Abstract
The Ca2+-activated Cl− channel (CaCC) TMEM16A/Anoctamin 1 (ANO1) is expressed in gastrointestinal epithelia and smooth muscle cells where it mediates secretion and intestinal motility. However, ANO1 Cl− conductance has never been reported to play a role in skeletal muscle. Here we show that ANO1 is robustly expressed in the highly evolved skeletal musculature of the euteleost species zebrafish. We characterised ANO1 as bonafide CaCC which is activated close to maximum by Ca2+ ions released from the SR during excitation-contraction (EC) coupling. Consequently, our study addressed the question about the physiological advantage of implementation of ANO1 into the euteleost skeletal-muscle EC coupling machinery. Our results reveal that Cl− influx through ANO1 plays an essential role in restricting the width of skeletal-muscle action potentials (APs) by accelerating the repolarisation phase. Resulting slimmer APs enable higher AP-frequencies and apparently tighter controlled, faster and stronger muscle contractions, crucial for high speed movements.
The Ca2+-activated Cl- channel TMEM16A/Anoctamin 1 (ANO1) mediates secretion and intestinal motility in gastrointestinal epithelia and smooth muscle cells. Here, the authors show a role for ANO1 in zebrafish skeletal muscle which is activated by SR Ca2+ release during excitation-contraction coupling.
Introduction
Excitation-contraction (EC) coupling in vertebrate skeletal muscle is initiated at the neuromuscular junction by an action potential (AP) from a single somatic efferent motor neuron. This neuronal AP causes the release of acetylcholine which binds to nicotinic acetylcholine receptors in the motor endplate of the muscle fibre, inducing influx of Na+ ions. This in turn, causes a depolarising excitatory postsynaptic potential which as soon exceeds a certain threshold level, triggers a sarcolemmal AP. Subsequently, these depolarisations travel downwards into specific sarcolemmal invaginations, the transvers (t)-tubules, where they are detected by the voltage-sensing α1S subunit of the dihydropyridine receptor (DHPR). This induces a conformational change in the DHPR which, via allosteric coupling, is transduced to the sarcoplasmic Ca2+ release channel or ryanodine receptor type-1 (RyR1)1. Opening of RyR1 leads to a massive release of Ca2+ ions from sarcoplasmic reticulum (SR) stores into the cytoplasmic gap2,3 of this triadic junction, which bind to troponin C of the thin filaments, finally inducing muscle contraction via actin-myosin cross-bridge interactions.
Previous studies on EC coupling in zebrafish (Danio rerio) skeletal muscle revealed that the skeletal musculature of euteleost fishes is evolutionary highly advanced compared to mammals4,5. Similar to mammals, teleost skeletal muscles are composed of two major types of muscle fibres, classified as slow- (type I) and fast-twitch (type II) fibres. However, in contrast to mammals, where slow- and fast-twitch fibres are intermingled, teleost axial musculature displays a clear separation, with slow (oxidative/red) muscles found on the lateral surface and fast (glycolytic/white) muscles forming the deeper layers. Additionally, the selective expression of each of the two isoforms of DHPRα1S subunit4 and RyR16 in red and white zebrafish skeletal muscles resulted in the formation of two different muscle-type specific DHPR-RyR1 couplons. Again in contrast, mammals express only one type of couplon, regardless if it is in type I or type II fibres. This couplon-specification in zebrafish is just one example of how the 3rd round of teleost-specific whole-genome duplication (Ts3R) at the basis of the teleost clade7,8 resulted in isoform formation during phylogenetic organ differentiation and hence in physiological specialisation4,5. Ts3R was also the initiation point of the loss of Ca2+ conductance through both the DHPRα1S isoforms5, interestingly, by involving distinct point mutations4. Notably, our investigations on the zebrafish skeletal muscle EC coupling machinery enabled us to detect another intriguing physiological and biophysical difference in EC coupling between euteleost and mammalian skeletal muscle—the unexpected participation of a Ca2+-activated Cl− channel (CaCC).
CaCC currents are involved in multiple physiological processes, ranging from sensory transduction9, epithelial secretion10, to smooth muscle contraction11. CaCC opening in smooth muscle cells results in membrane depolarisation due to Cl− efflux, since in contrast to skeletal muscle cells the intracellular Cl− concentration in smooth muscle cells is high due to active accumulation by Cl−/HCO3− exchange and Na+, K+, Cl− co-transportation11. Smooth muscle CaCCs are activated by different sources of intracellular Ca2+ increase, viz. via Ca2+ entry through voltage-gated Ca2+ channels, Ca2+ release from intracellular stores as a result of G-protein-coupled pathways, or Ca2+-induced Ca2+ release through ryanodine receptors. Despite their crucial role in a wide range of biological processes, the molecular identity of CaCCs remained elusive until the year 2008, when three independent research groups12–14 identified ‘Transmembrane protein with unknown function 16’ (TMEM16A), which is part of the 10-member mammalian TMEM16 family15, to be accountable for CaCC currents. Hydropathy analysis indicated that the anion-selective channel embraces 8 (octa) transmembrane helices with cytosolic C- and N-terminal ends, and hence was referred to as anoctamin 1 (ANO1)14. In general, ANO proteins are homo-dimers16,17, and in the case of ANO1 each subunit is activated independently18,19, suggesting a double-barrelled channel similar to the ClC Cl− channel20. Newer reports, based on crystal structure analyses and cryo-electron microscopy, proposed that the membrane-spanning domain of ANO1 consists of 10 transmembrane α-helices21,22. Hallmark biophysical features of ANO1 currents are voltage- and Ca2+-dependent activation23,24 with an outward rectification at lower micromolar Ca2+ concentrations and a linear current–voltage (I–V) relationship at higher Ca2+ concentrations25,26.
In the present study, we identify and characterise ANO1 expression pattern, subcellular distribution, and current properties in zebrafish skeletal muscle by implementing molecular, immunocytochemical, and biophysical approaches. Experiments on myotubes derived from the DHPRβ1-null zebrafish mutant relaxed which lack SR Ca2+ release27, reveal that Cl− influx via sarcolemmal ANO1 channels is activated by Ca2+ ions released through ryanodine receptors. Interestingly, our results demonstrate that this ANO1-mediated Cl− influx accelerates the repolarisation phase and thereby decreases the duration of the skeletal muscle AP, apparently acting synergistically with the canonical K+ efflux repolarisation mechanism. Furthermore, we show that evolution of this accelerated AP repolarisation mechanism in the euteleost species Danio rerio allows enhanced muscle stimulation frequencies during AP trains, consequently expected to generate tighter muscle control as well as increased force production28–32—an all over improvement in muscle properties especially vital for the aquatic pray-predator environment.
Results
SR Ca2+ release awakes zebrafish skeletal muscle Cl− current
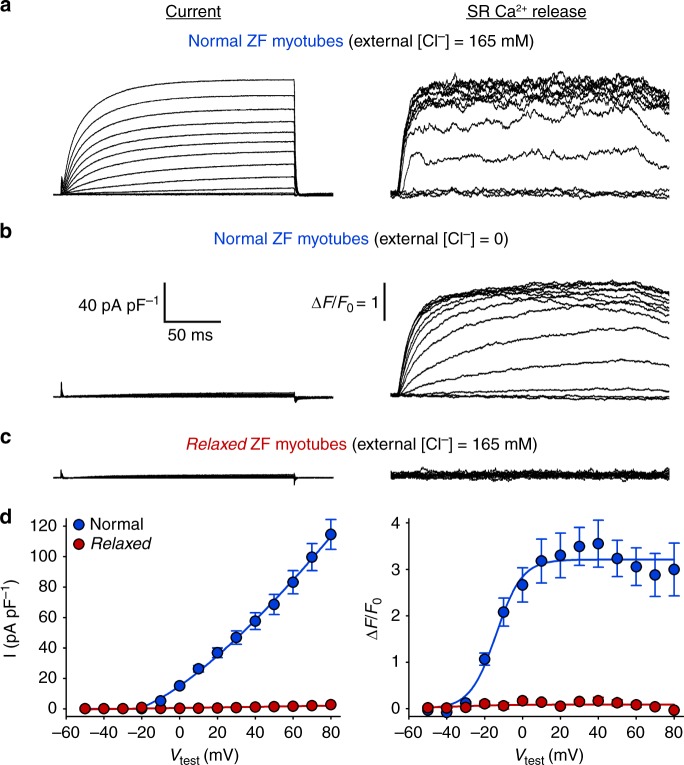
Patch-clamp recordings from freshly dissociated zebrafish skeletal myotubes (Fig. 1a) displayed a very distinct picture compared to mouse myotubes, even under identical experimental conditions30,33. Although the standard depolarisation protocols elicited the expected robust SR Ca2+ release in zebrafish myotubes, the archetypal slow DHPR Ca2+ inward current was missing4, and surprisingly was ‘replaced’ by a huge outward current (Fig. 1a). To test if this outward current could be massive Cl− influx, we measured whole-cell currents under Cl− free conditions34. And indeed, under Cl−-free conditions the outward current nearly extinguished whereas SR Ca2+ release remained intact (P > 0.05) (Fig. 1b). To assess if this Cl− current was Ca2+-dependent or more precisely dependent on Ca2+ released from the SR during EC coupling, we repeated the recordings under standard Cl− conditions (165 mM Cl− in external and 4 mM Cl− in internal solution), but on myotubes isolated from the DHPRβ1-null zebrafish mutant relaxed, which lacks skeletal muscle EC coupling27. As demonstrated in Fig. 1c, d the lack of considerable Cl− current (left panels) is concordant with the lack of SR Ca2+ release (right panels). Overall, integrating the above results we identified this molecular participant of the zebrafish skeletal muscle EC coupling mechanism as a Ca2+-activated Cl− channel (CaCC). The voltage dependence of the zebrafish skeletal muscle CaCC current (Fig. 1d) strongly resembles Anoctamin (ANO) currents described in mammalian smooth muscles35,36.
Fig. 1.
SR Ca2+ release activates Cl− currents in zebrafish skeletal muscle. a–c Representative recordings from zebrafish myotubes elicited by 200-ms depolarising test potentials between −50 and +80 mV. a Robust outward currents (left traces) and intracellular SR Ca2+ release (right traces) were recorded from normal myotubes under standard external solution containing 165 mM Cl−. b In contrast, under Cl− free conditions normal myotubes displayed only marginal currents (I = 2.99 ± 0.20 pA pF−1 at +80 mV, n = 5) (left traces) but unaltered (P > 0.05) SR Ca2+ release ((ΔF/F0)max = 3.01 ± 0.24, n = 5) (right traces). Scale bars, (left) 50 ms (horizontal), 40 pA pF−1 (vertical); (right) ∆F/F0 = 1 (vertical). c Representative recordings from relaxed zebrafish myotubes with standard external solution containing 165 mM Cl− showed neither considerable outward currents (left traces) nor SR Ca2+ release (right traces), identifying this outward current as SR Ca2+-release-activated Cl− current. d Left graph, plots of current–voltage relationship under standard Cl− conditions (165 mM) from normal (I = 114.55 ± 9.81 pA pF−1 at +80 mV, n = 5) and relaxed myotubes (I = 2.70 ± 0.02 pA pF−1 at +80 mV, n = 5), and (right graph) voltage dependence of maximal Ca2+ release from normal ((ΔF/F0)max = 3.23 ± 0.45, n = 5) and relaxed ((ΔF/F0)max = 0.11 ± 0.05, n = 5) myotubes. Data are presented as mean ± s.e.m.; P determined by unpaired Student’s t-test
Zebrafish mutant relaxed contains functional CaCCs
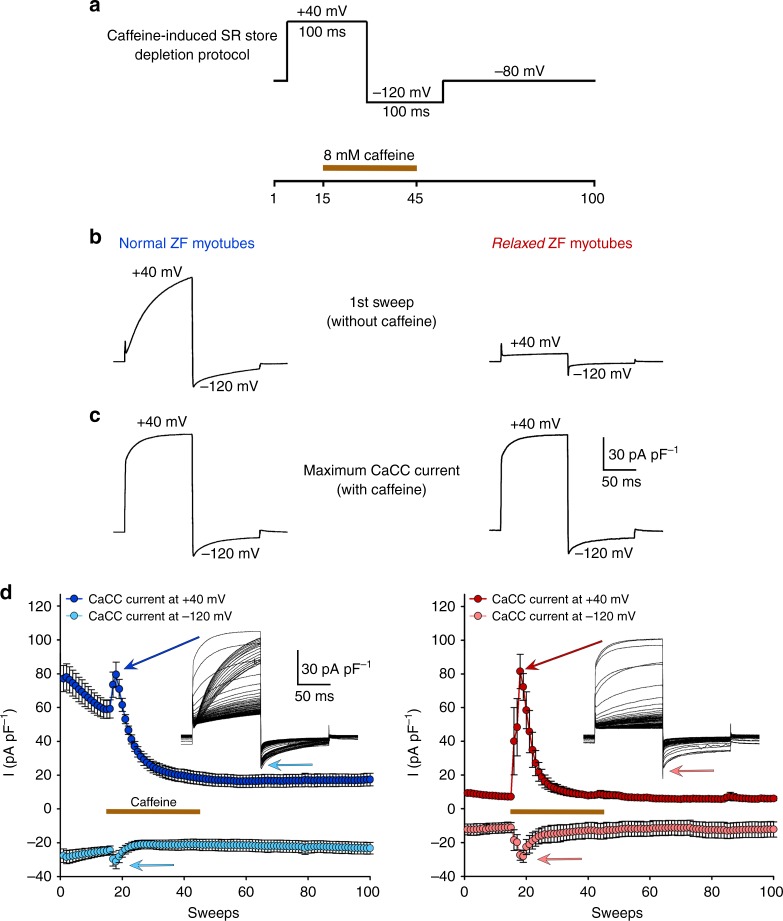
As a first step, to validate if zebrafish relaxed myotubes are a suitable system for studying skeletal muscle CaCCs, we tested the physiological availability of CaCCs in relaxed myotubes by SR store depletion experiments in the presence of the RyR1 agonist caffeine, using the pulse protocol depicted in Fig. 2a. As expected, even before application of caffeine we recorded robust CaCC outward currents at +40 mV and smaller inward currents at −120 mV from normal control myotubes, but very marginal currents from relaxed myotubes (Fig. 2b). We observed a pronounced current rundown in normal myotubes (Fig. 2d), which is a characteristic of ANO currents and is most probably produced by phosphorylation of the channel by Ca2+/calmodulin-dependent protein kinase II (CamKII)37,38.
Fig. 2.
Relaxed myotubes express functional CaCCs and contain intact SR Ca2+ stores. a Test-pulse protocol from a holding potential of −80 mV to +40 mV for 100 ms followed by a 100-ms pulse to −120 mV. The entire protocol was repeated 100 times. Brown bar indicates the application of 8 mM caffeine to induce SR Ca2+ store depletion between 15th and 45th sweep, which was terminated by perfusion with caffeine-free standard bath solution. b Representative sweep of CaCC currents before application of caffeine showing robust CaCC outward current at +40 mV (Imax = 77.12 ± 7.69 pA pF−1, n = 5) and smaller inward current at −120 mV (Imax = −27.85 ± 3.99 pA pF−1, n = 5) from normal control myotubes (left trace), in contrast to only marginal outward (P < 0.001) and inward (P < 0.05) currents (Imax = 9.32 ± 0.97 and −12.27 ± 3.20 pA pF−1, n = 5, respectively) from relaxed myotubes (right trace). c Representative sweep of CaCC currents after application of 8 mM caffeine, showing indistinguishable (P > 0.05) CaCC currents at +40 and −120 mV between normal (Imax = 79.50 ± 7.29 and −31.12 ± 4.22 pA pF−1, respectively, n = 5) (left trace) and relaxed (Imax = 81.42 ± 10.14 and −28.18 ± 3.52 pA pF−1, respectively, n = 5) (right trace) myotubes. Scale bars, 50 ms (horizontal), 30 pA pF−1 (vertical). d Plots of CaCC currents vs. sweeps at +40 and −120 mV with corresponding representative CaCC current traces (insets) from normal (left graph) and relaxed (right graph) myotubes. After caffeine application, the maximal CaCC outward current at +40 mV is indicated by dark blue or dark red arrows and the maximal inward current at −120 mV by light blue or light red arrows, from normal or relaxed myotubes, respectively. Data are presented as mean ± s.e.m.; P determined by unpaired Student’s t-test
Notably, application of 8 mM caffeine after the 14th sweep of the pulse protocol (Fig. 2a) induced comparable (P > 0.05) augmentation of CaCC currents, in both normal as well as relaxed myotubes (Fig. 2c, d). These results unambiguously demonstrate that relaxed myotubes (i) contain intact SR Ca2+ stores and (ii) express functional CaCCs which can be activated by intracellular Ca2+ ions. Thus, zebrafish relaxed myotubes serve as an ideal experimental system for studying CaCCs under SR Ca2+-release free conditions.
Two ANO1 isoforms are expressed in zebrafish skeletal muscle
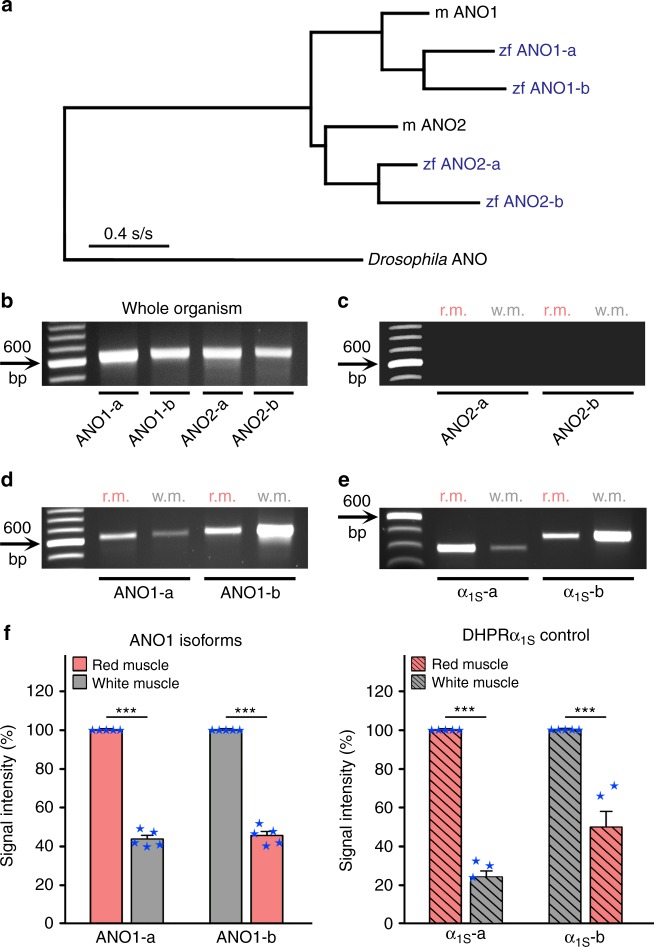
Since strict Ca2+-dependence (Fig. 1c, d; Fig. 2), nearly-linear outward voltage dependence (Fig. 1d, left graph), and pronounced current rundown (Fig. 2d) are characteristics for Cl− currents of the ANO channel family, the apparent question arose which ANO isoform(s) is/are expressed in zebrafish skeletal muscle. Out of the 10 isoforms of the ANO protein family, only ANO1 and ANO2 are verified CaCCs (Supplementary Fig. 1a), while the other 8 ANO proteins, either work as scramblases or have unknown functions39,40. As a result of the Ts3R genome duplication7,8 zebrafish has two ANO1 and two ANO2 genes. The pedigree in Fig. 3a, with the Drosophila ANO protein as outgroup, indicates that the predicted translational products of the two ANO1 and two ANO2 zebrafish isoforms cluster well with their respective mouse counterparts but show a higher degree of phylogenetic advance.
Fig. 3.
Two distinct ANO1 isoforms are expressed in red and white skeletal muscle of zebrafish. a Likelihood-based phylogenetic tree (Treefinder, version 2011) from aligned amino acid sequences of mouse (m) ANO1 (GenBank accession no. NP_848757), zebrafish (zf) isoforms ANO1-a (accession no. XP_009301746) and ANO1-b (accession no. XP_017209649), mouse ANO2 (accession no. NP_705817), zebrafish isoforms ANO2-a (accession no. XP_009298253) and ANO2-b (accession no. XM_009293432), with the ancestral Drosophila ANO channel (accession no. NP_648535) as outgroup, shows species-independent isoform clustering. Likelihood score, −16,906.7; edge lengths optimised. Scale bar, evolutionary distance in substitutions per site (s/s). b RT-PCR amplification from first strands of whole adult zebrafish with primers specific for all 4 ANO channel isoforms (Supplementary Table 1) showed signals with similar intensity (P > 0.05, n = 5) for RNAs that were isolated from adult normal zebrafish. c RT-PCR amplification with ANO2-specific primers under identical PCR conditions did not show any signal, neither from red muscle (r.m.) nor white muscle (w.m.). d In contrast, ANO1-specific primers amplified PCR fragments from both muscle types but to different extents. e Similarly, control PCR amplifications with DHPα1S-specific primers detected both, α1S-a and α1S-b in both tissues but again to different extents. f Left graph, quantification of band intensities of ANO1 isoforms revealed that ANO1-a is predominantly expressed in the red muscle compared (P < 0.001) to white muscle (43.75 ± 1.89%, n = 5), and ANO1-b is mainly expressed in the white muscle compared (P < 0.001) to red muscle (45.56 ± 2.08%, n = 5). Right graph, DHPRα1S-a and α1S-b, known to be exclusively expressed in red and white muscles, respectively4, displayed a similar cross-contamination pattern for DHPRα1S-a in white (24.21 ± 3.12%; n = 5) and DHPRα1S-b in red muscle (49.90 ± 8.09%; n = 5). Bars represent mean ± s.e.m. and overlaying blue stars indicate individual data points; *** P < 0.001 determined by unpaired Student’s t-test
ANO1 and ANO2 isoform-specific RT-PCR primers were designed (Supplementary Table 1) to amplify DNA fragments coding for a region between transmembrane α-helices 4 and 9 containing amino acid residues N650, E654, E702, E705, E734, and D738, supposed to delineate the ANO Ca2+-binding site21,41. Supplementary Figure 1b depicts the positions of these six residues on the proposed 10-helix membrane folding model of ANO channels21. Amino acid sequence alignment of ANO1 isoforms of zebrafish and mouse shows conservation of these six residues in both the ANO1 isoforms of zebrafish (Supplementary Fig. 1c). PCR amplification of ANO1 and ANO2 isoforms from first strands of whole adult zebrafish confirmed the accuracy of the four primer pairs and amplified fragments from all targeted ANO isoforms (Fig. 3b) to a similar extent (P > 0.05). On the contrary, RT-PCR amplification of ANO1 and ANO2 isoform fragments with first strands from red and white skeletal musculature showed no signals for both ANO2 isoforms (Fig. 3c) but signals for both ANO1 isoforms (Fig. 3d). More specifically, ANO1-a isoform-specific primers predominantly amplified the DNA fragment from red muscle and ANO1-b-specific primers from white muscle. Furthermore, RT-PCR amplification by using two additional ANO2 pan-primer pairs confirmed the non-existence of ANO2 isoforms in skeletal muscle (Supplementary Fig. 2). In order to test the purity of the tissue sample preparations, positive control PCRs with DHPRα1S-a and DHPRα1S-b isoform-specific primers and identical first strands were performed (Fig. 3e). Since α1S-a and α1S-b isoforms are exclusively expressed in red and white muscle, respectively4, the respective weaker amplification products in Fig. 3d, e are merely due to cross-contaminated red and white muscle preparations from the relatively small-sized zebrafish. Hence, from the very similar amplification intensity profile of ANO1 and DHPRα1S isoforms (Fig. 3f) we can conclude that ANO1-a is predominantly expressed in the red and ANO1-b in the white skeletal musculature of zebrafish.
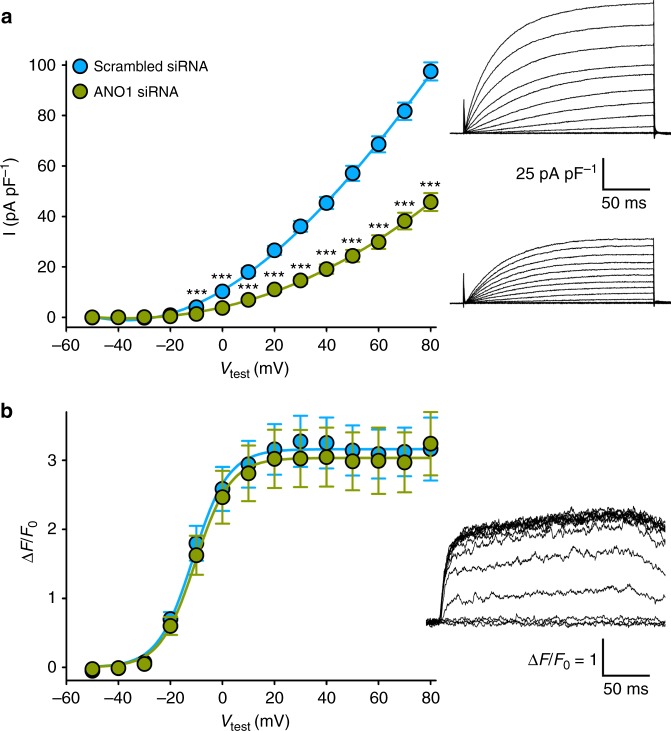
To confirm that ANO1 isoforms are indeed the channels responsible for Ca2+-activated Cl− conductance in zebrafish skeletal muscle, we implemented a short interfering (siRNA) knock-down strategy previously described to work well in zebrafish42,43, since zebrafish ANO1 KO model strains are not available to date. Using the BLOCK-iT™ RNAi Designer algorithm (ThermoFisher Scientific) we designed a series of siRNA expressing DNA constructs targeting the 5′-untranslated region as well as the open reading frame of both ANO1 isoforms. Out of the ten siRNAs expressed in normal zebrafish myotubes seven showed significant (P < 0.05) knock-down of Ca2+-activated Cl− currents from 20.6 to 53.1% (n = 8–34) compared to a control scrambled siRNA (n = 28) (see Method section). The strongest current knock-down (53.1 ± 3.6%, n = 34) was obtained with siRNA targeted against a region, showing 91% homology between ANO1-a and ANO1-b isoforms (nucleotide positions 2199–2219 and 2073–2093, respectively). In contrast to the highly significant (P < 0.001) ANO1 current reduction (Fig. 4a), SR Ca2+ release was indistinguishable (P > 0.05) from control scrambled siRNA expressing myotubes (Fig. 4b), indicating that the observed ANO1 current reduction cannot be attributed to putative siRNA off-target effects hampering SR Ca2+ release. Altogether, our results explicitly ascertain that the channel responsible for zebrafish skeletal muscle Ca2+-activated Cl− conductance is ANO1.
Fig. 4.
ANO1 isoforms are accountable for CaCC conductance in zebrafish skeletal muscle. a Plots of current–voltage relationship from normal myotubes expressing control scrambled siRNA (I = 97.47 ± 3.59 pA pF−1 at +80 mV, n = 28) or ANO1-specific (nt position 2073–2093; ANO1-b numbering) siRNA (I = 45.71 ± 3.52 pA pF−1 at +80 mV, n = 34) showing highly significant (P < 0.001) siRNA knock-down effects between test potentials of −10 to +80 mV. Representative recordings of corresponding ANO1 currents (250-ms pulse protocol) are depicted on the right. Scale bars, 50 ms (horizontal), 25 pA pF−1 (vertical). b Plots of voltage dependence recordings of maximal Ca2+ release from normal myotubes expressing control scrambled siRNA ((ΔF/F0)max = 3.12 ± 0.35, n = 16) or ANO1-specific siRNA ((ΔF/F0)max = 3.18 ± 0.40, n = 16). Representative SR Ca2+ release recording from a myotube expressing ANO1-specific siRNA is depicted on the right. Scale bars, 50 ms (horizontal), ΔF/F0 = 1 (vertical). Bars represent mean ± s.e.m.; ***P < 0.001 determined by unpaired Student’s t-test
ANO1 is localised in the sarcolemma
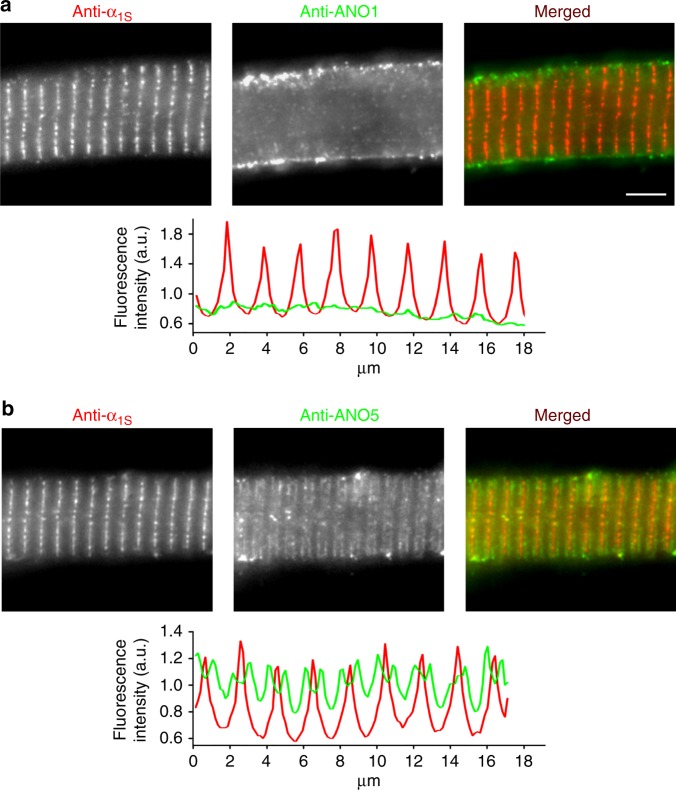
Since ANO1 is activated by SR Ca2+ release, which is initiated by the allosteric interaction between DHPR and RyR1, we next tested if ANO1 channels are located closely to these Ca2+ release sites in the triadic junctions or reside in the sarcolemma, or are rather distributed in both the subcellular domains. Immunolocalisation assays on zebrafish skeletal myotubes with anti-ANO1 and anti-DHPRα1S antibodies suggest that ANO1 does not reside in the triads like DHPRα1S, but is exclusively expressed in the sarcolemma (Fig. 5a).
Fig. 5.
ANO1 is localised in the zebrafish skeletal muscle surface membrane. Representative images of normal zebrafish myotubes double immunolabelled for DHPRα1S subunit (anti-α1S) and ANO1 (anti-ANO1) or ANO5 (anti-ANO5) isoforms showing their respective subcellular distribution. a ANO1 is expressed in the sarcolemma (centre image) and does not co-localise with DHPRα1S (left image) in the triads (merged image). Scale bar, 5 µm. Fluorescence intensity profile (bottom) obtained by measuring the average fluorescence intensity along a horizontal line on the merged image demonstrates regular t-tubular periodicity of the DHPRα1S (red) but a complete lack of a structured ANO1 signal (green). b Double immunofluorescence labelling of DHPRα1S (left image) and ANO5 (centre image) under similar conditions clearly revealed anti-ANO5 signal on both sides of the t-tubular membrane (merged image) and thus indicating SR membrane localisation of ANO5. Fluorescence intensity profile displays distinct but regular spatial periodicity with one red peak (DHPR signal) flanked by two green peaks (ANO5 signal)
To test for the accuracy of our fixation procedure and resolution of our immunolocalisation approach for ANO protein detection, we immunostained zebrafish myotubes for another member of the ANO family expected to be present in the skeletal muscles of all vertebrate species, namely ANO5 (Supplementary Fig. 1a). Several lines of evidence suggest that ANO5, expressed in skeletal muscles of human and mouse44 is essential for the development and maintenance of skeletal muscle45. Recessive mutations in ANO5 cause severe myopathies like gnathodiaphyseal dysplasia, GDD45,46, limb-girdle muscular dystrophy type-2L, LGMD2L47, and Miyoshi muscular dystrophy-3, MMD347,48. Immunolabeling of zebrafish myotubes with anti-ANO5 antibody showed a similar expression pattern as observed previously for some SR targeted proteins like calsequestrin and the Ca2+ sensor D1ER49–51. Consequently, we can conclude that ANO5 is expressed in the triadic SR membrane juxtaposing the t-tubules which were immunostained by anti-DHPRα1S antibody (Fig. 5b). The strong and clear signal of ANO5 in zebrafish myotubes suggests that with identical cell preparation, fixation, and fluorescent staining procedures ANO1 would have certainly been detected in the triads if it would not solely reside in the sarcolemma.
To further confirm the specificity of ANO1 surface expression, we performed immunolocalisation assays on zebrafish skeletal myotubes expressing siRNA 2073–2093 (ANO1-b numbering) which was most effective in knocking-down the ANO1 current (Fig. 4a). Quantification of the sarcolemmal ANO1 fluorescence signal showed a significant (P < 0.001) reduction of ANO1 in myotubes expressing the ANO1-specific shRNA construct (61.4 ± 1.8%, n = 128) compared to control myotubes (100.0 ± 3.7%, n = 161) expressing the scrambled shRNA construct (Supplementary Fig. 3). Altogether, results from the above experiments indicate the exclusive sarcolemmal localisation of ANO1 channels in zebrafish skeletal myotubes.
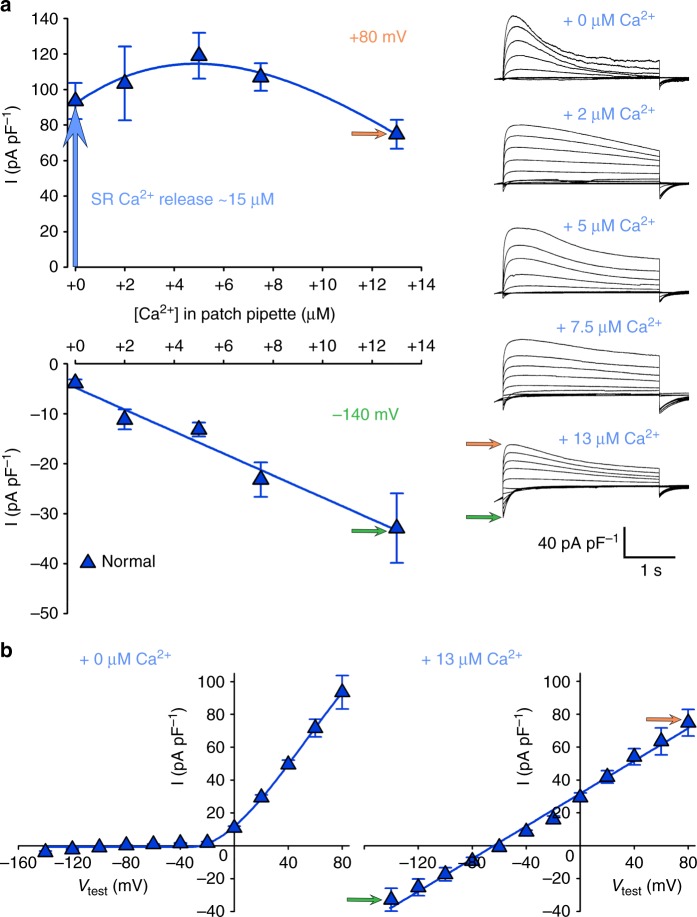
SR Ca2+ release awakes ANO1 outward current close to maximum
To elucidate if ANO1 currents are activated only marginally or to a substantial extent by ~ 15 µM of Ca2+ ions released during EC coupling52, we carried out ANO1 Ca2+-dependence experiments by raising the Ca2+ concentration in the cytosol via perfusion with the patch pipette solution. With a 3-s depolarisation protocol from −140 to +80 mV in 20-mV increments we observed that at +80 mV under normal SR Ca2+ release conditions (i.e., +0 Ca2+ addition) the ANO1 current reached 93.47 ± 10.15 pA pF−1 (n = 5), which is already ~80% of the maximum current yielded after cytosolic addition of +5 µM Ca2+ (118.97 ± 12.92 pA pF−1, n = 9) (Fig. 6a). While the ANO1 outward current, recorded at +80 mV shows a bell shaped Ca2+ dependence (Fig. 6a, upper graph), the ANO1 inward current at −140 mV displays a linear Ca2+ concentration–current relationship (Fig. 6a, lower graph). These specific current properties sufficiently explain the ANO1 current–voltage (I–V) relationship during depolarisation-induced SR Ca2+ release under physiological conditions (Fig. 6b, left graph). The cytosolic Ca2+ concentration at negative potentials, below or at the initiation of SR Ca2+ release around −40 mV (Supplementary Fig. 4a) is apparently too low to induce considerable ANO1 currents. Only intracellular addition of micromolar Ca2+ can elicit ANO1 inward currents at negative potentials (Fig. 6a, lower graph), which finally yields a linear I–V curve at +13 µM of Ca2+ addition (Fig. 6b, right graph).
Fig. 6.
ANO1 outward current is activated close to maximum by SR Ca2+ release. a Ca2+-concentration dependence experiments of ANO1 currents were performed by elevating cytosolic Ca2+ concentrations via perfusion with the patch pipette solution from the physiological level (+0, n = 5) to +2 (n = 5), +5 (n = 9), +7.5 (n = 5), and +13 µM (n = 8). Plots of Ca2+ dependence of the outward Cl− current at +80 mV (top graph) and Ca2+ dependence of the inward Cl− current at −140 mV (bottom graph) are shown. Vertical blue arrow indicates Cl− current stimulation by SR Ca2+ release, which raises cytosolic Ca2+ to ~ 15 µM52. Representative recordings of ANO1 currents (3-s pulse protocol) at different elevations of intracellular Ca2+ concentrations are depicted on the right. Scale bars, 1 s (horizontal), 40 pA pF−1 (vertical). b Current–voltage relationship under physiological conditions i.e., +0 Ca2+ addition (n = 5, left graph) and after addition of +13 µM Ca2+ to the cytosol (n = 8, right graph). Orange arrow indicates Imax at +80 mV and green arrow at −140 mV. Data are presented as mean ± s.e.m.; P determined by unpaired Student’s t-test
Zebrafish skeletal muscle ANO1 channel is a bona fide CaCC
Similar to mammalian ANO1 channels24,41, zebrafish skeletal muscle ANO1 is gated synergistically by intracellular Ca2+ ions and membrane potential, showing strong outward rectification at low intracellular Ca2+ concentrations—a biophysical hallmark of CaCCs. Under physiological conditions (+0 cytosolic Ca2+ addition), the skeletal muscle ANO1 I–V curve shows a zone of combined Ca2+- and voltage dependence between −40 and +10 mV and a pure voltage dependence zone between +10 and +80 mV, defined by the raising phase or plateau phase of SR Ca2+ release, respectively (Supplementary Fig. 4a). Outward rectification was still observed after cytosolic addition of low (+5 µM) Ca2+ ions via the patch pipette (Supplementary Fig. 4b). Regardless of whether Ca2+ ions were added to the cytosol of normal or relaxed myotubes, inward currents were very small compared to the respective outward currents and consequently lead to a pronounced nick in the I–V curves (Supplementary Fig. 4b). As expected, outward currents recorded from normal myotubes were more pronounced compared to relaxed myotubes, due to additional current amplification by SR Ca2+ release. Moreover, a linear I–V curve at high [Ca2+] (+13 µM Ca2+ addition) (Fig. 6b, right graph) depicts the competence of the skeletal muscle ANO1 channel for voltage-independent pore opening i.e., entry into a constitutively active mode.
Altogether, outwardly rectifying as well as ligand/voltage-gating properties of zebrafish skeletal muscle ANO1 channels classify them as bona fide voltage-sensitive Ca2+-activated Cl− channels, analogous to those expressed in mammalian gastrointestinal epithelia or smooth muscles40.
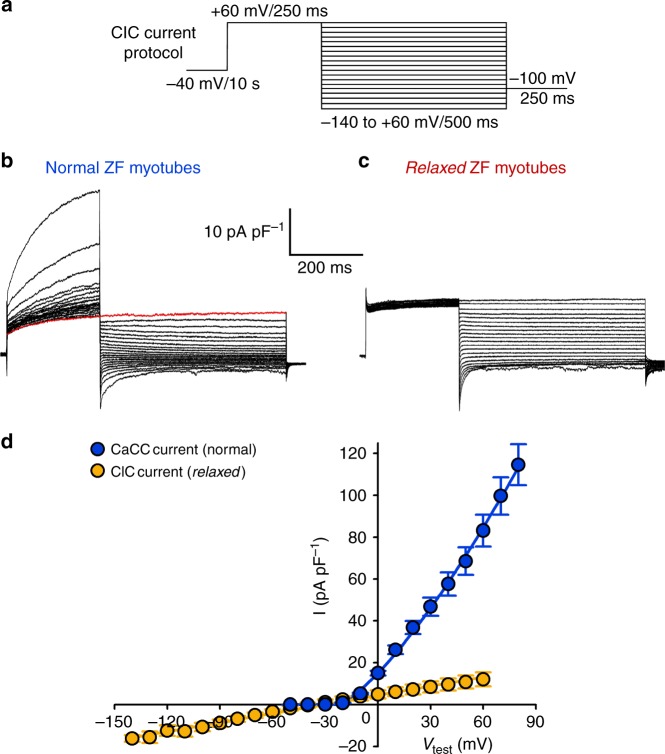
Zebrafish skeletal muscle ANO1 coexists with ClC
Did the evolutionary innovative concept of the Ca2+-activated Cl−-channel ANO1 expression in zebrafish skeletal muscle lead to extinction of the solely voltage-gated, canonical skeletal muscle Cl− channel ClC-1 found in mammals53–55? ClC-1 and ClC-2 mRNAs were previously identified in zebrafish skeletal muscle56 but putative ClC currents remained uncharacterised. To investigate this, we attempted to record ClC currents in zebrafish myotubes using a voltage-clamp protocol previously described for recording ClC-1 currents in mouse FDB fibres57. To fully activate ClC currents57, normal zebrafish myotubes were stimulated with a 250-ms prepulse from a holding potential of −40 to +60 mV (Fig. 7a) and as expected, we observed robust ANO1 currents (Fig. 7b, left trace). During the 500-ms test pulses to positive test potentials, the expected ‘contamination’ with ANO1 currents could be minimised by starting the recordings with negative test potentials and hence taking advantage of the pronounced ANO1 current rundown as depicted in Fig. 2d. These recording conditions allowed us to measure a current even at +60 mV in normal myotubes (red trace) which mainly reflects the ClC current (Fig. 7b). However, unquestionably non-contaminated ClC recordings could be harvested only from relaxed myotubes (Fig. 7c), where ANO1 was not activated due to the lack of SR Ca2+ release (Fig. 1c). Consistent with these findings, we observed a dramatic reduction in peak ClC current density (77% at −140 mV and 66% at +60 mV) in the presence of 1 mM 9AC, a blocker of ClC-1 channels57 (Supplementary Fig. 5).
Fig. 7.
Zebrafish skeletal muscle expresses ClC current beside ANO1 current. a To elicit ClC currents, a pulse protocol from −140 to +60 mV in 10-mV increments, preceded by a 250-ms prepulse to +60 mV to fully activate ClC currents, as described previously57 was used. b Representative recording from normal myotubes (n = 8) demonstrating ClC currents contaminated by ANO1 currents at positive test potentials. Minimisation of contamination in order to harvest close-to-accurate ClC current recordings at positive potentials was achieved by starting the recordings with negative test potentials and thus taking advantage of the ANO1-specific CamKII-induced current rundown. Red trace indicates the last current recorded at +60 mV. Scale bars, 200 ms (horizontal), 10 pA pF−1 (vertical). c Representative non-contaminated ClC recording from relaxed myotubes (n = 6), lacking the SR Ca2+ release-activated ANO1 currents. d Overlay of the clean ClC I–V curve from relaxed myotubes (n = 6) and the ANO1 I–V curve from normal myotubes (n = 5) (from Fig. 1d), displays the significant difference (P < 0.001) between ClC and ANO1 (CaCC) Cl− currents, starting from the test potential (Vtest) of 0 mV. At +60 mV, ClC displays I = 12.03 ± 3.38 pA pF−1 while ANO1 reaches I = 83.12 ± 7.62 pA pF−1. Data are presented as mean ± s.e.m.; P determined by unpaired Student’s t-test
As shown in Fig. 7d, the zebrafish ClC current displays a linear I–V relationship with an inward component at potentials below −40 mV and an outward current above −40 mV. Notably, in contrast to ANO1, the ClC current does not show outward rectification. ClC currents at potentials above −10 mV are significantly (P < 0.001) smaller than the corresponding ANO1 currents and hence might have only a minor contribution to the total skeletal muscle Cl− influx during membrane depolarisation. On the contrary, the ClC inward current component at negative potentials, accounting for 80% of the total membrane conductance in resting human muscle and thus ensuring electrical stability, is a characteristic of skeletal muscle ClC55. Lastly, this ClC inward current explains slight Cl− currents that we found in some of our experiments, like in ANO1-current free relaxed myotubes (Fig. 2b, d, right panel) or in normal myotubes at −140 mV without cytosolic Ca2+ addition (Fig. 6a, lower graph).
Skeletal muscle ANO1 current accelerates AP repolarisation
We were interested why the euteleost species zebrafish evolved such pronounced CaCC currents in skeletal muscle—a phenomenon that has not been reported from mammals. Our prime working hypothesis was that this CaCC current, that immediately follows EC coupling-induced SR Ca2+ release (Fig. 1a, Supplementary Fig. 4a), plays a role in acceleration of the skeletal muscle action potential (AP) termination. Conventionally, voltage-gated Na+ influx and delayed K+ efflux contribute to the depolarisation and repolarisation phase of an AP, respectively58. However, a number of studies in cardiac and neuronal tissues showed that also CaCC currents are involved in shaping of the AP repolarisation phase59–62. Application of the CaCC blocker 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS) or reduction of the Cl− concentration induced significant broadening of cardiac muscle APs in pig ventricular myocytes59 as well as in rabbit ventricular and atrial myocytes60,61. Broadening of AP width was also observed in hippocampal pyramidal neuronal slices upon ANO2 current reduction, either by applying CaCC blockers niflumic acid (NFA) and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) or by silencing ANO2 expression with short hairpin RNAs62.
Consequently, to investigate a putative role of the zebrafish skeletal muscle ANO1 in shaping of the AP repolarisation phase, we performed whole-cell current-clamp electrophysiology on relaxed and normal myotubes. Interestingly, congruent to our hypothesis, the AP½ width recorded from relaxed myotubes, which lack the SR Ca2+ release-activated CaCC current (Figs 1 and 2), was with 2.84 ± 0.28 ms (n = 13) nearly double as wide (P < 0.001) as recorded from normal myotubes with 1.60 ± 0.14 ms (n = 14) (Fig. 8a). However, the AP time to peak was comparable (P > 0.05) between relaxed and normal myotubes (1.28 ± 0.12 ms, n = 13 and 1.28 ± 0.07 ms, n = 14, respectively) (Fig. 8a). Finally, to confirm the results obtained from relaxed myotubes under standard Cl− conditions, we performed current-clamp recordings on normal myotubes under Cl−-free conditions (see Methods). The AP½ width (2.91 ± 0.57 ms, n = 10) as well as the AP time to peak (1.17 ± 0.05 ms, n = 10) was comparable (P > 0.05) between normal myotubes under Cl− free conditions and relaxed myotubes (Fig. 8b). Thus, both series of experiments strongly indicate that the ANO1 Cl− influx in zebrafish skeletal muscle plays a substantial role in modulating the AP by accelerating the repolarisation phase.
Fig. 8.
The ANO1 current accelerates skeletal muscle AP repolarisation. a Overlay of representative AP recordings from normal (dark blue) and relaxed (red) myotubes under standard external Cl− conditions (142 mM). Bar graphs depict AP time to peak and AP½ width of normal (n = 14) compared to relaxed (n = 13) myotubes. Scale bars, 5 ms (horizontal), 20 mV (vertical). b Overlay of representative AP recordings from normal myotubes in Cl−-free external solution ([Cl−] = 0) (light blue) and relaxed myotubes in standard external Cl− solution (red). Bar graphs show AP time to peak and AP½ width of normal myotubes in Cl−-free external solution (n = 10) compared to relaxed myotubes under standard Cl− conditions (n = 13). Bars represent mean ± s.e.m. and overlaying orange stars indicate individual data points; ***P < 0.001 determined by unpaired Student’s t-test
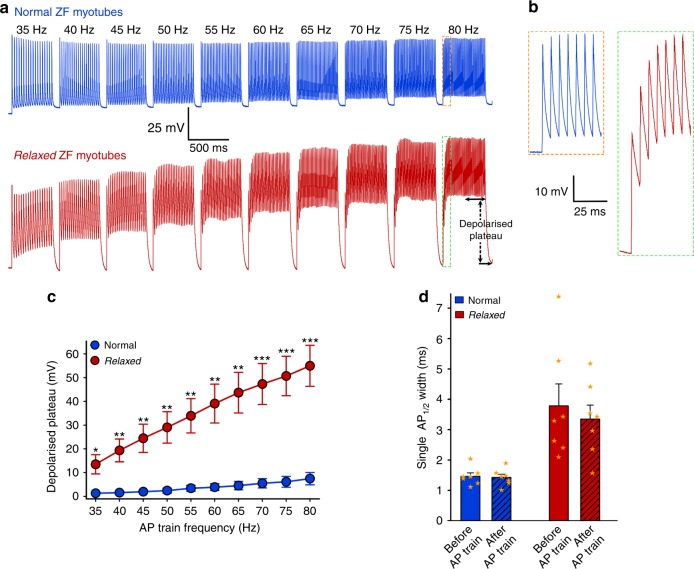
Only ANO1-current accelerated APs enable proper spike trains
Apparently, single APs have only limited importance in skeletal muscle functioning. Sustained muscle contractions are evoked only under conditions when motor neurons emit higher frequency APs, resulting in fusion of contractions (tetanic contraction). Muscle contractions start to sum up beyond stimulation frequencies of 5–20 Hz until the response forms a smooth ramped increase of tetanic contraction, which—depending on species and muscle type—reaches 90% of its maximum fusion rate (complete tetanus) at 20–60 Hz. Increased stimulation frequency leads to increased force production and a maximum force of 90% is generated around 60–80 Hz28–31. The usual firing rate of vertebrate motor neurons during voluntary muscle contraction is within this tetanic range32.
To investigate the physiological impact of the skeletal muscle AP width on trains of APs, we performed whole-cell current-clamp recordings in the physiological frequency range from normal and relaxed myotubes. Myotubes were subjected to increasing stimulation frequencies between 35 and 80 Hz, in 5-Hz increments to elicit 500-ms long trains of APs with 100-ms recovery intervals. As shown in Fig. 9, there are significant differences (P < 0.05) in the kinetics of AP trains at all spike frequencies between normal and relaxed myotubes. This is due to piling up (Fig. 9b) of the broader APs in relaxed myotubes (Fig. 9d), which leads to the development of a high depolarised plateau63, evidently because the sarcolemma is unable to adequately repolarise between the broadened APs. The formation of these depolarised plateaus can already be observed at lowest firing rates (Fig. 9a, c). On the contrary, depolarised plateaus—as the manifestation of membrane potential derailment in relaxed myotubes—hardly develop in normal myotubes even at the highest tested AP train frequencies (Fig. 9a, c). These results clearly demonstrate the crucial influence of the ANO1 current on the electrical membrane stability.
Fig. 9.
The ANO1 current facilitates proper skeletal muscle spike trains. a Representative recordings of 500-ms trains of APs from normal (upper traces) and relaxed (lower traces) myotubes at increasing stimulations frequencies from 35 to 80 Hz in 5-Hz increments with 100-ms inter-train intervals. Relaxed myotubes display pronounced depolarised plateaus in a frequency-dependent mode due to the summation of broader APs. For calculating the size of depolarised plateaus, the second half of the 500-ms AP trains (indicated by a horizontal double-headed arrow) was taken into consideration. Vertical dashed arrow symbolizes the size of the depolarised plateau at 80 Hz. Scale bars, 500 ms (horizontal), 25 mV (vertical). b Train of APs on an expanded time base showing piling up of broader APs in relaxed myotubes (red trace) compared to normal myotubes (blue trace) during the initial phase of the 80-Hz train. Scale bars, 25 ms (horizontal), 10 mV (vertical). c Depolarised plateau sizes were significantly different at all tested frequencies between relaxed (n = 8) and normal (n = 7) myotubes. d Mean single AP½ width before and after the AP train recordings from the same set of normal and relaxed myotubes. Mean AP½ width was indistinguishable (P > 0.05) when recorded before or after the trains of APs in normal (1.46 ± 0.11 ms; 1.43 ± 0.10 ms; n = 7) as well as in relaxed (3.79 ± 0.72 ms; 3.35 ± 0.46 ms; n = 7) myotubes. However, the difference in AP½ width was highly significant (P < 0.01) between normal and relaxed myotubes, regardless if recorded before or after the trains of APs. Data are presented as mean ± s.e.m. and overlaying orange stars indicate individual data points; *P < 0.05, **P < 0.01, ***P < 0.001 determined by unpaired Student’s t-test
Overall, our data show that zebrafish skeletal muscle contains at least two distinct Cl− channels, namely ANO1 and ClC. While ClC is most likely crucial for its canonical function of stabilising the sarcolemmal resting potential, it seems not to play a major role in shaping the AP because above −10 mV ClC currents are significantly (P < 0.001) smaller than ANO1 currents (Fig. 7d) and thus are not sufficient to support ANO1 in accelerating the AP repolarisation phase. Furthermore, this is supported by the striking similarity in AP kinetics (similar AP½ width) between normal myotubes under Cl− free recording conditions (where evidently neither ANO1 nor ClC can conduct Cl− ions) and relaxed myotubes under regular Cl− conditions (where ClC conductance is intact) (Fig. 8b).
Discussion
ANO1, a Ca2+-activated Cl− channel (CaCC) has never been reported to functionally express in skeletal muscle. In this study we identified and characterised the expression pattern, subcellular distribution and physiological role of ANO1 in zebrafish skeletal muscle. Immunocytochemical results provide direct evidence for the sarcolemmal localisation of ANO1 and RT-PCR amplification experiments revealed the expression profile of the two ANO1 isoforms, with ANO1-a in superficial slow/red and ANO1-b in deep fast/white skeletal musculature of zebrafish. Using a broad range of experiments, we characterised ANO1 as a bona fide CaCC which is gated synergistically by membrane potential and intracellular Ca2+ concentration and is activated close to maximum by the SR Ca2+ release during excitation-contraction coupling. Furthermore, ANO1 coexists with ClC in zebrafish skeletal muscle but only ANO1-current accelerated APs enable proper spike trains crucial for high speed muscle contractions. ClC seems not to play a role in shaping the AP, rather stabilises the sarcolemmal resting potential.
Subsuming these findings, we postulate a model (Fig. 10) where depolarisation-induced SR Ca2+ release, activated via the DHPR-RyR1 interaction, leads to more or less in-parallel binding of Ca2+ ions to (i) troponin C for initiation of muscle contraction and (ii) ANO1 for induction of a transient Cl− influx. This Ca2+-release-induced Cl− influx, together with the delayed K+ efflux via the voltage-gated (KV) channels, causes a quick drop in the membrane potential (Vm) during the decline phase of the AP, thereby repolarising the sarcolemma and returning the electrochemical gradient to the resting state. Due to the rapid decline of cytosolic Ca2+ by the fast pumping back action of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA)64, ANO1 is prohibited from Cl− efflux at negative membrane potentials and thus is strictly outwardly rectifying. The resulting short-term APs, due to the ANO1-KV synergistic action, are evidently the basis of electrical membrane stability that enables accelerated muscle stimulation rates for high speed (tetanic) muscle contractions with increased force production28–32. Altogether, this is crucial for high speed swimming which is vital in the aquatic pray-predator context. Apparently, the phylogenetically highest advanced skeletal muscles of euteleost species4,5, like zebrafish, developed this innovative ‘high-speed gear’ by adding ANO1-mediated Ca2+ release-induced Cl− influx to the skeletal muscle EC coupling machinery.
Fig. 10.
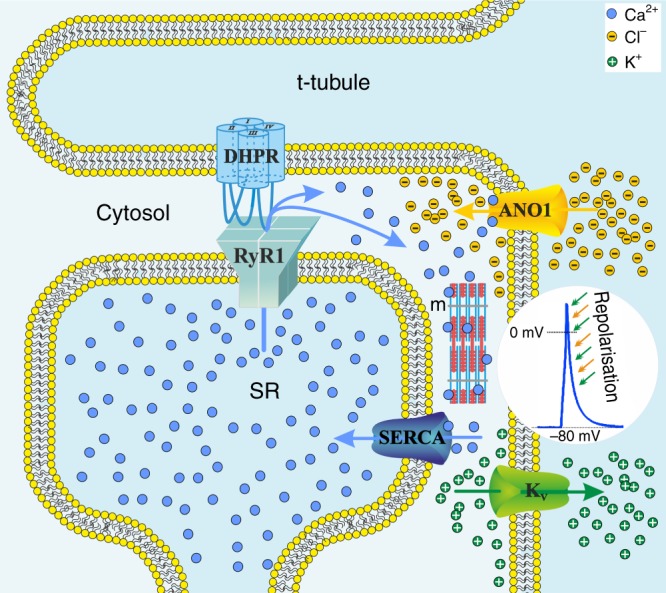
Model of ANO1 activation by SR Ca2+ release as the basis for acceleration of AP repolarisation. Schematic representation of the skeletal muscle triad with sarcolemmal t-tubular invagination (t-tubule) adjacent to the sarcoplasmic reticulum Ca2+ store (SR) and membrane localisation of some selected channels and pumps involved in cytosolic (Cytosol) Ca2+ handling. Initially, depolarisation-induced conformational changes in the DHPR are transmitted to the RyR1, which leads to pore opening and release of Ca2+ ions (blue spheres) from the SR stores. Cytosolic Ca2+ activates contraction of muscle fibres (m) and also binds to intracellular ANO1 Ca2+ binding sites19, to activate massive Cl− influx (yellow spheres) into the cytosol. Cl− influx together with simultaneous K+ efflux (green spheres) via the voltage-gated K+ channels (KV), synergistically and rapidly reduces the membrane potential to accelerate AP repolarisation (inset, green and yellow arrows). Finally, the SERCA pumps back Ca2+ into the SR and thus resetting the system
Future investigations on a double transgenic zebrafish strain that selectively expresses fluorescent proteins mCherry and GFP in slow/red and fast/white muscles, respectively, will enable us to gain deeper insights into this mechanism of AP acceleration by studying the putatively distinct input of the muscle-type specific (Fig. 3) ANO1 isoforms, identified in this study. According to our model, AP acceleration is expected to be more pronounced in the fast/white musculature which is dedicated to burst activities and thus dependent on high frequency activation.
Methods
Zebrafish care
Care and maintenance of adult zebrafish, wild-type (wt) and heterozygous for the DHPRβ1-null mutation relaxed (redts25)27, obtained from the Max Planck Institute (Tübingen, Germany), was according to the established procedures65,66 and was approved by the Tierethik-Beirat of the Medical University of Innsbruck and Bundesministerium für Wissenschaft, Forschung und Wirtschaft.
RT-PCR detection
Total RNA was extracted from superficial slow (red) and deep fast (white) skeletal muscle of multiple adult zebrafish individually, using the RNeasy Mini Kit (Qiagen) and reverse-transcribed using random primers and M-MLV reverse transcriptase (Promega). PCR primer sequences for amplification of DNA fragments from the two isoforms of ANO1 and ANO2, as well as from DHPRα1S-a and α1S-b subunits, used as positive controls to determine the purity of the muscle tissue preparations4, were designed according to the sequences deposited in GenBank database (Supplementary Table 1). Quantification of the band intensities was done using ImageJ (open source). Identity and fidelity of the RT-PCR products was confirmed by sequence analysis (Eurofins, Germany).
Primary culture of myotubes
Myoblasts from 2-dpf relaxed zebrafish, homozygous or heterozygous for the DHPRβ1-null mutation27, or wt zebrafish were isolated and cultured as described67. Homozygous relaxed mutants were identified by their inability to move in response to tactile stimulation and motile ‘normal’ siblings (heterozygous and wt) were used as controls. Myotubes were cultured for 4–6 days in a humidified 28.5 °C incubator in L-15 medium supplemented with 3% foetal calf serum, 3% horse serum, 4 mM L-glutamine and 4 U/ml penicillin/streptomycin.
siRNA knock-down
Short hairpin RNA (shRNA) constructs that are intracellularly processed into short interfering RNAs (siRNAs), making them putatively suitable to inhibit gene expression, were designed according to described procedures for zebrafish42,43. 21 nucleotides (nt) long targeting sequences against ANO1-a and ANO1-b RNA were designed using BLOCK-iT™ RNAi Designer software (https://rnaidesigner.thermofisher.com/rnaiexpress/) and only hits with the highest score (5 stars) were considered. The siRNA sequences used were as follows: −156 to −136, 1725–1745 (ANO1-a nt numbering); 653–673, 914–934, 1434–1454, 1496–1516, 1593–1613, 2073–2093, 2271–2291, and 2327–2347 (ANO1-b nt numbering). Knock-down effects of ANO1-targeting sequences were compared to a scrambled control sequence (TCACAATAGTACCAAGCATGA) that showed no homology when blasted to the zebrafish genome (https://blast.ncbi.nlm.nih.gov/Blast.cgi). For construction of a plasmid apt for expressing siRNAs under the control of a CMV promoter, the SalI-NotI DNA fragment containing the original zebrafish miR30 hairpin region from plasmid pmE-actin-exon+intron-miR30-CFP43 was inserted into the corresponding polylinker sites of expression vector pCI-neo (Promega). Following deletion of the BbsI RE site in the pCI-neo backbone by replacing nts G957/A958 with TT via fusion PCR, the ANO1 or control scrambled siRNA target sequences could be inserted into the corresponding BbsI sites of the miR30 hairpin region. Sense and antisense 71-mer shRNA-coding oligonucleotides in a typical passenger-loop-guide assembly42,43 were synthesised and annealed at a decreasing temperature gradient. Integrity of all DNA constructs was confirmed by sequence analysis (Eurofins, Germany). 1-dpf wt zebrafish myoblasts were transfected with shRNA constructs using AMAXATM nucleofector kit34 and subsequently the cultured myotubes were used for immunostaining and electrophysiological recordings34,67.
Immunostaining
Six days old cultured normal myotubes were washed with 1x PBS, fixed in 4% paraformaldehyde both supplemented with 100 µM N-benzyl-p-toluene sulphonamide (BTS) and blocked by incubating in 5% goat or rabbit serum, as described in detail34. Primary and secondary antibodies, diluted in PBS supplemented with 0.2% BSA and Triton X-100 were as follows: mouse monoclonal antibody 1A against DHPRα1S (1:2,000, MA3-920, Affinity Bioreagents), rabbit polyclonal anti-ANO1 (1:50, ab84115, Abcam), goat polyclonal anti-ANO5 (1:500, sc-169628, Santa Cruz), rabbit anti-GFP (1:5,000, A11122, Invitrogen), secondary goat anti-mouse Alexa Fluor 594, goat anti-rabbit Alexa Fluor 488 (both 1:4,000, A11032 and A11034, respectively, Invitrogen), rabbit anti-goat Cy3 and rabbit anti-mouse FITC (1:500, C2821, and 1:2,000, F9137, respectively, Sigma).
Images were recorded with a cooled CCD camera (Diagnostic Instruments) and MetaVue image processing software (v 6.2, Universal Imaging, PA). Quantification of ANO1 surface membrane expression after siRNA knock-down was determined by measuring the average fluorescence intensity along the periphery of CFP positive myotubes, obtained from at least two different cultures using the MetaVue software.
Voltage-clamp electrophysiology
CaCC currents were recorded from cultured myotubes simultaneously with intracellular SR Ca2+ release by using 0.2 mM Fluo-4 in the patch pipette (internal) solution, and were evoked by a 200-ms pulse protocol from +80 to −50 mV in 10-mV steps from a holding potential of −80 mV, as described67. BTS Myosin-II blocker (100 µM) was continuously present in the bath (external) solution. The standard external solution, with a total Cl− concentration of 165 mM, contained (in mM): 10 CaCl2, 145 TEA-Cl and 10 HEPES, pH 7.4 with TEA-OH. For Cl− free external solution, 10 mM CaCl2 and 145 mM TEA-Cl were replaced by 10 mM Ca(OH)2 and 100 mM Aspartate, respectively. The standard Cl− containing internal solution used was as follows (in mM): 145 Cs-aspartate, 2 MgCl2, 10 HEPES, 0.1 Cs2-EGTA, and 2 Mg-ATP (pH 7.4 with CsOH). The Cl− free internal solution contained (in mM): 100 Cs-aspartate, 10 HEPES, 0.5 Cs2-EGTA, and 3 Mg-ATP (pH 7.4 with CsOH).
To test the integrity of CaCC in the DHPRβ1-null relaxed zebrafish, recordings of CaCC currents following caffeine-induced SR Ca2+ store depletion were performed. From a holding potential of −80 mV, 2-dpf myotubes were voltage-clamped at +40 mV for 100 ms followed by a 100-ms step to −120 mV (Fig. 2a). The entire protocol was repeated 100 times and between the 15th and 45th sweep the myotubes were perfused with the standard bath solution supplemented with 8 mM of the RyR agonist caffeine (Merck). Changes in CaCC currents at +40 mV (outward current) and at −120 mV (inward current) before and after application of caffeine were analysed.
To investigate the Ca2+-dependence of CaCC currents, intracellular Ca2+ concentrations were altered via the patch pipette solution. Using the MaxChelator simulation program (http://maxchelator.stanford.edu), pipette solutions for free Ca2+ concentrations ([Ca2+]) of 0, 2, 5, 7.5 and 13 μM were adjusted (Supplementary Table 2). Due to the unknown kinetics of the CaCC currents under different Ca2+ concentrations contributed by the patch pipette solution in addition to the normal SR Ca2+ release, a prolonged 3-s pulse protocol from +80 to −140 mV in 20-mV steps was used.
ClC currents were recorded by applying the voltage-step protocol described by57 and depicted in Fig. 7a, using standard Cl−-containing external and internal solutions. To confirm that the Cl− current recordings from relaxed myotubes are indeed currents through the skeletal muscle ClC channel, 1 mM 9-anthracene carboxylic acid (9AC), a blocker of ClC-1 channels57 was added to the external recording solution.
All recordings were performed at room temperature in the whole-cell configuration using the Axopatch 200B amplifier (Axon Instruments Inc., CA), filtered at 1 kHz and digitised at 5 kHz. Data were analysed using ClampFit (v10.0; Axon Instruments) and SigmaPlot (v10.0; Systat Software, Inc.).
Current-clamp electrophysiology
Action potentials (APs) were recorded from single myotubes in a perforated patch configuration by using 120 µg/ml of amphotericin B in the patch pipette solution under standard Cl− and Cl−-free conditions. The standard Cl−-containing bath solution used was as follows (in mM): 130 NaCl, 4 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose (pH 7.4 with NaOH). For Cl−-free bath solution NaCl, KCl, MgCl2 and CaCl2 were replaced by Na-aspartate, K-aspartate, Mg-aspartate and Ca(OH)2, respectively. The standard Cl− containing internal solution consisted of (in mM): 135 K-aspartate, 8 NaCl, 2 MgCl2, 20 HEPES, and 5 EGTA (pH 7.4 with NaOH). For Cl− free internal solution NaCl and MgCl2 were replaced by Na-aspartate and Mg-aspartate, respectively. Recordings were initiated after amphotericin B lowered the access resistance below 15 MΩ. A small hyperpolarising current was injected to set the membrane potential to −80 mV and APs were elicited by injecting a current of 3.8 nA for 1 ms. Identical current injections were applied to record 500-ms trains of APs from 35 to 80 Hz, in 5-Hz increments bracketed by 100-ms recovery intervals. Data were acquired with an EPC10 amplifier (HEKA Elektronik, Germany) at a sampling rate of 30 kHz, low-pass-filtered at 3 kHz and analysed using FitMaster (v2x73.2, HEKA Elektronik, Germany) and SigmaPlot 10.0 (Systat Software, Inc.).
General experimental design and statistical analyses
Sample sizes of zebrafish myotubes or tissues are based on previous publications4,5,27,34,67, hence, power calculations were not necessary. For siRNA transfection or drug administration assays, myotube cultures according to genotype were arbitrarily allocated to the specific sample groups without the use of an explicit randomisation procedure. Experiments did not require blinding and thus were not performed under blinded conditions. In our study, no data points or samples were excluded from analysis. Based on our previous publications4,5,27,30,33,34,67 all statistical analyses are considered appropriate. n-values represent the number of independent experiments on zebrafish myotubes or tissues, as specified. Variance is similar between comparison groups. All results are expressed as means ± s.e.m. Statistical significance was determined by using unpaired Student’s t-test. P < 0.05 was considered statistically significant and * indicates P < 0.05, **P < 0.01, and ***P < 0.001.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Dr Petronel Tuluc for support in establishing current-clamp recordings and analysis, Dr Hazel Sive for the miR30 backbone clone, Birgit Kagerbauer and Robert B. Janssen for excellent technical assistance. This study was supported by the Austrian Science Fund (FWF) Grant (P23229-B09) and graduate program (W1101-B12) to M.G. and P27392-B21 to M.G. and A.D.
Author contributions
A.D. and M.G. designed research; S.F.N., A.D. and M.G. performed experiments; A.D. and M.G. wrote the manuscript.
Data availability
Data supporting the findings of this study are available from the corresponding authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contributions to the peer review of this work. Peer Reviewer Reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Anamika Dayal, Shu Fun J. Ng.
Contributor Information
Anamika Dayal, Email: anamika.dayal@i-med.ac.at.
Manfred Grabner, Email: manfred.grabner@i-med.ac.at.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-07918-z.
References
- 1.Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(β-aminoethyl ether)-N,N′-tetracetic acid. Biochim. Biophys. Acta. 1972;267:605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- 2.Schneider MF, Chandler WK. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973;242:244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- 3.Rios E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987;325:717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- 4.Schredelseker J, Shrivastav M, Dayal A, Grabner M. Non-Ca2+-conducting Ca2+ channels in fish skeletal muscle excitation-contraction coupling. Proc. Natl Acad. Sci. USA. 2010;107:5658–5663. doi: 10.1073/pnas.0912153107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrötter K, Dayal A, Grabner M. The mammalian skeletal muscle DHPR has larger Ca2+ conductance and is phylogenetically ancient to the early ray-finned fish sterlet (Acipenser ruthenus) Cell Calcium. 2017;61:22–31. doi: 10.1016/j.ceca.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirata H, et al. Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease. Development. 2007;134:2771–2781. doi: 10.1242/dev.004531. [DOI] [PubMed] [Google Scholar]
- 7.Meyer A, Van de Peer Y. From 2R to 3R: evidence for a fish-specific genome duplication (FSGD) Bioessays. 2005;27:937–945. doi: 10.1002/bies.20293. [DOI] [PubMed] [Google Scholar]
- 8.Glasauer SM, Neuhauss SC. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. Genom. 2014;289:1045–1060. doi: 10.1007/s00438-014-0889-2. [DOI] [PubMed] [Google Scholar]
- 9.Mayer M. A calcium‐activated chloride current generates the after‐depolarisation of rat sensory neurones in culture. J. Physiol. 1985;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarran R, et al. Regulation of murine airway surface liquid volume by CFTR and Ca2+-activated Cl− conductances. J. Gen. Physiol. 2002;120:407–418. doi: 10.1085/jgp.20028599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulley S, Jaggar JH. Cl- channels in smooth muscle cells. Pflüg. Arch. 2014;466:861–872. doi: 10.1007/s00424-013-1357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caputo A, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YD, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 15.Milenkovic VM, Brockmann M, Stöhr H, Weber BH, Strauss O. Evolution and functional divergence of the anoctamin family of membrane proteins. BMC Evol. Biol. 2010;10:319. doi: 10.1186/1471-2148-10-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallah, G. et al. TMEM16A(a)/anoctamin-1 shares a homodimeric architecture with CLC chloride channels. Mol. Cell. Proteomics10, 10.1074/mcp.M110.004697 (2011). [DOI] [PMC free article] [PubMed]
- 17.Sheridan JT, et al. Characterisation of the oligomeric structure of the Ca2+-activated Cl− channel Ano1/TMEM16A. J. Biol. Chem. 2011;286:1381–1388. doi: 10.1074/jbc.M110.174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeng G, Aggarwal M, Yu WP, Chen TY. Independent activation of distinct pores in dimeric TMEM16A channels. J. Gen. Physiol. 2016;148:393–404. doi: 10.1085/jgp.201611651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim NK, Lam AK, Dutzler R. Independent activation of ion conduction pores in the double-barreled calcium-activated chloride channel TMEM16A. J. Gen. Physiol. 2016;148:375–392. doi: 10.1085/jgp.201611650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 21.Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature. 2014;516:207–212. doi: 10.1038/nature13984. [DOI] [PubMed] [Google Scholar]
- 22.Paulino, C. et al. Structural basis for anion conduction in the calcium-activated chloride channel TMEM16A. eLife6, 10.7554/eLife.26232 (2017). [DOI] [PMC free article] [PubMed]
- 23.Arreola J, Melvin JE, Begenisich T. Activation of calcium-dependent chloride channels in rat parotid acinar cells. J. Gen. Physiol. 1996;108:35–47. doi: 10.1085/jgp.108.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao Q, et al. Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop. Proc. Natl Acad. Sci. USA. 2011;108:8891–8896. doi: 10.1073/pnas.1102147108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilius B. Properties of volume-regulated anion channels in mammalian cells. Prog. Biophys. Mol. Biol. 1997;68:69–119. doi: 10.1016/S0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- 26.Kuruma A, Hartzell HC. Bimodal control of a Ca2+-activated Cl− channel by different Ca2+ signals. J. Gen. Physiol. 2000;115:59–80. doi: 10.1085/jgp.115.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schredelseker J, et al. The β1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc. Natl Acad. Sci. USA. 2005;102:17219–17224. doi: 10.1073/pnas.0508710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe S, Kitawaka T, Oka H. Mathematical equation of fusion index of tetanic contraction of skeletal muscles. J. Electromyogr. Kinesiol. 2010;20:284–289. doi: 10.1016/j.jelekin.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Mròwczynski W, Celichowski J, Krutki P. Interspecies differences in the force-frequency relationship pf the medial gastrocnemius motor units. J. Physiol. Pharmacol. 2006;57:491–501. [PubMed] [Google Scholar]
- 30.Dayal A, et al. The Ca2+ influx through the mammalian skeletal muscle dihydropyridine receptor is irrelevant for muscle performance. Nat. Commun. 2017;8:475. doi: 10.1038/s41467-017-00629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altringham JD, Johnston IA. The mechanical properties of polyneuronally innervated, myotomal muscle fibres isolated from a teleost fish (Myoxocephalus Scorpius) Pflüg. Arch. 1988;412:524–529. doi: 10.1007/BF00582542. [DOI] [PubMed] [Google Scholar]
- 32.Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. J. Neurophys. 1977;40:1432–1443. doi: 10.1152/jn.1977.40.6.1432. [DOI] [PubMed] [Google Scholar]
- 33.Weiss RG, et al. Functional analysis of the R1086H malignant hyperthermia mutation in the DHPR reveals an unexpected influence of the III-IV loop on skeletal muscle EC coupling. Am. J. Physiol. Cell. Physiol. 2004;287:C1094–C1102. doi: 10.1152/ajpcell.00173.2004. [DOI] [PubMed] [Google Scholar]
- 34.Schredelseker J, Dayal A, Schwerte T, Franzini-Armstrong C, Grabner M. Proper restoration of excitation-contraction coupling in the dihydropyridine receptor β1-null zebrafish relaxed is an exclusive function of the β1a subunit. J. Biol. Chem. 2009;284:1242–1251. doi: 10.1074/jbc.M807767200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis AJ, et al. Expression profile and protein translation of TMEM16A in murine smooth muscle. Am. J. Physiol. Cell. Physiol. 2010;299:C948–C959. doi: 10.1152/ajpcell.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manoury B, Tamuleviciute A, Tammaro P. TMEM16A/Anoctamin 1 protein mediates calcium-activated chloride currents in pulmonary arterial smooth muscle cells. J. Physiol. 2010;588:2305–2314. doi: 10.1113/jphysiol.2010.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YX, Kotlikoff MI. Inactivation of calcium-activated chloride channels in smooth muscle by calcium/calmodulin-dependent protein kinase. Proc. Natl Acad. Sci. USA. 1997;94:14918–14923. doi: 10.1073/pnas.94.26.14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenwood IA, Ledoux J, Leblanc N. Differential regulation of Ca2+-activated Cl− currents in rabbit arterial and portal vein smooth muscle cells by Ca2+-calmodulin-dependent kinase. J. Physiol. 2001;534:395–408. doi: 10.1111/j.1469-7793.2001.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berg J, Yang H, Jan LY. Ca2+-activated Cl− channels at a glance. J. Cell. Sci. 2012;125:1367–1371. doi: 10.1242/jcs.093260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins) Physiol. Rev. 2014;94:419–459. doi: 10.1152/physrev.00039.2011. [DOI] [PubMed] [Google Scholar]
- 41.Ma KE, Wang H, Yu J, Wei M, Xiao Q. New Insights on the Regulation of Ca2+-Activated Chloride Channel TMEM16A. J. Cell. Physiol. 2017;232:707–716. doi: 10.1002/jcp.25621. [DOI] [PubMed] [Google Scholar]
- 42.Dong, M. et al. Heritable and lineage-specific gene knockdown in zebrafish embryo. PLoS. ONE. 4, 10.1371/journal.pone.0006125 (2009). [DOI] [PMC free article] [PubMed]
- 43.De Rienzo G, Gutzman JH, Sive H. Efficient shRNA-mediated inhibition of gene expression in zebrafish. Zebrafish. 2012;9:97–107. doi: 10.1089/zeb.2012.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, et al. Genetic disruption of Ano5 in mice does not recapitulate human ANO5-deficient muscular dystrophy. Skelet. Muscle. 2015;21:43. doi: 10.1186/s13395-015-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marconi C, et al. A novel missense mutation in ANO5/TMEM16E is causative for gnathodiaphyseal dyplasia in a large Italian pedigree. Eur. J. Hum. Genet. 2013;21:613–619. doi: 10.1038/ejhg.2012.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsutsumi S, et al. The novel gene encoding a putative transmembrane protein is mutated in gnathodiaphyseal dysplasia (GDD) Am. J. Hum. Genet. 2004;74:1255–1261. doi: 10.1086/421527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolduc V, et al. Recessive mutations in the putative Ca2+-activated Cl− channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am. J. Hum. Genet. 2010;86:213–221. doi: 10.1016/j.ajhg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linssen WH, et al. Genetic heterogeneity in Miyoshi-type distal muscular dystrophy. Neuromuscul. Disord. 1998;8:317–320. doi: 10.1016/S0960-8966(98)00020-0. [DOI] [PubMed] [Google Scholar]
- 49.Franzini-Armstrong C, Kenney LJ, Varriano-Marston E. The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J. Cell. Biol. 1987;105:49–56. doi: 10.1083/jcb.105.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franzini‐Armstrong C, Protasi F, Ramesh V. Comparative ultrastructure of Ca2+ release units in skeletal and cardiac muscle. Ann. N. Y. Acad. Sci. 1998;853:20–30. doi: 10.1111/j.1749-6632.1998.tb08253.x. [DOI] [PubMed] [Google Scholar]
- 51.Canato M, et al. Massive alterations of sarcoplasmic reticulum free Ca2+ in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe. Proc. Natl Acad. Sci. USA. 2010;107:22326–22331. doi: 10.1073/pnas.1009168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baylor S, Hollingworth S. Sarcoplasmic reticulum Ca2+ release compared in slow‐twitch and fast‐twitch fibres of mouse muscle. J. Physiol. 2003;551:125–138. doi: 10.1113/jphysiol.2003.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aromataris EC, Rychkov GY. ClC-1 chloride channel: matching its properties to a role in skeletal muscle. Clin. Exp. Pharmacol. Physiol. 2006;33:1118–1123. doi: 10.1111/j.1440-1681.2006.04502.x. [DOI] [PubMed] [Google Scholar]
- 54.Stölting G, Fischer M, Fahlke C. CLC channel function and dysfunction in health and disease. Front. Physiol. 2014;5:378. doi: 10.3389/fphys.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pedersen TH, de Paoli F, Nielsen OB. Increased excitability of acidified skeletal muscle: role of chloride conductance. J. Gen. Physiol. 2005;125:237–246. doi: 10.1085/jgp.200409173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang YF, Yan JJ, Tseng YC, Chen RD, Hwang PP. Molecular physiology of an extra-renal Cl− uptake mechanism for body fluid Cl− homeostasis. Int. J. Biol. Sci. 2015;11:1190–1203. doi: 10.7150/ijbs.11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lueck JD, Rossi AE, Thornton CA, Campbell KP, Dirksen RT. Sarcolemmal-restricted localization of functional ClC-1 channels in mouse skeletal muscle. J. Gen. Physiol. 2010;136:597–613. doi: 10.1085/jgp.201010526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li GR, Sun H, To J, Tse HF, Lau CP. Demonstration of Ca2+-activated transient outward Cl− current and delayed rectifier K+ currents in Swine atrial myocytes. J. Mol. Cell. Cardiol. 2004;36:495–504. doi: 10.1016/j.yjmcc.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Guo D, et al. Ca2+-activated Cl− current contributes to action potential alternations in left ventricular hypertrophy rabbit. Am. J. Physio. Heart Circ. Physiol. 2008;64:H97–H104. doi: 10.1152/ajpheart.01032.2007. [DOI] [PubMed] [Google Scholar]
- 61.Kanaporis G, Blatter LA. Ca2+‐activated Cl− current determines action potential morphology during Ca2+ alternans in atrial myocytes. J. Physiol. 2016;594:699–714. doi: 10.1113/JP271887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang WC, et al. Calcium-activated chloride channels (CaCCs) regulate action potential and synaptic response in hippocampal neurons. Neuron. 2012;74:179–192. doi: 10.1016/j.neuron.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JH, Kushmerick C, Gersdorff H. Presynaptic resurgent Na+ currents sculpt the action potential waveform and increase firing reliability at a CNS nerve terminal. J. Neurosci. 2010;17:15479–15490. doi: 10.1523/JNEUROSCI.3982-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chemaly ER, Troncone L, Lebeche D. SERCA control of cell death and survival. Cell Calcium. 2018;69:46–61. doi: 10.1016/j.ceca.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westerfield, M. The Zebrafish Book: A Guide For The Laboratory Use Of Zebrafish, Danio rerio. 4th edn (Univ of Oregon Press, 2000).
- 66.Nüsslein-Volhard, C. & Dahm, R. Zebrafish (Oxford. Univ. Press, 2002).
- 67.Dayal A, Schredelseker J, Franzini-Armstrong C, Grabner M. Skeletal muscle excitation-contraction coupling is independent of a conserved heptad repeat motif in the C-terminus of the DHPRβ1a subunit. Cell Calcium. 2010;47:500–506. doi: 10.1016/j.ceca.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available from the corresponding authors upon reasonable request.