Abstract
Objectives:
Recent studies indicate that glucose metabolism is altered in rheumatoid arthritis (RA) fibroblast-like synoviocytes (FLS). Hexokinases (HK)s catalyze the first step in glucose metabolism and HK2 constitutes the principal HK inducible isoform. We hypothesize that HK2 contributes to the synovial lining hypertrophy and plays a critical role in bone and cartilage damage.
Methods:
HK1 and HK2 expression were determined in RA and osteoarthritis (OA) synovial tissue by immunohistochemistry. RA FLS were transfected with either HK1 or HK2 siRNA, or infected with either adenovirus (ad)-GFP, ad-HK1 or ad-HK2. FLS migration and invasion were assessed. To study the role of HK2 in vivo, 108 particles of ad-HK2 or ad-GFP were injected into the knee of WT mice. K/BxN serum-transfer arthritis was induced in HK2F/F mice harboring Col1a1-Cre (HK2Col1), to delete HK2 in non-hematopoietic cells.
Results:
HK2 is particular of RA histopathology (9/9 RA; 1/8 OA) and colocalizes with FLS markers. Silencing HK2 in RA FLS resulted in a less invasive and migratory phenotype. Consistently, overexpression of HK2 resulted in an increased ability to migrate and invade. It also increased extracellular lactate production. Intra-articular injection of ad-HK2 in normal knees dramatically increased synovial lining thickness, FLS activation and proliferation. HK2 was highly expressed in the synovial lining after K/BxN serum transfer arthritis. HK2Col1 mice significantly showed decreased arthritis severity, bone and cartilage damage.
Conclusion:
HK2 is specifically expressed in RA synovial lining and regulates FLS aggressive functions. HK2 might be an attractive selective metabolic target safer than global glycolysis for RA treatment.
Keywords: rheumatoid arthritis, fibroblast-like synoviocyte, glucose metabolism, exokinase
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic autoimmune disease that leads to chronic inflammation and progressive joint destruction(1). One of the apparent histopathological changes of the joint in RA is the hypertrophic synovial lining(2, 3). Fibroblast-like synoviocytes (FLS) are stromal cells that give structure to the synovial lining and are contributing to RA pathology by invading and promoting cartilage destruction, expressing metalloproteases (MMP), and inducing chronic inflammation by secreting pro-inflammatory cytokines and chemokines(2, 4, 5). There is an unmet need to target FLS aggressive phenotype in RA in combination with current therapies.
Different approaches have highlighted underlying mechanisms that explain RA FLS behavior, including activation of signaling pathways(6, 7)), and epigenetic modifications(8, 9). RA FLS metabolism has gained attention as recent studies have pointed out to modifications in RA FLS cell metabolism(3, 10). Many key signaling pathways that are activated by the inflamed joint microenvironment converge to adapt cell metabolism in order to support FLS activation and aggressive phenotype, suggesting that the study of metabolic changes in RA FLS and key players could potentially lead to the identification of new therapeutic agents(3, 10–12).
Among other metabolic changes, our recent work and others have highlighted a critical role of glucose metabolism in activated FLS(13, 14). The high level of activity in glycolytic inflamed tissues is manifested by the use of positron emission tomography (FDG-PET), which can be observed by PET in swollen joints(15, 16). Also, synovial fluid from RA patients has higher levels of lactate and less glucose levels than osteoarthritis (OA) synovial fluid(17, 18). This dependency of activated cells on accelerated glucose metabolism could render them more vulnerable to the disruption of glucose metabolism and support that targeting glucose metabolism might be a reasonable complementary approach for RA patients(11).
Hexokinases (HKs) catalyze the first committed step in glucose metabolism(19, 20). By catalyzing the phosphorylation of glucose to G6P, hexokinases promote and sustain a concentration gradient that facilitates glucose entry into cells and the initiation of all major pathways of glucose utilization. HK1 is constitutively expressed in most mammalian adult tissues. HK2, which also has high affinity for glucose and harbors two catalytic domains, constitutes the principal inducible isoform(21). The identification of isoform-specific contributors to elevated cell glucose metabolism without compromising systemic homeostasis or normal metabolic functions could make targeting cell metabolic changes an approach feasible.
Here, we test the hypothesis that HK2 is a specific isoform contributing to elevated cell glucose metabolism in RA FLS and is a key regulator of aggressive FLS phenotype. We show that HK2 expression is elevated in the RA synovial tissue and overexpression of HK2 in the murine synovial lining promotes hypertrophy of healthy synovium as well as RA FLS activation. HK2 deletion in FLS decreases its aggressive phenotype, and HK2 deletion in Col1a1-expressing cells ameliorates disease severity of arthritis. Taken together, our data suggest that HK2 is involved in FLS activation and synovial hypertrophy and could play a role in RA.
Methods
Human synovium and FLS:
Human synovium or FLS were extracted from patients with RA or OA undergoing total joint replacement. All RA patients met the American College of Rheumatology 1987 revised criteria for seropositive RA as previously described(22, 23). FLS were used between p4 and p9 passages.
More detailed methods are provided as supplementary methods
RESULTS
HK2 expression is mainly induced in the inflamed synovial lining of RA patients
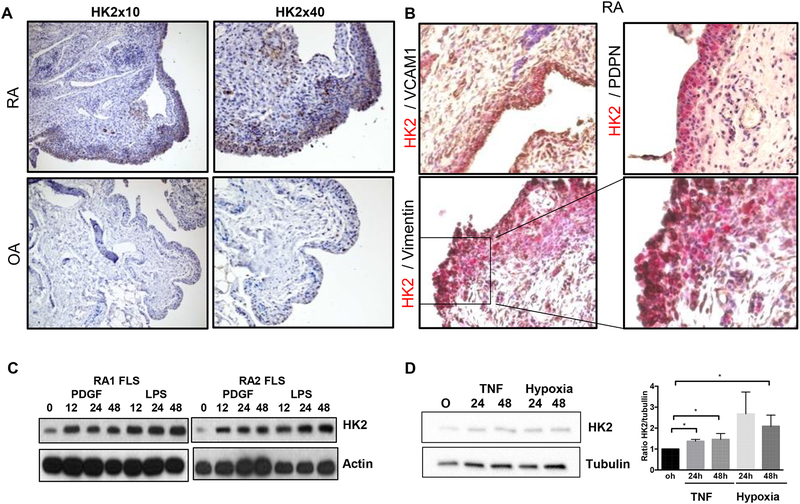
We first studied the expression of both HK isoforms in synovium to determine whether or not HK2 is an inducible isoform in synovial tissue. Immunohistochemistry (IHC) analysis of HK2 expression in RA synovium revealed that HK2 was detected in RA synovial tissue, very predominant in the lining but also in sublining (Fig. 1A). Of interest, while HK2 expression was abundant in all RA synovial samples tested (9 different RA donors), both lining and sublining were negative for HK2 expression in most OA synovial samples (7 out of 8 different OA donors) (Fig. 1A). HK1 expression, as expected, was uniformly present in both OA and RA synovial tissue (Supplementary Fig. 1A). Of note, when cultured in vitro, both RA and OA FLS cell lines expressed HK2 at basal level, although HK2 expression level was higher in RA than in OA FLS (Supplementary Fig. 1B).
Figure 1. HK2 expression is expressed in the inflamed synovial lining of RA patients.
A) Representative IHC images of HK2 staining in RA and OA synovium. B) Representative IHC images of double-color staining of HK2 (red) and vimentin (black), VCAM1 (black) or PDPN (black) in a RA human synovium. C) Immunoblot (IB) of the indicated proteins in RA FLS at baseline and after PDGF or LPS stimulation for 24 and 48 hrs. D) IB and quantification of the indicated proteins in RA FLS after TNF or hypoxia (1%) stimulation. Results are average of 3 different RA FLS lines. Values are the mean ± SEM * =p<0.05.
We then performed a double-color immunohistochemistry (IHC) to determine whether or not FLS expressed HK2 in the RA synovium. We identified FLS as cells positive for vimentin, vascular cell adhesion protein 1 (VCAM1) or podoplanin (PDPN)(2, 24). As shown in Fig. 1B, HK2 positive staining colocalized with all the different FLS markers in the lining. Of interest, other cells in the sublining of some RA samples, which were negative for FLS markers, did expressed HK2, suggesting that other synovial cell types could also upregulate HK2 expression in RA synovial tissue.
Effect of HK2 modulation in aggressive functions of human FLS
In order to investigate the role of HK2 in RA FLS functions, we conducted in vitro studies. We first studied whether inflammatory mediators implicated in FLS inflammatory response upregulated HK2 expression. As shown in Fig. 1C and D, stimulation of RA FLS with PDGF, LPS, TNF or hypoxia increased the expression of HK2 protein. TNF and hypoxia stimulation also increased HK2 protein levels in OA FLS (Supplementary Fig. 1C).
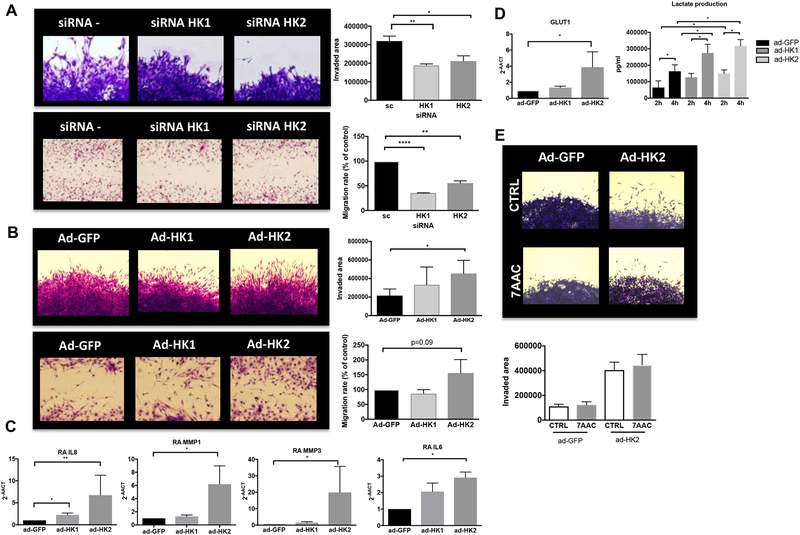
We then silenced HK1 and HK2 expression in those cell lines. Supplementary Fig. 2A and B, shows HK1 and HK2 expression by qPCR and immunoblotting (IB) after HK silencing in RA FLS. As glycolysis inhibition was shown to induce cell death in FLS(25), we confirmed that HK1 or HK2 silencing did not change cell viability or shape (supplementary Fig. 2C). As shown in Fig. 2A, RA FLS after HK silencing were less invasive (HK1: p<0.001; HK2: p<0.05) and migration measured by the length of the scar was also diminished (HK1: p<0.0001; HK2 p<0.001). Yet, HK silencing did not modify IL-6, IL-8 or MMP expression (supplementary Fig. 2D).
Figure 2. Effect of HK2 modulation in human RA FLS migration and invasion.
A) RA FLS were transfected with either siRNA control, siRNA HK1 or siRNA HK2 as detailed in methods. Representative images of invasion area (upper panels) and migration (lower panels) are shown. Quantification, as detailed in methods, of area of invasion and migration after PDGF stimulation for 24 hrs are shown. Results are average of 3 different RA FLS lines. Values are the mean ± SEM. B) RA FLS were infected with Ad-GFP, Ad-hHK1 or Ad-hHK2 as detailed in methods. Representative images of invasion area (upper panels) and migration (lower panels) are shown. Quantification, as detailed in methods, of area of invasion and migration after PDGF stimulation for 24 hrs. are shown. Results are average of 3 different RA FLS lines. Values are the mean ± SEM. C) A) qPCR analysis of the indicated genes in RA FLS 72 hrs. after ad-GFP, ad-HK1 and ad-HK2 infection. Results are average of 5 different RA FLS lines. Values are shown as mean ± SEM. * =p<0.05; **=p<0.01. D) left: RNA levels of GLUT1 in RA FLS, 72 hrs. after ad-GFP, ad-HK1 or ad-HK2 infection. Results are average of 3 different RA FLS lines. Values are the mean ± SEM. Right: RA FLS were infected with ad-GFP, ad-HK1 or ad-HK2. Media was changed 48 hrs. after infection and lactate was measured in the supernatants at 2 hrs. and at 4 hrs. Data are pooled from thee technical replicates, and 3 different RA FLS lines. Values are the mean ± SEM. E) RA FLS were infected with Ad-GFP or Ad-hHK2 as detailed in methods. Representing images and quantification are shown of area of invasion after PDGF stimulation for 24 hrs. in the presence or absence of 10uM 7AAC. Results are average of 3 different RA FLS lines. Values are the mean ± SEM. * =p<0.05, **p<0.01, ***p<0.001.
We also tested the effect of HK2 overexpression in RA FLS by infecting with adenovirus carrying human HK1 or HK2 (Ad-huHK1 or Ad-huHK2). Supplementary Fig. 3A and B shows HK1 and HK2 expression by qPCR and IB after Ad-HK infection in RA FLS. After HK2 overexpression, RA FLS were more invasive than after HK1 overexpression (HK2: p=0.01, Fig. 2B). HK2 overexpression also decreased the length of the scar in the migration assay in FLS although did not reach significance (Fig. 2B), and increased RNA levels of the pro-inflammatory cytokine IL-6, IL-8 and metalloproteinases (MMP) (Fig. 2C). Yet, it did not induce any change in proliferation (Supplementary Fig. 3C) Interestingly, both HK1 and HK2 overexpression increased RA FLS extracellular lactate production but only HK2 overexpression also increased GLUT1 mRNA levels (Fig. 2D). Yet, inhibition of the extracellular lactate transporters after HK2 overexpression by 7AAC, which specifically inhibits monocarboxylate transporter 1 and 4 (MTC1/4), did not abolish HK2 invasive phenotype in RA FLS (Fig. 2E).
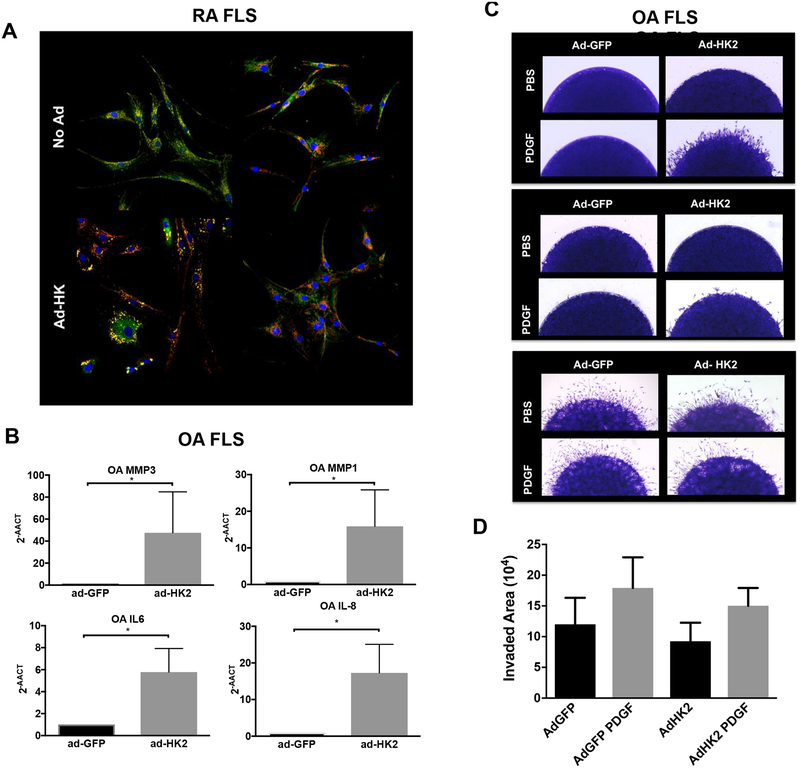
HKs can bind to mitochondria through their N-terminal hydrophobic regions. Confocal studies were conducted to determine HKs intracellular distribution in FLS. Of interest, these studies showed that the distribution of HKs in non-infected FLS was different between HK1 and HK2. While HK1 was colocalized with or adjacent to mitochondria, HK2 was only partially mitochondria-bounded and it also displayed diffuse cytoplasmic distribution (Fig. 3A and supplementary Fig. 4). Notably, after HK1 overexpression, the intensity of HK1 colocalization with mitochondria and mitochondria distribution differed considerably from HK2 distribution and colocalization after HK2 overexpression, which may explain the discrepancies observed after HK1 and HK2 overexpression. (Figure 3A and supplementary Fig. 4)
Figure 3. Intracellular distribution of HKs and OA FLS phenotype after HKs overexpression.
A) Intracellular distribution of HKs (in green) and mitochondria (Tom20; in red) in FLS at baseline (upper panels) and after Ad- infection (lower panels) examined by confocal microscopy. Figure shows overlapping images (yellow, colocalization of HKs with Tom20). Representative images of three different RA FLS are shown. B) qPCR analysis of the indicated genes in OA FLS 72 hrs. after ad-GFP and ad-HK2 infection. Results are average of 3 different OA FLS lines. Values are shown as mean ± SEM. * =p<0.05. C) OA FLS were infected with Ad-GFP or Ad-hHK2 as detailed in methods. Representative images of invasion area of 3 different OA FLS cell lines are shown. D) OA FLS were infected with Ad-GFP or Ad-hHK2 as detailed in methods. Quantification, as detailed in methods, of area of invasion after PDGF stimulation for 24 hrs. is shown. Results are average of 4 different OA FLS lines. Values are the mean ± SEM.
We finally addressed whether HK2 overexpression could induce an inflammatory phenotype in OA FLS. As shown in Figure 3B, overexpression of HK2 induced an increase of RNA levels of the pro-inflammatory cytokine IL-6, IL-8 and metalloproteinases (MMP) in OA cell lines. Of interest, the overexpression of HK2 induced an invasive phenotype compared to ad-GFP but not in all OA FLS cell lines tested. As opposed to the homogeneity of the HK2 overexpression effect on RA FLS, the invasive phenotype after HK2 overexpression varied among OA FLS cell lines (Figure 3C), and overall quantification (Figure 3D) did not show significant differences, suggesting that HK2 expression contributes but is not sufficient to completely recapitulate the aggressive phenotype of RA FLS.
HK2 overexpression leads to a synovial lining hypertrophy in murine healthy joints
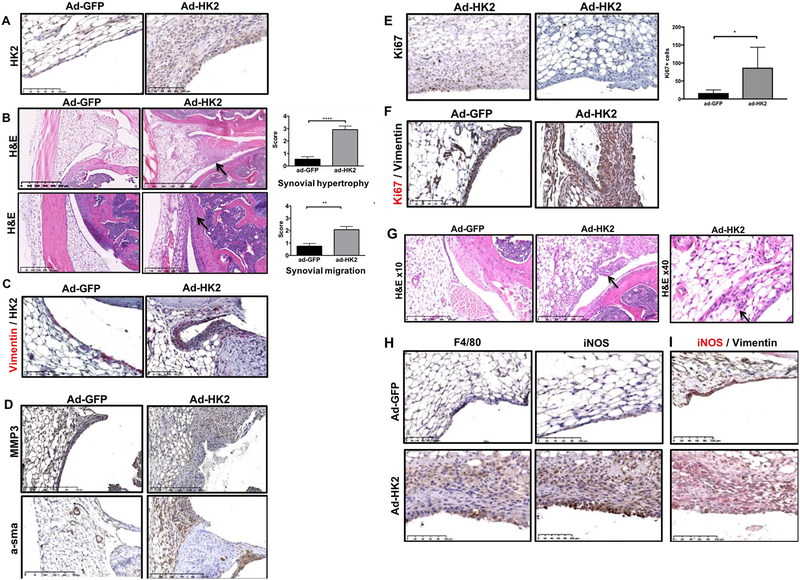
In order to assess the effect of HK2 overexpression in synovial tissue in vivo, we intra-articularly injected adenovirus carrying murine HK2 (Ad-mHK2) into murine knees, and adenovirus carrying GFP (Ad-GFP) as a control in the contralateral knee of wild-type (WT) healthy mice. Overexpression of HK2 in the synovial lining was effective since HK2 expression was increased in Ad-mHK2 injected joints compare to Ad-GFP injected joints (Fig. 4A). Interestingly, we observed that intra-articular injection of ad-mHK2 in normal knees dramatically increased synovial lining thickness (mean± SEM: ad-GFP: 0.54±0.21; ad-HK2: 2.91±0.28; p<0.00001) (Fig. 4B). We also observed synovial lining from ad-HK2 injected knee migrating towards the cartilage compared to ad-GFP-injected knees (mean± SEM: ad-GFP: 0.75±0.22; ad-HK2: 2.03±0.29; p=0.0015) (Fig. 4B). Double-color IHC with vimentin confirmed HK2 expression in FLS after ad-mHK2 injection (Fig. 4C).
Figure 4. HK2 overexpression leads to a hypertrophic synovia in healthy joints.
Control WT mice (n=13) were injected intraarticularly in one knee with 108 ad-mHK2 and ad-GFP in contralateral knee. A) Representative image of HK2 staining of the indicated mice. B) Upper panels: Representative images of hematoxylin & Eosin (H&E) showing hypertrophy (arrow indicated synovial hypertrophy), and quantification (mean± SEM: ad-GFP: 0.54±0.21; ad-HK2: 2.91±0.28; p<0.00001) are shown. Lower panels: Representative images of hematoxylin & Eosin (H&E) showing synovial attachment to cartilage (arrow indicated synovial migration), and quantification (mean± SEM: ad-GFP: 0.75±0.22; ad-HK2: 2.03±0.29; p=0.0015) are shown. C) Representative IHC image of double color staining of anti-vimentin (red) and anti-HK2 (black) of the indicated mice. D) Representative IHC image of anti-alpha-sma and MMP3 of the indicated mice. E) Representative Ki67 staining of the indicated mice and quantification of Ki67 positive cells in the synovium of ad-GFP or ad-HK2 injected knees (mean± SEM: ad-GFP: 16.5±8.7; ad-HK2: 87.14±56.64, p=0.05, n=6 per group). F) Representative IHC image of double color staining of anti-vimentin (black) and anti-Ki67 (red) of the indicated mice. G) Representative images of hematoxylin & Eosin (H&E) showing sublining infiltration (100× magnification in upper images and 400× magnification in lower images) of indicated mice. (quantification: mean± SEM: ad-GFP: 0.08±0.08; ad-HK2: 0.92±0.29; p=0.02). H) Representative IHC image of F4/80, iNOS and vimentin (black) and iNOS (red) staining of the synovial of the indicated mice.
We then tested if HK2 overexpression modified FLS activation and proliferation by staining for anti-alpha smooth muscle actin (α-sma), a myofibroblastic marker, MMP3 and Ki67, a marker of proliferative cells. Fig. 4D shows that anti α -sma staining was increased in ad-mHK2 injected knees when compared with ad-GFP injected knees. MMP3 staining was also widely present in the hypertrophic lining in ad-mHK2 synovium (Fig. 4D). Of interest, intra-articular injection of ad-mHK2 also increased the number of positive Ki67 cells in the synovium (Fig. 4E) mean± SEM: ad-GFP: 16.5±8.7; ad-HK2: 87.14±56.64, p=0.05. Double color IHC with vimentin showed some Ki67 positive cells in vimentin positive cells, suggesting that HK2 overexpression in FLS induced FLS proliferation in vivo (Fig. 4F).
We also determined whether the overexpression of Ad-mHK2 in the lining induced the recruitment of infiltrating cells. Although the response was more heterogeneous than the synovial hypertrophy induction by HK2 overexpression, we observed a mild sublining infiltration in 6 out of 13 knees after ad-mHK2 injection, compared to none after ad-GFP injection (Mean± SEM: ad-GFP: 0.08±0.08; ad-HK2: 0.92±0.29; p=0.02) (Fig. 4G). When observed at higher magnification, most of the infiltrating cells were mononuclear cells (Fig. 4G). We then stained for F4/80, a marker of monocyte-macrophage lineage, and for iNOS, which stains activated myeloid cells. We observed that both sublining infiltrating cells were positive for F4/80 and iNOS. Double-color IHC with vimentin and iNOS confirmed that most of vimentin-positive cells were negative for iNOS, suggesting that the infiltrating cells were indeed activated myelomonocitic cells (Fig. 4H).
HK2 expression in murine joints is also increased in synovial FLS after arthritis induction.
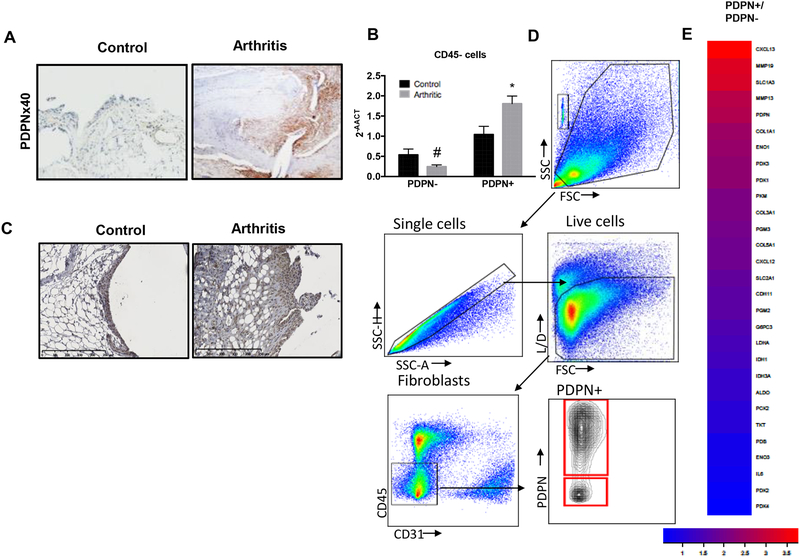
The effect of HK2 on FLS migration and invasion, led us to evaluate the role of HK2 in the K/BxN arthritis model. The K/BxN passive serum transfer model is FLS dependent and requires only innate immunity(26, 27). PDPN, glycoprotein highly expressed in activated RA FLS, is also expressed in the arthritic murine lining (Fig. 5A). As we recently published(14), several genes related to glucose metabolism were upregulated early in the course of the arthritis, at day 5 after K/BxN serum transfer, in enriched arthritic CD45negPDPNpos cells. We confirmed that HK2 expression had higher expression in enriched arthritic CD45negPDPNpos cells (1.04 ± 0.4 in normal joints vs 1.8 ± 0.3 in arthritic joints: p=0.03; 1.8 ± 0.3 in arthritic CD45negPDPNpos cells vs 0.25 ± 0.09 in arthritic CD45negPDPNneg cells: p<0.01) (Fig. 5B). HK2 was highly and uniformly expressed in the invasive lining in arthritic joints (Fig. 5C). Several glucose metabolism related genes together with other markers of activated FLS, were also increased at the peak of the arthritis in CD45negPDPNpos cells compared to CD45negPDPNneg (Fig. 5D and E). Specifically, GLUT1, ENO1 but also pyruvate kinase muscle isoenzyme 2 (PKM2), Pyruvate Dehydrogenase Kinase (PDK) 1 and 3 were upregulated in this population.
Figure 5. Specific deletion of HK2 in Col1a1 expressing cells ameliorates arthritis in an animal model of inflammatory arthritis.
Serum passive K/BxN arthritis was induced after intraperitoneally injection of 150 μl of K/BxN mouse serum on day 0. A) Representative IHC images at day 9 after serum injection of PDPN expression in synovial tissue of arthritic WT mice. B) Gene expression level of HK2 in isolated CD45negPDPNpos cells from control mice or KRN induced arthritic mice. HK2 expression is increased in arthritic CD45negPDPNpos (1.04 ± 0.4 in normal joints vs 1.8 ± 0.3 in arthritic joints; p=0.03). C) Representative IHC images at day 9 after serum injection of HK2 expression in synovial tissue of arthritic WT mice versus control WT mice. D) Single cells from arthritic joints were enriched for CD45-PDPN+ as described in methods. Shown sorted populations for further RNAseq analysis. E) Heat map of gene expression level of isolated CD45negPDPNpos cells compare to CD45negPDPNneg from arthritic joints as described in methods.
Conditional knockout of HK2 in Col1a1-expressing cells has an ameliorated arthritic disease
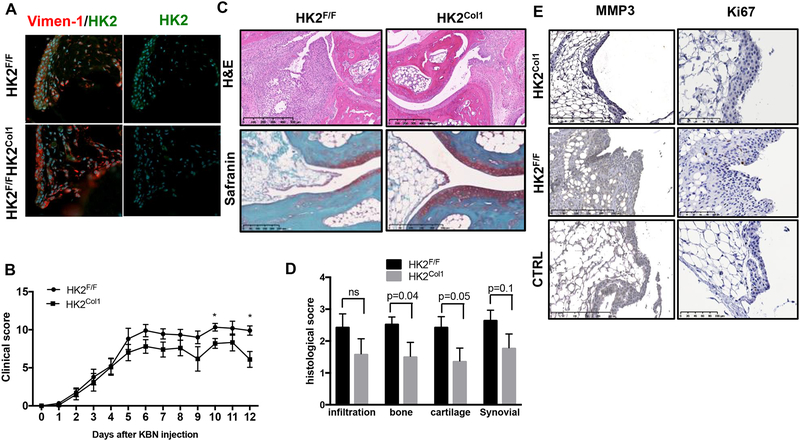
As there is no available Cre-recombinase expressing strain that deletes specifically in FLS, we generated HK2F/F Col1a1-Cre (HK2Col1) mice to delete HK2 in collagen type I expressing cells including FLS. As shown in Fig. 6A, HK2 was deleted in HK2Col1 joint FLS. We then conducted the arthritic model of K/BxN serum-transfer arthritis. Deletion of HK2 in Col1a1 expressing cells significantly decreased arthritis severity (clinical score at day 12 was 9±2.5 in WT mice vs. 6.1±3.2 in HK2F/FCol1a1-Cre; p=0.02) (Fig. 6B). Histopathological studies at day 12 showed that bone erosion (p=0.04) and cartilage damage (p=0.05), but not less cellular infiltration was reduced in HK2Col1 mice compared to littermate control (Fig. 6C, D). Of note, MMP3 and Ki67 expression that were upregulated in K/BxN arthritis synovium were decreased in HK2Col1 mice compared to littermate control (Fig. 6 E).
Figure 6. Specific deletion of HK2 in Col1a1 expressing cells ameliorates arthritis in an animal model of inflammatory arthritis.
Serum passive K/BxN arthritis was induced after intraperitoneally injection of 150 μl of K/BxN mouse serum on day 0. A). Immunofluorescence of the murine synovium (vimentin in red; HK2 in green; DAPI blue) in the indicated mice, showing that HK2Col1synovial cells lack HK2 in murine FLS compared to control HK2F/F B) Clinical arthritis scores were determined in HK2F/F and HK2Col1 mice after arthritis induction. Values are the mean ± SEM of 10 mice per group. * =p<0.05. C) Sections of the ankle joints of HK2F/F and HK2Col1 mice were stained with hematoxylin and eosin (H&E) or Safranin O on day 12 after arthritis induction. D) Histologic scores were determined on day 12 after serum transfer in HK2F/F and HK2Col1 mice. E) Representative IHC images of MMP3 and Ki67 of the indicated mice.
DISCUSSION
The concept of metabolic reprogramming to improve immunotherapy slowly is being translated into autoimmune diseases to complement current therapies(28). Yet, there are little data about targeting metabolic changes in RA. We here showed that targeting the first step in glucose metabolism could be a potential selective metabolic therapy for RA and regulates FLS aggressive behavior.
Although previous works have demonstrated a role of glucose metabolism in cell activation and function(29–31), inhibiting general glucose metabolism is not desirable in the overall body. Thus, there is a need of finding specific metabolic targets that are induced in activated cells such as FLS. HKs are the first enzymatic step in glucose metabolism. While HK1 is a ubiquitously expressed enzyme in all living cells, HK2 is an inducible form and only expressed in adulthood in some organs. Our data suggests that HK2 constitutes an attractive potential selective target for arthritis therapy. Indirect evidence of HK2 redundancy in adulthood is the fact that global HK2 ablation in adult mice was well tolerated, and HK2-deficient mice were indistinguishable from control mice both morphologically, and in terms of growth, body weight and glucose homeostasis(19). Adult tissues that abundantly express HK2 also express relatively high levels of HK1, which might play a compensatory or redundant role in the absence of HK2.
Here, we have observed that HK2 is not expressed in most of OA patient’s synovial tissue. Consistent with the idea of up-regulation secondary to synovial inflammation, we observed that its expression was induced by inflammatory and activation signals including LPS, PDGF, TNF and hypoxia. In K/BxN model of mice, its expression is also elevated in inflamed joints but not in healthy joints. Interestingly, HK2 mRNA expression levels are also increased in TNF or IL-1β- stimulated RA FLS as published in Geo Datasets (Array GSE49604, GSE516, GSE2676), together with other glucose metabolism related genes. While both HK1 and HK2 silencing reduced migratory and invasive phenotype of RA FLS, only the overexpression of HK2 contributed to aggressive phenotype of FLS since its overexpression increased the invasiveness of these cells and induced IL-6, MMPs, and IL-8 mRNA expression. Other studies performed in cancer cells observed similar effects. When HK2 was silenced, breast cancer cells were less migratory after in vitro TNF stimulation(32), and pancreatic cells were less metastatic in an in vivo model(33). Although extracellular lactate was shown to be involved in cancer cell invasive phenotype(33), in RA FLS, the inhibition of lactate transporters (MTC1 and 4) did not reverse the HK2-dependent invasive phenotype. Interestingly, although both HK2 and HK1 similarly increase extracellular lactate, only HK2 and not HK1 overexpression triggers a FLS aggressive phenotype, suggesting the involvement of glycolysis independent mechanisms. Confocal studies revealed that HK1 and HK2 differ in their intracellular colocalization, but further mechanistic studies are warranted. Of note, HK2 deletion was not sufficient to modulate IL-6 or TNF expression, which might indicate that targeting HK2 will not change pro-inflammatory cytokine profile and could potentially complement current immunotherapies.
In addition, HK2 overexpression in the synovium of a healthy murine knee transformed the thin lining into a hypertrophic synovium and it also enhanced FLS activation and migration towards cartilage. HK2, therefore, led a healthy normal synovial lining into a hypertrophic and aggressive synovium upregulating α-sma and MMP3 expression, and also stimulating proliferation as shown by the presence of Ki-67 cells in vimentin positive cells. Yet, HK2 overexpression did not increase proliferation in vitro, suggesting that other mediators are involved in the proliferation observed in the in vivo experiments. The myofibroblastic marker α-sma might explain the migratory phenotype since α-sma has been associated with higher contractile profile in fibroblasts(34). Whether or not HK2 also plays a role in synovial macrophage recruitment, activation and differentiation requires further studies. Although, double-color IHC showed that most of HK2 positive cells colocalized with vimentin positive cells, suggesting FLS HK2 involvement in recruiting and activating synovial macrophages, we cannot rule out a direct activation of macrophages by the Ad-HK2.
Partial ablation of HK2 in FLS slightly ameliorated the clinical signs of arthritis animal model. We did not see a significant effect on inflammation, probably because as shown in vitro, deletion of HK2 in FLS was not sufficient to modulate the expression of inflammatory mediators. Yet, histological scores of bone and cartilage damage were significantly improved in HK2Col1 mice suggesting a predominant role of HK2 in synovial migration and invasion. Col1a1-Cre does not delete only in FLS so the effect of HK2 deletion in other cells including chondrocytes and osteoblasts needs further attention. Although our conditional mice explains the effect of HK2 in non-hematopoietic cells, double staining IHC suggest that human RA synovial cells other than FLS also expresses HK2, so further experiments are needed to study the effect of HK2 in other synovial cell types.
This is the first study to identify an isoform-specific contributor to metabolism changes in RA synovial tissue that could be selectively targeted without compromising systemic homeostasis or corresponding metabolic functions in normal cells, offering a safer and novel additional approach for combination therapy in RA joint disease independent of systemic immunosuppression. Given HK2 selective overexpression in RA inflamed synovium, and its restricted distribution of expression in normal adult tissues, HK2 constitutes an attractive potential selective target for arthritis therapy and safer than global glycolysis inhibition.
Supplementary Material
ACKNOWLEDGMENTS
M.G. was supported by NIH 1K08AR064834, R03AR068094 and Rheumatology Research Foundation. R.G.C work was supported by a fellowship from the Boehringer-Ingelheim Foundation. A.C was supported by a Wellcome Trust Clinical Career Development Fellowship (WT104551MA). Additional support was provided by grants DK-054441–15 and CA-188652 to A.N.M., R56 HL097037 to S.M.; and R01 CA206167, and the VA merit award BX000733 to N.H. This manuscript is based on work previously presented at 2016 and 2017 ACR conferences and published as conference abstract. This work was also supported in part by the Erickson Family. We finally thank to Tissue Technology Shared Resources (TTSR), and microscope core: NINDS P30 NS047101.
REFERENCES
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. [DOI] [PubMed] [Google Scholar]
- 2.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bustamante MF, Garcia-Carbonell R, Whisenant KD, Guma M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumann E, Lefevre S, Zimmermann B, Gay S, Muller-Ladner U. Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol Med. 2010;16(10):458–68. [DOI] [PubMed] [Google Scholar]
- 6.Hammaker D, Firestein GS. “Go upstream, young man”: lessons learned from the p38 saga. Ann Rheum Dis. 2010;69 Suppl 1:i77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammaker D, Sweeney S, Firestein GS. Signal transduction networks in rheumatoid arthritis. Ann Rheum Dis. 2003;62 Suppl 2:ii86–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araki Y, Tsuzuki Wada T, Aizaki Y, Sato K, Yokota K, Fujimoto K, et al. Histone Methylation and STAT-3 Differentially Regulate Interleukin-6-Induced Matrix Metalloproteinase Gene Activation in Rheumatoid Arthritis Synovial Fibroblasts. Arthritis Rheumatol. 2016;68(5):1111–23. [DOI] [PubMed] [Google Scholar]
- 9.Bottini N, Firestein GS. Epigenetics in rheumatoid arthritis: a primer for rheumatologists. Curr Rheumatol Rep. 2013;15(11):372. [DOI] [PubMed] [Google Scholar]
- 10.Falconer J, Murphy AN, Young S, Clark AR, Tiziani S, Guma M, et al. Synovial cell metabolism and chronic inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semerano L, Romeo PH, Boissier MC. Metabolomics for rheumatic diseases: has the time come? Ann Rheum Dis. 2015;74(7):1325–6. [DOI] [PubMed] [Google Scholar]
- 12.Guma M, Sanchez-Lopez E, Lodi A, Garcia-Carbonell R, Tiziani S, Karin M, et al. Choline kinase inhibition in rheumatoid arthritis. Ann Rheum Dis. 2015;74(7):1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fearon U, Canavan M, Biniecka M, Veale DJ. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat Rev Rheumatol. 2016;12(7):385–97. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Carbonell R, Divakaruni AS, Lodi A, Vicente-Suarez I, Saha A, Cheroutre H, et al. Critical Role of Glucose Metabolism in Rheumatoid Arthritis Fibroblast-like Synoviocytes. Arthritis Rheumatol. 2016;68(7):1614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijayant V, Sarma M, Aurangabadkar H, Bichile L, Basu S. Potential of (18)F-FDG-PET as a valuable adjunct to clinical and response assessment in rheumatoid arthritis and seronegative spondyloarthropathies. World J Radiol. 2012;4(12):462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogel WV, van Riel PL, Oyen WJ. FDG-PET/CT can visualise the extent of inflammation in rheumatoid arthritis of the tarsus. Eur J Nucl Med Mol Imaging. 2007;34(3):439. [DOI] [PubMed] [Google Scholar]
- 17.Bushinsky DA, Frick KK. The effects of acid on bone. Curr Opin Nephrol Hypertens. 2000;9(4):369–79. [DOI] [PubMed] [Google Scholar]
- 18.Treuhaft PS DJ MC. Synovial fluid pH, lactate, oxygen and carbon dioxide partial pressure in various joint diseases. Arthritis Rheum. 1971;14(4):475–84. [DOI] [PubMed] [Google Scholar]
- 19.Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer cell. 2013;24(2):213–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206(Pt 12):2049–57. [DOI] [PubMed] [Google Scholar]
- 21.Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25(34):4683–96. [DOI] [PubMed] [Google Scholar]
- 22.Alvaro-Gracia JM, Zvaifler NJ, Brown CB, Kaushansky K, Firestein GS. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J Immunol. 1991;146(10):3365–71. [PubMed] [Google Scholar]
- 23.Alvaro-Gracia JM, Zvaifler NJ, Firestein GS. Cytokines in chronic inflammatory arthritis. V. Mutual antagonism between interferon-gamma and tumor necrosis factor-alpha on HLA-DR expression, proliferation, collagenase production, and granulocyte macrophage colony-stimulating factor production by rheumatoid arthritis synoviocytes. J Clin Invest. 1990;86(6):1790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekwall AK, Eisler T, Anderberg C, Jin C, Karlsson N, Brisslert M, et al. The tumour-associated glycoprotein podoplanin is expressed in fibroblast-like synoviocytes of the hyperplastic synovial lining layer in rheumatoid arthritis. Arthritis Res Ther. 2011;13(2):R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Rey MJ, Valin A, Usategui A, Garcia-Herrero CM, Sanchez-Arago M, Cuezva JM, et al. Hif-1alpha Knockdown Reduces Glycolytic Metabolism and Induces Cell Death of Human Synovial Fibroblasts Under Normoxic Conditions. Sci Rep. 2017;7(1):3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyburz D, Corr M. The KRN mouse model of inflammatory arthritis. Springer Semin Immunopathol. 2003;25(1):79–90. [DOI] [PubMed] [Google Scholar]
- 27.Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315(5814):1006–10. [DOI] [PubMed] [Google Scholar]
- 28.Guma M, Tiziani S, Firestein GS. Metabolomics in rheumatic diseases: desperately seeking biomarkers. Nat Rev Rheumatol. 2016;12(5):269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galvan-Pena S, O’Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van den Bossche J, O’Neill LA, Menon D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017;38(6):395–406. [DOI] [PubMed] [Google Scholar]
- 31.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982;70(3):550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Y, Yang Y, Yuan F, Huang J, Xu W, Mao B, et al. TNFalpha-YAP/p65-HK2 axis mediates breast cancer cell migration. Oncogenesis. 2017;6(9):e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson M, Marayati R, Moffitt R, Yeh JJ. Hexokinase 2 promotes tumor growth and metastasis by regulating lactate production in pancreatic cancer. Oncotarget. 2017;8(34):56081–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinde AV, Humeres C, Frangogiannis NG. The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta. 2017;1863(1):298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.