Abstract
Novel cancer therapeutics contribute to a steadily declining cancer mortality. However, several of these new therapies target pathways also involved in the cardiovascular system thus causing cardiotoxic side effects such as chemotherapy-induced heart failure (CIHF). This might limit the applicability of these effective treatments in a relevant number of patients. Furthermore, given the improving cancer survival rates, chemotherapy-induced cardiotoxic complications receive increasing attention given their potential impact on long-term morbidity and mortality. The understanding of molecular mechanisms that underlie CIHF is crucial for future improvement of pharmacodynamics of these therapeutics but also for developing specific interventions to prevent CIHF. Here, we discuss molecular mechanisms underlying CIHF of novel cancer therapeutics including a short synopsis on clinical management of patients suffering from CIHF.
Keywords: Chemotherapy, targeted therapy, cardiotoxicity, heart failure (HF)
Introduction
Cardiotoxicity is generally regarded one of the most frequent and serious complications of cancer therapies. Some authors refer to all cardiovascular complications of cancer therapies as “cardiotoxicity”. Chemotherapy-induced heart failure (CIHF) or cancer therapeutics related cardiac dysfunction (CTRCD) are often used synonymously, and are defined clinically by a reduction in left-ventricular ejection fraction (LVEF) below certain cut-offs. The current consensus statement of the European Society of Cardiology (ESC) on cancer treatments and cardiovascular toxicity defines “cardiotoxicity” as a ≥10% reduction in LVEF below 50%, assessed via echocardiography (1). This review will follow this definition but will generally focus on CIHF resulting from myocardial dysfunction. Historically, 2 distinct types of CIHF have been described (2): type 1 or the anthracycline like type, and type 2 or the trastuzumab like type. This classification was derived from mechanistic and clinical differences in the effects of the two leading substances causing cardiotoxicity. Yet, it has to be acknowledged that characteristics initially assigned to this classification, i.e., irreversibility of type 1 and reversibility of type 2 cardiotoxicity, are no longer valid since both types show relevant overlap. Additionally, several other classical chemotherapeutics are associated with an increased risk of CIHF and this list is steadily increasing with developments of novel cancer therapeutics such as so called targeted therapies or immunotherapeutics (Table 1). Facing this increasing diversity some authors postulate new classifications of cardiotoxicity based on pharmacodynamic properties and mechanistic pathophysiology. The latter seems reasonable given that new insight in molecular mechanisms might not only determine the clinical course of the complications, but also offer new strategies of preventive and therapeutic interventions.
Table 1. Chemotherapeutic agents associated with CIHF and their molecular targets, modified from (3).
| Class | Agent | Molecular mechanism | Incidence of CIHF in % |
|---|---|---|---|
| TKIs based on monoclonal antibodies | Bevacizumab | VEGF-A inhibition | 2–4 |
| Trastuzumab | Inhibition of HER2/HER2 dimerization by binding of extracellular subdomain IV | Up to 27 when combined with anthracyclines | |
| Ado-trastuzumab emtansine | Trastuzumab conjugated to cytotoxic tubulin inhibitor emtansine | 1.8 | |
| Pertuzumab | Inhibition of HER2/HER3 dimerization by binding of extracellular subdomain II | 4.5–14.5 | |
| Small molecule TKIs | Sunitinib | Several VEGFR, PDGF-R, GCSF-R, c-KIT, RET, FLT3, AMPK | 1–27 |
| Sorafenib | VEGFR-2 and -3, PDGF-R, c-Kit, Raf kinase family | 1.9–11 | |
| Lapatinib | HER2 and HER1/EGFR | 0.9–4.9 | |
| Dasatinib | Bcr-abl, c-KIT, Src, ephrins | 8–9 | |
| Dabrafenib | B-Raf | Up to 10 | |
| Imatinib | Bcr-abl, PDGF-R, M-CSF-R | <1 | |
| Nilotinib | Bcr-abl, c-KIT | <1 | |
| Ponatinib | BCR-abl, FGF-R, VEGFR-2, PDGF-R, FLT3 | 3–15 | |
| Trametinib | MEK1 and MEK2 | Up to 10 | |
| Proteasome inhibitors | Bortezomib | Reversible inhibition of the 20S proteasome | 2–5 |
| Carfilzomib | Irreversible inhibition of the 20S proteasome | 7 |
PDGF-R, platelet-derived growth factor receptor; GCSF-R, granulocyte colony-stimulating factor receptor; c-KIT, mast/stem cell growth factor receptor; FGF-R, fibroblast growth factor receptor; FLT3, Fms like tyrosine kinase 3; AMPK, adenosine monophosphate-activated protein kinase; Src, proto-oncogene tyrosine-protein kinase; Ephrins, Eph family receptor ligands; Bcr-abl, kinase product of the Philadelphia translocation; MEK, mitogen-activated protein kinase; M-CSF-R, macrophage colony-stimulating factor receptor.
This review will discuss the most important novel cancer therapeutics causing CIHF, elucidate the underlying molecular mechanisms and provide a quick synopsis of the current guidelines concerning clinical practice. Check point inhibitors are omitted here as they will be discussed in detail in a separate contribution of this focused issue.
HER2 inhibitors/EGF dependent therapeutics
The human epidermal growth factor receptor 2 (HER2 or ErbB2 in nonhumans) is overexpressed in 25–30% of breast cancer cases and indicates higher malignancy with significantly shortened overall survival and shorter time to relapse (3). As a member of the ErbB-family (ErbB 1–4) of transmembrane growth factor receptor tyrosine kinases (RTK), its activation leads to receptor dimerization and downstream activation of oncogenic targets (4). Less than 15 years after the first description of HER2 (5), the FDA approval of trastuzumab, a monoclonal antibody directed against the extracellular subdomain IV of HER2, marked the dawn of targeted cancer therapeutics. Although classical chemotherapeutic side effects such as myelosuppression or alopecia were not severely increased, the pivotal phase III trial showed dramatically increased rates of LV-dysfunction (6). CIHF upon treatment with trastuzumab is generally considered to be reversible (2), however this concept has recently been questioned (7).
In vitro and in vivo evidence point toward a crucial role of ErbB2-signaling in the fetal and adult murine heart: germline knock-out (KO) of ErbB2 in mice led to severely impaired embryonic development with significant cardiac malformations in unviable animals (8), whereas mice with a cardiac specific conditional ErbB2-KO developed classical features of dilated cardiomyopathy (9) and were especially susceptible to triggers of heart failure (HF) such as pressure-overload or anthracycline therapy (10). The importance of ErbB2 and ErbB4 for cardiac homeostasis is further supported by the observation of their reduced expression in human failing heart in comparison to healthy controls (11). Binding of the ErbB ligand neuregulin-1 leads to dimerization of ErbB2/ErbB4, thereby promoting a variety of cardioprotective effects: translocation of ErbB2 into the mitochondria regulates energy homeostasis (12), phosphoinositide 3-kinase-Akt (PI3K) activation increases cardiomyocyte survival (13), Src-Focal adhesion kinase (FAK) signaling improves myocardial ultrastructure (14) and regulation of Ca2+-homeostasis together with endothelial nitric oxide (NO) synthase (eNOS) activation enhance cardiac inotropy (12-15). The beneficial effects of neuregulin-1 have been demonstrated in various animal models of HF (16) and humans (17), making it a potential candidate for future HF therapy. Finally, there is evidence of apoptosis induction through HER2 inhibition. A cardiac specific conditional KO of ErbB2 showed increased apoptosis of cardiomyocytes in the ventricle, which was ameliorated after overexpression of the anti-apoptotic Bcl-XL (10). Mice treated with trastuzumab exhibited apoptosis corresponding with increase in caspase-3/7 activation (18) whereas a significant change in the ratio of pro- and antiapoptotic proteins after trastuzumab treatment has been shown in vitro (19).
In conclusion, downstream signaling of HER2/ErbB2 is essential for fetal development and stress-resistance in the adult heart. Thus, it is pathophysiologically plausible that trastuzumab-induced blockage of signaling downstream of HER2/ErbB2 can lead to the development of cardiotoxicity particularly in the context of other stressors such as age, cardiovascular comorbidity or co-medication with anthracyclines (Figure 1).
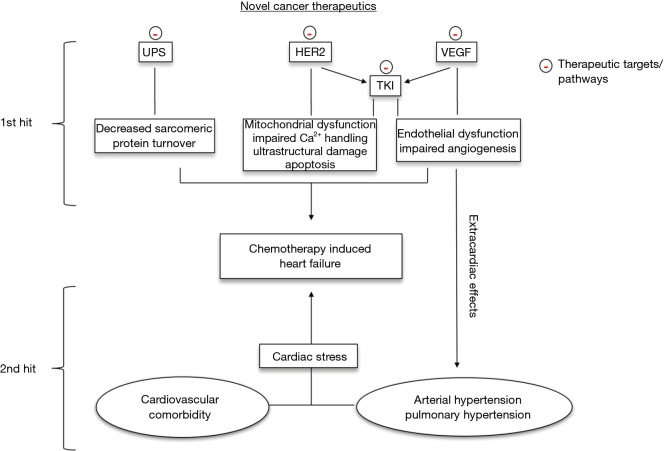
Figure 1.
Mechanisms underlying chemotherapy induced heart failure (CIHF). 1st hit: interference with different pathways (among others: VEGF, vascular endothelial growth factor; HER2, human epidermal growth factor receptor 2), associated sub-receptor tyrosine kinases (TKI) and basic cellular systems (UPS, ubiquitin-proteasome-system) leads to distinct molecular effects predisposing to cardiotoxicity. 2nd hit: together with the individual cardiovascular comorbidity, systemic extracardiac effects of certain therapeutics increase cardiac stress. Both hits are essential for the development of CIHF.
Based on these findings alternative ways of inhibiting HER2-signalling in cancer cells have been investigated. Ado-trastuzumab emtansine, pertuzumab and lapatinib accomplish HER2-antagonism by targeting alternative receptor-epitopes or acting in a ligand-independent manner and have demonstrated a reduced incidence of cardiotoxicity (20-22). However it must be noted, that patients in these trials were highly-selected: they were younger, had a higher baseline-LVEF and less cardiovascular co-morbidities (23). Also, ado-trastuzumab emtansine and lapatinib were initiated as part of a second-line therapy in patients with disease-progression after previous trastuzumab treatment and excluded patients that had developed severe CIHF under trastuzumab. Furthermore, lapatinib which serves as a tyrosine kinase inhibitor (TKI) for HER1 and HER2 receptors might have distinct cardioprotective effects through activation of 5’AMP-activated protein kinase which might protect from ischemic injury (24). Thus, based on clinical data a final appraisal on differences in cardiotoxicity across these substances possibly indicating differential molecular effects is currently difficult.
VEGF dependent therapeutics
Limited oxygen supply leads to expression of hypoxia-inducible-factor (HIF-1), a transcription factor promoting over 60 target genes including erythropoietin and vascular endothelial growth factor (VEGF) (25). VEGF signaling is essential for angiogenesis in malignant disease but also in rheumatic disorders and physiological tissue function (26). Since the first identification of VEGF by Senger and colleagues in 1983, five members of the VEGF-family (VEGF-A, VEGF-B, VEGF-C, VEGF-D and PGF) and three VEGF-receptors of the RTK-superfamily (VEGFR-1, VEGFR-2 and VEGFR-3) have been described (27,28). Whereas VEGF-C, VEGF-D and VEGFR-3 are primarily involved in lymphangiogenesis, alternatively spliced isoforms of VEGF-A mainly activate VEGFR-1 and VEGFR-2, leading to dimerization and autophosphorylation, which results in pro-angiogenic signaling (26).
The concept of anti-angiogenesis to treat cancer was thought of and proposed even before the discovery of VEGF (29) and led to FDA approval of bevacizumab, the first class member of VEGF-inhibitors, in 2004. Bevacizumab is a monoclonal antibody targeting all isoforms of circulating VEGF-A, that has been used successfully in selected patients suffering from colorectal, breast, lung, ovarian, cervix and brain cancers (30-35). Second generation VEGF-inhibitors like sorafenib and sunitinib belong to the class of so called small molecules and inhibit angiogenic signaling in an antibody-independent manner. Both target intracellular domains of multiple both non-receptor and receptor tyrosine-kinases, including VEGFR-1-3.
Arterial hypertension (aHTN) is the most common side effect of all anti-angiogenic agents and, dependent on cut off values for definition of aHTN, has been reported in 70% of patients treated with bevacizumab (36) and 24% to 22% of patients receiving sorafenib and sunitinib, respectively (37,38). Remarkably, aHTN is not a classical side effect but rather a marker of efficacy of anti-angiogenic regimes and it has been demonstrated that the incidence of aHTN correlates with progression free survival in patients treated with sunitinib (39,40). Pathophysiologically, aHTN typically precedes VEGF-dependent CIHF.
Endothelial dysfunction seems to play a central role in the pathophysiology of antiangiogenic-induced aHTN. VEGF leads to upregulation of endothelial and inducible NO synthase, thereby increasing levels of NO within the endothelium up to 5-fold (41,42). Infusion of VEGF induces systemic vasodilation in rabbits, resulting in an immediate decrease in systemic blood pressure and this effect has been shown to be NO-dependent (43). Sunitinib-mediated VEGF-inhibition increased Endothelin-1 serum levels in humans and rats up to 3-fold, resulting in 15–30 mmHg rise in systolic blood pressure and an attenuation of nocturnal dipping. These effects were completely reversible after discontinuation of sunitinib treatment (44). Lankhorst and colleagues have shown that treatment with endothelin-receptor blockers such as macicentan prevented sunitinib-induced aHTH, further supporting the critical role of endothelin-signaling in the pathophysiology of anti-angiogenetic induced aHTN (45). If untreated, chronically increased cardiac afterload leads to adaptive hypertrophic remodeling in the myocardium, finally terminating in hypertensive cardiomyopathy and HF. Similar to tumors, the increased oxygen demand in the myocardium is met by angiogenesis and results in HIF-1-signaling and consecutive activation of the VEGF-pathway (46). Sano and colleagues demonstrated that HIF inhibition via p53 led to left-ventricular systolic dysfunction in a model of chronic pressure overload (47). If this adaptive VEGF-signaling is blocked by cancer therapeutics, it becomes evident how the clinical endpoint of CIHF is reached.
Off-target effect contribution of multi-faceted TKIs such as sunitinib and sorafenib are difficult to define given the pleiotropy of tyrosine kinases in the organism. Sunitinib can induce myocyte apoptosis through ribosomal S6 kinase and thus contribute to the development of myocardial dysfunction (48). Among others, sunitinib inhibits the kinase domain of the platelet-derived growth factor receptor and the adenosine monophosphate-activated protein kinase, and thus prevents cardiomyocytes from adequately responding to stress (49). Furthermore, sunitinib impairs myocardial function in-vitro through mitochondrial damage (50).
Multi-faceted TKIs
Besides sunitinib and sorafenib, many other small molecule chemotherapeutic agents operate by targeting the kinase activity of several receptor and non-RTK. This is achieved by interfering with the vital ATP-pocket, which is highly conserved within the human kinome (51). Their small size of ≤500 Da, a fraction the size of antibody-based agents, enables them to easily translocate through plasma membranes (52).
By targeting the fusion protein product of the Philadelphia chromosome, Bcr-abl, the first small molecule TKI imatinib revolutionized therapy of chronic myelogenous leukemia, turning a fatal disease into a chronic condition (53,54). Five-year follow up of over 1,100 patients reported only one case of drug-related CIHF (55) and prospective studies have reported a low incidence of subclinical LV-dysfunction, similar to the expected incidence within the population (56). In contrast to this, experimental data from in vitro and in vivo animal studies have demonstrated imatinib-associated cardiotoxicity, which was rescued after retrovirally-mediated overexpression of c-Abl (50). This discrepancy is likely explained by artificially high doses of imatinib used for these experiments. To overcome imatinib-resistance due to point mutations at the ATP-binding site of Bcr-abl, more potent compounds have been developed: dasatinib, nilotinib and ponatinib each differ from imatinib in their affinity to Bcr-abl and other tyrosine kinases (57). Whereas this results in specific profiles of off-target effects, i.e., dasatinib associated pulmonary artery hypertension (58), nilotinib-associated prolongation of QT-interval (59) and ponatinib associated arterial hypertension (60), none has been reported to significantly increase incidence of CIHF.
Other multifaceted TKIs associated with CIHF include trametinib and dabrafenib, both involved in the RAS-RAF-MEK-ERK pathway and approved for treatment of melanoma and non-small cell lung cancer. A decrease of 10% in LVEF has been observed in up to 10% of patients treated with trametinib alone or in combination with dabrafenib (61,62). However, this is often asymptomatic and usually LVEF resolves to baseline levels after discontinuation of treatment (63). Experimental data for trametinib are lacking so far. However, due to its high selectivity for MEK1/2 and MEK1/2 having only one substrate (ERK1/2), mechanisms can be deduced from knowledge on this pathway in the heart. Mice genetically lacking ERK1/2 show normal cardiac phenotype (64) under rest, but high susceptibility for stressors such as ischemia or pressure overload with larger infarction areas and accelerated transition from hypertrophy to HF (65). Cardiotoxic effects following this “two-hit” hypothesis seem common for targeted therapeutics since already discussed above for inhibitors of the VEGF and HER2 pathway.
Proteasome inhibitors
Regulation of the eukaryotic cells’ proteome is essential for its function, including cancer cells and cardiac cells. The two major components that ensure a balanced protein turnover are the ubiquitin-proteasome system (UPS) and autophagy based on lysosomal degradation. In the UPS a process termed ubiquitination binds ubiquitin-molecules to a target protein, which is then degraded in the proteasome. Interestingly, it has been demonstrated that the activity of the proteasome is highest in heart and kidney, underlying the importance of a balanced proteome in these organs (66). Cardiomyocytes are end-differentiated cells that lack significant regenerative potential, making the heart more susceptible to protein turnover interventions like proteasome-inhibition (67,68). Aggregation of misfolded proteins has been detected in different forms of hypertrophic and dilated cardiomyopathy (69). The paramount example is desmin-related cardiomyopathies, that most often are caused by mutations in the desmin-associated chaperone a-B-crystallin (70). On the other hand, it has been shown that deliberate inhibition of the proteasome can reduce pathological hypertrophy in animal models of hypertensive heart disease (71).
Bortezomib is a specific reversible inhibitor of chymotrypsin-like activity at the 20S proteasome that has shown to significantly increase median overall survival in patients suffering from relapsing multiple myeloma (MM) (72). It was approved as a first-line therapy in MM in 2008. Toxic effects on the myocardium have been reported in vitro and in vivo (73,74), however large clinical trials have not shown a significant increase in CIHF (72). The significance of these findings remains unclear, as bortezomib induced HF often occurs in patients previously treated with combination chemotherapy that included anthracyclines (73). Carfilzomib is another proteasome inhibitor used for relapsed and bortezomib-refractory MM and has shown new-onset or worsening HF in 4–7% of patients (75-77). It differs mechanistically from its class member as it is more specific to the chymotrypsin-like catalytic site by targeting the ß5-subunit and inhibits the proteasome function in an irreversible manner (75). Importantly, is has been shown that this catalytic subunit is crucial for attenuating doxorubicin-induced cytotoxic effects in vitro (78).
Clinical practice directions
In 2016, the European Society of Cardiology published a comprehensive position paper on the management of cardiovascular toxicity in cancer patients (1). It provides crucial support in assessing the individual risk for cardiotoxicity in each patient before initiation of treatment including evaluation of cardiovascular risk factors, latent or manifest cardiovascular disease and myocardial dysfunction, monitoring during treatment using biomarkers and imaging techniques and implementation of primary or secondary prevention interventions as well as long-term surveillance. Interestingly, none of these interventions are specifically using or addressing molecular mechanisms of the cancer therapeutics. On the contrary, most recommendations are applied generally to cardiotoxicity independently of underlying therapeutics. For instance, manifest cardiotoxicity is treated according to general HF guidelines. This underlines our insufficient knowledge of molecular mechanisms of cancer therapeutics and calls for further study. The most effective prevention of cardiotoxicity today is the treatment of cardiovascular risk factors and diseases according to current guidelines. This is somehow reasonable and in line with our molecular understanding of anthracycline and trastuzumab toxicity which follows the multiple-hit concept and impaired stress tolerance through disruption of cardioprotective signaling pathways. A major challenge with respect to novel cancer therapeutics such as TKI with their pleiotropic effects is the accurate preclinical prediction of off-target effects involving the cardiovascular system as well as the individual risk prediction in the patient before therapy. Approaches using cardiomyocytes from pluripotent, inducible human stem cells might be one potential model for these objectives but still suffers several limitations precluding routine clinical application (79,80).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Zamorano JL, Lancellotti P, Rodriguez Munoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail 2017;19:9-42. 10.1002/ejhf.654 [DOI] [PubMed] [Google Scholar]
- 2.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 2005;23:2900-2. 10.1200/JCO.2005.05.827 [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. 10.1126/science.3798106 [DOI] [PubMed] [Google Scholar]
- 4.Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014;25:282-303. 10.1016/j.ccr.2014.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science 1985;229:974-6. 10.1126/science.2992089 [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 7.Riccio G, Coppola C, Piscopo G, et al. Trastuzumab and target-therapy side effects: Is still valid to differentiate anthracycline Type I from Type II cardiomyopathies? Hum Vaccin Immunother 2016;12:1124-31. 10.1080/21645515.2015.1125056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KF, Simon H, Chen H, et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 1995;378:394-8. 10.1038/378394a0 [DOI] [PubMed] [Google Scholar]
- 9.Ozcelik C, Erdmann B, Pilz B, et al. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci U S A 2002;99:8880-5. 10.1073/pnas.122249299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 2002;8:459-65. 10.1038/nm0502-459 [DOI] [PubMed] [Google Scholar]
- 11.Rohrbach S, Niemann B, Silber RE, et al. Neuregulin receptors erbB2 and erbB4 in failing human myocardium -- depressed expression and attenuated activation. Basic Res Cardiol 2005;100:240-9. 10.1007/s00395-005-0514-4 [DOI] [PubMed] [Google Scholar]
- 12.Lemmens K, Fransen P, Sys SU, et al. Neuregulin-1 induces a negative inotropic effect in cardiac muscle: role of nitric oxide synthase. Circulation 2004;109:324-6. 10.1161/01.CIR.0000114521.88547.5E [DOI] [PubMed] [Google Scholar]
- 13.Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol 2006;41:228-35. 10.1016/j.yjmcc.2006.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bersell K, Arab S, Haring B, et al. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 2009;138:257-70. 10.1016/j.cell.2009.04.060 [DOI] [PubMed] [Google Scholar]
- 15.Ding Y, Liu Z, Desai S, et al. Receptor tyrosine kinase ErbB2 translocates into mitochondria and regulates cellular metabolism. Nat Commun 2012;3:1271. 10.1038/ncomms2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Gu X, Li Z, et al. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol 2006;48:1438-47. 10.1016/j.jacc.2006.05.057 [DOI] [PubMed] [Google Scholar]
- 17.Gao R, Zhang J, Cheng L, et al. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol 2010;55:1907-14. 10.1016/j.jacc.2009.12.044 [DOI] [PubMed] [Google Scholar]
- 18.ElZarrad MK, Mukhopadhyay P, Mohan N, et al. Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. PLoS One 2013;8:e79543. 10.1371/journal.pone.0079543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grazette LP, Boecker W, Matsui T, et al. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J Am Coll Cardiol 2004;44:2231-8. 10.1016/j.jacc.2004.08.066 [DOI] [PubMed] [Google Scholar]
- 20.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. 10.1056/NEJMoa1209124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733-43. 10.1056/NEJMoa064320 [DOI] [PubMed] [Google Scholar]
- 22.Swain SM, Ewer MS, Cortes J, et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist 2013;18:257-64. 10.1634/theoncologist.2012-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponde NF, Lambertini M, de Azambuja E. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open 2016;1:e000073. 10.1136/esmoopen-2016-000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terai K, Hiramoto Y, Masaki M, et al. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol 2005;25:9554-75. 10.1128/MCB.25.21.9554-9575.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams JM, Difazio LT, Rolandelli RH, et al. HIF-1: a key mediator in hypoxia. Acta Physiol Hung 2009;96:19-28. 10.1556/APhysiol.96.2009.1.2 [DOI] [PubMed] [Google Scholar]
- 26.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011;2:1097-105. 10.1177/1947601911423031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieran MW, Kalluri R, Cho YJ. The VEGF pathway in cancer and disease: responses, resistance, and the path forward. Cold Spring Harb Perspect Med 2012;2:a006593. 10.1101/cshperspect.a006593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983;219:983-5. 10.1126/science.6823562 [DOI] [PubMed] [Google Scholar]
- 29.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182-6. 10.1056/NEJM197111182852108 [DOI] [PubMed] [Google Scholar]
- 30.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 31.Lauro S, Onesti CE, Righini R, et al. The use of bevacizumab in non-small cell lung cancer: an update. Anticancer Res 2014;34:1537-45. [PubMed] [Google Scholar]
- 32.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357:2666-76. 10.1056/NEJMoa072113 [DOI] [PubMed] [Google Scholar]
- 33.Narita Y. Drug review: Safety and efficacy of bevacizumab for glioblastoma and other brain tumors. Jpn J Clin Oncol 2013;43:587-95. 10.1093/jjco/hyt051 [DOI] [PubMed] [Google Scholar]
- 34.Tewari KS, Sill MW, Long HJ, 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014;370:734-43. 10.1056/NEJMoa1309748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia A, Singh H. Bevacizumab and ovarian cancer. Ther Adv Med Oncol 2013;5:133-41. 10.1177/1758834012467661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izzedine H, Ederhy S, Goldwasser F, et al. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol 2009;20:807-15. 10.1093/annonc/mdn713 [DOI] [PubMed] [Google Scholar]
- 37.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol 2009;48:9-17. 10.1080/02841860802314720 [DOI] [PubMed] [Google Scholar]
- 38.Wu S, Chen JJ, Kudelka A, et al. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol 2008;9:117-23. 10.1016/S1470-2045(08)70003-2 [DOI] [PubMed] [Google Scholar]
- 39.Robinson ES, Khankin EV, Karumanchi SA, et al. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol 2010;30:591-601. 10.1016/j.semnephrol.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2011;103:763-73. 10.1093/jnci/djr128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouloumie A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res 1999;41:773-80. 10.1016/S0008-6363(98)00228-4 [DOI] [PubMed] [Google Scholar]
- 42.Facemire CS, Nixon AB, Griffiths R, et al. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 2009;54:652-8. 10.1161/HYPERTENSIONAHA.109.129973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horowitz JR, Rivard A, van der Zee R, et al. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension. Evidence for a maintenance role in quiescent adult endothelium. Arterioscler Thromb Vasc Biol 1997;17:2793-800. 10.1161/01.ATV.17.11.2793 [DOI] [PubMed] [Google Scholar]
- 44.Kappers MH, van Esch JH, Sluiter W, et al. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 2010;56:675-81. 10.1161/HYPERTENSIONAHA.109.149690 [DOI] [PubMed] [Google Scholar]
- 45.Lankhorst S, Kappers MH, van Esch JH, et al. Treatment of hypertension and renal injury induced by the angiogenesis inhibitor sunitinib: preclinical study. Hypertension 2014;64:1282-9. 10.1161/HYPERTENSIONAHA.114.04187 [DOI] [PubMed] [Google Scholar]
- 46.Oka T, Akazawa H, Naito AT, et al. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res 2014;114:565-71. 10.1161/CIRCRESAHA.114.300507 [DOI] [PubMed] [Google Scholar]
- 47.Sano M, Minamino T, Toko H, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 2007;446:444-8. 10.1038/nature05602 [DOI] [PubMed] [Google Scholar]
- 48.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer 2007;7:332-44. 10.1038/nrc2106 [DOI] [PubMed] [Google Scholar]
- 49.Chintalgattu V, Ai D, Langley RR, et al. Cardiomyocyte PDGFR-beta signaling is an essential component of the mouse cardiac response to load-induced stress. J Clin Invest 2010;120:472-84. 10.1172/JCI39434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerkela R, Woulfe KC, Durand JB, et al. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin Transl Sci 2009;2:15-25. 10.1111/j.1752-8062.2008.00090.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng H, Force T. Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ Res 2010;106:21-34. 10.1161/CIRCRESAHA.109.206920 [DOI] [PubMed] [Google Scholar]
- 52.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer 2006;6:714-27. 10.1038/nrc1913 [DOI] [PubMed] [Google Scholar]
- 53.Trela E, Glowacki S, Blasiak J. Therapy of chronic myeloid leukemia: twilight of the imatinib era? ISRN Oncol 2014;2014:596483. 10.1155/2014/596483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003;348:994-1004. 10.1056/NEJMoa022457 [DOI] [PubMed] [Google Scholar]
- 55.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006;355:2408-17. 10.1056/NEJMoa062867 [DOI] [PubMed] [Google Scholar]
- 56.Estabragh ZR, Knight K, Watmough SJ, et al. A prospective evaluation of cardiac function in patients with chronic myeloid leukaemia treated with imatinib. Leuk Res 2011;35:49-51. 10.1016/j.leukres.2010.08.020 [DOI] [PubMed] [Google Scholar]
- 57.Bitencourt R, Zalcberg I, Louro ID. Imatinib resistance: a review of alternative inhibitors in chronic myeloid leukemia. Rev Bras Hematol Hemoter 2011;33:470-5. 10.5581/1516-8484.20110124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ozgur Yurttas N, Eskazan AE. Dasatinib-induced pulmonary arterial hypertension. Br J Clin Pharmacol 2018;84:835-45. 10.1111/bcp.13508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Novartis. Nilotinib prescribing information. Novartis Pharmaceuticals Corporation. 2007 2007
- 60.Muller MC, Cervantes F, Hjorth-Hansen H, et al. Ponatinib in chronic myeloid leukemia (CML): Consensus on patient treatment and management from a European expert panel. Crit Rev Oncol Hematol 2017;120:52-9. 10.1016/j.critrevonc.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 61.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30-9. 10.1056/NEJMoa1412690 [DOI] [PubMed] [Google Scholar]
- 62.Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015;386:444-51. 10.1016/S0140-6736(15)60898-4 [DOI] [PubMed] [Google Scholar]
- 63.Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol 2015;7:122-36. 10.1177/1758834014566428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lips DJ, Bueno OF, Wilkins BJ, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation 2004;109:1938-41. 10.1161/01.CIR.0000127126.73759.23 [DOI] [PubMed] [Google Scholar]
- 65.Purcell NH, Wilkins BJ, York A, et al. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A 2007;104:14074-9. 10.1073/pnas.0610906104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel MB, Majetschak M. Distribution and interrelationship of ubiquitin proteasome pathway component activities and ubiquitin pools in various porcine tissues. Physiol Res 2007;56:341-50. [DOI] [PubMed] [Google Scholar]
- 67.Mearini G, Schlossarek S, Willis MS, et al. The ubiquitin-proteasome system in cardiac dysfunction. Biochim Biophys Acta 2008;1782:749-63. 10.1016/j.bbadis.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 68.Carrier L. Too much of a good thing is bad: proteasome inhibition induces stressed hearts to fail. Cardiovasc Res 2010;88:389-90. 10.1093/cvr/cvq315 [DOI] [PubMed] [Google Scholar]
- 69.Willis MS, Patterson C. Proteotoxicity and cardiac dysfunction--Alzheimer's disease of the heart? N Engl J Med 2013;368:455-64. 10.1056/NEJMra1106180 [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Osinska H, Dorn GW, 2nd, et al. Mouse model of desmin-related cardiomyopathy. Circulation 2001;103:2402-7. 10.1161/01.CIR.103.19.2402 [DOI] [PubMed] [Google Scholar]
- 71.Meiners S, Dreger H, Fechner M, et al. Suppression of cardiomyocyte hypertrophy by inhibition of the ubiquitin-proteasome system. Hypertension 2008;51:302-8. 10.1161/HYPERTENSIONAHA.107.097816 [DOI] [PubMed] [Google Scholar]
- 72.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 2003;348:2609-17. 10.1056/NEJMoa030288 [DOI] [PubMed] [Google Scholar]
- 73.Nowis D, Maczewski M, Mackiewicz U, et al. Cardiotoxicity of the anticancer therapeutic agent bortezomib. Am J Pathol 2010;176:2658-68. 10.2353/ajpath.2010.090690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orciuol O, Buda G, Cecconi N, et al. Unexpected cardiotoxicity in haematological bortezomib treated patients. Br J Haematol 2007;138:396-7. 10.1111/j.1365-2141.2007.06659.x [DOI] [PubMed] [Google Scholar]
- 75.Grandin EW, Ky B, Cornell RF, et al. Patterns of cardiac toxicity associated with irreversible proteasome inhibition in the treatment of multiple myeloma. J Card Fail 2015;21:138-44. 10.1016/j.cardfail.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 76.Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood 2012;120:2817-25. 10.1182/blood-2012-05-425934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siegel D, Martin T, Nooka A, et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica 2013;98:1753-61. 10.3324/haematol.2013.089334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spur EM, Althof N, Respondek D, et al. Inhibition of chymotryptic-like standard proteasome activity exacerbates doxorubicin-induced cytotoxicity in primary cardiomyocytes. Toxicology 2016;353-354:34-47. 10.1016/j.tox.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 79.Chen IY, Matsa E, Wu JC. Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat Rev Cardiol 2016;13:333-49. 10.1038/nrcardio.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshida Y, Yamanaka S. Induced Pluripotent Stem Cells 10 Years Later: For Cardiac Applications. Circ Res 2017;120:1958-68. 10.1161/CIRCRESAHA.117.311080 [DOI] [PubMed] [Google Scholar]