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leitao MM, Jr., Malhotra V, Briscoe G, et al. Postoperative pain medication requirements in patients undergoing computer-assisted (“Robotic”) and standard laparoscopic procedures for newly diagnosed endometrial cancer. Ann Surg Oncol. 20(11)13;20:3561–3567. [DOI] [PubMed] [Google Scholar]