Abstract
Syzygium cumini L. Skeels (Myretacae family) is a native plant of the Indian subcontinent which has wide socio-economical importance and is well known for its ant diabetic activity. The present study aimed to investigate the antibiofilm activity of purified fraction (EA) from S. cumini leaf extract against P. aeruginosa and S. aureus. The EA did not show any effect on growth of P. aeruginosa and S. aureus at the concentration of 900 µg/ml. At this concentration EA showed biofilm inhibition up to 86 ± 1.19% (***P < 0.0001) and 86.40 ± 1.19% (***P < 0.0001) in P. aeruginosa and S. aureus respectively. SEM examination also confirmed the reduction in biofilm formation. Further EA also disrupted some virulence phenotypes in P. aeruginosa and S. aureus. Bioactive compounds detected by GC–MS showed their possible molecular interaction with RhlG/NADP active-site complex (PDB ID: 2B4Q), LasR-TP4 complex (PDB ID: 3JPU) and Pseudaminidase (PDB ID: 2W38) from P. aeruginosa. The in vitro biofilm inhibition, virulence factor inhibition and the mode of interaction of bioactive components in Syzygium cumini with QS proteins of bacteria reported in this study might be an affordable and effective alternative method of controlling quorum sensing/biofilm-associated infections.
Electronic supplementary material
The online version of this article (10.1007/s12088-018-0770-9) contains supplementary material, which is available to authorized users.
Keywords: Anti-biofilm, Syzygium cumini, Pseudomonas aeruginosa, Pyocyanin, Swarming motility, Swimming motility
Introduction
Biofilms are unique microenvironment for microbial growth, where microorganisms are stuck to each other to the substratum and encapsulated by self produced matrix comprised of biological polymers such as exopolysaccharide, protein, and DNA [1, 2]. Micro-organisms residing within the biofilm become highly resistance to conventional antibiotic drug therapy, disinfectants, and thus make the micro-organisms prone to evade host immune system [3]. Biofilm formation involves the expression of biofilm formation related genes through the accumulation of signaling molecules that eventually mediate inter-cellular communication commonly called as quorum sensing (QS) [4, 5]. Several gram negative micro-organisms use homoserine lactone as a signal molecule to communicate with each other which also regulate the virulence expression. Over the years, microbial infections associated with biofilms become difficult to treat using conventional antibiotics due to development of antibiotic resistance [6–9]. Bacteria coordinate their cell density via communication through a typical quorum-sensing system which initiates the formation of biofilm matrix. Quorum sensing enables micro-organisms to maintain their density and also crucial in the production of virulence factor until the density reaches to sufficient amount to surmounting the host defense [10]. The QS inhibition may lead to the inhibition of the virulence of the micro-organisms as well as the biofouling caused by the micro-organisms [11]. Several lactonase genes have been identified in Bacillus which may have quorum quenching activity [12–14]. Pseudomonas aeruginosa and S. aureus are the common pathogens responsible for nosocomial infections. Antibiofilm compounds against P. aeruginosa which have been reported in plants mainly include furanones [15], ursine triterpenes [16] corosolic acid and acetic acid [17] and 3-indolylacetonitrile [18].
Several plant-derived molecules have been used to target the biofilm formation of S. aureus with those identified, including diterpenoids [19], oleic acid [20], and tannic acid [21]. Emergence of resistance against conventional antibiotics in pathogens is a critical concern worldwide and there is an urgent need to find out some alternatives to deal with this problem. One of the best ways is to reduce the pathogenicity rather direct killing of the micro-organisms. For example protein phosphatase is an enzyme which plays an important role in virulence of the pathogenic bacateria and can be a potent target to reduce the pathogenecity rather direct killing of the bacteria [22]. This has prompted researchers to identify alternatives and natural plant products that preferentially target specific receptors/activators of QS to combat biofilm-associated infections. Biofilm formation of S. aureus could also be inhibited by targeting the quorum sensing of the bacterium [23].
Syzygium cumini (L.) Skeels is commonly known in India as ‘jambo or jamun’, belonging to Myrtaceae family.There are several reports worldwide which validate the medicinal property of the plant as a potential antimicrobial agent. The seed of plant, fruit and the whole plant extract is found to be an excellent good antimicrobial source against the common pathogenic bacteria as well as fungus [24]. The lethal sepsis-induced in mice by the microbes is also improved by the use of the plant extract [25]. The Antiquorum sensing activity of the plant has already been reported [26]. These scientific validations give a glimpse that this plant retains some chemicals which have activity against the microorganisms.
The present study describes the anti-biofilm activity and inhibition of certain virulence phenotypes which are regulated by quorum sensing in P. aeruginosa by the active fraction (EA) of S. cumini. In-silico studies also demonstrated the interaction of the compounds in EA with the quorum sensing/biofilm related proteins. So this study gives a combined approach of in vitro and in silico to find out the compound which may act as an inhibitor of QS-mediated virulence along with the antibiofilm activity.
Materials and Methodology
Chemicals
All the solvents, silica gel, TLC plates were purchased from Merck, Mumbai, India. Luria–Bertani (LB), Muller Hinton Broth (MHB), soyabean casean digest medium, agar powder (bacteriological), and gentamicin from Himedia was used.
Bacterial Strains and Growth Condition
Staphylococcus aureus MTCC 3160 and Pseudomonas aeruginosa MCC 2081 used in the present study was routinely cultured in LB broth. MCC strain was procured from Microbial Culture collection, NCCS, Pune India and MTCC strain was procured from Microbial Type Culture Collection, CSIR-Institute of Microbial Technology, Chandigarh, India.
Plant Identification and Processing
The leaves of the plant were collected from Tezpur University campus, Tezpur, Assam between April and May 2012. The herbarium sample was identified by the Botanical Survey of India, Shillong as Syzygium cumini L. Skeels (No. BSI/ERC/2015/Plant identification/799). The voucher specimen (KG # 5) has been deposited in the Departmental herbarium, Tezpur University. Fresh leaves of the plant species were plucked from the tree. The leaves were first washed with sterile water, then surface sterilized by 70% ethanol followed by bleaching with 5% aqueous sodium hypochlorite solution. Finally, the leaves were again washed with sterile double distilled water to get rid of any impurities. After surface sterilization, the healthy leaves were air dried to maintain their natural phytoconstituents.
Extraction and Fractionation
Powder of leaves (500 g) was soaked in methanol (2 l) for 24 h under continuous stirring. The extract was centrifuged at 5000 rpm for 20 min to remove the plant material followed by filtration with Whatman Filter paper and concentrated using a vacuum evaporator and lyophilized to yield a crude methanolic extract. The dried extract was re-suspended in an appropriate volume of methanol and bio-assay guided partial fractionation of the extract was done by thin-layer chromatography (TLC) and column chromatography. In column chromatography, a series of fractions with n-hexane, ethyl acetate and n-butanol were eluted. Each fraction was concentrated, lyophilized and subjected to antibiofilm assay. Ethyl acetate fraction (EA) has the potential to inhibit the formation of biofilm and it was used for further experiments.
Antimicrobial Activity
EA was tested for antimicrobial activity against P. aeruginosa and S. aureus by method described elsewhere with slight modification [27]. Briefly, 106–107 colony-forming units/mL of microorganisms were spread uniformly on Muller Hinton Agar (MHA) plates and EA (10 mg/ml) was loaded on the wells. Antibiotic gentamicin was used as a control.
Growth Curve Analysis
Staphylococcus aureus and P. aeruginosa were cultured in the presence of different concentrations of EA to study the effect of EA on growth of the micro-organisms. Briefly, test inoculum (100 µl of 106–107 CFU/ml) was added to the test tube already containing EA in 10 ml of MHB. The test tubes were incubated at 37 °C and the OD were recorded at 600 nm at 2 h intervals up to 24 h.
Effect of EA on Biofilm Formation
The method described elsewhere [28] was used to check the efficiency of EA to inhibit biofilm formation of P. aeruginosa and S. aureus. In brief 106–107 CFU/ml bacterial culture was filled in the wells of 96-well-flat bottom tissue culture plate. 50 µl of EA from 125 to 900 µg/ml were added in corresponding wells of the plate and the plate was incubated at 37 °C for 24 h. To remove planktonic bacteria, the wells were washed thrice with phosphate buffer saline (PBS, pH 7.4) finally, crystal violet (0.1%, w/v) was used to stain the cells in biofilm for 1 h. The Excess bound stain on wells was removed by washing the plate with (PBS pH 7.4) and the plate was air dried. To solubilise the bound crystal violet, 100% ethanol was used and the optical densities (OD) were determined with a plate reader (Thermo Scientific Multiskan GO) at 570 nm. The inhibition of biofilm formation was calculated by using the formula
where ODcontrol is the absorbance without the addition of EA.
Scanning Electron Microscopy: Biofilm Visualization
Scanning electron microscopy (SEM) was done to see the effect of EA on biofilm inhibition, briefly bacterial culture was added in a tissue culture plate (24 well) having a sterile coverslip on the bottom of the plate and 900 µg/ml of EA was added in the well and incubated for 24 h at 37º C. Biofilm on coverslip was fixed for 8 h at 4 °C with 2% glutaraldehyde followed by dehydration with the graded concentration of ethanol. The formed biofilm was analyzed by Scanning Electron Microscope (SEM) (JEOL JSM-6390LV, Tokyo, Japan).
Anti-quorum Sensing Activity of EA
Qualitative Assay
To evaluate the effect of EA on quorum sensing, the qualitative well diffusion method was adopted as described by Rahman et al. [29]. 100 µl of overnight grown Chromobacterium violaceum was spread uniformly on the MHA plate and an 8 mm well was prepared at the centre of the plate. 100 µl of the EA was added into the corresponding wells of the plate followed by incubation at 37 °C for 24 h.
Inhibition of Violacein Production
Inhibition of violacein production in C. violaceum, the method described by Vattem, D. A. et al. [30] was used with slight modifications. Briefly, C. violaceum ATCC 12472 was inoculated in Erlenmeyer flasks containing TSB and different concentration of EA ranging from 150 to 900 µg/ml. The flask was incubated at 30 °C for 30 h. After incubation period 1 ml culture was centrifuged at 10,000 rpm for 10 min. The precipitated culture with pigment was resuspended in 100% Dimethylsulfoxide (DMSO) and vortexed. The absorbance was determined at 585 nm by UV–Vis spectrophotometer and violacein inhibition was calculated.
Effect of EA on Pyocyanin Production
Pseudomonas aeruginosa was grown overnight and the culture was diluted in fresh LB medium. EA at the final concentration 125, 300, 600 and 900 µg/ml was added to the culture and incubated at 37 °C for 24 h. After incubation the culture was centrifuged at 10,000 rpm for 15 min. The culture supernatant was filtered by 0.22 μm syringe filters (Millipore) to obtain cell-free supernatant and the pyocyanin was extracted by adding 4.5 ml of chloroform to 7.5 ml of culture supernatant. At this point, the color changes to green blue. 3 ml of the bottom blue layer was mixed with 1.5 ml of 0.2 M HCl. The resulting pink color indicated the pyocyanin production and it was measured at 520 nm by UV–Vis spectrophotometer (Thermo Fischer Scientific, Evolution 201).
Effect of EA on Swimming and Swarming Motility
The effect of EA on motility of P. aeruginosa was checked by the method described by Vattem et al. [30] with slight modification. Different concentrations of EA were mixed with swarm agar medium and swim agar medium and P. aeruginosa was point inoculated and incubated at 37 °C for 48 h. The effect of EA on swarming and swimming motility was determined by swarm and swim pattern of treated groups in comparison with control.
Staphyloxanthin Biosynthesis Inhibition Assay
To study the pigment staphyloxanthin inhibition by S. aureus, the bacterium was grown in TSB (5 ml) in the presence and absence of EA for 24 h. The bacterial culture was centrifuged at 6000 rpm for 10 min and washed in PBS. Methanol was used to extract staphyloxanthin from the pallet, and the OD was measured at 450 nm using a spectrophotometer (Thermo Fischer Scientific, Evolution 201).
GC–MS Analysis
The compounds present in EA fraction were identified by gas chromatography–mass spectrometry (GC–MS). A volume of 1 µl of EA was injected into GC–MS coupled with Mass Spectrometer. The temperature was initially held at 80 °C for 2 min, then increased from 80 °C to 250 °C at 10 °C per minute, and held for 5 min at 250 °C. Helium with a flow rate of 1 ml/min was used as the carrier gas. The total run time was 30 min. The peaks were analyzed by mass spectral reference library (Wiley NIST).
Molecular Docking Studies
The possible interaction of the studied compounds within EA and QS signaling protein was evaluated using the Molegro Virtual Docker (MVD). The docking study was carried out against RhlG/NADP active-site complex (PDB ID: 2B4Q), LasR-TP4 complex (PDB ID: 3JPU) and Pseudaminidase from Pseudomonas aeruginosa (PDB ID: 2W38). Initially, MVD was used for the binding cavity prediction of said proteins. 0.8 Å resolution discrete grid of 1.4 Å radiuses positioned to check any overlap with any of the spheres. These locations were then confirmed whether it is a position of the cavity. Finally, these regions were determined for neighboring region and ranked based on their size [31]. ChemOffice 2010 (Cambridge Soft, USA) was used to generate the 3D optimized structures of the bioactive compounds determined using GC–MS and imported in the MVD. The iteration was set for maximum with 1500 having an evolution size of 50 for at least 20 docking engine runs. The best orientation of each compound was chosen for docking scores and subsequent analysis.
In Vitro Cytotoxicity Evaluation by MTT Assay
The murine monocytic macrophage cells (RAW 264.7) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Initially, 1 × 104 cells were counted and seeded in 96-well tissue culture plate at 37 °C and 5% CO2 for the period of 24 h. To find out the effect of EA on morphology and cell viability, the cells were treated with different concentration of EA for 24 h followed by analysis with an inverted microscope (Zeiss, Model AXIOVERT A1). After the incubation, MTT solution was added to the wells and kept at 37 °C. Mitochondrial dehydrogenases in the metabolically active cells reduced the MTT to purple formazan. This insoluble dark purple formazan was dissolved in denaturing buffer and the absorbance was taken at 580 nm. The cell viability was calculated by using the formula:
Statistical Analysis
Graph pad prism (version 5.0) was used for statistical analysis. One-way analysis of variance (ANOVA) and multiple comparison tests were used for P values determination.
Results
Antimicrobial Activity by Well Diffusion Method and Growth Curve Analysis
The result of the well diffusion assay indicated that the EA did not have any direct antimicrobial activity against S. aureus and P. aeruginosa up to concentration of 2 mg/ml. Supporting antimicrobial result from well diffusion assay, growth curve of the microbes also indicated that the EA did not have any effect on the growth of the microbes. Up to a concentration of 900 µg/ml, the EA does not have any effect on the growth of S. aureus (Fig. S1a) and P. aeruginosa (Fig. S1b).
Effect of EA on Biofilm Formation
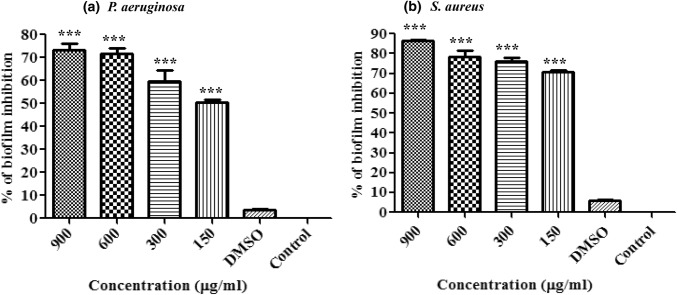
The present study revealed the increase in biofilm inhibition with an increase in EA concentration. The EA showed 50.60 ± 2.11% (***P < 0.0001), 59.72 ± 8.38% (***P < 0.0001), 71.68 ± 4.16% (***P < 0.0001) and 73.07 ± 5.13% (***P < 0.0001) (125, 300, 600, 900 µg ml−1) reduction in the biofilm production by P. aeruginosa (Fig. 1a). Further EA also showed 70.60 ± 2.11 (***P < 0.0001), 76.05 ± 3.06 (***P < 0.0001), 78.35 ± 5.30 (***P < 0.0001) and 86.40 ± 1.19% (***P < 0.0001) (at 125, 300, 600, 900 µg ml−1 respectively) inhibition in biofilm formation by S. aureus (Fig. 1b). 1% DMSO (control) did not show any significant anti-biofilm activity against both the microbes. SEM images (Fig S2) also showed the reduction in biofilm formation by S. aureus (Fig. S2a) and P. aeruginosa (Fig. S2b).
Fig. 1.
Effect of EA on biofilm inhibirion. Biofilm inhibition was assessed by crystal violet staining. The results are expressed as percentage biofilm inhibition for Staphylococcus aureus (a) and Pseudomonas aeruginosa (b). Values are represented as mean ± SD, n = 3. *P < 0.05; **P < 0.001; ***P < 0.0001
Anti-quorum Sensing Activity of EA
Further, this partially purified fraction was evaluated for its quorum sensing inhibition activity in QS reporter strain Chromobacterium violaceum ATCC 12472, and we have observed a clear hollow zone around the well containing EA (Fig. S3a). The violacein inhibition was also quantified and found that the EA inhibited the violacein pigment production in a dose-dependent manner (Fig. S3b).
Effect of EA on Motility in P. aeruginosa
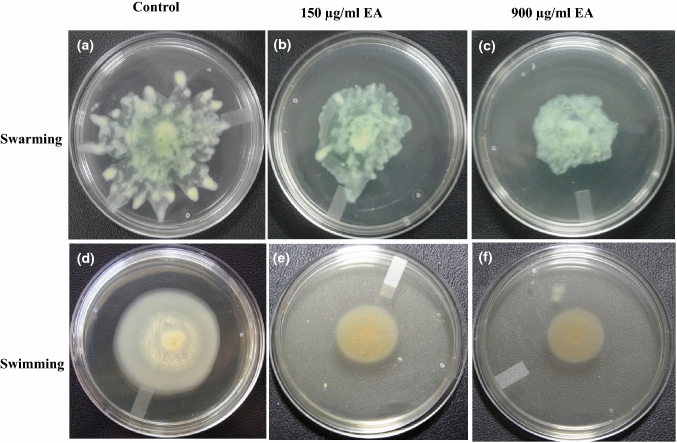
Motility in P. aeruginosa is appendages mediated movement, which enables bacteria to move or to adhere to the surface. This adherence leads to the formation of biofilm and also virulence of the bacteria. In the present study, EA reduced the motility (swarming and swimming) of P. aeruginosa (Fig. 2).
Fig. 2.
Effect of EA on Swarming and swimming motility in P. aeruginosa. Dose-dependent effect of EA on swarming motility (a control, b 150 µg/ml, c 900 µg/ml) and swimming motility (d control, e 150 µg/ml, f 900 µg/ml) in Pseudomonas aeruginosa
Pyocyanin Analysis
Pseudomonas aeruginosa showed inhibition of pyocyanin production when grown in presence of EA at different concentrations. Pre treatment with EA (concentration 150, 300, 600, 900 µg/ml) produced a significant reduction in pyocyanin concentration in a dose-dependent manner (Fig. 3). Upon treatment with lower concentration of EA (150 µg/ml) the absorbance of the pyocyanin production was reduced to 1.4505 ± 0.03 which is low as compared to control. But when treated with the higher concentration (900 µg/ml) the absorbance of the pyocyanin produced was reduced to 0.551 ± 0.039 which significantly lower in comparison to control.
Fig. 3.
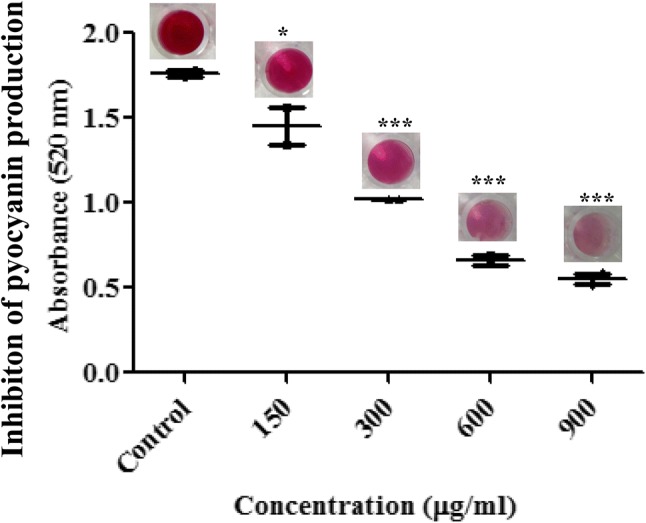
Inhibition of pyocyanin production by different concentration of EA in Pseudomonas aeruginosa. Values are represented as mean ± SD, n = 3. *P < 0.05; **P < 0.001; ***P < 0.0001
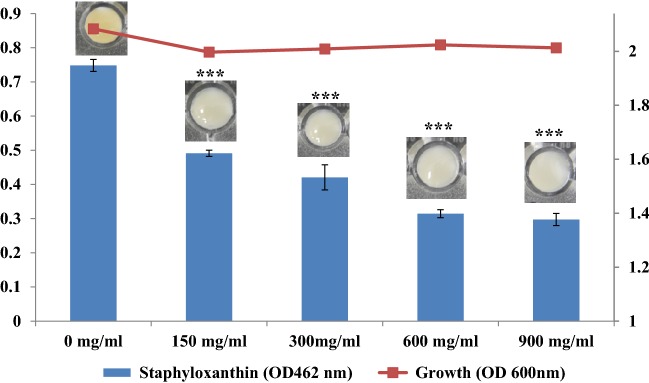
Staphyloxanthin Biosynthesis Inhibition by EA
Staphyloxanthin, a golden color carotenoid pigment produced by S. aureus is a key virulence factor of this pathogen. We examined whether EA could inhibit the synthesis of this pigment. When treated with the lower concentration of EA (150 µg/ml) the absorbance of the staphyloxanhin was reduced to 0.491 ± 0.01, while upon treatment with highest concentration used (900 µg/ml) the absorbance was reduced to 0.297 ± 0.02 which is significantly lower to the absorbance of the control which was 0.748 ± 0.01. The results indicated that S. aureus when grown in presence of different concentration of EA the pigment production inhibited significantly compared to the untreated cells (Fig. 4).
Fig. 4.
Reduction of Staphylococcus aureus pigmentation after treatment with EA at different concentration (150, 300, 600 and 900 µg/ml) for 24 h. Values are represented as mean ± SD, n = 3. *P < 0.05; **P < 0.001; ***P < 0.0001
Identification of Bioactive Compounds and Molecular Docking
Total 12 compounds have been identified by GC–MS analysis (Fig. S4). Among the phytochemicals, 3-N-Hexylthiane s–s-dioxide, 3-N-Hexylthiolane s s-dioxide, Dodecane 1-Fluoro- and Heptacosanoic acid were the most abundant compounds (Fig. S4). Interestingly, the molecular docking carried out against the compounds obtained from the GC–MS result shown in Table S1(a) (PDB ID: 2B4Q) Table S1(b) (PDB ID: 2W38) and Table S1(c) (PDB ID: 3JPU) revealed that Compound 9 (Heptacosanoic acid); compound 2 (3-N-Hexylthiane s, s-Dioxide) and Compound 11 (3-Methyl 2-(2-Oxopropyl) Furan) exhibiting favourable molecular interaction as evidenced from the Rerank score, MolDock score and H bond energy. The snaps shots of the molecular interaction are shown in Fig. S5. The molecular interaction of the compound with the biofilm associated proteins confirmed that the S. cumini partially purified extract is a potentially rich anti-biofilm agents.
Cytotoxicity Activity of Extract
The in vitro toxicity of EA was accessed by using RAW 264.7 cell line. The stability of the cells is a good indicator of compounds for the screening of cytotoxicity. Fig. S6 (a) represents the percentage of viable RAW cells treated with different concentration of extract. All concentration ranging from 100 to 1000 µg/ml, did not show any significant effect on cell viability. The effect of EA on cell morphology upon treatment with different concentrations of EA was observed using an inverted microscope, and it was found that the EA did not show any morphological changes upon treatment Fig. S6b (I–VII).
Discussion
The plant extract could be a promising tool for compromising the biofilm associated infection [32] as the battle of survival of micro-organisms against the antibiotics is a major concern [33].
When the micro-organisms were grwon in the presence of EA, it shows up to 86.40 ± 1.19% (***P < 0.0001) and 73.07 ± 5.13% (***P < 0.0001) reduction in formation of biofilm of S. aureus and P. aeruginosa respectively as evident from crystal violet staining method which is supported by the Scanning Electron Microscope images which correlates with earlier findings [30]. Quorum sensing is a process in which chemical signals secreted by microbes itself help in the organization of biofilm into complex structures [34] and is activated when microbes reaches a optimum cell density [35] which is also important for the production of virulence factors like swarming motility, swimming motility and pyocyanin production, etc. in P. aeruginosa. Now inhibition of quorum sensing is seen as the potential alternative of antibiotics to deal with the virulence of pathogenic micro-organisms [36, 37].
Swarming motility, a quorum sensing mediated phenomenon, helps the bacterial movement and colonization of the cells in nutrient rich surroundings [38] and any interference with the swarming motility affect the biofilm formation [39]. It is evident from the results that the swarming motility in P. aeruginosa was reduced remarkably (in comparison to control) by the treatment with different concentrations of EA. In addition to swarming motility, flagella-mediated swimming motility is known for its role in cell-to-surface attachment which leads to biofilm formation and other virulence phenotypes. From Fig. 2, it is clear that the swimming and swarming motility of P. aeruginosa were reduced significantly in dose dependent manner in comparison to control upon treatment with different concentration of EA.
Pseudomonas aeruginosa produces a green phenazine i.e. pyocyanin, a major virulence determinant of P. aeruginosa, functioning as a redox active toxin and also promote release of extracellular DNA (eDNA) which help in the biofilm formation [40]. In the present study the pyocyanin production by P. aeruginosa is inhibited significantly with the increase of EA concentration.
Staphylococcus aureus is responsible for causing different clinically and community-acquired infections. Staphyloxanthin a pigment produced by S. aureus, help the bacterium to survive in very harsh environmental condition and is one of the virulence factors during infections [41]. To see the effect of EA on S. aureus pigment production, staphyloxanthin was extracted from EA-treated S. aureus and it was found that the treated S. aureus cells showed less pigment production in comparison to control.
From GC–MS analysis of EA, different photochemical have been identified based on the mass spectra. Further, the molecular docking simulation carried out against the bacterial enzyme (PDB ID: 2B4Q, 2W38 and 3JPU) comes to a conclusion that Heptacosanoic acid (compound 9), 3-N-Hexylthiane s, s-Dioxide (compound 2) and 3-Methyl 2-(2-Oxopropyl) Furan (Compound 11) is most likely to form molecular interaction with the enzyme (2B4Q, 2W38 and 3JPU) on active site as evidenced from the MolDock Score and Rerank Score Table S1, S2 and S3. This molecular interaction concludes that these compounds will form a strong interaction with the bacterial proteins.
RhlG/NADP active-site complex (PDB ID: 2B4Q) is essential for the rhamnolipid formation which regulates the swarming motility in P. aeruginosa and LasR is also related in quorum sensing of the bacteria. Studies also show that mutation in sialidase (Pseudaminidase) gene resulted in the reduction of biofilm formation [42]. Overall the molecular docking analysis revealed that Heptacosanoic acid, 3-N-Hexylthiane s, s-Dioxide and 3-Methyl 2-(2-Oxopropyl) is likely to involve in inhibition of biofilm formation and quorum sensing.
Many QS inhibitors have been identified from different medicinal plants of Southern Florida [43]. Hence, biofilm formation and virulence in the micro-organisms could be inhibited by searching alternative strategies like compounds isolated from plants and micro-organisms which could inhibit the QS signaling of bacteria rather direct killing [44]. The present study demonstrated the anti-biofilm and quorum sensing related virulence inhibition potential and the presence of possible QS inhibitors in the extracts of S. cumini.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The author K. Gupta acknowledges to Department of Biotechnology, Government of India for financial support (DBT-JRF, Ref. No. DBT-JRF/2011-12/315 dated 19/10/2011). The authors also express their gratitude to Botanical Survey of India (BSI), Shillong, India, to help in the identification of plant sample and SAIC Tezpur University for SEM images.
Abbreviations
- EA
Ethyl acetate active fraction from crude extract
- QS
Quorum sensing
- PBS
Phosphate buffer saline
- OD
Optical density
- P. aeruginosa
Pseudomonas aeruginosa
- S. aureus
Staphylococcus aureus
References
- 1.Kalia VC, Prakash J, Koul S, Ray S. Simple and rapid method for detecting biofilm forming bacteria. Indian J Microbiol. 2017;57:109–111. doi: 10.1007/s12088-016-0616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das MC, Sandhu P, Gupta P, Rudrapaul P, De Tribedi UC. Attenuation of Pseudomonas aeruginosa biofilm formation by Vitexin: a combinatorial study with azithromycin and gentamicin. Sci Rep. 2016;22:23347. doi: 10.1038/srep23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalia VC. Microbes, antimicrobials and resistance: the battle goes on. Indian J Microbiol. 2014;54:1–2. doi: 10.1007/s12088-013-0443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 5.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31:224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Kalia VC. Microbes: the most friendly beings? In: Kalia VC, editor. Quorum sensing vs quorum quenching: a battle with no end in sight. New Delhi: Springer; 2015. pp. 1–5. [Google Scholar]
- 7.Kalia VC, Wood TK, Kumar P. Evolution of resistance to quorum-sensing inhibitors. Microb Ecol. 2014;68:13–23. doi: 10.1007/s00248-013-0316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Koul S, Prakash J, Mishra A, Kalia VC. Potential emergence of multi-quorum sensing inhibitor resistant (MQSIR) bacteria. Indian J Microbiol. 2016;56:1–18. doi: 10.1007/s12088-015-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalia VC. In search of versatile organisms for quorum sensing inhibitors: acyl homoserine lactones (AHL) acylase and AHLlactonase. FEMS Microbiol Lett. 2014;359:143. doi: 10.1111/1574-6968.12585. [DOI] [PubMed] [Google Scholar]
- 11.Kalia VC, Kumar P, Pandian STK, Sharma P. Biofouling control by quorum quenching. In: Kim SK, editor. Springer handbook of marine biotechnology (vol 15) Berlin: Springer; 2015. pp. 431–440. [Google Scholar]
- 12.Huma N, Shankar P, Kushwah J, Bhushan A, Joshi J, Mukherjee T, Raju SC, Purohit HJ, Kalia VC. Diversity and polymorphism in AHL-lactonase gene (aiiA) of Bacillus. J Microbiol Biotechnol. 2011;21:1001–1011. doi: 10.4014/jmb.1105.05056. [DOI] [PubMed] [Google Scholar]
- 13.Kalia VC, Raju SC, Purohit HJ. Genomic analysis reveals versatile organisms for quorum quenching enzymes: acyl-homoserine lactone-acylase and –lactonase. Open Microbiol J. 2011;5:1–13. doi: 10.2174/1874285801105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar P, Patel SKS, Lee JK, Kalia VC. Extending the limits of Bacillus for novel biotechnological applications. Biotechnol Adv. 2013;31:1543–1561. doi: 10.1016/j.biotechadv.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Hoiby N, Givskov M. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu JF, Garo E, Goering MG, Pasmore M, Yoo HD, Esser T, Sestrich J, Cremin PA, Hough GW, Perrone P, Lee YS, Le NT, O’Neil-Johnson M, Costerton JW, Eldridge GR. Bacterial biofilm inhibitors from Diospyros dendo. J Nat Prod. 2006;69:118–120. doi: 10.1021/np049600s. [DOI] [PubMed] [Google Scholar]
- 17.Garo E, Eldridge GR, Goering MG, DeLancey Pulcini E, Hamilton MA, Costerton JW, James GA. Asiatic acid and corosolic acid enhance the susceptibility of Pseudomonas aeruginosa biofilms to tobramycin. Antimicrob Agents Chemother. 2007;51:1813–1817. doi: 10.1128/AAC.01037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Cho MH, Lee J. 3-Indolylacetonitrile decreases Escherichia coli O157:H7 biofilm formation and Pseudomonas aeruginosa virulence. Environ Microbiol. 2011;13:62–73. doi: 10.1111/j.1462-2920.2010.02308.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuzma L, Rozalski M, Walencka E, Rozalska B, Wysokinska H. Antimicrobial activity of diterpenoids from hairy roots of Salvia sclarea L.: salvipisone as a potential anti-biofilm agent active against antibiotic resistant Staphylococci. Phytomedicine. 2007;14:31–35. doi: 10.1016/j.phymed.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Stenz L, Francois P, Fischer A, Huyghe A, Tangomo M, Her-nandez D, Cassat J, Linder P, Schrenzel J. Impact of oleic acid (cis-9-octadecenoic acid) on bacterial viability and biofilm production in Staphylococcus aureus. FEMS Microbiol Lett. 2008;287:149–155. doi: 10.1111/j.1574-6968.2008.01316.x. [DOI] [PubMed] [Google Scholar]
- 21.Payne DE, Martin NR, Parzych KR, Rickard AH, Underwood A, Boles BR. Tannic acid inhibits Staphylococcus aureus surface colonization in an IsaA dependent manner. Infect Immun. 2013;81:496–504. doi: 10.1128/IAI.00877-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sajid A, Arora G, Singhal A, Kalia VC, Singh Y. Protein phosphatases of pathogenic bacteria: role in physiology and virulence. Annu Rev Microbiol. 2015;69:527–547. doi: 10.1146/annurev-micro-020415-111342. [DOI] [PubMed] [Google Scholar]
- 23.Kalia VC, Koul S, Ray S, Prakash S. Targeting quorum sensing mediated Staphylococcus aureus biofilms. In: Kalia VC, editor. Biotechnological applications of quorum sensing inhibitors. Berlin: Springer; 2018. pp. 23–32. [Google Scholar]
- 24.Duraipandiyan V, Ayyanar M, Ignacimuthu S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement Altern Med. 2006;6:35. doi: 10.1186/1472-6882-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maciel MCG, Farias JC, Maluf MJ, Gomes EA, Pereira PVS, Aragão-Filho WC, Frazão JB, Costa GC, Sousa SM, Silva LA, Amaral FMM, Russo M, Guerra RNM, Nascimento FRF. Syzygium jambolanum treatment improves survival in lethal sepsis induced in mice. BMC Complement Altern Med. 2008;8:1–7. doi: 10.1186/1472-6882-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gopu V, Kothandapani S, Shetty PH. Quorum quenching activity of Syzygium cumini (L.) Skeels and its anthocyanin malvidin against Klebsiella pneumoniae. Microb Pathog. 2015;79:61–69. doi: 10.1016/j.micpath.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Gupta K, Hazarika SN, Saikia D, Namsa ND, Mandal M. One step green synthesis and anti-microbial and anti-biofilm properties of Psidium guajava L. leaf extract-mediated silver nanoparticles. Mater Lett. 2014;125:67–70. doi: 10.1016/j.matlet.2014.03.134. [DOI] [Google Scholar]
- 28.Gupta K, Barua S, Hazarika NS, Manhar AK, Nath D, Karak N, Namsa ND, Mukhopadhyay R, Kalia VC, Mandal M. Green silver nanoparticles: enhanced antimicrobial and anti biofilm activity with effects on DNA replication and cell cytotoxicity. RSC Adv. 2014;4:52845–52855. doi: 10.1039/C4RA08791G. [DOI] [Google Scholar]
- 29.Rahman MRT, Lou Z, Yu F, Wang P, Wang H. Anti-quorum sensing and anti-biofilm activity of Amomum tsaoko (Amommum tsao-ko Crevost et Lemarie) on foodborne pathogens. Saudi J Biol Sci. 2017;24:324–330. doi: 10.1016/j.sjbs.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vattem DA, Mihalik K, Crixell SH, McLean RJ. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia. 2007;78:302–310. doi: 10.1016/j.fitote.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Thomsen R, Christensen MH. MolDock: a new technique for high-accuracy molecular docking. J Med Chem. 2006;49:3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- 32.Wojnicz D, Kucharska AZ, Sokoł-Łętowska A, Kicia M, Tichaczek-Goska D. Medicinal plants extracts affect virulence factors expression and biofilm formation by the uropathogenic Escherichia coli. Urolo Res. 2012;40:683–697. doi: 10.1007/s00240-012-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalia VC, Kumar P. The battle: quorum-sensing inhibitors versus evolution of bacterial resistance. In: Kalia VC, editor. Quorum sensing vs quorum quenching: a battle with no end in sight. New Delhi: Springer; 2015. pp. 385–391. [Google Scholar]
- 34.Kalia VC, Prakash S, Koul S, Ray S. Quorum sensing and its inhibition: Biotechnological applications. In: Kalia VC, editor. Quorum sensing and its biotechnological applications. New Delhi: Springer; 2018. pp. 3–16. [Google Scholar]
- 35.Koul S, Kalia VC. Multiplicity of quorum quenching enzymes: a potential mechanism to limit quorum sensing bacterial population. Indian J Microbiol. 2017;57:100–108. doi: 10.1007/s12088-016-0633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalia VC, Kumar P. Potential applications of quorum sensing inhibitors in diverse fields. In: Kalia VC, editor. Quorum sensing vs quorum quenching: a battle with no end in sight. New Delhi: Springer; 2015. pp. 359–370. [Google Scholar]
- 37.Kumar P, Koul S, Patel SKS, Lee JK, Kalia VC. Heterologous expression of quorum sensing inhibitory genes in diverse organisms. In: Kalia VC, editor. Quorum sensing vs quorum quenching: a battle with no end in sight. New Delhi: Springer; 2015. pp. 343–356. [Google Scholar]
- 38.Verstraeten N, Braeken K, Debkumari B, Fauvart M, Fransaer J, Vermant J, Michiels J. Living on a surface: swarming and biofilm formation. Trends Microbiol. 2008;16:496–506. doi: 10.1016/j.tim.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Fuente-Núñez CDL, Korolik V, Bains M, Nguyen U, Breidenstein EBM, Horsman S, Lewenza S, Burrows L, Hancock REW. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob Agents Chemother. 2012;56:2696–2704. doi: 10.1128/AAC.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das T, Manefield M. Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS ONE. 2012;7:e46718. doi: 10.1371/journal.pone.0046718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahlon AK, Roy S, Sharma A. Molecular docking studies to map the binding site of squalene synthase inhibitors on dehydrosqualene synthase of Staphylococcus aureus. J Biomol Struct Dyn. 2010;28:201–210. doi: 10.1080/07391102.2010.10507353. [DOI] [PubMed] [Google Scholar]
- 42.Soong G, Muir A, Gomez MI, Waks J, Reddy B, Planet P, Singh PK, Kaneko Y, Wolfgang MC, Hsiao YS, Tong L, Prince A. Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J Clin Invest. 2006;116:2297–2305. doi: 10.1172/JCI27920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adonizio A, Kong KF, Mathee K. Vattem DA, Mihalik K, Crixell SH, McLean RJ (2007) Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother. 2008;52:198–203. doi: 10.1128/AAC.00612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalia VC, Patel SKS, Kang YC, Lee JK. Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotechnol Adv. 2018 doi: 10.1016/j.biotechadv.2018.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.