Abstract
In this study, novel, hollow superparamagnetic copper ferrite (CuFe2O4) nanoparticles (NPs) were synthesized by a low-temperature hydrothermal method. The hollow magnetic spheres were characterized by field emission scanning electron microscopy and high resolution transmission electron microscopy to confirm their morphology and size. The hollow NPs were demonstrated as the support for biological materials by the immobilization of Thermomyces lanuginosus lipase on the inner and outer surfaces of the hollow spheres. The immobilization of the enzyme was confirmed by Fourier Transform Infra-red spectroscopy and confocal laser scanning microscopy. The immobilized enzyme was shown to have an immobilization efficiency of 84.5%, with approximately 176 mg g−1 of enzyme loading, for the hollow-NPs support. The immobilized enzyme exhibited high storage and temperature stability. The reusability of the immobilized lipase was more than 80% after 10 cycles of repeated use.
Keywords: Immobilization, Hollow nanosphere, Lipase, Magnetic nanoparticles, Storage stability
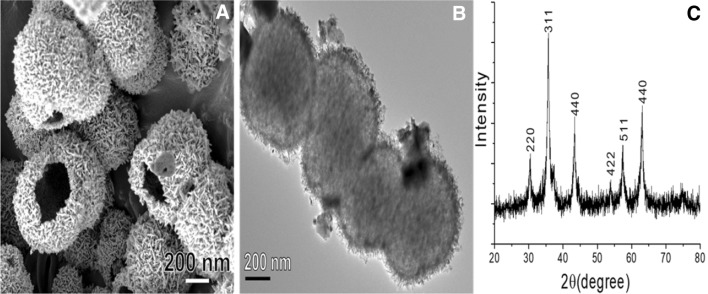
Thermomyces lanuginosus lipase is an industrially important enzyme catalyzing various reactions including esterification, hydrolysis, and polymerization, among others. Therefore, lipase has been used in the industrial manufacture of various products such as food, biodiesel, detergent, pharmaceuticals, leather, textiles, and cosmetics [1]. Due to its high cost, low stability in some environmental conditions, and its non-reusability, large-scale application of lipase in industry is rare. In the present study, we achieved the immobilization of this biocatalyst on a suitable support, which could provide a solution for the above-mentioned problems by facilitating reusability and sustainable stability and thus making the process economic. Various methods (via covalent bonding, adsorption, ionic interactions, etc.) and supports (organic and inorganic) have been reported for the immobilization of enzymes [2–6]. Among the different supports, functionalized magnetic NPs have been considered as an ideal support for lipase carrier, enabling easy separation by external magnetic field. Various structured magnetic NPs for lipase have been demonstrated, for a vast variety of applications, and with long-term stability [7–9]. Akyl et al. [10] used dense spherical magnetic Fe3O4 NPs for the immobilization of lipase during the synthesis of different forms of lipids. The chitosan functionalized Fe3O4 NPs were used for the immobilization of Thermomyces lanuginosus lipase for ascorbyl palmitate synthesis [11]. Liu et al. demonstrated use of hollow Fe3O4 NPs for lipase support for high stability at 60 °C [12]. Here, hydrothermally synthesized CuFe2O4 hollow NPs are presented as an effective and efficient support for lipase immobilization. Cu(NO3)2 and Fe(NO3)3 were dissolved in glycerol and propanol in a 50-mL Teflon-lined hydrothermal reactor and heated at 180 °C for 22 h to form hollow CuFe2O4 NPs after calcination at 600 °C for 2 h. The field emission scanning electron microscopy (FE-SEM) images revealed that the as-prepared magnetic NPs are hollow and spherical in structure, with plate-like rough surfaces that acted as sites for the entry and attachment of the enzymes (Fig. 1a). The transmission electron microscopy (TEM) images confirm the formation of spherical approximately 400-nm-diameter NPs (Fig. 1b). X-ray diffraction (XRD) measurements indicate that the hollow CuFe2O4 NPs are crystalline in nature (Fig. 1c); all the obtained peaks shown in Fig. 1c were indexed and correspond to the XRD pattern of CuFe2O4 (JCPDS card no. 34-0425).
Fig. 1.
Physical characterizations of hollow CuFe2O4 nanoparticles; a morphology studies using field emission scanning electron microscopy (FE-SEM) images, b structural studies with high resolution transmission electron microscopy (Hr-TEM) images, and c crystallinity studies by an XRD spectra
Immobilization of lipase (Thermomyces lanuginosus, Sigma-Aldrich, St. Louis, MO, USA) was carried out by exposing 10 mg of glutaraldehyde-factionalized CuFe2O4 NPs (0.6 M) to lipase (3 mg mL−1) at 4 °C with stirring at 150 rpm for 24 h, during which time the enzyme was covalently attached to the NPs. The NPs were collected by use of an external magnetic field and the concentration of the supernatant enzyme was determined via the Bradford method. Enzyme activity, immobilization yield (IY), and immobilization efficiency (IE) were calculated as previously reported [13, 14]. The dense CuFe2O4 NPs prepared by the co-precipitation method were used as the control and were functionalized for enzyme immobilization using a method previously described for hollow CuFe2O4 NPs [15]. The maximum amount of lipase on the dense CuFe2O4 NPs was 96 mg g−1 with an activity recovery value of 74% of initial free lipase activity. In the case of the hollow CuFe2O4 NPs, the maximum protein loading on the support was 176 mg g−1 with 84.5% immobilization efficiency. This increased protein loading may be due to the unique morphology of the hollow NPs which provides inner and outer surfaces for attachment sites for the proteins [16]. The decrease in the activity of immobilized lipase compared to free enzyme is perhaps due to a less effective interaction between the enzymes and particles, with a less active enzyme-particle composite formed. In a report of lipase immobilization on hollow Fe3O4 NPs, the protein loading value was 143.88 mg g−1, and the maximum activity recovery was 73.25% of the initial activity [17].
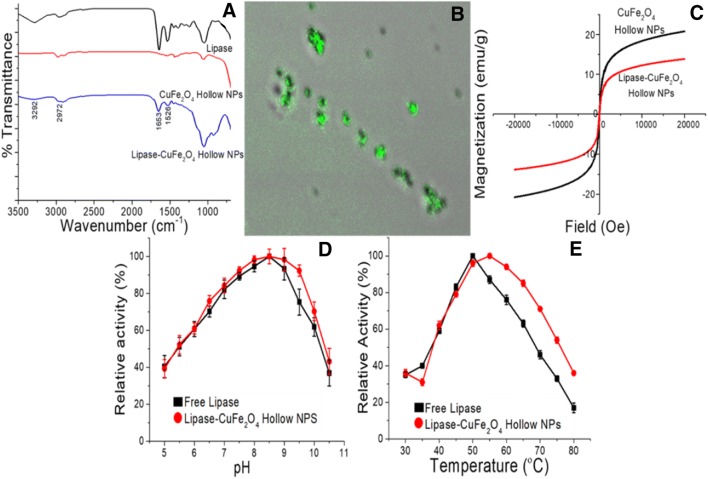
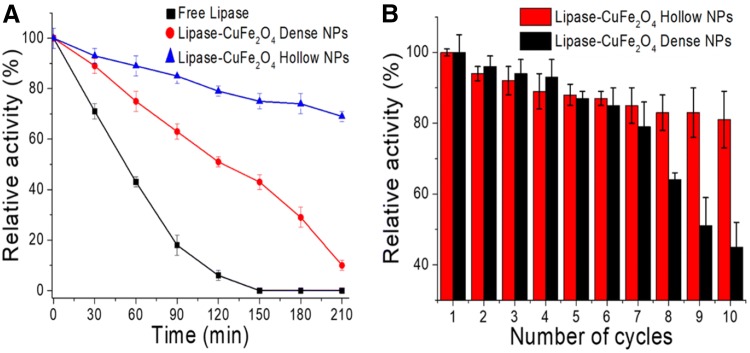
The FT-IR spectra (Fig. 2a) indicated strong absorbance at 1653 and 1526 cm−1, which is characteristic of the amide groups of the protein and was absent in the case of the free CuFe2O4 hollow NPs [18, 19] The presence of the amide group on the support confirmed the binding of the enzyme to the NPs. Further, the N–H bending vibration and stretching in the amide group in lipase appeared at 3297 cm−1 for both the free and immobilized lipase [20]. The immobilized enzyme was observed by confocal laser electron microscopy (CLSM) by labelling the lipase enzyme with fluorescein isothiocyanate (FITC), a uniform distribution of enzymes on the surface of NPs is apparent (Fig. 2b). The magnetizations of the CuFe2O4 hollow particles before and after enzyme immobilization were 22 and 12 emu g−1 (Fig. 2c), respectively, indicating their superparamagnetic nature. The change in the magnetization property was due to the presence of organic content on the surface of the magnetic NPs. The optimum temperature for free and immobilized lipase was 50 °C, and the immobilized lipase retained high activity at this temperature (Fig. 2d). Though optimum pH for both free and immobilized lipase was 8.5; the immobilized lipase has a relatively high activity in a high pH range (Fig. 2e). The CuFe2O4-hollow- and CuFe2O4-dense-nanoparticle immobilized lipase retained 79% and 15%, respectively, of their original activity at 70 °C after 210 min; the hollow NPs compare well with free lipase (Fig. 3a). After 10 cycles of repeated reactions for p-NPP hydrolysis, the hollow CuFe2O4-immobilized lipase showed 85% of its initial activity at 50 °C, while the dense CuFe2O4-bound lipase retained 43% of its initial activity after the same treatment (Fig. 3b). Thus, the hollow-CuFe2O4-immobilized lipase preparation is very stable and can be reused multiple times.
Fig. 2.
Physico-chemical characteristics of free and immobilized lipase; a FT-IR spectroscopy of free and immobilized lipase, b CLSM images of FITC-labelled immobilized lipase, c magnetization studies of CuFe2O4 hollow magnetic nanoparticles with and without the immobilized enzyme, relative activity of lipase free (■, black filled square) and immobilized lipase (●, red filled circle) as a function of d pH and e temperature (color figure online)
Fig. 3.
Stability and reusability of free and immobilized enzyme; a stability studies free lipase (■, black filled square), lipase-CuFe2O4 dense NPs (●, red filled circle) and lipase-CuFe2O4 hollow NPs (▲, blue filled triangle), b reusability of lipase immobilized on hollow and dense CuFe2O4 nanoparticles (color figure online)
In summary, we have demonstrated hydrothermally synthesized novel magnetic hollow CuFe2O4 NPs for the effective immobilization of the enzyme lipase that provide stability and reusability at 70 °C that is significantly improved with respect to those of a dense CuFe2O4 NPs support. The as-prepared structure could also be developed for use as the support for other industrially important enzymes, namely, laccase, dehydrogenase, peroxidase, etc., and hence we expect that it will be used in industry in the near future.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2018H1D3A2001746, 2015R1D1A1A01061279, 2015R1D1A1A01061227, 2013M3A6A8073184). This work was supported by the Energy Efficiency & Resources Core Technology Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resource from the Ministry of Trade, Industry and Energy, Republic of Korea (20153030091450). This paper was written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2017.
Contributor Information
In-Won Kim, Email: inwon@konkuk.ac.kr.
Jung-Kul Lee, Email: jkrhee@konkuk.ac.kr.
References
- 1.Houde A, Kademi A, Leblanc D. Lipases and their industrial applications: an overview. Appl Biochem Biotechnol. 2004;118:155–170. doi: 10.1385/ABAB:118:1-3:155. [DOI] [PubMed] [Google Scholar]
- 2.Patel SKS, Kalia VC, Choi J-H, Haw J-R, Kim I-W, Lee JK. Immobilization of laccase on SiO2 nanocarriers improves its stability and reusability. J Microbiol Biotechnol. 2014;24:639–647. doi: 10.4014/jmb.1401.01025. [DOI] [PubMed] [Google Scholar]
- 3.Kim TS, Patel SKS, Selvaraj C, Jung W-S, Pan C-H, Kang YC, Lee JK. A highly efficient sorbitol dehydrogenase from Gluconobacter oxydans G624 and improvement of its stability through immobilization. Sci Rep. 2016;6:33438. doi: 10.1038/srep33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SKS, Otari SV, Kang YC, Lee JK. Protein-inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in l-xylulose production. RSC Adv. 2017;7:3488–3494. doi: 10.1039/C6RA24404A. [DOI] [Google Scholar]
- 5.Kumar A, Patel SKS, Mardan B, Pagolu R, Lestari R, Jeong SH, Kim T, Haw JR, Kim SY, Kim IW, Lee JK. Immobilization of xylanase using a protein-inorganic hybrid system. J Microbiol Biotechnol. 2018;28:638–644. doi: 10.4014/jmb.1710.10037. [DOI] [PubMed] [Google Scholar]
- 6.Gao J, Lu CL, Wang Y, Wang SS, Shen JJ, Zhang JX, Zhang YW. Rapid immobilization of cellulase onto graphene oxide with a hydrophobic spacer. Catalysts. 2018;8:180. doi: 10.3390/catal8050180. [DOI] [Google Scholar]
- 7.Liu W, Zhou F, Zhang XY, Li Y, Wang XY, Xu XM, Zhang YW. Preparation of magnetic Fe3O4@SiO2 nanoparticles for immobilization of lipase. J Nanosci Nanotechnol. 2014;14:3068–3072. doi: 10.1166/jnn.2014.8567. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Liu W, Tao QL, Jiang XP, Liu CH, Zeng S, Zhang YW. Immobilization of lipase by adsorption onto magnetic nanoparticles in organic solvents. J Nanosci Nanotechnol. 2016;16:601–607. doi: 10.1166/jnn.2016.10694. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang MY, Zhou QL, Wang XY, Zhang JX, Xue L, Wang R, Zhang JX, Zhang YW. Immobilization of lipase onto dopamine functionalized magnetic nanoparticles. Nanosci Nanotechnol Lett. 2016;8:251–254. doi: 10.1166/nnl.2016.2127. [DOI] [Google Scholar]
- 10.Akil E, Carvalho T, Bárea B, Finotelli P, Lecomte J, Torres AG, Amaral P, Villeneuve P. Accessing regio-and typo-selectivity of Yarrowia lipolytica lipase in its free form and immobilized onto magnetic nanoparticles. Biochem Eng J. 2016;109:101–111. doi: 10.1016/j.bej.2015.12.019. [DOI] [Google Scholar]
- 11.Wang XY, Jiang XP, Li Y, Zeng S, Zhang YW. Preparation Fe3O4@chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. Int J Biol Macromol. 2015;75:44–50. doi: 10.1016/j.ijbiomac.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Liu X. Preparation of porous hollow Fe3O4/P(GMA–DVB–St) microspheres and application for lipase immobilization. Bioprocess Biosyst Eng. 2018;4:771–779. doi: 10.1007/s00449-018-1910-7. [DOI] [PubMed] [Google Scholar]
- 13.Patel SKS, Choi SH, Kang YC, Lee JK. Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl Mater Interfaces. 2017;9:2213–2222. doi: 10.1021/acsami.6b05165. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Kim IW, Patel SKS, Lee JK. Synthesis of protein-inorganic nanohybrids with improved catalytic properties using Co3(PO4)2 Indian. J Microbiol. 2018;58:100–104. doi: 10.1007/s12088-017-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dandia A, Jain AK, Sharma S. CuFe2O4 nanoparticles as a highly efficient and magnetically recoverable catalyst for the synthesis of medicinally privileged spiropyrimidine scaffolds. RSC Adv. 2012;3:2924–2934. doi: 10.1039/c2ra22477a. [DOI] [Google Scholar]
- 16.Patel SKS, Choi SH, Kang YC, Lee JK. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk-shell particles: a promising support for enzyme immobilization. Nanoscale. 2016;8:6728–6738. doi: 10.1039/C6NR00346J. [DOI] [PubMed] [Google Scholar]
- 17.Ramanathan T, Fisher FT, Ruoff RS, Brinson LC. Amino-functionalized carbon nanotubes for binding to polymers and biological systems. Chem Mater. 2005;17:1290–1295. doi: 10.1021/cm048357f. [DOI] [Google Scholar]
- 18.Anwar MZ, Kim DJ, Kumar A, Patel SKS, Otari S, Mardina P, Jeong JH, Sohn JH, Kim JH, Park JT, Lee JK. SnO2 hollow nanotubes: a novel and efficient support matrix for enzyme immobilization. Sci Rep. 2017;7:15333. doi: 10.1038/s41598-017-15550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuang MY, Wang C, Xu MQ, Ling XM, Shen JJ, Zhang YW. Using concanavalinA as a spacer for immobilization of E-coli onto magnetic nanoparticles. Int J Biol Macromol. 2017;104:63–69. doi: 10.1016/j.ijbiomac.2017.05.150. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Wang XY, Jiang XP, Ye JJ, Zhang YW, Zhang XY. Fabrication of graphene oxide decorated with Fe3O4@SiO2 for immobilization of cellulase. J Nanopart Res. 2015;17:8. doi: 10.1007/s11051-014-2826-z. [DOI] [Google Scholar]