Abstract
Macrophages play a key role in the pathophysiology of rheumatoid arthritis (RA). Notably, positive correlations have been reported between synovial macrophage infiltration and disease activity as well as therapy outcome in RA patients. Hence, macrophages can serve as an important target for both imaging disease activity and drug delivery in RA. Folate receptor β (FRβ) is a glycosylphosphatidyl (GPI)-anchored plasma membrane protein being expressed on myeloid cells and activated macrophages. FRβ harbors a nanomolar binding affinity for folic acid allowing this receptor to be exploited for RA disease imaging (e.g., folate-conjugated PET tracers) and therapeutic targeting (e.g., folate antagonists and folate-conjugated drugs). This review provides an overview of these emerging applications in RA by summarizing and discussing properties of FRβ, expression of FRβ in relation to macrophage polarization, FRβ-targeted in vivo imaging modalities, and FRβ-directed drug targeting.
Keywords: Rheumatoid arthritis, Macrophages, Folate receptor, Folate-conjugated drugs, Imaging, Positron emission tomography (PET)
Rheumatoid arthritis
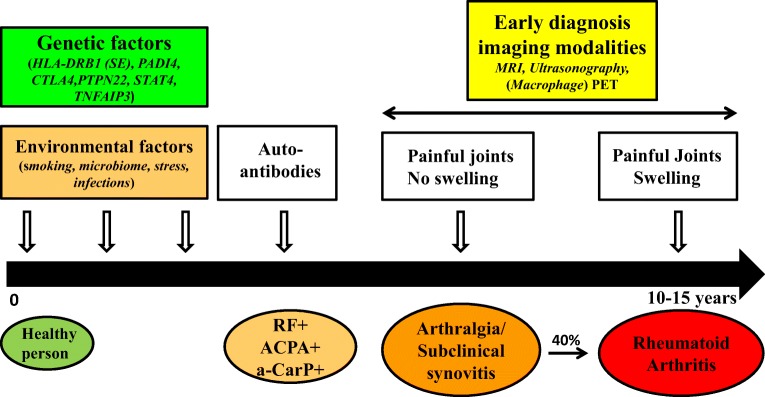
Rheumatoid arthritis (RA) is an autoimmune disease, which affects approximately 0.5–1.0% of the world population [1]. Although the exact etiology of RA is unknown, the currently accepted hypothesis consists of two stages [2]. In genetically susceptible individuals, the first stage of development of RA consists of accelerated citrullination of proteins in extra-articular sites, e.g., due to smoking or infection, including formation of rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA), and anti-carbamylated proteins (a-CarP) [3–6]. Only 40% of ACPA-positive arthralgia individuals will eventually develop RA [7]. A second trigger seems to be needed for development of clinical disease. Up to 15 years later, the second trigger could be an unrelated episode of otherwise self-limiting synovial inflammation and associated locally induced citrullination. In the presence of pre-existing anti-citrullinated protein/peptide antibodies, this event may induce chronic synovitis evolving into clinical RA through binding of the antibodies to autoantigens in the joints [8–10] (Fig. 1).
Fig. 1.
Onset of rheumatoid arthritis and positioning of macrophage imaging for early disease monitoring. Early in a time frame spanning 10–15 years, combined genetic and environmental factors can trigger in a healthy person the formation of autoantibodies which can lead to joint complaints without swelling (arthralgia). Following an unknown second hit, 40% of arthralgia patients ultimately develop RA. The subclinical stage of arthritis provides a window of opportunity early diagnosis with imaging modalities
To detect development of (subclinical) synovitis, advanced imaging techniques may have diagnostic value on top of detection of ACPA. Application of ultrasonography and MRI techniques in preclinical RA have been discussed in recent reports [11, 12], while application of positron emission tomography (PET) will be discussed in detail below. RA’s main characteristics include (chronic) inflamed synovium and joint destruction, which, when left untreated, can lead to permanent joint deformities and comorbidities, such as cardiovascular disease and osteoporosis [10]. Early identification and treatment of RA is currently recommended to prevent further joint damage and disability [13]. To this end, the European League Against Rheumatism (EULAR) guidelines indicate treatment with classical disease-modifying anti-rheumatic drugs (DMARDs) (e.g., methotrexate (MTX)), biological DMARDs (e.g., infliximab, rituximab, tocilizumab, and secukinumab), and targeted synthetic DMARDs (e.g., Janus kinase inhibitors), either as monotherapy or in combination therapy [14]. Despite this wide spectrum of potential therapeutic agents that are currently available, response to treatment usually varies between 50 and 70%. This is probably related to factors such as the heterogeneous character of RA, the stage of the disease, and the presence of anti-drug antibodies. To increase treatment efficacy and to reduce costs, monitoring tools, e.g., imaging, are needed in order to select responders and non-responders in an early phase of treatment.
Immune cells and RA
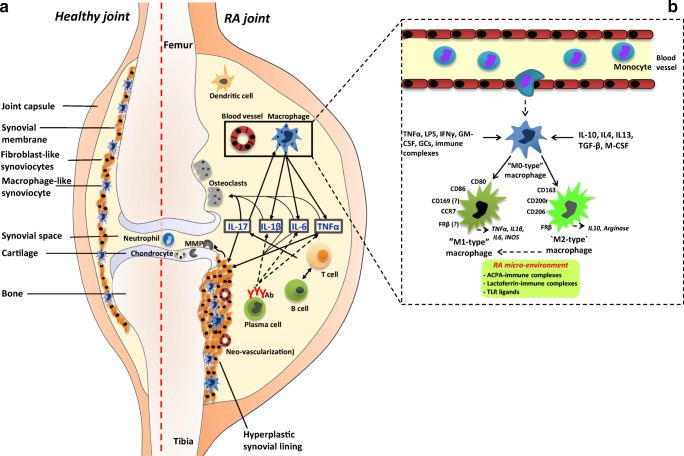
In RA, the inflamed synovium harbors several immune cell types, especially B and T lymphocytes, dendritic cells, neutrophils, and macrophages [8–10] (Fig. 2a). As dominant producers of tumor necrosis factor alpha (TNFα), macrophages are known to play a central role in RA disease progression [15–19], macrophage production of IL1β, IL-6, and TNFα mediates proliferation and activation of fibroblast-like synoviocytes [20]. These promote formation and activation of osteoclasts and chondrocytes, which drive bone and cartilage destruction [8–10, 18, 20], being hallmarks of RA disease (Fig. 2a). Cytokine networks involving a.o. IL15, IL17, IL18, IL21, IL23, and IFNγ mediate interactions among macrophages and B cells, T cells, and dendritic cells to induce pro-inflammatory effects (reviewed in [8, 9, 21, 22]). For example, IL17 release by T cells triggers activation of synovial fibroblasts and osteoclasts [8, 21], whereas B cells/plasma cells primarily release autoantibodies such as rheumatoid factor and ACPAs to promote T cell activation [23, 24]. Macrophages in inflamed synovium are thought to be mainly derived from influx of circulating monocytes [16, 17] (Fig. 2b). Following differentiation of monocytes into macrophages, various cytokines and immune complexes can skew them in subcategories designed M1-type (pro-inflammatory) and M2-type (anti-inflammatory) macrophages, featuring characteristic cluster of differentiation (CD) membrane marker expression and release of cytokines, chemokines, and degrading enzymes [18, 19] (Fig. 2b). M1-type and M2-type macrophages do not represent static states as in an RA synovial microenvironment; M2-type macrophages can acquire M1-type properties of producing pro-inflammatory cytokines like TNFα, IL1β, and IL-6 [15–27]. Folate receptor β (FRβ) has been identified as an emerging macrophage marker. FRβ properties and clinical exploitation will be discussed in more detail in the following sections. Together, given the prominent role of macrophages in RA pathophysiology, their non-invasive visualization can hold promise for early RA disease monitoring.
Fig. 2.
Pathogenesis of RA and the role of macrophages. a Schematic representation of a healthy (left) and its changes in RA (right). The healthy joint shows the synovium and synovial space between two bone ends covered with a cartilage layer. The synovial membrane separating the capsule and the synovial space consists of a thin cell layer of fibroblast-like synoviocytes (FLS) and macrophage-like synoviocytes (MLS). The RA joint features a hyperplastic synovial lining, neovascularization, and infiltration of various types of immune cells (macrophages, T cells, B cells, antibody-producing plasma cells, dendritic cells, neutrophils). The release of pro-inflammatory cytokines (a.o. TNFα, IL-1β, IL-6, and IL-17) triggers a cascade of events, proliferation and activation of FLS, activation of osteoclasts and chondrocytes, and induction of bone and cartilage destruction (via matrix metalloproteases (MMPs)), being hallmarks of RA disease. bMagnification inset: Synovial macrophages are derived from influx of monocytes which, depending on stimuli by various cytokines and immune complexes, can differentiate into macrophage subtypes called M1-type and M2-type macrophages, representing the extremes of a spectrum of pro-inflammatory and anti-inflammatory macrophages, respectively. M1- and M2-type macrophages can be distinguished by membrane marker expression and cytokine release profiles. Components of the RA synovial microenvironment can alter macrophage polarization
Macrophage PET imaging in RA
In RA, synovial macrophage infiltration is a hallmark of the disease, reflecting disease activity in early and established stages, being a sensitive biomarker for assessment of response to therapy [28–30]. Therefore, macrophage imaging could serve as an important clinical and diagnostic tool as well as a tool for guiding therapy in RA. Positron emission tomography (PET) is a non-invasive, in vivo imaging modality, with high sensitivity to detect active arthritis both at early or advanced stages of RA [31, 32]. It also has the ability to quantify tracer uptake, which is essential for intervention studies, i.e., for monitoring disease activity and therapy response in the whole body [33–36]. While ultrasound and MRI cover mostly detection of anatomical changes in synovial tissue [37], PET imaging allows for quantitative detection and monitoring of molecular targets. Various PET tracers have been developed to image RA. Initial macrophage-directed PET studies used [18F]FDG (measuring glucose metabolism in inflammatory sites) to visualize inflamed RA joints with results corresponding to clinical findings, thus providing evidence for the usefulness of PET in detecting synovitis [38–40]. This tracer showed high sensitivity, but low specificity for arthritis imaging [38]. Subsequently, PET studies were extended by using more macrophage-specific tracers (Table 1).
Table 1.
PET tracers for macrophage imaging in rheumatoid arthritis
| Name | PET isotope | Half-life (min) | Binding target | Use | Reference |
|---|---|---|---|---|---|
| FDG | 18F | 110 | Glucose transporter | Glucose metabolism | [39, 40] |
| (R)-PK11195 | 11C | 20 | TSPO | Neuro-inflammation/RA | [41–44] |
| DPA713 | 11C | 20 | TSPO | Neuro-inflammation/RA | [42, 45] |
| DPA714 | 18F | 110 | TSPO | Neuro-inflammation/RA | [42, 46, 47] |
| PEG-Folate receptor | 18F | 110 | Folate receptor | RA, arthrosclerosis | [48–50] |
The first class of potential macrophage tracers was targeted towards the 18-kDa translocator protein (TSPO, formerly known as peripheral benzodiazepine receptor), an outer mitochondrial membrane protein that is upregulated in activated macrophages [51, 52]. (R)-[11C]PK11195 is the prototypical TSPO tracer that was employed in preclinical RA models [41, 42, 53–56] after successful application for imaging of activated microglia in neuroinflammatory diseases (reviewed in [57, 58]. In a clinical setting, significantly higher (R)-[11C]PK11195 uptake was observed in severely inflamed joints of RA patients than in moderately or mildly inflamed joints, which correlated with the extent of macrophage infiltration in excised synovial tissue [43]. In addition, subclinical disease activity could be shown when contralateral uninflamed knee joints of RA were compared with non-inflamed joints of healthy controls [43]. However, (R)-[11C]PK11195 showed limitations in detecting subclinical synovitis in RA. In particular, considerable background uptake was seen in periarticular tissue both in a rat model of arthritis [48] and in RA patients [35]. To overcome these limitations, a second generation of TSPO tracers was developed, with [11C]DPA713 and [18F]DPA714 [50, 51] having been evaluated in preclinical RA models [42, 59]. Herein, both [11C]DPA713 and [18F]DPA714 were superior to (R)-[11C]PK11195, but this still needs to be confirmed in a clinical setting.
In search for novel macrophage PET tracers in RA, macrophage markers identified on activated microglia can be helpful, e.g., CB2R and A2AR (G protein-coupled receptors), P2X7R (purinergic ion channel receptor), or matrix metalloproteinases [60].
The focus of the present review is on another emerging (activated) macrophage marker, i.e., the folate receptor β (FRβ), which potentially could also be exploited for imaging and therapeutic targeting purposes in RA [61, 62].
Folate receptors (general properties)
Folate receptors (FR) belong to a family of two other proteins, i.e., reduced folate carrier (RFC) and proton-coupled folate transporter (PCFT). RFC and PCFT have an established function in membrane transport/internalization of folates required for a variety of biosynthetic reactions and DNA synthesis [63–66] (Table 2).
Table 2.
Overview and expression profiling and transport kinetic features of folate transporters
| Cellular (anti) folate uptake systems | |||
|---|---|---|---|
| PCFT (proton-coupled folate transporter) | RFC (reduced folate carrier) | FR (folate receptor α,β,γ isoform) | |
| Membrane orientation | Transmembrane | Transmembrane | GPI - anchored |
| Localization | Enterocytes | Immune cells Tumor cells |
Kidney (FRα) Tumor cells (FRα) Myeloid cells/activated Macrophages (FRβ) Hematopoietic cells (FRγ, soluble, secreted form) |
| pH optimum | 5.0–5.5 | 7.2–8.0 | 7.4–8.0 |
| Affinity folic acid | Km 1–5 μM | Km 200–400 μM | Kd 0.1–1 nM |
| Affinity 5-methyl-THF | Km 2–10 μM | Km 1–5 μM | Kd 5–10 nM |
| Affinity MTX | Km 2–10 μM | Km 2–10 μM | Kd 50–100 nM |
FR, RFC, and PCFT differ in membrane orientation, folate substrate affinity, pH optimum, and tissue distribution [63, 66–68] (Table 2). While RFC and PCFT are transmembrane carrier proteins, FR is anchored to the plasma membrane via a glycosylphosphatidylinositol (GPI) anchor [69]. At least 3 isoforms of FR exist, FRα, FRβ, and FRγ, of which the latter is a soluble secreted form because it lacks a GPI-anchoring signal [70]. FRα and FRβ display high binding affinity for folic acid (Kd 0.1–1.0 nM), but low binding affinity for the folate antagonist methotrexate (MTX) [63, 68, 71, 72]. FRs internalize their substrates via a process of receptor-mediated endocytosis [73, 74] or potocytosis [75]. FRα has a relatively broad tissue distribution profile in normal cells (e.g., kidney) and cancer cells (e.g., ovarian carcinoma cells) [76], whereas FRβ expression is restricted to hematopoietic cells of the myeloid lineage [77, 78]. In fact, FRβ is expressed on monocytes [79], activated macrophages of RA patients [80, 81], tumor-associated macrophages [82], and acute myeloid leukemia (AML) cells [83]. A number of substances have been reported to upregulate FRβ expression, e.g., retinoic acid [84] and curcumin [85], whereas a pluripotent growth factor like activin A downregulates FRβ expression [86].
Given the fact that RFC is constitutively expressed on immune cells [87, 88], including macrophages [86, 89], and exhibits a much greater folate transport capacity than FRβ [68, 81], it is still an unresolved issue whether the primary function of FRβ in macrophages is folate transport rather than other homeostatic or immune-regulatory functions. In rapidly proliferating cancer cells, folate transporters (Table 2) facilitate folate uptake to promote DNA synthesis [66–68]. However, in inflamed RA synovium, increased numbers of macrophages are mainly derived from influx of circulating monocytes (Fig. 2b) following enhanced myelopoiesis [16]. Moreover, RA synovium macrophages display only modest cell proliferation [90, 91], thus suggesting a role for FRβ in folate uptake for macrophage proliferation may not be of primary importance. In this regard, alternative functions for FRβ have been suggested, although they still lack experimental evidence: (a) delivery of folates for biopterin metabolism, which facilitates reactive oxygen species (ROS) production in macrophages [92]; (b) FRβ-mediated scavenging of folates from sites of inflammation to deprive pathogens from nutrients [80]; or (c) involvement in signaling processes consistent with the notion that FR, as GPI-anchored protein, is localized in specialized cholesterol-rich membrane invaginations called caveolae, which harbor multiple proteins involved in signaling processes [63, 66]. With respect to the latter, a recent study reported that FRβ on macrophages had a functional interaction with CD11/CD18 to regulate cellular adhesion to collagen [93].
Beyond RA synovium, FRβ expression has been identified on macrophages in inflamed atherosclerotic lesions [94–97], accounting for cardiovascular comorbidities in RA, and tumor-associated macrophages [82, 98–100], thus underscoring that FRβ plays a role on macrophages regulating inflammatory processes. Lastly, in mice, FRβ expression has been noted on LyC6 myeloid-derived suppressor cells (MDSC), a myeloid subset capable of suppressing T cell activity [101]. So far, expression of FRβ on human MDSC counterparts has not been examined.
Role of folate receptor β in rheumatoid arthritis
Consistent with FRβ being expressed in hematopoietic cells of the myeloid lineage [77, 78], peripheral blood monocytes (PBMs) from healthy donors and RA patients express FRβ. Based on their CD14/CD16 expression, 3 subclasses of PBMs were identified, classical (CD14+/CD16−), non-classical (CD14−/CD16+), and intermediate (CD14+/CD16+) monocytes, of which the pro-inflammatory classical monocytes expressed FRβ and were capable of binding folate-linked molecules [79]. This finding provides a rationale for targeting pro-inflammatory FRβ+ monocytes to suppress their infiltration into sites of inflammation, e.g., RA synovium [79].
FRβ-positive macrophages were originally identified in RA synovial fluid and assigned a functional role in methotrexate transport [102]. A study by van der Heijden et al. [81] showed that FRβ mRNA expression in synovial fluid macrophages and synovial tissue from RA patients was two orders of magnitude higher than that of T cells from the same patient. Immunohistochemical evaluation of synovial biopsies from RA patients confirmed strong FRβ staining of CD68-positive macrophages both in synovial lining and sublining [81]. Importantly, a study by Xia et al. [80] revealed that especially activated macrophages rather than quiescent macrophages, in RA synovial fluid, had high FRβ expression and concomitant folate conjugate binding activity.
Macrophage FRβ expression is not only restricted to RA, but has also been reported in other arthritis-related diseases. In temporal artery biopsies of giant cell arteritis patients, severe inflammation coincided with FRβ-positive macrophages in the adventitia [103]. In two murine models of systemic lupus erythematosis, the number of FRβ-positive macrophages correlated with disease activity [104]. Also, in two experimental models of autoimmune uveitis and autoimmune encephalomyelitis in rats, FRβ-positive macrophages were detected at local and systemic sites (e.g., peritoneal cavity) of inflammation [105]. Lastly, several studies reported the presence of FRβ on macrophages in knee sections of osteoarthritis patients [106, 107].
Folate receptor β and macrophage polarization
Macrophage heterogeneity is a common feature in RA-inflamed synovial tissue [16–19]. Microenvironmental factors may affect both activation status and skewing of macrophages into various subsets with distinct immunophenotypes and specialized immune-regulatory and homeostatic functions. Polarization of macrophages covers the broad spectrum from pro-inflammatory to anti-inflammatory macrophages, which have been designated “M1-type” (classical activation, pro-inflammatory) macrophages and “M2-type” (alternatively activated, anti-inflammatory) macrophages, respectively [108]. Whereas M1- and M2-type macrophages represent the extremes of polarization, macrophages harbor plasticity of skewing in either direction. There are many markers that may help to differentiate M1/M2 macrophages. M1 macrophages are involved in tumor inhibition and are resistant to pathogens, whereas M2 macrophages promote tumor growth and have immunoregulatory properties [109]. Classical activation stimuli for M1-type macrophages include IFNγ, LPS, and GM-CSF; those for M2-type macrophages include M-CSF, IL-4, IL-10, IL13, glucocorticoids, and immune complexes [110, 111]. Immunophenotypically, M1-stimulated macrophages display increased cell surface expression of CD80 (provides a costimulatory signal necessary for T cell activation and survival) and CD64 (Fc-gamma receptor 1, FcγRI), while M2-stimulated macrophages have increased expression of CD163 (hemoglobin scavenger receptor), CD206 (mannose receptor), CD200R (orexin receptor 2), and CD32 (FcγRIIa) [112]. CD68 is acknowledged as one of the most common markers for identifying human macrophages [112], although its expression can also be detected on fibroblasts [113]. CD169 (Siglec-1) is a macrophage marker that is implicated in immune tolerance and antigen presentation [114]. Although CD169 has been found on activated macrophages in inflammatory diseases [115, 116], its function in RA is still unknown.
During the past decade, several studies have explored FRβ expression in the context of macrophage polarization. Initially, studies from Puig-Kroger et al. [117] showed that FRβ was preferentially expressed on M2-type macrophages following in vitro skewing of monocytes with M-CSF compared with M1-type macrophages with GM-CSF. Moreover, RA synovial fluid macrophages showed an activin A-dependent skewing to pro-inflammatory M1 macrophages and reduced expression of FRβ [118]. In synovial tissue of osteoarthritis patients, however, FRβ expression was not exclusively observed on either M1- or M2-type macrophages [119]. Some recent studies add complexity to this issue by reporting that M-CSF-polarized FRβ-expressing M2 macrophages demonstrated a high pro-inflammatory response to TLR ligands and complex IgG and/or autoantibodies to citrullinated protein immune complexes (ACPA-IC) as commonly present in RA [25, 26]. Together, these data suggest that FRβ is differentially expressed on in vitro M-CSF skewed M2-type monocyte-derived macrophages, which is in line with FRβ expression on tumor-associated macrophages [82, 99, 100]. However, in RA (and OA) synovium, inflammatory conditions alter macrophage phenotypes along with FRβ expression (Fig. 2b).
Imaging folate receptor β in rheumatoid arthritis
The high binding affinity of folate receptors for folic acid has been exploited for the design of multiple imaging agents [120] to either detect FRα expression in tumors [121, 122] and FRβ-expressing macrophages in RA [62, 123]. Subsequently, macrophage FRβ imaging has also been applied in macrophage implicated inflammation-related diseases, e.g., asthma [124–126] and cardiovascular diseases [94, 97]. The first folate macrophage imaging study in rats with adjuvant-induced arthritis was performed using [99mTc]folic acid to generate the single-photon emitting tracer [99mTc]EC20, which enabled visualization of arthritic joints in a rat model [127]. Isolated macrophages from the arthritic rats also showed high FR binding capacity for folate-FITC [127]. Subsequently, [99mTc]EC20 was successfully used to assess disease activity in RA patients with established disease [128, 129] as well as OA patients [107]. In RA patients, the [99mTc]EC20 distribution corresponded with clinical predictors of disease activity [128]. Notably, in a subset of RA patients, [99mTc]EC20 scans detected actively involved joints more accurately than clinical assessments of arthritis [128].
Further development of folate imaging agents also focused on PET tracers, which could be used for detection of (sub)clinical arthritis as well as for more accurate therapy monitoring. To this end, a folate PET tracer, [18F]-fluoro-PEG-folate, was synthesized in a two-step procedure and evaluated in an antigen-induced arthritis model in rats [48]. Uptake of [18F]-fluoro-PEG-folate was significantly higher in arthritic than in non-inflamed control knees, and also arthritic knee to bone and arthritic knee to blood ratios were higher for [18F]-fluoro-PEG-folate than (R)-[11C]PK11195 [48]. In addition, using [18F]-fluoro-PEG-folate PET, it was possible to monitor therapeutic effects of MTX in arthritic rats [49] and to monitor systemic inflammatory effects in an arthritic rat model [50]. Based on these encouraging preclinical results, [18F]-fluoro-PEG-folate was taken to a clinical setting in which this tracer could readily visualize arthritic joints in RA patients [130]. Recently, a novel folate-based PET tracer was synthesized in a faster (< 1 h) one-step procedure, i.e., [18F]-folate-PEG-NOTA-Al [131], which warrants further (pre)clinical evaluation.
Next to folate PET imaging agents, recent progress has been made in the development of folate conjugates of (near infrared) fluorescent probes that can be used for fluorescent and optical imaging purposes [58, 132, 133]. Thus far, these approaches have mostly been applied in a cancer research setting for fluorescence-guided surgery of FRα-positive tumors [134] or macrophage FRβ expression in tumors [135]. Recently, OTL-38, a novel near-infrared fluorescent folate-conjugated imaging agent, showed feasibility of imaging FRα-positive tumors [136]. OTL-38 was also examined in animal models of various inflammatory diseases including RA [137]. Interestingly, the uptake of OLT-38 in inflamed joints of the animals was shown to precede changes in clinical symptoms [137]. However, it should be noted that optical techniques have their limitations. Firstly, the penetrating power of near-infrared light is limited, so that only relatively superficial processes can be imaged. In other words, although imaging in small laboratory animals is possible, translation to the human is difficult and restricted to intraoperative imaging and possibly small hand/foot joints in RA. Secondly, as the amount of light collected by a probe depends on the depth of the source (e.g., tumor) within the body, quantification is very difficult and awaits further developments. Therefore, at this stage, optical imaging is less suited for monitoring quantitative follow-up of therapeutic interventions in vivo in humans.
Therapeutic targeting of folate receptor β in rheumatoid arthritis
FRs have not only been exploited for imaging, but also for therapeutic targeting in cancer and inflammation [65, 66]. Targeting of FRα-expressing tumors has included folate-conjugated (a) radionuclides (α-emitters) for cancer treatment; (b) anti-cancer drugs; (c) nanoparticles containing either anticancer drugs, siRNAs, miRNAs, or genes; or (d) folate antagonists for which FRα has a high affinity [65, 68, 138].
For FRβ, similar targeting approaches are applicable [139]. Table 3 provides a selection of approaches that have been reported for targeting FRβ-expressing macrophages in RA and RA-related diseases as well as for FRβ-expressing tumor-associated macrophages and FRβ-expressing acute myeloid leukemia cells. Conceivably, applications in the cancer setting may be translatable to the RA setting. Table 3 describes several modalities for FRβ targeting, including folate antagonists, folate-conjugated immunotoxins, folate-conjugated drugs, folate-conjugated nanoparticles containing drugs or genetic material, and via chimeric antigen receptor (CAR) T cells. With respect to antifolates, several drugs inhibiting key enzymes in folate metabolisms, e.g., dihydrofolate reductase (DHFR), thymidylate synthase (TS), and glycinamide ribonucleotide formyltransferase (GARTFase) [87], were evaluated for FR-targeting and anti-arthritic activity in vitro or in arthritic animals. In general, FR has a low affinity for DHFR inhibitors, including MTX, as compared with TS and GARTFase inhibitors [68, 81]. Antifolates with selectivity for FRα and FRβ rather than other folate transporters (RFC or PCFT) include BGC-945 and selected GARTFase inhibitors. As illustrated in Table 3, folic acid conjugation to a variety of (anti-inflammatory) drugs, drug-containing liposomes, proteins, siRNAs, and miRNAs provided a bona fide vehicle for targeted delivery to FR-positive tumor cells and activated macrophages in different autoimmune inflammatory animal models. CAR T cell therapies with T cells transduced with a high affinity FRβ-specific single chain antibody represent a novel approach for selective targeting and lysis of FRβ-positive AML cells [166, 167]. Experimental therapeutics with anti-FRβ CAR T cells has as yet not been explored in relation to FRβ-positive macrophages targeting in auto-immune inflammatory diseases.
Table 3.
FRβ therapeutic targeting in rheumatoid arthritis
| Category | Remarks | Reference |
|---|---|---|
| Antifolates | ||
| MTX | DHFR inhibitor, low FR affinity, high RFC/PCFT affinity | [102] |
| CH-1504 | DHFR inhibitor, low FR affinity, high RFC affinity | [140] |
| EC0746 | Aminopterin-folate conjugate DHFR inhibitor, activity in RA mouse model | [141] |
| EC0746 | Aminopterin-folate conjugate DHFR inhibitor, activity in animal uveitis and encephalomyelitis model | [105] |
| BGC945 | TS inhibitor, FRα/β specific | [81, 142] |
| ALIMTA/pemetrexed | TS inhibitor, moderate FR affinity, high RFC/PCFT affinity | [143] |
| LY309887 | GARTFase inhibitor, high FR and RFC affinity, activity in mouse RA model | [144] |
| LY329201 and LY309886 | GARTFase inhibitors, in vitro activity, and activity in rat RA model | [145] |
| Divers compounds | GARTFase inhibitors, FRβ selective, in vitro activity | [146] |
| Immunotoxins | ||
| Anti-FRβ-PE38 | Recombinant immunotoxin dsFv anti-FRβ-Pseudomonas endotoxin A (PE38). Reduction RA synovial macrophages and fibroblasts | [147–149] |
| Anti-FRβ-PE38 | Targeting FRβ-positive tumor-associated macrophages in mouse glioma | [150] |
| Anti-FRβ-PE38 | Targeting FRβ-positive macrophages mouse atherosclerotic lesions | [151] |
| Folate-conjugated nanoparticles | ||
| G5 dendrimer MTX | Targeting mouse primary FRβ macrophages | [152] |
| Liposomes + MTX | Activity to FRβ-positive macrophages in mouse collagen-induced arthritis | [153] |
| Dextran-MTX | Activity to FRβ-positive macrophages in mouse collagen-induced arthritis | [154] |
| Liposomes + anti-inflammatory drugs | Targeting activated macrophages in inflammatory diseases | [155] |
| NFkB decoy | Delivery to murine macrophages | [156] |
| G5 dendrimers MTX | Targeting FRβ-positive tumor-associated macrophages | [157] |
| Liposomes + zoledronate | Targeting FRβ-positive tumor-associated macrophages | [158] |
| HSA-nanodrug | Targeting FRβ-positive AML cells | [159] |
| Liposomes + Dox | Targeting FRβ-positive AML cells | [160] |
| Folate drug conjugates | ||
| FA-Everolimus (EC0565) | Targeting FRβ-positive rat macrophages | [161] |
| FDG-FA | Targeting FRα-positive tumors and FRβ-positive macrophages | [162] |
| Gene delivery (miRNA, siRNA) | ||
| FA-liposomes +MCL1-siRNA | Delivery to activated macrophages | [163] |
| FA-micelles/hydrogels | Gene delivery to activated macrophages | [164] |
| FolamiRs | FA-conjugated microRNAs for delivery to FR-positive cells | [165] |
| CAR T cells | ||
| High affinity FRβ-specific CAR T cells | For eradication FRβ-positive AML cells | [166, 167] |
Although studies described in Table 3 underscore the suitability of macrophage FRβ targeting and imaging in RA models, several points may be considered to guide future research directions. One consideration relates to the choice of the RA animal model. For most anti-rheumatic drugs, it takes time to evaluate their action on arthritis activity when using synovial macrophage infiltration as a biomarker. Therefore, especially in the case of (sub)clinical arthritis, most existing animal models of RA may not be optimal from this perspective as they are either short-term acute models or models with severe bone destruction and/or poly-articular distribution [168, 169]. Instead, for (sub)clinical arthritis studies, antigen-induced arthritis models may be more suitable as they are more chronic and resemble human RA in terms of synovial macrophage infiltration and moderate systemic inflammation [44]. Also regarding animal studies, it is well documented that plasma levels of naturally circulating folates in rodents are 10-fold higher than in humans (≈ 100 nM vs 10 nM, respectively) [44, 170], which may increase competitive binding with an experimental folate-conjugated drug for FRβ. Lastly, FRβ expression and folate binding capacity is very much dependent on the activation status of macrophages [80], which may vary between animal models and stages of disease progression.
Optimal FRβ targeting will also benefit from information about receptor density, occupancy and kinetics (recycling rates), and levels of co-expression of any other folate transporters on target cells. In target cells with dual expression of RFC and FR, the first transporter is often dominant in internalizing natural folates and small molecule antifolates. FR can fully compensate for this when RFC expression/activity is low [171]. Since RFC, in contrast to FR, has a poor affinity for folic acid drug conjugates, FR is their sole route of cell entry and thus receptor density and recycling rates determine intracellular drug delivery to concentrations eliciting a therapeutic effect [74, 172].
Conclusion
There is growing evidence that FRβ expression on activated macrophages represent an important biomarker in various autoimmune inflammatory diseases, including RA. FRβ expression in relation to macrophage polarization warrants further investigations under conditions mimicking inflamed RA synovium. FRβ holds promise as a target for imaging with various modalities including PET and optical imaging with rationally designed tracers. This will allow disease monitoring studies and, ideally, early identification of arthritis and PET-guided therapy response monitoring. With respect to therapy, FRβ serves as an excellent target for delivery of therapeutics to macrophages; these may include folate antagonist and folate-conjugated drugs.
In conclusion, FRβ expression on activated macrophages may be exploited to guide future diagnostics, targeted therapies, and therapy response monitoring in RA.
Abbreviations
- CD
cluster of differentiation
- CTLA4
cytotoxic T lymphocyte antigen 4
- DMARDs
disease-modifying anti-rheumatic drugs
- FLS
fibroblast-like synoviocytes
- FRβ
folate receptor β
- GC
glucocorticoids
- GPI
glycosylphosphatidylinositol
- GM-CSF
granulocyte macrophage-colony-stimulating factor
- HLA-DRB1
human leucocyte antigen DRB1
- iNOS
inducible nitric oxide synthase
- IL
interleukin
- “M1-type” macrophage
pro-inflammatory macrophages
- “M2-type” macrophages
anti-inflammatory macrophages
- MLS
macrophage-like synoviocytes
- M-CSF
macrophage-colony-stimulating factor
- MTX
methotrexate
- MRI
magnetic resonance imaging
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PADI4
peptidyl arginine deaminase type 4
- PET
positron emission tomography
- PTPN22
protein tyrosine phosphatase, non-receptor type 22
- RA
rheumatoid arthritis
- SE
shared epitope
- STAT4
signal transducer and activator of transcription 4
- TGFβ
transforming growth factor β
- TLR
toll-like receptor
- TNFα
tumor necrosis factor α
- TNFAIP3
TNF alpha-induced protein 3
Funding information
This study was supported by the Center for Translational Molecular Medicine (CTMM; project - TRACER), VU University Medical Center - Cancer Center Amsterdam (CCA—PV13/87), the Dutch Arthritis Association (NRF 09-01-404), and ZonMw translational project (Positron Emission Tomography and macrophage targeting to select individuals at risk for rheumatoid arthritis; 95104012).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, Williams B, Gabriel S, Lassere M, Johns N, Buchbinder R, Woolf A, March L. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1316–1322. doi: 10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- 2.Klareskog L, Rönnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol. 2008;26:651–675. doi: 10.1146/annurev.immunol.26.021607.090244. [DOI] [PubMed] [Google Scholar]
- 3.Nielen MMJ, Van Schaardenburg D, Reesink HW, Van De Stadt RJ, Van Der Horst-Bruinsma IE, De Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 4.Rantapää-Dahlqvist S, De Jong BAW, Berglin E, Hallmans G, Wadell G, Stenlund H, van Venrooij WJ. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 5.Van De Stadt LA, MHMT DK, Van De Stadt RJ, Wolbink G, Dijkmans BA, Hamann D, van Schaardenburg D. Development of the anti-citrullinated protein antibody repertoire prior to the onset of rheumatoid arthritis. Arthritis Rheum. 2011;63:3226–3233. doi: 10.1002/art.30537. [DOI] [PubMed] [Google Scholar]
- 6.Shi J, Van De Stadt LA, Levarht EWN, Huizinga TWJ, Hamann D, Van Schaardenburg D, Toes RE, Trouw LA. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis. 2014;73:780–783. doi: 10.1136/annrheumdis-2013-204154. [DOI] [PubMed] [Google Scholar]
- 7.Bos WH, Wolbink GJ, Boers M, Tijhuis GJ, De Vries N, Van Der Horst-Bruinsma IE, Tak PP, van de Stadt RJ, van der Laken CJ, Dijkmans BA, van Schaardenburg D. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis. 2010;69:490–494. doi: 10.1136/ard.2008.105759. [DOI] [PubMed] [Google Scholar]
- 8.Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology. 2012;51:3–11. doi: 10.1093/rheumatology/kes113. [DOI] [PubMed] [Google Scholar]
- 9.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 10.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2018;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 11.Ten Cate DF, Luime JJ, Swen N, Gerards AH, De Jager MH, Basoski NM, Johanna MWH, Cees JH, Johannes WGJ. Role of ultrasonography in diagnosing early rheumatoid arthritis and remission of rheumatoid arthritis - a systematic review of the literature. Arthritis Res Ther. 2013;15:R4. doi: 10.1186/ar4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Agostino MA, Haavardsholm EA, van der Laken CJ. Diagnosis and management of rheumatoid arthritis; what is the current role of established and new imaging techniques in clinical practice? Best Pract Res Clin Rheumatol. 2016;30:586–607. doi: 10.1016/j.berh.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Combe B, Landewe R, Daien CI, Hua C, Aletaha D, Álvaro-Gracia JM, Bakkers M, Brodin N, Burmester GR, Codreanu C, Conway R, Dougados M, Emery P, Ferraccioli G, Fonseca J, Raza K, Silva-Fernández L, Smolen JS, Skingle D, Szekanecz Z, Kvien TK, van der Helm-van Mil A, van Vollenhoven R. Update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis. 2016;76:948–959. doi: 10.1136/annrheumdis-2016-210602. [DOI] [PubMed] [Google Scholar]
- 14.Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, Nam J, Ramiro S, Voshaar M, van Vollenhoven R, Aletaha D, Aringer M, Boers M, Buckley CD, Buttgereit F, Bykerk V, Cardiel M, Combe B, Cutolo M, van Eijk-Hustings Y, Emery P, Finckh A, Gabay C, Gomez-Reino J, Gossec L, Gottenberg JE, Hazes JMW, Huizinga T, Jani M, Karateev D, Kouloumas M, Kvien T, Li Z, Mariette X, McInnes I, Mysler E, Nash P, Pavelka K, Poór G, Richez C, van Riel P, Rubbert-Roth A, Saag K, da Silva J, Stamm T, Takeuchi T, Westhovens R, de Wit M, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 15.Davignon JL, Hayder M, Baron M, Boyer JF, Constantin A, Apparailly F, Poupot R, Cantagrel A. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology. 2013;52:590–598. doi: 10.1093/rheumatology/kes304. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton JA, Tak PP. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum. 2009;60:1210–1221. doi: 10.1002/art.24505. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy A, Fearon U, Veale DJ, Godson C. Macrophages in synovial inflammation. Front Immunol. 2011;2:1–9. doi: 10.3389/fimmu.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. 2016;12:472–485. doi: 10.1038/nrrheum.2016.91. [DOI] [PubMed] [Google Scholar]
- 19.Orr C, Vieira-Sousa E, Boyle DL, Buch MH, Buckley CD, Canete JD, Catrina AI, Choy EHS, Emery P, Fearon U, Filer A, Gerlag D, Humby F, Isaacs JD, Just SA, Lauwerys BR, Le Goff B, Manzo A, McGarry T, McInnes IB, Najm A, Pitzalis C, Pratt A, Smith M, Tak PP, Thurlings R, Fonseca JE, Veale DJ, Tas SW. Synovial tissue research: a state-of-the-art review. Nat Rev Rheumatol. 2017;13:463–475. doi: 10.1038/nrrheum.2017.115. [DOI] [PubMed] [Google Scholar]
- 20.Tu J, Hong W, Zhang P, Wang X, Körner H, Wei W. Ontology and function of fibroblast-like and macrophage-like synoviocytes: how do they talk to each other and can they be targeted for rheumatoid arthritis therapy? Front Immunol. 2018;9:1467. doi: 10.3389/fimmu.2018.01467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan FM, Mcinnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann K, Clauder AK, Manz RA. Targeting B cells and plasma cells in autoimmune diseases. Front Immunol. 2018;9:835. doi: 10.3389/fimmu.2018.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverman GJ, Carson DA. Roles of B cells in rheumatoid arthritis. Arthritis Res Ther. 2003;5:1–6. doi: 10.1186/ar1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerkman PF, Fabre E, van der Voort EI, Zaldumbide A, Rombouts Y, Rispens T, Wolbink G, Hoeben RC, Spits H, Baeten DL, Huizinga TW, Toes RE, Scherer HU. Identification and characterisation of citrullinated antigen-specific B cells in peripheral blood of patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1170–1176. doi: 10.1136/annrheumdis-2014-207182. [DOI] [PubMed] [Google Scholar]
- 25.Vogelpoel LTC, Hansen IS, Rispens T, Muller FJM, van Capel TMM, Turina MC, Vos JB, Baeten DL, Kapsenberg ML, de Jong EC, den Dunen J. Fc gamma receptor-TLR cross-talk elicits pro-inflammatory cytokine production by human M2 macrophages. Nat Commun. 2014;5:5444. doi: 10.1038/ncomms6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clavel C, Ceccato L, Anquetil F, Serre G, Sebbag M. Among human macrophages polarised to different phenotypes, the M-CSF-oriented cells present the highest pro-inflammatory response to the rheumatoid arthritis-specific immune complexes containing ACPA. Ann Rheum Dis. 2016;75:2184–2191. doi: 10.1136/annrheumdis-2015-208887. [DOI] [PubMed] [Google Scholar]
- 27.Gao CH, Dong HL, Tai L, Gao XM. Lactoferrin-containing immunocomplexes drive the conversion of human macrophages from M2- into M1-like phenotype. Front Immunol. 2018;9:37. doi: 10.3389/fimmu.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith MD, Kraan MC, Slavotinek J, Au V, Weedon H, Parker A, Coleman M, Roberts-Thomson PJ, Ahern MJ. Treatment-induced remission in rheumatoid arthritis patients is characterized by a reduction in macrophage content of synovial biopsies. Rheumatology. 2001;40:367–374. doi: 10.1093/rheumatology/40.4.367. [DOI] [PubMed] [Google Scholar]
- 29.Jahangier ZN, Jacobs JWG, Kraan MC, Wenting MJG, Smeets TJ, Bijlsma JW, Lafeber FPJG, Tak PP. Pretreatment macrophage infiltration of the synovium predicts the clinical effect of both radiation synovectomy and intra-articular glucocorticoids. Ann Rheum Dis. 2006;65:1286–1292. doi: 10.1136/ard.2005.042333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haringman JJ, Gerlag DM, Zwinderman AH, Smeets TJM, Kraan MC, Baeten D, McInnes IB, Bresnihan B, Tak PP. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:834–838. doi: 10.1136/ard.2004.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs AH, Tavitian B. INMiND consortium. Noninvasive molecular imaging of neuroinflammation. J Cereb Blood Flow Metab. 2012;32:1393–1415. doi: 10.1038/jcbfm.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lammertsma AA. Forward to the past: the case for quantitative PET imaging. J Nucl Med. 2017;58:1019–1024. doi: 10.2967/jnumed.116.188029. [DOI] [PubMed] [Google Scholar]
- 33.Beckers C, Ribbens C, Marcelis S. Assessment of disease activity in rheumatoid. J Nucl Med. 2004;45:956–965. [PubMed] [Google Scholar]
- 34.Bruijnen STG, Gent YYJ, Voskuyl AE, Hoekstra OS, van der Laken CJ. Present role of positron emission tomography in the diagnosis and monitoring of peripheral inflammatory arthritis: a systematic review. Arthritis Care Res. 2014;66:120–130. doi: 10.1002/acr.22184. [DOI] [PubMed] [Google Scholar]
- 35.Gent YY, Voskuyl AE, Kloet RW, van Schaardenburg D, Hoekstra OS, Dijkmans BA, Lammertsma AA, van der Laken CJ. Macrophage positron emission tomography imaging as a biomarker for preclinical rheumatoid arthritis: findings of a prospective pilot study. Arthritis Rheum. 2012;64:62–66. doi: 10.1002/art.30655. [DOI] [PubMed] [Google Scholar]
- 36.Roivainen A, Hautaniemi S, Möttönen T, Nuutila P, Oikonen V, Parkkola R, Seneca N, Seppänen M, Yli-Kerttula T. Correlation of 18F-FDG PET/CT assessments with disease activity and markers of inflammation in patients with early rheumatoid arthritis following the initiation of combination therapy with triple oral antirheumatic drugs. Eur J Nucl Med Mol Imaging. 2013;40:403–410. doi: 10.1007/s00259-012-2282-x. [DOI] [PubMed] [Google Scholar]
- 37.Gent YY, ter Wee MM, Voskuyl AE, den Uyl D, Ahmadi N, Dowling C, van Kuijk C, Hoekstra OS, Boers M, Lems WF, van der Laken CJ. Subclinical synovitis detected by macrophage PET, but not MRI, is related to short-term flare of clinical disease activity in early RA patients: an exploratory study. Arthritis Res Ther. 2015;17:266. doi: 10.1186/s13075-015-0770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elzinga EH, Van Der Laken CJ, Comans EFI, Lammertsma AA, Dijkmans BAC, Voskuyl AE. 2-Deoxy-2-[F-18]fluoro-D-glucose joint uptake on positron emission tomography images: rheumatoid arthritis versus osteoarthritis. Mol Imaging Biol. 2007;9:357–360. doi: 10.1007/s11307-007-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elzinga EH, van der Laken CJ, Comans EFI, Boellaard R, Hoekstra OS, Dijkmans BAC, Lammertsma AA, Voskuyl AE. 18F-FDG PET as a tool to predict the clinical outcome of infliximab treatment of rheumatoid arthritis: an explorative study. J Nucl Med. 2011;52:77–80. doi: 10.2967/jnumed.110.076711. [DOI] [PubMed] [Google Scholar]
- 40.Goerres GW, Forster A, Uebelhart D, Seifert B, Treyer V, Michel B, von Schulthess GK, Kaim AH. F-18 FDG whole-body PET for the assessment of disease activity in patients with rheumatoid arthritis. Clin Nucl Med. 2006;31:386–390. doi: 10.1097/01.rlu.0000222678.95218.42. [DOI] [PubMed] [Google Scholar]
- 41.Kropholler MA, Boellaard R, Elzinga EH, van der Laken CJ, Maruyama K, Kloet RW, Voskuyl AE, Dijkmans BA, Lammertsma AA. Quantification of (R)-[11C]PK11195 binding in rheumatoid arthritis. Eur J Nucl Med Mol Imaging. 2009;36:624–631. doi: 10.1007/s00259-008-0987-7. [DOI] [PubMed] [Google Scholar]
- 42.Gent YY, Weijers K, Molthoff CF, Windhorst AD, Huisman MC, Kassiou M, Jansen G, Lammertsma AA, van der Laken CJ. Promising potential of new generation translocator protein tracers providing enhanced contrast of arthritis imaging by positron emission tomography in a rat model of arthritis. Arthritis Res Ther. 2014;16:R70. doi: 10.1186/ar4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Laken CJ, Elzinga EH, Kropholler MA, Molthoff CFM, van der Heijden JW, Maruyama K, Boellaard R, Dijkmans BA, Lammertsma AA, Voskuyl AE. Noninvasive imaging of macrophages in rheumatoid synovitis using 11C-(R)-PK11195 and positron emission tomography. Arthritis Rheum. 2008;58:3350–3355. doi: 10.1002/art.23955. [DOI] [PubMed] [Google Scholar]
- 44.Chandrupatla DMSH, Weijers K, Gent YYJ, de Greeuw I, Lammertsma AA, Jansen G, van der Laken CJ, Molthoff CF. Sustained macrophage infiltration upon multiple intra-articular injections: an improved rat model of rheumatoid arthritis for PET guided therapy evaluation. Biomed Res Int. 2015;2015:509295. doi: 10.1155/2015/509295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chauveau F, Boutin H, Van Camp N, Dolle F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging. 2008;35:2304–2319. doi: 10.1007/s00259-008-0908-9. [DOI] [PubMed] [Google Scholar]
- 46.James ML, Fulton RR, Henderson DJ, Eberl S, Meikle SR, Thomson S, Allan RD, Dolle F, Fulham MJ, Kassiou M. Synthesis and in vivo evaluation of a novel peripheral benzodiazepine receptor PET radioligand. Bioorg Med Chem. 2005;13:6188–6194. doi: 10.1016/j.bmc.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 47.James ML, Fulton RR, Vercoullie J, Henderson DJ, Garreau L, Chalon S, Dolle F, Costa B, Guilloteau D, Kassiou M. DPA-714, a new translocator protein-specific ligand: synthesis, radiofluorination, and pharmacologic characterization. J Nucl Med. 2008;49:814–822. doi: 10.2967/jnumed.107.046151. [DOI] [PubMed] [Google Scholar]
- 48.Gent YYJ, Weijers K, Molthoff CFM, Windhorst AD, Huisman MC, Smith DE, Kularatne SA, Jansen G, Low PS, Lammertsma AA, van der Laken CJ. Evaluation of the novel folate receptor ligand [18F] fluoro-PEG-folate for macrophage targeting in a rat model of arthritis. Arthritis Res Ther. 2013;15:R37. doi: 10.1186/ar4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandrupatla DMSH, Jansen G, Vos R, Verlaan M, Chen Q, Low PS, Windhorst AD, Lammertsma AA, van der Laken CJ, Molthoff CFM. In-vivo monitoring of anti-folate therapy in arthritic rats using [18F]fluoro-PEG-folate and positron emission tomography. Arthritis Res Ther. 2017;19:114. doi: 10.1186/s13075-017-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandrupatla DM, Jansen G, Mantel E, Low PS, Matsuyama T, Musters R, Windhorst AD, Lammertsma AA, Molthoff CF, van der Laken CJ. Imaging and methotrexate response monitoring of systemic inflammation in arthritic rats employing the macrophage PET tracer [18F]fluoro-PEG-folate. Contrast Media Mol Imaging. 2018;2018:8092781. doi: 10.1155/2018/8092781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narayan N, Owen D, Mandhair H, Smyth E, Carlucci F, Saleem A, Gunn R, Rabiner EIA, Wells L, Dakin S, Sabokbar A, Taylor P. Translocator protein as an imaging marker of macrophage and stromal activation in RA pannus. J Nucl Med. 2018;59:1125–1132. doi: 10.2967/jnumed.117.202200. [DOI] [PubMed] [Google Scholar]
- 52.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere J-J, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Nozaki S, Ozaki N, Suzuki S, Goto M, Mawatari A, Nakatani Y, Hayashinaka E, Wada Y, Doi H, Watanabe Y. Development of diagnostic techniques for early rheumatoid arthritis using positron emission tomography with [11C]PK11195 and [11C]ketoprofen tracers. Mol Imaging Biol. 2017;19:746–753. doi: 10.1007/s11307-016-1039-5. [DOI] [PubMed] [Google Scholar]
- 54.Folkersma H, Foster Dingley JC, van Berckel BNM, Rozemuller A, Boellaard R, Huisman MC, Lammertsma AA, Van der Top WP, Molthoff CF. Increased cerebral (R)-[(11)C]PK11195 uptake and glutamate release in a rat model of traumatic brain injury: a longitudinal pilot study. J Neuroinflammation. 2011;8:67. doi: 10.1186/1742-2094-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chauveau F, Van Camp N, Dolle F, Kuhnast B, Hinnen F, Damont A, Boutin H, James M, Kassiou M, Tavitian B. Comparative evaluation of the translocator protein radioligands 11C-DPA-713, 18F-DPA-714, and 11C-PK11195 in a rat model of acute neuroinflammation. J Nucl Med. 2009;50:468–476. doi: 10.2967/jnumed.108.058669. [DOI] [PubMed] [Google Scholar]
- 56.Doorduin J, Klein HC, Dierckx RA, James M, Kassiou M, de Vries EFJ. [11C]-DPA-713 and [18F]-DPA-714 as new PET tracers for TSPO: a comparison with [11C]-(R)-PK11195 in a rat model of herpes encephalitis. Mol Imaging Biol. 2009;11:386–398. doi: 10.1007/s11307-009-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vivash L, O'Brien TJ. Imaging microglial activation with TSPO PET: lighting up neurologic diseases? J Nucl Med. 2016;57:165–168. doi: 10.2967/jnumed.114.141713. [DOI] [PubMed] [Google Scholar]
- 58.Narayan N, Owen DR, Taylor PC. Advances in positron emission tomography for the imaging of rheumatoid arthritis. Rheumatology (Oxford) 2017;56:1837–1846. doi: 10.1093/rheumatology/kew484. [DOI] [PubMed] [Google Scholar]
- 59.Pottier G, Bernards N, Dollé F, Boisgard R. [18F]DPA-714 as a biomarker for positron emission tomography imaging of rheumatoid arthritis in an animal model. Arthritis Res Ther. 2014;16:R69. doi: 10.1186/ar4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tronel C, Largeau B, Ribeiro MJS, Guilloteau D, Dupont AC, Arlicot N. Molecular targets for PET imaging of activated microglia: the current situation and future expectations. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed]
- 61.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008;41:120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 62.Yi Y-S. Folate receptor-targeted diagnostics and therapeutics for inflammatory diseases. Immune Netw. 2016;16:337–343. doi: 10.4110/in.2016.16.6.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56:1067–1084. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Gonen N, Assaraf YG. Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance. Drug Resist Updat. 2012;15:183–210. doi: 10.1016/j.drup.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Assaraf YG, Leamon CP, Reddy JA. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist Updat. 2014;17:89–95. doi: 10.1016/j.drup.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Jansen G, Peters GJ. Novel insights in folate receptors and transporters: implications for disease and treatment of immune diseases and cancer. Pteridines. 2015;26:41–53. [Google Scholar]
- 67.Matherly LH, Hou Z, Gangjee A. The promise and challenges of exploiting the proton-coupled folate transporter for selective therapeutic targeting of cancer. Cancer Chemother Pharmacol. 2017;81:1–15. doi: 10.1007/s00280-017-3473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Westerhof GR, Schornagel JH, Kathmann I, Jackman AL, Rosowsky A, Forsch R, Hynes JB, Boyle FT, Peters GJ, Pinedo HM, Jansen G. Carrier- and receptor-mediated transport of folate antagonists targeting folate-dependent enzymes: correlates of molecular-structure and biological activity. Mol Pharmacol. 1995;48:459–471. [PubMed] [Google Scholar]
- 69.Wu M, Fan J, Gunning W, Ratnam M. Clustering of GPI-anchored folate receptor independent of both cross-linking and association with caveolin. J Membr Biol. 1997;159:137–147. doi: 10.1007/s002329900277. [DOI] [PubMed] [Google Scholar]
- 70.Shen F, Wu M, Ross JF, Miller D, Ratnam M. Folate receptor type γ is primarily a secretory protein due to lack of an efficient signal for glycosylphosphatidylinositol modification: protein characterization and cell type specificity. Biochemistry. 1995;34:5660–5665. doi: 10.1021/bi00016a042. [DOI] [PubMed] [Google Scholar]
- 71.Maziarz KM, Monaco HL, Shen F, Ratnam M. Complete mapping of divergent amino acids responsible for differential ligand binding of folate receptors alpha and beta. J Biol Chem. 1999;274:11086–11091. doi: 10.1074/jbc.274.16.11086. [DOI] [PubMed] [Google Scholar]
- 72.Wibowo AS, Singh M, Reeder KM, Carter JJ, Kovach AR, Meng W, Ratnam M, Zhang F, Dann CE. Structures of human folate receptors reveal biological trafficking states and diversity in folate and antifolate recognition. Proc Natl Acad Sci. 2013;110:15180–15188. doi: 10.1073/pnas.1308827110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rijnboutt S, Jansen G, Posthuma G, Hynes JB, Schornagel JH, Strous GJ. Endocytosis of GPI-linked membrane folate receptor-α. J Cell Biol. 1996;132:35–47. doi: 10.1083/jcb.132.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varghese B, Vlashi E, Xia W, Ayala Lopez W, Paulos CM, Reddy J, Xu LC, Low PS. Folate receptor-β in activated macrophages: ligand binding and receptor recycling kinetics. Mol Pharm. 2014;11:3609–3616. doi: 10.1021/mp500348e. [DOI] [PubMed] [Google Scholar]
- 75.Anderson RG, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- 76.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 77.Ross JF, Wang H, Behm FG, Mathew P, Wu M, Booth R, Ratnam M. Folate receptor type beta is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer. 1999;85:348–357. doi: 10.1002/(sici)1097-0142(19990115)85:2<348::aid-cncr12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 78.Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Cancer 1994;2432–43. [DOI] [PubMed]
- 79.Shen J, Hilgenbrink AR, Xia W, Feng Y, Dimitrov DS, Lockwood MB, Amato RJ, Low PS. Folate receptor-β constitutes a marker for human proinflammatory monocytes. J Leukoc Biol. 2014;96:563–570. doi: 10.1189/jlb.2AB0713-372R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113:438–446. doi: 10.1182/blood-2008-04-150789. [DOI] [PubMed] [Google Scholar]
- 81.van der Heijden JW, Oerlemans R, Dijkmans BAC, Qi H, van der Laken CJ, Lems WF, Jackman AL, Kraan MC, Tak PP, Ratnam M, Jansen G. Folate receptor beta as a potential delivery route for novel folate antagonists to macrophages in the synovial tissue of rheumatoid arthritis patients. Arthritis Rheum. 2009;60:12–21. doi: 10.1002/art.24219. [DOI] [PubMed] [Google Scholar]
- 82.Shen J, Putt KS, Visscher DW, Murphy L, Cohen C, Singhal S, Sandusky G, Feng Y, Dimitrov DS, Low PS. Assessment of folate receptor-β expression in human neoplastic tissues. Oncotarget. 2015;6:14700–14709. doi: 10.18632/oncotarget.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H, Zheng X, Behm FG, Ratnam M. Differentiation-independent retinoid induction of folate receptor type beta, a potential tumor target in myeloid leukemia. Blood. 2000;96:3529–3536. [PubMed] [Google Scholar]
- 84.Qi H, Ratnam M. Synergistic induction of folate receptor beta by all-trans retinoic acid and histone deacetylase inhibitors in acute myelogenous leukemia cells: mechanism and utility in enhancing selective growth inhibition by antifolates. Cancer Res. 2006;66:5875–5882. doi: 10.1158/0008-5472.CAN-05-4048. [DOI] [PubMed] [Google Scholar]
- 85.Dhanasekaran S, Biswal BK, Sumantran VN, Verma RS. Augmented sensitivity to methotrexate by curcumin induced overexpression of folate receptor in KG-1 cells. Biochimie. 2013;95:1567–1573. doi: 10.1016/j.biochi.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 86.Samaniego R, Palacios BS, Domiguez-Soto A, Vidal C, Salas A, Matsuyama T, Sánchez-Torres C, de la Torre I, Miranda-Carús ME, Sánchez-Mateos P, Puig-Kröger A. Macrophage uptake and accumulation of folates are polarization-dependent in vitro and in vivo and are regulated by activin. J Leukoc Biol. 2014;95:797–808. doi: 10.1189/jlb.0613345. [DOI] [PubMed] [Google Scholar]
- 87.Blits M, Jansen G, Assaraf YG, Van De Wiel MA, Lems WF, Nurmohamed MT, van Schaardenburg D, Voskuyl AE, Wolbink GJ, Vosslamber S, Verweij CL. Methotrexate normalizes up-regulated folate pathway genes in rheumatoid arthritis. Arthritis Rheum. 2013;65:2791–2802. doi: 10.1002/art.38094. [DOI] [PubMed] [Google Scholar]
- 88.van der Heijden JW, Assaraf YG, Gerards AH, Oerlemans R, Lems WF, Scheper RJ, Dijkmans BA, Jansen G. Methotrexate analogues display enhanced inhibition of TNF-α production in whole blood from RA patients. Scand J Rheumatol. 2014;43:9–16. doi: 10.3109/03009742.2013.797490. [DOI] [PubMed] [Google Scholar]
- 89.Municio C, Soler Palacios B, Estrada-Capetillo L, Benguria A, Dopazo A, García-Lorenzo E, Fernández-Arroyo S, Joven J, Miranda-Carús ME, González-Álvaro I, Puig-Kröger A. Methotrexate selectively targets human proinflammatory macrophages through a thymidylate synthase/p53 axis. Ann Rheum Dis. 2016;75:2157–2165. doi: 10.1136/annrheumdis-2015-208736. [DOI] [PubMed] [Google Scholar]
- 90.Ceponis A, Konttinen YT, Imai S, Tamulaitiene M, Li TF, Xu JW, Hietanen J, Santavirta S, Fassbender HG. Synovial lining, endothelial and inflammatory mononuclear cell proliferation in synovial membranes in psoriatic and reactive arthritis: a comparative quantitative morphometric study. Br J Rheumatol. 1998;37:170–178. doi: 10.1093/rheumatology/37.2.170. [DOI] [PubMed] [Google Scholar]
- 91.Lalor PA, Mapp PI, Hall PA, Revell PA. Proliferative activity of cells in the synovium as demonstrated by a monoclonal antibody, Ki67. Rheumatol Int. 1987;7:183–186. doi: 10.1007/BF00541375. [DOI] [PubMed] [Google Scholar]
- 92.Brown PM, Praat AG, Isaacs JD. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol. 2016;12:731–742. doi: 10.1038/nrrheum.2016.175. [DOI] [PubMed] [Google Scholar]
- 93.Machacek C, Supper V, Leksa V, Mitulovic G, Spittler A, Drbal K, Suchanek M, Ohradanova-Repic A, Stockinger H. Folate receptor beta regulates integrin CD11b/CD18 adhesion of a macrophage subset to collagen. J Immunol. 2016;197:2229–2238. doi: 10.4049/jimmunol.1501878. [DOI] [PubMed] [Google Scholar]
- 94.Ayala-Lopez W, Xia W, Varghese B, Low PS. Imaging of atherosclerosis in apoliprotein E knockout mice: targeting of a folate-conjugated radiopharmaceutical to activated macrophages. J Nucl Med. 2010;51:768–774. doi: 10.2967/jnumed.109.071324. [DOI] [PubMed] [Google Scholar]
- 95.Jager NA, Westra J, Golestani R, van Dam GM, Low PS, Tio RA, Slart RH, Boersma HH, Bijl M, Zeebregts CJ. Folate receptor-β imaging using 99mTc-folate to explore distribution of polarized macrophage populations in human atherosclerotic plaque. J Nucl Med. 2014;55:1945–1951. doi: 10.2967/jnumed.114.143180. [DOI] [PubMed] [Google Scholar]
- 96.Winkel LCJ, Groen HC, van Thiel BS, Muller C, van der Steen, AFW, Wentzel JJ, de Jong M, Van der Heiden K. Folate receptor-targeted single-photon emission computed tomography/computed tomography to detect activated macrophages in atherosclerosis: can it distinguish vulnerable from stable atherosclerotic plaques? Mol Imaging 2014;13. [DOI] [PubMed]
- 97.Muller A, Beck K, Rancic Z, Muller C, Fischer CR, Betzel T, Kaufmann PA, Schibli R, Krämer SD, Ametamey SM. Imaging atherosclerotic plaque inflammation via folate receptor targeting using a novel 18F-folate radiotracer. Mol Imaging. 2014;13:1–11. [PubMed] [Google Scholar]
- 98.O’Shannessy DJ, Somers EB, Wang LC, Wang H, Hsu R. Expression of folate receptors alpha and beta in normal and cancerous gynecologic tissues: correlation of expression of the beta isoform with macrophage markers. J Ovarian Res. 2015;8:1–9. doi: 10.1186/s13048-015-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kurahara H, Takao S, Kuwahata T, Nagai T, Ding Q, Maeda K, Shinchi H, Mataki Y, Maemura K, Matsuyama T, Natsugoe S. Clinical significance of folate receptor b-expressing tumor-associated macrophages in pancreatic cancer. Ann Surg Oncol. 2012;19:2264–2271. doi: 10.1245/s10434-012-2263-0. [DOI] [PubMed] [Google Scholar]
- 100.Shen J, Hu Y, Putt KS, Singhal S, Han H, Visscher DW, Murphy LM, Low PS. Assessment of folate receptor alpha and beta expression in selection of lung and pancreatic cancer patients for receptor targeted therapies. Oncotarget. 2017;9:4485–4495. doi: 10.18632/oncotarget.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haverkamp JM, Crist SA, Elzey BD, Cimen C, Ratliff TL. In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. Eur J Immunol. 2011;41:749–759. doi: 10.1002/eji.201041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakashima-Matsushita N, Homma T, Yu S, Matsuda T, Sunahara N, Nakamura T, Tsukano M, Ratnam M, Matsuama T. Selective expression of folate receptor beta and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:1609–1616. doi: 10.1002/1529-0131(199908)42:8<1609::AID-ANR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 103.Albano-Aluquin S, Malysz J, Aluquin VR, Ratnam M, Olsen N. An immunohistochemical analysis of folate receptor beta expression and distribution in giant cell arteritis - a pilot study. Am J Clin Exp Immunol. 2017;6:107–114. [PMC free article] [PubMed] [Google Scholar]
- 104.Varghese B, Haase N, Low PS. Depletion of folate-receptor-positive macrophages leads to alleviation of symptoms and prolonged survival in two murine models of systemic lupus erythematosus. Mol Pharm. 2007;4:679–685. doi: 10.1021/mp0700615. [DOI] [PubMed] [Google Scholar]
- 105.Lu Y, Wollak KN, Cross VA, Westrick E, Wheeler LW, Stinnette TW, Vaughn JF, Hahn SJ, Xu LC, Vlahov IR, Leamon CP. Folate receptor-targeted aminopterin therapy is highly effective and specific in experimental models of autoimmune uveitis and autoimmune encephalomyelitis. Clin Immunol. 2014;150:64–77. doi: 10.1016/j.clim.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 106.Siebelt M, Korthagen N, Wei W, Groen H, Bastiaansen-Jenniskens Y, Müller C, Waarsing JH, de Jong M, Weinans H. Triamcinolone acetonide activates an anti-inflammatory and folate receptor-positive macrophage that prevents osteophytosis in vivo. Arthritis Res Ther. 2015;17:1–13. doi: 10.1186/s13075-015-0865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Piscaer TM, Müller C, Mindt TL, Lubberts E, Verhaar J, Krenning EP, Schibli R, De Jong M, Weinans H. Imaging of activated macrophages in experimental osteoarthritis using folate-targeted animal single-photon-emission computed tomography/computed tomography. Arthritis Rheum. 2011;63:1898–1907. doi: 10.1002/art.30363. [DOI] [PubMed] [Google Scholar]
- 108.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front Immunol. 2014;5:1–9. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murray P, Allen J, Biswas S, Fisher E, Gilroy D, Goerdt S, Gordon S, Hamilton J, Ivashkiv L, Lawrence T, Locati M. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;17:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA, Tak PP, Baeten DL. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 111.Kittan NA, Allen RM, Dhaliwal A, Cavassani KA, Schaller M, Gallagher KA. Cytokine induced phenotypic and epigenetic signatures are key to establishing specific macrophage phenotypes. PLoS One. 2013;8:1–15. doi: 10.1371/journal.pone.0078045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ambarus CA, Noordenbos T, de Hair MJ, Tak PP, Baeten DL. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res Ther. 2012;14:R74. doi: 10.1186/ar3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gottfried E, Kunz-Schughart LA, Weber A, Rehli M, Peuker A, Müller A, Kastenberger M, Brockhoff G, Andreesen R, Kreutz M. Expression of CD68 in non-myeloid cell types. Scand J Immunol. 2008;67:453–463. doi: 10.1111/j.1365-3083.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 114.Crocker PR, Gordon S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J Exp Med. 1986;164:1862–1875. doi: 10.1084/jem.164.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hiemstra IH, Beijer MR, Veninga H, Vrijland K, Borg EGF, Olivier BJ, Mebius RE, Kraal G, den Haan JM. The identification and developmental requirements of colonic CD169+ macrophages. Immunology. 2014;142:269–278. doi: 10.1111/imm.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.York MR, Nagai T, Mangini AJ, Lemaire R, Van Seventer JM, Lafyatis R. A macrophage marker, siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007;56:1010–1020. doi: 10.1002/art.22382. [DOI] [PubMed] [Google Scholar]
- 117.Puig-Kröger A, Sierra-Filardi E, Domínguez-Soto A, Samaniego R, Corcuera MT, Gómez-Aguado F, Ratnam M, Sánchez-Mateos P, Corbí AL. Folate receptor β is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 2009;69:9395–9403. doi: 10.1158/0008-5472.CAN-09-2050. [DOI] [PubMed] [Google Scholar]
- 118.Palacios BS, Estrada-Capetillo L, Izquierdo E, Criado G, Nieto C, Municio C, Municio C, González-Alvaro I, Sánchez-Mateos P, Pablos JL, Corbí AL, Puig-Kröger A. Macrophages from the synovium of active rheumatoid arthritis exhibit an activin A-dependent pro-inflammatory profile. J Pathol. 2015;235:515–526. doi: 10.1002/path.4466. [DOI] [PubMed] [Google Scholar]
- 119.Tsuneyoshi Y, Tanaka M, Nagai T, Sunahara N, Matsuda T, Sonoda T, Ijiri K, Komiya S, Matsuyama T. Functional folate receptor beta-expressing macrophages in osteoarthritis synovium and their M1/M2 expression profiles. Scand J Rheumatol. 2012;41:132–140. doi: 10.3109/03009742.2011.605391. [DOI] [PubMed] [Google Scholar]
- 120.Srinivasarao M, Galliford CV, Low PS. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat Rev Drug Discov Nature. 2015;14:203–19. [DOI] [PubMed]
- 121.Bettio A, Honer M, Müller C, Brühlmeier M, Müller U, Schibli R, Groehn V, Schubiger AP, Ametamey SM. Synthesis and preclinical evaluation of a folic acid derivative labeled with 18F for PET imaging of folate receptor-positive tumors. J Nucl Med. 2006;47:1153–1160. [PubMed] [Google Scholar]
- 122.Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13:256–262. doi: 10.1016/j.cbpa.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 123.Paulos CM, Turk MJ, Breur GJ, Low PS. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv Drug Deliv Rev. 2004;56:1205–1217. doi: 10.1016/j.addr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 124.Han W, Zaynagetdinov R, Yull FE, Polosukhin VV, Gleaves LA, Tanjore H, Young LR, Peterson TE, Manning HC, Prince LS, Blackwell TS. Molecular imaging of folate receptor α-positive macrophages during acute lung inflammation. Am J Respir Cell Mol Biol. 2015;53:50–59. doi: 10.1165/rcmb.2014-0289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang FP, Fan YQ, Li SY, Mao H. Biomarkers of in vivo fluorescence imaging in allergic airway inflammation. Mol Cell Probes. 2016;30:100–105. doi: 10.1016/j.mcp.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 126.Shen J, Chelvam V, Cresswell G, Low PS. Use of folate-conjugated imaging agents to target alternatively activated macrophages in a murine model of asthma. Mol Pharm. 2013;10:1918–1927. doi: 10.1021/mp3006962. [DOI] [PubMed] [Google Scholar]
- 127.Turk MJ, Breur GJ, Widmer WR, Paulos CM, Xu LC, Grote LA, Low PS. Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis. Arthritis Rheum. 2002;46:1947–1955. doi: 10.1002/art.10405. [DOI] [PubMed] [Google Scholar]
- 128.Matteson EL, Lowe VJ, Prendergast FG, Crowson CS, Moder KG, Morgenstern DE, Messmann RA, Low PS. Assessment of disease activity in rheumatoid arthritis using a novel folate targeted radiopharmaceutical Folatescan. Clin Exp Rheumatol. 2009;27:253–259. [PMC free article] [PubMed] [Google Scholar]
- 129.Henne WA, Rothenbuhler R, Ayala-Lopez W, Xia W, Varghese B, Low PS. Imaging sites of infection using a 99mTc-labeled folate conjugate targeted to folate receptor positive macrophages. Mol Pharm. 2012;9:1435–1440. doi: 10.1021/mp3000138. [DOI] [PubMed] [Google Scholar]
- 130.Verweij N, Bruijnen S, Gent Y, Huisman M, Jansen G, Molthoff C, Chen Q, Low P, Windhorst A, Lammertsma A, Hoekstra O, Voskuyl A, van der Laken C. Rheumatoid arthritis imaging on PET-CT using a novel folate receptor ligand for macrophage targeting. Arthritis Rheumatol. 2017;69 (abstract).
- 131.Chen Q, Meng X, McQuade P, Rubins D, Lin S-A, Zeng Z, Haley H, Miller P, González Trotter D, Low PS. Synthesis and preclinical evaluation of folate-NOTA-Al 18F for PET imaging of folate receptor-positive tumors. Mol Pharm. 2016;13:1520–1527. doi: 10.1021/acs.molpharmaceut.5b00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Van Dam GM, Themelis G, Crane LMA, Harlaar NJ, Pleijhuis RG, Kelder W. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 133.Zheng X, Xing D, Zhou F, Wu B, Chen WR. Indocyanine green-containing nanostructure as near infrared dual-functional targeting probes for optical imaging and photothermal therapy. Mol Pharm. 2011;8:447–456. doi: 10.1021/mp100301t. [DOI] [PubMed] [Google Scholar]
- 134.Snoeks TJA, Van Driel PBAA, Keereweer S, Aime S, Brindle KM, van Dam GM, Löwik CW, Ntziachristos V, Vahrmeijer AL. Towards a successful clinical implementation of fluorescence-guided surgery. Mol Imaging Biol. 2014;16:147–151. doi: 10.1007/s11307-013-0707-y. [DOI] [PubMed] [Google Scholar]
- 135.Sun JY, Shen J, Thibodeaux J, Huang G, Wang Y, Gao J, Low PS, Dimitrov DS, Sumer BD. In vivo optical imaging of folate receptor-beta in head and neck squamous cell carcinoma. Laryngoscope. 2014;124:E312–E319. doi: 10.1002/lary.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boogerd LSF, Hoogstins CES, Gaarenstroom KN, de Kroon CD, Beltman JJ, Bosse T, Stelloo E, Vuyk J, Low PS, Burggraaf J, Vahrmeijer AL. Folate receptor-α targeted near-infrared fluorescence imaging in high-risk endometrial cancer patients: a tissue microarray and clinical feasibility study. Oncotarget. 2018;9:791–801. doi: 10.18632/oncotarget.23155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kelderhouse LE, Mahalingam S, Low PS. Predicting response to therapy for autoimmune and inflammatory diseases using a folate receptor-targeted near-infrared fluorescent imaging agent. Mol Imaging Biol. 2016;18:201–208. doi: 10.1007/s11307-015-0876-y. [DOI] [PubMed] [Google Scholar]
- 138.Sznol M, Lin SL, Bermudes D, Zheng L, King I, Kirn D. Advances in synergistic combinations of Chinese herbal medicine for the treatment of cancer. Current Cancer Drug Target. 2000;105:1027–1030. doi: 10.2174/1568009616666151207105851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nogueira E, Gomes AC, Preto A, Cavaco-Paulo A. Folate-targeted nanoparticles for rheumatoid arthritis therapy. Nanomedicine. 2016;12:1113–1126. doi: 10.1016/j.nano.2015.12.365. [DOI] [PubMed] [Google Scholar]
- 140.Castaneda O, Nair MG. Controlled trial of methotrexate versus CH-1504 in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:862–864. [PubMed] [Google Scholar]
- 141.Lu Y, Stinnette TW, Westrick E, Klein PJ, Gehrke MA, Cross VA, Vlahov IR, Low PS, Leamon CP. Treatment of experimental adjuvant arthritis with a novel folate receptor-targeted folic acid-aminopterin conjugate. Arthritis Res Ther. 2011;13:R56. doi: 10.1186/ar3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gibbs DD, Theti DS, Wood N, Green M, Raynaud F, Valenti M, Forster MD, Mitchell F, Bavetsias V, Henderson E, Jackman AL. BGC 945, a novel tumor-selective thymidylate synthase inhibitor targeted to α-folate receptor-overexpressing tumors. Cancer Res. 2005;65:11721–11728. doi: 10.1158/0008-5472.CAN-05-2034. [DOI] [PubMed] [Google Scholar]
- 143.Karatas A, Koca SS, Ozgen M, Dagli AF, Erman F, Sahin N, Sahin K, Isik A. Pemetrexed ameliorates experimental arthritis in rats. Inflammation. 2015;38:9–15. doi: 10.1007/s10753-014-0002-3. [DOI] [PubMed] [Google Scholar]
- 144.Nagayoshi R, Nakamura M, Ijiri K, Yoshida H, Komiya S, Matsuyama T. LY309887, antifolate via the folate receptor suppresses murine type II collagen-induced arthritis. Clin Exp Rheumatol. 2003;21:719–725. [PubMed] [Google Scholar]
- 145.Chintalacharuvu S, Evans GF, Shih C, Bryant HU, Sandusky GE, Zuckerman SH. Inhibition of glycinamide ribonucleotide formyltransferase results in selective inhibition of macrophage cytokine secretion in vitro and in vivo efficacy in rat adjuvant arthritis. Clin Exp Rheumatol. 2005;23:438–446. [PubMed] [Google Scholar]
- 146.Golani LK, George C, Zhao S, Raghavan S, Orr S, Wallace A, Wilson MR, Hou Z, Matherly LH, Gangjee A. Structure-activity profiles of novel 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolates with modified amino acids for cellular uptake by folate receptors alpha and beta and the proton-coupled folate transporter. J Med Chem. 2014;57:8152–8166. doi: 10.1021/jm501113m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nagai T, Kyo A, Hasui K, Takao S, Matsuyama T. Efficacy of an immunotoxin to folate receptor beta in the intra-articular treatment of antigen-induced arthritis. Arthritis Res Ther. 2012;14:R106. doi: 10.1186/ar3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nagai T, Tanaka M, Tsuneyoshi Y, Matsushita K, Sunahara N, Matsuda T, Yoshida H, Komiya S, Onda M, Matsuyama T. In vitro and in vivo efficacy of a recombinant immunotoxin against folate receptor β on the activation and proliferation of rheumatoid arthritis synovial cells. Arthritis Rheum. 2006;54:3126–3134. doi: 10.1002/art.22082. [DOI] [PubMed] [Google Scholar]
- 149.Nagayoshi R, Nagai T, Matsushita K, Sato K, Sunahara N, Matsuda T, Nakamura T, Komiya S, Onda M, Matsuyama T. Effectiveness of anti-folate receptor β antibody conjugated with truncated Pseudomonas exotoxin in the targeting of rheumatoid arthritis synovial macrophages. Arthritis Rheum. 2005;52:2666–2675. doi: 10.1002/art.21228. [DOI] [PubMed] [Google Scholar]
- 150.Nagai T, Tanaka M, Tsuneyoshi Y, Xu B, Michie SA, Hasui K, Hirano H, Arita K, Matsuyama T. Targeting tumor-associated macrophages in an experimental glioma model with a recombinant immunotoxin to folate receptor β. Cancer Immunol Immunother. 2009;58:1577–1586. doi: 10.1007/s00262-009-0667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Furusho Y, Miyata M, Matsuyama T, Nagai T, Li H, Akasaki Y, Hamada N, Miyauchi T, Ikeda Y, Shirasawa T, Ide K, Tei C. Novel therapy for atherosclerosis using recombinant immunotoxin against folate receptor β-expressing macrophages. J Am Heart Assoc. 2012;1:e003079. doi: 10.1161/JAHA.112.003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thomas TP, Goonewardena SN, Majoros IJ, Kotlyar A, Cao Z, Leroueil PR. Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis Rheum. 2011;63:2671–2680. doi: 10.1002/art.30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nogueira E, Lager F, Le Roux D, Nogueira P, Freitas J, Charvet C. Enhancing methotrexate tolerance with folate tagged liposomes in arthritic mice. J Biomed Nanotechnol. 2015;11:2243–2252. doi: 10.1166/jbn.2015.2170. [DOI] [PubMed] [Google Scholar]
- 154.Yang M, Ding J, Zhang Y, Chang F, Wang J, Gao Z, Zhuang X, Chen X. Activated macrophage-targeted dextran–methotrexate/folate conjugate prevents deterioration of collagen-induced arthritis in mice. J Mater Chem B. 2016;4:2102–2113. doi: 10.1039/c5tb02479j. [DOI] [PubMed] [Google Scholar]
- 155.Poh S, Chelvam V, Ayala-López W, Putt KS, Low PS. Selective liposome targeting of folate receptor positive immune cells in inflammatory diseases. Nanomedicine. 2018;14:1033–1043. doi: 10.1016/j.nano.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 156.Hattori Y, Sakaguchi M, Maitani Y. Folate-linked lipid-based nanoparticles deliver a NFkappa B decoy into activated murine macrophage-like RAW264.7 cells. Biol Pharm Bull. 2006;29:1516–1520. doi: 10.1248/bpb.29.1516. [DOI] [PubMed] [Google Scholar]
- 157.Penn CA, Yang K, Zong H, Lim J-Y, Cole A, Yang D, Baker J, Goonewardena SN, Buckanovich RJ. Therapeutic impact of nanoparticle therapy targeting tumor-associated macrophages. Mol Cancer Ther. 2018;17:96–106. doi: 10.1158/1535-7163.MCT-17-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hattori Y, Yamashita J, Sakaida C, Kawano K, Yonemochi E. Evaluation of antitumor effect of zoledronic acid entrapped in folate-linked liposome for targeting to tumor-associated macrophages. J Liposome Res. 2015;25:131–140. doi: 10.3109/08982104.2014.954128. [DOI] [PubMed] [Google Scholar]
- 159.Yongbo P, Zilong Z, Teng L, Xiong L, Xiaoxiao H, Xiaoping W, Zhang X, Tan W. Smart human-serum-albumin–As2O3 nanodrug with self-amplified folate receptor-targeting ability for chronic myeloid leukemia treatment. Angew Chem. 2017;56:10845–10849. doi: 10.1002/anie.201701366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pan XQ, Zheng X, Shi G, Wang H, Ratnam M, Lee RJ. Strategy for the treatment of acute myelogenous leukemia based on folate receptor beta-targeted liposomal doxorubicin combined with receptor induction using all-trans retinoic acid. Blood. 2002;100:594–602. doi: 10.1182/blood.v100.2.594. [DOI] [PubMed] [Google Scholar]
- 161.Lu Y, Parker N, Kleindl PJ, Cross VA, Wollak K, Westrick E, Stinnette TW, Gehrke MA, Wang K, Santhapuram HK, You F, Hahn SJ, Vaughn JF, Klein PJ, Vlahov IR, Low PS, Leamon CP. Antiinflammatory activity of a novel folic acid targeted conjugate of the mTOR inhibitor everolimus. Mol Med. 2015;21:584–596. doi: 10.2119/molmed.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fischer CR, Muller C, Reber J, Muller A, Kramer SD, Ametamey SM, Schibli R. [18F]fluoro-deoxy-glucose folate: a novel PET radiotracer with improved in vivo properties for folate receptor targeting. Bioconjug Chem. 2012;23:805–813. doi: 10.1021/bc200660z. [DOI] [PubMed] [Google Scholar]
- 163.Nogueira E, Freitas J, Loureiro A, Nogueira P, Gomes AC, Preto A, Carmo AM, Moreira A, Cavaco-Paulo A. Neutral PEGylated liposomal formulation for efficient folate-mediated delivery of MCL1 siRNA to activated macrophages. Colloids Surf B: Biointerfaces. 2017;155:459–465. doi: 10.1016/j.colsurfb.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 164.Mohammadi M, Li Y, Abebe DG, Xie Y, Kandil R, Kraus T, Gomez-Lopez N, Fujiwara T, Merkel OM. Folate receptor targeted three-layered micelles and hydrogels for gene delivery to activated macrophages. J Control Release. 2016;244:269–279. doi: 10.1016/j.jconrel.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 165.Orellana EA, Tenneti S, Rangasamy L, Lyle LT, Low PS, Kasinski AL. FolamiRs: ligand-targeted, vehicle-free delivery of microRNAs for the treatment of cancer. Sci Transl Med. 2017;9:401. doi: 10.1126/scitranslmed.aam9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lynn RC, Feng Y, Schutsky K, Poussin M, Kalota A, Dimitrov DS, Powell DJ., Jr High-affinity FRβ-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia. 2016;30:1355–1364. doi: 10.1038/leu.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lynn RC, Poussin M, Kalota A, Feng Y, Low PS, Dimitrov DS, Powell DJ., Jr Targeting of folate receptor β on acute myeloid leukemia blasts with chimeric antigen receptor – expressing T cells. Blood. 2015;125:3466–3477. doi: 10.1182/blood-2014-11-612721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Asquith DL, Miller AM, McInnes IB, Liew FY. Animal models of rheumatoid arthritis. Eur J Immunol. 2009;39:2040–2044. doi: 10.1002/eji.200939578. [DOI] [PubMed] [Google Scholar]
- 169.Choudhary N, Bhatt LK, Prabhavalkar KS. Experimental animal models for rheumatoid arthritis. Immunopharmacol Immunotoxicol. 2018;40:193–200. doi: 10.1080/08923973.2018.1434793. [DOI] [PubMed] [Google Scholar]
- 170.Peters GJ, Smitskamp-Wilms E, Smid K, Pinedo HM, Jansen G. Determinants of activity of the antifolate thymidylate synthase inhibitors Tomudex (ZD1694) and GW1843U89 against mono- and multilayered colon cancer cell lines under folate-restricted conditions. Cancer Res. 1999;59:5529–5535. [PubMed] [Google Scholar]
- 171.Mauritz R, Peters GJ, Kathmann I, Teshale H, Noordhuis P, Comijn EM, Pinedo HM, Jansen G. Dynamics of antifolate transport via the reduced folate carrier and the membrane folate receptor in murine leukaemia cells in vitro and in vivo. Cancer Chemother Pharmacol. 2008;62:937–948. doi: 10.1007/s00280-008-0683-0. [DOI] [PubMed] [Google Scholar]
- 172.Bandara NA, Hansen MJ, Low PS. Effect of receptor occupancy on folate receptor internalization. Mol Pharm. 2014;11:1007–1013. doi: 10.1021/mp400659t. [DOI] [PubMed] [Google Scholar]