Abstract
Methane (CH4) is a well-known and abundant feedstock for natural gas, and is readily available from various sources. In thermal plants, the CH4 generated from anthropogenic sources is converted into electrical energy via combustion. Microbial fuel cell (MFC) technology has proven to be an efficient strategy for the biological conversion of a many substrates, including biogas (CH4), to electricity. MFC technology uses gaseous substrate along with an enriched and selective microbial consortium. Predominantly, methanotrophs and electrochemically active Geobacter were utilized in a syntrophic association on the anode of an MFC. This review focuses on the exploitation of CH4 as a substrate for bioelectrogenesis via MFCs.
Keywords: Microbial fuel cells, Greenhouse gases, Anaerobic methane oxidation, Reverse methanogenesis, Methanol
Methane (CH4) and carbon dioxide (CO2)—the key components of greenhouse gases (GHG)—have the potential to provide a promising platform for generating renewable and sustainable value-added products through biological and bioelectrochemical processes. The major emissions of CH4 occur from natural and manmade anthropogenic activities. The natural sources include oceans, termites, and wetlands, which collectively account for approximately 36% of global CH4 emission. The remaining portion of CH4 emission (64%) majorly arises from human sources including the utilization, production, and transportation of fossil fuels. Minor quantities are also emitted from various sources, such as agriculture, landfill, and dairy farming (https://www.epa.gov/ghgemissions/overview-greenhouse-gases#methane). This has led to doubling of atmospheric CH4 levels over the last 150 years [1]. Besides, a comparative analysis of the impact of CH4 and CO2 on the environment as GHGs reveals that CH4 is 25 times more dangerous than CO2. This has led to the utilization of natural gas for electricity generation. However, current decline in power charges has led to a search of renewable and sustainable process for upgradation and valorization of CH4. Therefore, the generation of value-added products utilizing CH4 on-site can help in minimizing losses due to storage leaks and transportation [2]. Methane can be converted to liquid fuels or electricity via conventional technologies, such as chemical conversion or gas-turbine generator set. These technologies require a capital investment of billions of dollars as well as a large land area for operation. Direct use of CH4 to generate electricity in fuel cells is challenging, owing to the requirement of high thermal operation (650–1100 °C) and instability of catalyst [1]. Thus, biological conversion of CH4 to value-added products [methanol, electricity, or polyhydroxyalkanoates] seems an interesting and promising option [3, 4]. In addition, biological systems can offer adaptability/flexibility in scaling up of operation and provide an ability to integrate with the catalytic process that generates desired chemical products. However, there are several reviews available on the biological conversion of CH4 to chemicals [5–7]. Therefore, the present study provides a perspective on the conversion of CH4 to bioelectricity.
The biological conversion of CH4 to electricity has been challenging due to difficulties in microbial culturing, eventually affecting the process of anaerobic CH4 oxidation. The first report on the use of CH4 as a sole substrate was published in 1965, with pure cultures of Pseudomonas methanica [8]. Until 2011, no publications/reports were recorded on use of CH4 by a similar process. In 2011, Girguis and Reimers, procured a US patent on the use of CH4 as a feed for microbial fuel cells (MFCs) by using methanotrophs as a biocatalyst [9]. This turned the attention of the research fraternity to the bioelectrochemical conversion of CH4 to electricity using MFCs. A recent study from the Pennsylvania State University, USA and National Institute of Cardiology, Mexico reported direct oxidation of CH4 to electricity by reverse methanogenesis [10]. The anaerobic CH4-oxidizing bacteria (ANME) performed reverse methanogenesis. ANME strains were difficult to isolate due to their association with other bacteria that reduce metal oxides and other inorganic chemicals, such as nitrites and sulfates. Furthermore, ANME requires CH4 activation without oxygen-based radicals and a longer growth time, even after adaptation for several days under laboratory conditions. Due to all these factors, the use of CH4 in MFC has been challenging. Based on their previous work on a microbially engineered ANME strain (Methanosarcina acetivorans), Wood and his colleagues established a syntrophic association between M. acetivorans and Geobacter sulfurreducens [10]. They also analyzed the influence of undefined microbial inoculum, collected from a wastewater treatment plant. This mechanism generated sustainable and considerable amount of energy in the form of electricity. M. acetivorans synthesizes an Mcr (methyl-coenzyme M reductase) enzyme and can convert CH4 to acetate. G. sulfurreducens and other undefined microbial consortia can oxidize acetate to CO2 with simultaneous electricity generation. An eightfold improvement in power density (160 mW/m2; control—20 mW/m2) was recorded due to the presence of electroactive G. sulfurreducens species along with engineered M. acetivorans and sludge on the anode of double chamber MFC. Interestingly, the CH4-fed MFC exhibited a columbic efficiency (CE) of 90%, which suggested that most of the electrons generated during CH4 conversion are extracted in the form of current [10]. These values of CE were comparable with those obtained for conventional organic substrates in MFC [11]. Additional results exhibited a variable increase in current generation by use of external electron carriers. The use of cytochrome C and humic acids (0.5%) as mediators had shown 1.5- and 1.9-fold increase in power density, respectively, compared to control [12]. Further, an 11-fold increase in current generation was recorded by increasing the humic acid concentration from 0.5 to 3.3%, suggesting that internal electron carriers might be a rate-limiting factor in this system. However, further investigations need to be carried out to understand why G. sulfurreducens utilized extracellular electron transfer instead of using outer membrane or electrically conductive nanowires for pumping electrons to anode.
Similarly, other researchers have tried using CH4 as an oxidant in MFC with major interest in decoupling of denitrifying anaerobic CH4 oxidizing archaea (DAMO) [13]. This study demonstrated 25 mV of power generation. Interestingly, it provided an alternative for the successful separation of DAMO archaea to understand their physiological characteristics. After 45 days of operation, the MFC anode exhibited a 2.5-fold increase with DAMO archaea, with a 12-fold decrease in DAMO and simultaneous increase in ANME. Considerable increase in the abundance of the genera Geobacter and Ignavibacterium were observed on the anode. Recently, Chen and Smith had analyzed the use of CH4 as a sole substrate in a single chamber air cathode MFC operated continuously [14]. MFC operation with a 16-h hydraulic retention time exhibited 85% CH4 removal with a high power density of 62 mW/m2. Reverse transcription-quantitative polymerase chain reaction analysis exhibited higher abundance of methanotrophs and the genus Geobacter. Instead of direct conversion of CH4 to electricity in the anode chamber of MFC, few researchers had tried a two-stage system [15]. Initially CH4 is converted to liquid fuels (methanol), and later, methanol is converted to electricity. The two-stage system generated a power density of 426 mW/m2, which was found to be 6.8 times higher than single stage process (62 mW/m2). Methylophilus, Arcobacter, and Acetobacterium are the major genera found in methanol-fed MFC.
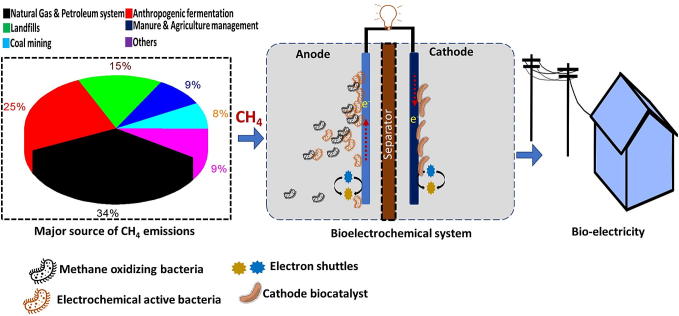
The long-term desire of anaerobic oxidation of CH4 to produce electricity has been fulfilled. The above-discussed electrochemical studies open new possibilities in employing MFC as a biological post-treatment strategy for energy recovery and to mitigate GHG emission from anthropogenic sources (Fig. 1). CH4 oxidation in MFC can also open a new approach for generation of value-added products and chemicals in microbial electrolysis cells (MECs). On the basis of current generation and the desired product, energy from MFC can be used for CO2 reduction to generate alcohols and other carboxylic acids, as well as hydrogen (H2), by using protons from the anode [16]. To further increase CH4 conversion in MFC, the association of ANME with other electrochemical active bacteria (EAB) needs to be analyzed. This can include either the analysis of intermediates or extracellular electron transfer between ANME and EAB. The next-generation microbial sequencing tools, such as pyrosequencing or meta-omics, can help in better understanding of microbial communities and gene expression, that are related to anaerobic oxidation of CH4 in natural systems [17]. Meanwhile, MFC reactor configurations can be modified/developed for the use of gaseous substrates. In addition, the economic evaluation of MFC for electricity generation should be performed with CH4-rich biogas (∼ $2.6 per 1000 ft3).
Fig. 1.
Bioelectrogenic conversion of methane to electricity using microbial fuel cell with electroactive microbes
Over the past few years, several aspects of MFC are being explored to enhance its performance. In this regard, the use of cheap natural gas for electricity seems interesting. However, the cost and energy conversion efficiency should be evaluated. The anode materials, such as gas diffusion electrodes, with specificity for methanotrophs should be tested to provide a better interface with syntrophic microbial community. In addition, there is a need to fabricate high conductive, scalable scaffolds, with better surface properties to enhance CH4 oxidation by methanotrophs and EAB. However, there are still several bottlenecks, such as long-term operation and limiting the diffusion of CH4 from the anode to cathode, which need to be evaluated to achieve a pilot scale of operation.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2018H1D3A2001746, 2013M3A6A8073184). This work was supported by the Energy Efficiency & Resources Core Technology Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (20153030091450). This research was supported by 2017 KU Brain Pool of Konkuk University.
Contributor Information
Jung-Kul Lee, Phone: (+82) 2-458-3504, Email: jkrhee@konkuk.ac.kr.
Vipin C. Kalia, Email: vckaliaku@gmail.com
References
- 1.Ren ZJ. Microbial fuel cells: running on gas. Nat Energy. 2017;2:17093. doi: 10.1038/nenergy.2017.93. [DOI] [Google Scholar]
- 2.Patel SKS, Mardina P, Kim D, Kim SY, Kalia VC, Kim IK, Lee JK. Improvement in methanol production by regulating the composition of synthetic gas mixture and raw biogas. Bioresour Technol. 2016;218:202–208. doi: 10.1016/j.biortech.2016.06.065. [DOI] [PubMed] [Google Scholar]
- 3.Patel SKS, Kondaveeti S, Otari SV, Pagolu RT, Jeong SH, Kim SC, Cho BK, Kang YC, Lee JK. Repeated batch methanol production from a simulated biogas mixture using immobilized Methylocystis bryophila. Energy. 2018;145:477–485. doi: 10.1016/j.energy.2017.12.142. [DOI] [Google Scholar]
- 4.Mardina P, Li J, Patel SK, Kim IW, Lee JK, Selvaraj C. Potential of immobilized whole-cell Methylocella tundrae as a biocatalyst for methanol production from methane. J Microbiol Biotechnol. 2016;26:1234–1241. doi: 10.4014/jmb.1602.02074. [DOI] [PubMed] [Google Scholar]
- 5.Fei Q, Guarnieri MT, Tao L, Laurens LM, Dowe N, Pienkos PT. Bioconversion of natural gas to liquid fuel: opportunities and challenges. Biotechnol Adv. 2014;32:596–614. doi: 10.1016/j.biotechadv.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Lebrero R, Chandran K. Biological conversion and revalorization of waste methane streams. Crit Rev Environ Sci Technol. 2017;47:2133–2157. doi: 10.1080/10643389.2017.1415059. [DOI] [Google Scholar]
- 7.Clomburg JM, Crumbley AM, Gonzalez R. Industrial biomanufacturing: the future of chemical production. Science. 2017;355:aag0804. doi: 10.1126/science.aag0804. [DOI] [PubMed] [Google Scholar]
- 8.Van Hees W. A bacterial methane fuel cell. J Electrochem Soc. 1965;112:258–262. doi: 10.1149/1.2423519. [DOI] [Google Scholar]
- 9.Girguis P, Reimers CE (2011) Methane-powered microbial fuel cells. 2011, Google Patents. US20110123835A1 https://patents.google.com/patent/US20110123835
- 10.McAnulty MJ, Poosaria VG, Kim KY, Jasso-Chávez R, Logan BE, Wood TK. Electricity from methane by reversing methanogenesis. Nat Commun. 2017;8:15419. doi: 10.1038/ncomms15419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pant D, Van Bogaert G, Diels L, Vanbroekhoven K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol. 2010;101:1533–1543. doi: 10.1016/j.biortech.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki R, Maeda T, Wood TK. Electron carriers increase electricity production in methane microbial fuel cells that reverse methanogenesis. Biotechnol Biofuels. 2018;11:211. doi: 10.1186/s13068-018-1208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding J, Lu YZ, Fu L, Ding ZW, Mu Y, Cheng SH, Zeng RJ. Decoupling of DAMO archaea from DAMO bacteria in a methane-driven microbial fuel cell. Water Res. 2017;110:112–119. doi: 10.1016/j.watres.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Smith AL. Methane-driven microbial fuel cells recover energy and mitigate dissolved methane emissions from anaerobic effluents. Environ Sci: Water Res Technol. 2018;4:67–79. [Google Scholar]
- 15.Myung J, Saikaly PE, Logan BE. A two-staged system to generate electricity in microbial fuel cells using methane. Chem Eng J. 2018;352:262–267. doi: 10.1016/j.cej.2018.07.017. [DOI] [Google Scholar]
- 16.Kondaveeti S, Min B. Bioelectrochemical reduction of volatile fatty acids in anaerobic digestion effluent for the production of biofuels. Water Res. 2015;87:137–144. doi: 10.1016/j.watres.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Kondaveeti S, Lee SH, Park HD, Min B. Bacterial communities in a bioelectrochemical denitrification system: the effects of supplemental electron acceptors. Water Res. 2014;51:25–36. doi: 10.1016/j.watres.2013.12.023. [DOI] [PubMed] [Google Scholar]