Abstract
Pseudomonas aeruginosa infections are a leading cause of death in patients suffering from respiratory diseases. The multidrug-resistant nature of Pseudomonas is potentiated by a process known as quorum sensing. The aim of this study was to reveal new inhibitors of a well-validated but quite unexplored target, enoyl-ACP reductase, which contributes acyl chain lengths of N-acyl homoserine lactones that are major signaling molecules in gram-negative bacteria. In the present study, the crystal structure of FabI (PDB, ID 4NR0) was used for the structure-based identification of quorum sensing inhibitors of Pseudomonas aeruginosa. Active site residues of FabI were identified from the complex of FabI with triclosan and these active site residues were further used to screen for potential inhibitors from natural database. Three-dimensional structures of the 75 natural compounds were retrieved from the ZINC database and screened using PyRX software against FabI. Thirty-eight molecules from the initial screening were sorted on the basis of binding energy, using the known inhibitor triclosan as a standard. These molecules were subjected to various secondary filters, such as Lipinski’s Rule of Five, ADME, and toxicity. Finally, eight lead-like molecules were obtained after their evaluation for drug-like characteristics. The present study will open a new window for designing QS inhibitors against P. aeruginosa.
Keywords: Microbial drug resistance, Quorum sensing, Enoyl-ACP reductase, Pseudomonas aeruginosa
Introduction
Pseudomonas aeruginosa is an important gram-negative bacterium associated with various nosocomial infections, such as cystic fibrosis, catheter-associated infections, as well as wound-related infections (Smith and Iglewski 2003; Taylor et al. 2014). P. aeruginosa harbors various traits, including virulence phenotypes and biofilm formation, which render it resistant to various antimicrobial treatments (Fuente-nu and Breidenstein 2011). P. aeruginosa forms microbial communities encased in an extracellular polysaccharide layer which protects it from various antimicrobial treatments (Høiby et al. 2001; Costerton and Stewart 1999). Quorum sensing regulates both biofilm formation and virulence factor production in P. aeruginosa.
Quorum sensing (QS) is a cell-to-cell communication process in which bacteria utilize small diffusible signaling molecules to communicate with each other as a function of cell density (Fuqua et al. 1994). As these autoinducers reach a particular threshold concentration, they bind to the regulators and modulate the expression of associated genes (Miller 2001). P. aeruginosa has three QS systems which are interconnected hierarchically to each other, i.e., las, rhl, and pqs; the lasIR system sits at the top in the hierarchy and regulates both rhlIR and PQS system (Lee and Zhang 2015). Two of the major signaling molecules in Pseudomonas aeruginosa, 3-oxo-dodecanoyl homoserine lactone and N-butanoyl homoserine lactone, belong to the N-acyl homoserine lactone family, whereas a third signaling molecule, PQS, belongs to the quinolone family (Lee and Zhang 2015).
QS is a viable target to eradicate the problem of antimicrobial resistance due to selection pressure by the excessive use of antimicrobial agents (LaSarre and Federle 2013). Therefore, various QS inhibition strategies have been employed to halt the communication process among the bacteria (LaSarre and Federle 2013). Most exploited are autoinducer mimics produced by various plants, in addition to signaling molecule degrading enzymes (Koh et al. 2013). However, pathways essential for the synthesis of QS molecules remain widely unexplored. More et al. reported that intermediates from Type II fatty acid synthesis are substrates for the synthesis of acyl homoserine lactone, along with AdoMeth (Moré et al. 1996). The Type II fatty acid pathway is highly conserved among prokaryotes. It is different from the Type I fatty acid synthesis pathway of other animals, including humans, as each step in the Type II pathway is catalyzed by various enzymes, when compared to the FAS complex of the Type I fatty acid synthesis pathway (Chirala et al. 1997; White et al. 2005). FabI is an enoyl reductase enzyme which catalyzes the critical last step of elongation in fatty acid synthesis (Lu and Tonge 2008). Mutational studies of P. aeruginosa FabI have revealed the significant role of enoyl-acyl carrier protein reductase in the synthesis of 3-oxo-C12 HSL molecules, as the level of 3-oxo-C12 HSL production is decreased when the FabI gene is mutated (Hoang and Schweizer 1999). There are very few reports on the projection of FabI as a target for the development of QS quenching agents.
Testing large number of compounds in vitro for their biological activities is a time-consuming and costly process, with very few chances of success. Computational and experimental methods have worked hand-in-hand in screening for biological activity. The rate of discovery of new drugs is enhanced because of the availability of crystal structure data of proteins, and various virtual screening and molecular docking software. In the present study, we have used the crystal structure of FabI of P. aeruginosa for the first time in a structure-based virtual screening SB-VS approach to identify potential lead compounds which could target enoyl-Acyl Carrier Protein (ACP) reductase (FabI), and may help in designing seven QS quenching agents.
Materials and methods
Macromolecule preparation
The crystal structure of P. aeruginosa enoyl-ACP reductase (FabI) at 1.4 Å resolution was extracted from the protein data bank (PDB, ID 4NR0, Lee et al. 2015). The protein structure obtained from PDB was not suitable for molecular docking in its native form bound to triclosan and NAD+. Therefore, optimization and minimization of protein structure were performed using Protein Preparation Wizard, initiating with the removal of water, and after that, the bound triclosan and NAD+.
Ligand preparation
A ZINC library, containing approximately 75 natural compounds that were available commercially, was selected to identify FabI inhibitors of natural origin (Irwin and Shoichet 2005). We selected the library of natural compounds on the basis of their evolutionary targets, as well as their antimicrobial properties. Structural coordinates of the compounds were retrieved from the ZINC database in SDF format. Therefore, they were converted from 2D to 3D using Open Babel software, taking care that, during format conversion, there were no changes to the structure of ligands.
Virtual screening
Virtual screening was performed using PyRX (Dallakyan and Olson 2015). PyRX was embedded with both Autodock (Morris et al. 2008) and Autodock Vina (Trott and Olson 2009), and its scoring function was based on the Lamarckian genetic algorithm. The present study was performed using Autodock. A grid box with dimensions X:36.4078, Y: 34.1745, Z:38.4251 was generated using active site residues (LEU150, ALA152, GLU153, ARG154, ALA157, GLU170, ARG174, GLY245, GLU246, ILE247, TYR249, ASN255, THR256) to stipulate the interface area of monomers. Results obtained were sorted by binding energy.
ADME and toxicity studies
Selected molecules were filtered using Lipinski’s Rule of Five (Lipinski 2004), and after that, ADME studies for the screened molecules were carried out using the Swiss ADME module (Daina et al. 2014; Daina and Zoete 2016). The Swiss ADME module analyzes pharmaceutical properties necessary for drug-like candidates, i.e., blood–brain barrier BBB permeability, GI absorption, skin permeation, octanol/water, and water/gas logPs. Toxicity studies of the screened molecules were carried out using the Lazar toxicity module (Maunz et al. 2013).
Molecular docking
Molecules filtered using primary and secondary filters were docked against FabI (enoyl-ACP reductase) to reveal the best inhibitor among the screened lead molecules. AutoDock was used for further docking of molecules against FabI. The Grid box defined interaction sites. Ligands were docked into this receptor grid using the Lamarckian genetic algorithm. The scoring function of AutoDock was based on the Amber force field, and results were analyzed and sorted by binding energy.
Results
The present study was carried out with the aim of identifying different inhibitors of P. aeruginosa by utilizing the Type II fatty acid pathway, whose intermediates play vital roles in the synthesis of N-acyl homoserine lactones as a target by using different primary and secondary filters, such as Lipinski’s Rule of Five, ADME, and toxicity prediction. Preliminary virtual screening for the lead molecules from the ZINC library against active site of FabI was performed using PyRX. Lead molecules obtained after the primary filter of binding energy were further refined using Lipinski’s Rule of Five as a secondary filter. Final screened hits were checked for their ADME characteristics and toxicity profiles. Molecular docking of the final hits against the active site of FabI using AutoDock was performed to understand molecular interactions involved.
Virtual screening
Primary virtual screening of the ZINC database was performed using PyRX to identify lead molecules at a more rapid rate. Grid box was generated on the active site of the enzyme to increase the specificity of the screening process. Molecules were further sorted by binding energy, and 38 molecules with binding energy less than − 7 were selected for secondary screening; others below it were excluded.
ADME and toxicity filters
All of the 38 molecules selected from the screening process were subjected to ADME studies using SWISS ADME. SWISS-ADME identifies the suitability of molecules as lead-like substances by different parameters, such as BBB permeability, GI absorption, octanol/water partition coefficient, skin permeation, and whether the molecule is a substrate for Pgp or CYP450 isoforms, Lipinski’s filter, PAINS, and several other parameters. Of 38 candidate molecules, only 14 passed the ADME studies and were found to be suitable lead-like compounds. These 14 compounds were further judged for their toxicity using the Lazar toxicity module. Of the 14 molecules, eight were found to be safe and could be utilized as lead-like molecules. Details of these eight molecules, along with their respective ZINC ID and different characteristics, are provided in Table 1. To choose the best inhibitor and discern possible molecular interactions among these eight molecules and FabI, they were further docked against the active site of FabI and compared with the known inhibitor triclosan.
Table 1.
ADME and toxicity properties of the final screened molecules
| S.no. | ZINC ID | Mass | H Donor | H acceptor | logP | GI absorption | BBB permeant | P-gp substarte | CYP1A2 inhibitor | CYP2C19 Inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | CYP3A4 inhibitor | LogKp (skin permeation) | PAINS | Leadlikenes | Carcinogenicit y (Rat) | Carcinogenicit y (Mouse) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ZINC03869685 | 302.24 | 5 | 7 | 1.54 | High | No | No | Yes | No | No | Yes | Yes | − 7.05 | 1 Alert: catechol_A | Yes | Inactive | Inactive |
| 2 | ZINC05842416 | 286.24 | 4 | 6 | 2.66 | High | No | No | Yes | No | No | Yes | Yes | − 6.16 | 1 Alert: catechol_A | Yes | Inactive | Inactive |
| 3 | ZINC05765089 | 258.23 | 3 | 5 | 2.81 | High | No | No | Yes | No | No | Yes | Yes | − 5.88 | 0 Alert | Yes | Inactive | Inactive |
| 4 | ZINC03871576 | 270.24 | 3 | 5 | 3.02 | High | No | No | Yes | No | No | Yes | Yes | − 5.8 | 0 Alert | Yes | Inactive | Inactive |
| 5 | ZINC02030112 | 300.31 | 1 | 5 | 3.17 | High | Yes | No | Yes | Yes | No | Yes | Yes | − 5.88 | 0 Alert | Yes | Inactive | Inactive |
| 6 | ZINC01721693 | 274.27 | 5 | 1 | 0.72 | High | No | Yes | No | No | No | No | No | − 7.46 | 0 Alert | Yes | Inactive | Inactive |
| 7 | ZINC00001785 | 272.25 | 3 | 5 | 2.52 | High | No | Yes | Yes | No | No | No | Yes | − 6.17 | 0 Alert | Yes | Inactive | Inactive |
| 8 | ZINC14815320 | 272.25 | 2 | 5 | 3.14 | High | No | No | Yes | No | Yes | Yes | Yes | − 5.73 | 0 Alert | Yes | Inactive | Inactive |
Molecular docking
Molecular docking was performed to select the best inhibitor among the final eight compounds, involving interactions between the receptor and ligand, binding energy, ADME, and toxicity profile. Molecules chosen for docking were ZINC03869685, ZINC05842416, ZINC05765089, ZINC03871576, ZINC02030112, ZINC01721693, ZINC00001785, and ZINC14815320. These compounds were further docked against the active site of the enzyme FabI, and similarly, a well-known inhibitor of fatty acid synthesis, triclosan, was also docked against the active site of the same for final selection by interaction and binding energies (Table 2).
Table 2.
Final screened molecules with residues involved in the hydrogen binding and binding energy
| S. no. | ZINC ID | Hydrogen bonds | Residues involved | Binding energy | KI (µM) |
|---|---|---|---|---|---|
| 1 | ZINC03869685 | 8 | GLY13, ILE20, ALA21, SER93, SER148, LYS 166 | − 7.15 | 5.71 |
| 2 | ZINC05842416 | 3 | THR155, MET209, SER191 | − 7.02 | 7.1 |
| 3 | ZINC05765089 | 2 | VAL14, GLY95 | − 7.34 | 4.18 |
| 4 | ZINC03871576 | 5 | ILE195, ALA97, TYR159 | − 7.4 | 3.77 |
| 5 | ZINC02030112 | 2 | ILE20, LYS166 | − 7.11 | 6.19 |
| 6 | ZINC01721693 | 5 | ALA192, ILE195, TYR159, MET162 | − 6.75 | 11.27 |
| 7 | ZINC00001785 | 2 | ILE195 | − 7.31 | 4.41 |
| 8 | ZINC14815320 | 2 | ALA192, LYS166 | − 6.61 | 14.17 |
| 9 | Triclosan | 1 | ILE195 | − 6.7 | 12.35 |
Insight into the interactions of screened leads and triclosan with FabI
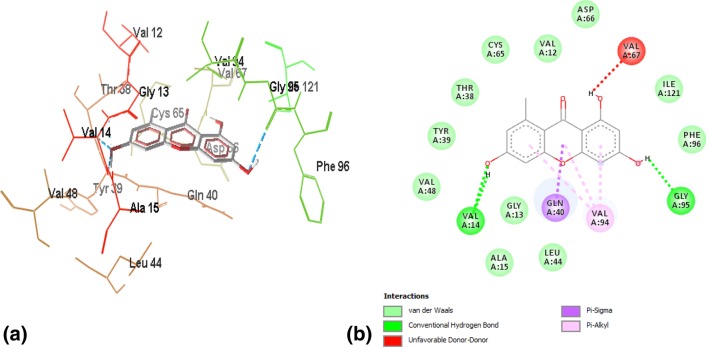
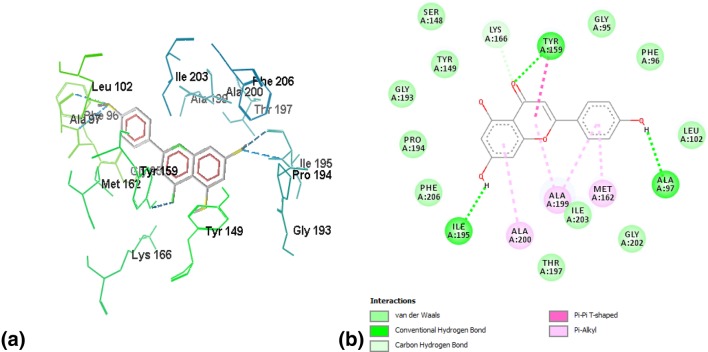
A single hydrogen bond strengthened interactions of the well-known inhibitor triclosan with FabI, with a binding energy of − 6.7. The hydrogen bond was formed between the hydrogen atom of ILE195 and the hydroxyl group of triclosan. Figure 1a, b demonstrates 3-D and 2-D plots of triclosan’s interaction profile with FabI.
Fig. 1.
a Wireframe model of binding interactions between triclosan and FabI. Hydrogen bonds are represented by blue dotted lines, whereas the residues other than ligand are the active site residues involved in different interactions. b 2-D plot of the interaction between triclosan and the active site of FabI. Triclosan is stabilized by one hydrogen bond with ILE195
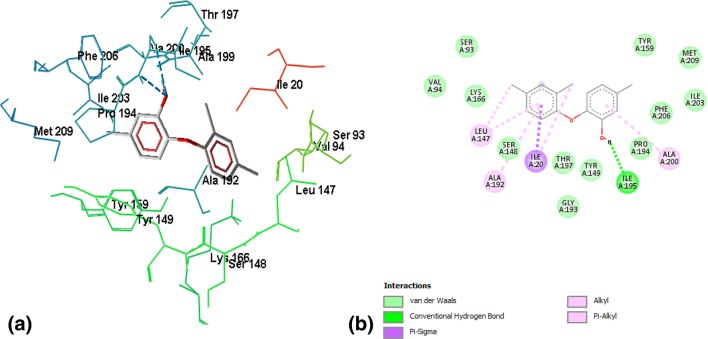
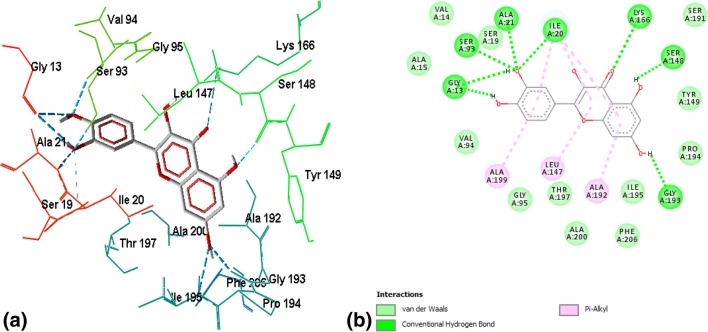
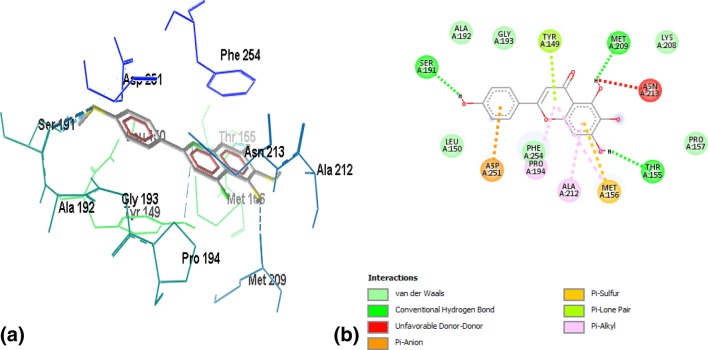
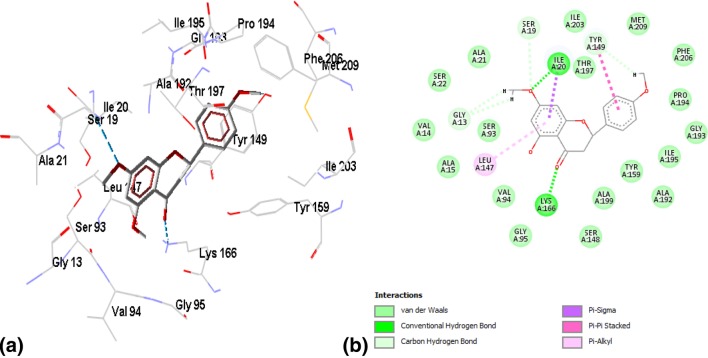
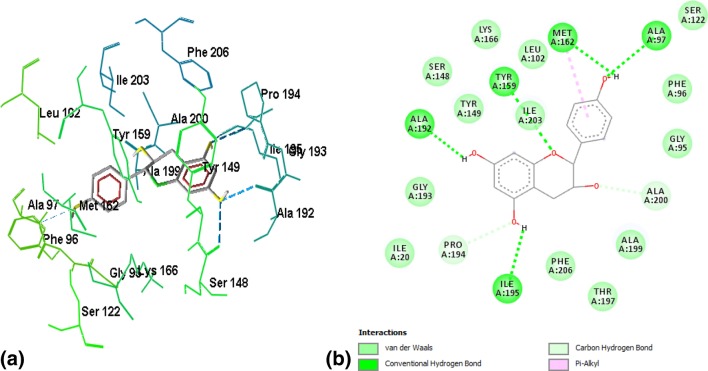
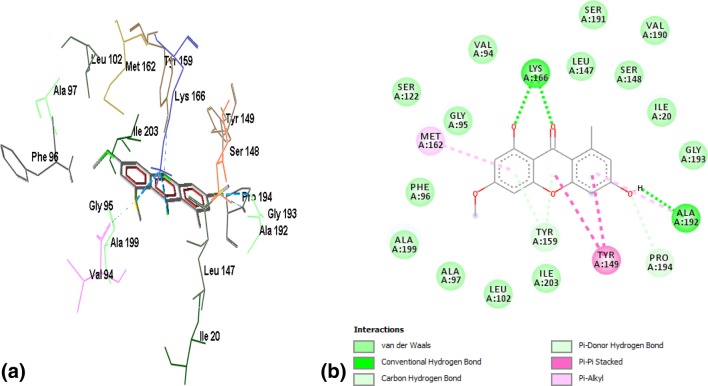
Binding of ZINC03869685 to the active site residues of FabI was stabilized by eight hydrogen bonds, with a binding energy of − 7.15, as shown in Fig. 2a, b. Amino acid residues involved in hydrogen bonding were GLY13, ILE20, ALA21, SER93, SER148, LYS 166, and GLY193. Figure 3a, b demonstrates the binding of ZINC05842416 to FabI. Three hydrogen bonds were observed in the stabilization of the complex, along with Van der Walls and alkyl interactions, with a binding energy of − 7.02. The complex of ZINC05765089 with FabI was stabilized by two hydrogen bonds contributed by VAL14 and GLY95, with a binding energy of − 7.34, as shown in Fig. 4a, b. The interaction profile of ZINC03871576 with FabI is shown in Fig. 5a, b. This complex was stabilized by three hydrogen bonds contributed by ILE195, ALA97, and TYR159 residues of the active site. The first hydrogen bond was formed between the NH3 group of ALA97 and the oxygen atom of the ligand, and the second hydrogen bond was formed between the NH3 and oxygen atoms of the ligand. The third hydrogen bond was observed between a hydrogen of ILE195 donating a group, and oxygen atoms of the ligand. A complex of ZINC 2030112 and FabI was stabilized by the formation of two hydrogen bonds, with a binding energy of − 7.11 (Fig. 6a, b). Amino acid residues involved in this hydrogen bond formation were LYS166 and ILE 20. Five hydrogen bonds were involved in the stabilization of the ZINC01721693-FabI complex, with a binding energy of − 6.75. The first hydrogen bond was formed between a hydrogen-donating group of the ligand and an oxygen-donating group of the macromolecule, whereas the second hydrogen bond was formed between the amino group of ILE195 and the oxygen atom of the ligand. The third hydrogen bond was contributed by ALA192, whereas the remaining two bonds were added by TYR159 and MET162, as shown in Fig. 7a, b. Analysis of the binding mode of ZINC00001785–FabI revealed that this complex had a binding energy of − 7.31 and was stabilized by two hydrogen bonds, as shown in Fig. 8a, b: both hydrogen bonds were formed by the oxygen-donating group of ILE195. ZINC14815320 binds to FabI by three hydrogen bonds; one with ALA192 and another two with LYS166, with a binding energy of − 6.61 (Fig. 9a, b).
Fig. 2.
a Interaction profile of ZINC03869685 in a wireframe model to reveal the residues involved in the stabilization of the complex. Eight hydrogen bonds represented by the blue dotted lines are responsible for the stabilization of the complex. b 2-D plot of the interaction between ZINC03869685 and active site residues of FabI. Alanine, glycine isoleucine, and serine residues of the active site are involved in hydrogen bond formation with the ligand
Fig. 3.
a Structural complex of ZINC05842416 bound to the active site of FabI. Active site residues involved in interactions are shown as a wireframe model, whereas ZINC05842416 is represented by a stick model. Hydrogen bonds are represented by blue dotted lines. b 2-D plot of interactions between ZINC05842416 and FabI. Amino acid residues involved in interactions are SER191, MET209, THR155
Fig. 4.
a Docked pose of ZINC05765089 bound to the active site of FabI. Residues in the wireframe model are the active site residues of FabI. b 2-D plot of ZINC05765089–FabI complex, where hydrogen bonds formed between the ligand and active site residues are shown as green dotted lines
Fig. 5.
a Wireframe model of the ZINC03871576 and FabI complex. b 2-D plot of ZINC03871576–FabI complex, where hydrogen bonds formed between the ligand and active site residues are shown as green dotted lines
Fig. 6.
a 3-D Representation of the ZINC 2030112–FabI complex, where FabI is shown as a wireframe model, whereas ZINC 2030112 is shown as a ball and stick one. Hydrogen bonds are shown as blue dotted lines. b 2-D representation of ZINC 2030112–FabI complex. The complex is stabilized by two hydrogen bonds: Lys166 and ILE 20
Fig. 7.
a Interaction profile of ZINC01721693–FabI complex in a wireframe model to reveal the residues involved in stabilization of the complex. Five hydrogen bonds represented by the blue dotted lines are responsible for the stabilization of the complex. b 2-D plot of interactions between ZINC01721693 and active site residues of FabI. Alanine, tyrosine, and methionine residues of the active site are involved in hydrogen bond formation with the ligand
Fig. 8.
a Structural complex of ZINC00001785 bound to the active site of FabI. Active site residues involved in interactions are shown in a wireframe model, whereas ZINC05842416 is represented by a stick model. Hydrogen bonds are represented by blue dotted lines. b 2-D plot of interactions between ZINC00001785 and FabI. The amino acid residue involved in the interaction is isoleucine 195
Fig. 9.
a Docked pose of ZINC14815320 bound to the active site of FabI. Residues in the wireframe model are the active site residues of FabI. b 2-D plot of ZINC14815320–FabI complex, where hydrogen bonds formed between the ligand and active site residues are shown as green dotted lines
Discussion
Increased antibiotic resistance has raised concerns to find alternative strategies to treat infections. Quorum sensing is an attractive option and has been exploited after its discovery for designing new drugs to treat infections. A major part of the QS inhibition strategies is focused on autoinducer mimics and receptor proteins, but the area of synthesis of acyl homoserine lactone has been left unexplored. The present investigation was carried out with the aim to identify molecules which can inhibit the major enoyl-ACP reductase enzyme involved in the generation of acyl chains of N-acyl homoserine lactones, which provide specificity to the signaling molecules. The proteins encoded by FabI belong to short-chain alcohol dehydrogenases or reductases superfamily that are characterized by the presence of a catalytic triad of tyrosine, lysine with phenylalanine or tyrosine as third residue (Parikh et al. 1999). Homology studies have revealed that FabI from different microorganisms are highly similar, except for variation in the free loop of amino acids which serve as the active site (Rozwarski et al. 1999; Stewart et al. 1999). Most of the molecules predicted in this study utilized isoleucine, tyrosine, alanine, glycine, and valine for the formation of hydrogen bonds with FabI, which suggested that most of the molecules predicted were antagonistic in nature, as most of the residues involved in hydrogen bonding are different from the signature motif residues.
Various structure–activity relationship and modelling studies have revealed that a sufficient number of interactions (hydrogen bonding, hydrophobic interactions, Van der Walls interactions) between the ligand and the ligand-binding sites of the receptor are required to circumvent any unfavorable interactions, such as steric hindrance by non-native ligands. Almost all of the molecules predicted in the present study formed 2–5 hydrogen bonds.
In an earlier study, Hoang and Schweizer characterized enoyl-acyl carrier protein reductase (FabI) from P. aeruginosa and tested for its role in the synthesis of acyl homoserine lactone using triclosan and found that FabI has a significant role in the synthesis of these signaling molecules (Hoang and Schweizer 1999). Similarly, in another study by Yang et al. the structure of FabI in P. aeruginosa was predicted using homology modelling, and two inhibitors, epigallocatechin and triclosan, were tested for their potential to quench QS in Pseudomonas aeruginosa. It was observed that epigallocatechin has a better affinity towards FabI then triclosan (Yang et al. 2010). According to one report by Scheiwzer, P. aeruginosa is resistant to triclosan, a well-known inhibitor of fatty acid synthesis, due to the presence of multidrug efflux pumps (Schweizer 2003). Therefore, in the present study, virtual screening was performed to identify alternative compounds of natural origin which could inhibit FabI.
Conclusion
The emergence of multidrug resistance in microorganisms such as P. aeruginosa poses a significant threat to human beings. Quorum sensing is a well-known communication process in P. aeruginosa, which controls various factors like biofilm formation, and virulence phenotypes, which ultimately contribute to drug resistance. Therefore, several targets of QS processes have been exploited to halt this process, but the area which is well validated, and which has remained widely unexplored, is the role of enoyl-ACP reductase in the synthesis, which may prove to be the best target as chain length in acyl homoserine lactone is a deciding factor for specificity in QS. Therefore, in the present study, we have performed virtual screening of a natural library against FabI to reveal new alternatives for QSI in P. aeruginosa. Screened molecules were further judged for their characteristics of being drug-like molecules. Being molecules of natural origin, identified lead molecules are safe and profitable; the present study will open new doors for deciding targets of QS inhibition.
Acknowledgements
Authors are thankful to the Department of Science and Technology (DST), Department of Biotechnology, Government of India for facilitation and support.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest.
References
- Chirala SS, Huang WY, Jayakumar A, et al. Animal fatty acid synthase: functional mapping and cloning and expression of the domain I constituent activities. Proc Natl Acad Sci USA. 1997;94:5588–5593. doi: 10.1073/pnas.94.11.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Stewart SPGEP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Daina A, Zoete V. A BOILED-egg to predict gastrointestinal absorption and brain penetration of small molecules. Chem Med Chem. 2016;11:1117–1121. doi: 10.1002/cmdc.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A, Michielin O, Zoete V. iLOGP: a simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J Chem Inf Model. 2014 doi: 10.1021/ci500467k. [DOI] [PubMed] [Google Scholar]
- Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Mol Biol. 2015;1263:243–250. doi: 10.1007/978-1-4939-2269-7. [DOI] [PubMed] [Google Scholar]
- Breidenstein Elena B.M., de la Fuente-Núñez César, Hancock Robert E.W. Pseudomonas aeruginosa: all roads lead to resistance. Trends in Microbiology. 2011;19(8):419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1111/j.1462-5822.2006.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TT, Schweizer HP. Characterization of Pseudomonas aeruginosa enoyl-acyl carrier protein reductase (FabI): a target for the antimicrobial triclosan and its role in acylated homoserine lactone synthesis. J Bacteriol. 1999;181:5489–5497. doi: 10.1128/jb.181.17.5489-5497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høiby N, Johansen HK, Moser C, et al. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect. 2001;3:23–35. doi: 10.1016/S1286-4579(00)01349-6. [DOI] [PubMed] [Google Scholar]
- Irwin JJ, Shoichet BK. ZINC-A free database of commercially available compounds for virtual screening. J Chem Inf Model. 2005;45:177–182. doi: 10.1021/ci049714+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh CL1, Sam CK, Yin WF, Tan LY, Krishnan T, Chong YM, Chan KG. Plant-derived natural products as sources of anti-quorum sensing compounds. Sensors. 2013;13:6217–6228. doi: 10.3390/s130506217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSarre B, Federle MJ. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev. 2013;77:73–111. doi: 10.1128/MMBR.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. 2015;6:26–41. doi: 10.1007/s13238-014-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Park AK, Chi YM, Jeong SW. Crystal structures of Pseudomonas aeruginosa Enoyl-ACP reductase (FabI) in the presence and absence of NAD + and triclosan. Bull Korean Chem Soc. 2015;36:322–326. doi: 10.1002/bkcs.10084. [DOI] [Google Scholar]
- Lipinski CA. Lead profiling lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;4:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Lu H, Tonge PJ. Inhibitors of Fabl, an enzyme drug target in the bacterial fatty acid biosynthesis pathway. Acc Chem Res. 2008;41:11–20. doi: 10.1021/ar700156e. [DOI] [PubMed] [Google Scholar]
- Maunz A, Gütlein M, Rautenberg M, et al. lazar: a modular predictive toxicology framework. Front Pharmacol. 2013;4:1–10. doi: 10.3389/fphar.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–169. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- Moré MI, Finger LD, Stryker JL, et al. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- Morris GM, Huey R, Olson AJ. Using AutoDock for ligand-receptor docking. Curr Protoc Bioinform. 2008;24:8–14. doi: 10.1002/0471250953.bi0814s24. [DOI] [PubMed] [Google Scholar]
- Parikh S, Moynihan DP, Xiao G, Tonge PJ. Roles of Tyrosine 158 and Lysine 165 in the catalytic mechanism of InhA, the enoyl-acp reductase from Mycobacterium tuberculosis. Biochemistry. 1999;38:13623–13634. doi: 10.1021/bi990529c. [DOI] [PubMed] [Google Scholar]
- Rozwarski DA, Vilchèze C, Sugantino M, et al. Crystal structure of the mycobacterium in complex with NAD + and a C16 fatty acyl substrate crystal structure of the mycobacterium tuberculosis enoyl-acp reductase, InhA, in complex with NAD+ and a C16 fatty acyl substrate. J Biol Chem. 1999;274:15582–15589. doi: 10.1074/jbc.274.22.15582. [DOI] [PubMed] [Google Scholar]
- Schweizer HP. Efflux as a mechanism of resistance to antimicrobials in Pseudomonas aeruginosa and related bacteria: unanswered questions. Genet Mol Res. 2003;2:48–62. [PubMed] [Google Scholar]
- Smith RS, Iglewski BH. Pseudomonas aeruginosa quorum sensing as a potential antimicrobial target. J Clin Investig. 2003;112:1460–1465. doi: 10.1172/JCI200320364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MJ, Parikh S, Xiao G, et al. Structural basis and mechanism of enoyl reductase inhibition by triclosan. J Mol Biol. 1999;290:859–865. doi: 10.1006/jmbi.1999.2907. [DOI] [PubMed] [Google Scholar]
- Taylor PK, Yeung ATY, Hancock REW. Antibiotic resistance in Pseudomonas aeruginosa biofilms: towards the development of novel anti-biofilm therapies. J Biotechnol. 2014;191:121–130. doi: 10.1016/j.jbiotec.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Trott O, Olson AJ. Software news and update AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2009;31:455–461. doi: 10.1002/jcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Zheng J, Zhang Y, Rock CO. Fatty acid biosynthesis. Rev Lit Arts Am. 2005;1:791–831. doi: 10.1146/annurev.biochem.74.082803.133524. [DOI] [PubMed] [Google Scholar]
- Yang L, Liu Y, Sternberg C, Molin S. Evaluation of enoyl-acyl carrier protein reductase inhibitors as Pseudomonas aeruginosa quorum-quenching reagents. Molecules. 2010;15:780–792. doi: 10.3390/molecules15020780. [DOI] [PMC free article] [PubMed] [Google Scholar]