Abstract
Pachychoroid is a relatively novel concept describing a phenotype characterized by attenuation of the choriocapillaris overlying dilated choroidal veins, and associated with progressive retinal pigment epithelium dysfunction and neovascularization. The emphasis in defining pachychoroid-related disorders has shifted away from simply an abnormally thick choroid (pachychoroid) toward a detailed morphological definition of a pathologic state (pachychoroid disease) with functional implications, which will be discussed in this review. Several clinical manifestations have been described to reside within the pachychoroid disease spectrum, including central serous chorioretinopathy, pachychoroid pigment epitheliopathy, pachychoroid neovasculopathy, polypoidal choroidal vasculopathy/aneurysmal type 1 neovascularization, focal choroidal excavation, peripapillary pachychoroid syndrome. These conditions all exhibit the characteristic choroidal alterations and are believed to represent different manifestations of a common pathogenic process. This review is based on both the current literature and the clinical experience of our individual authors, with an emphasis on the clinical and imaging features, management considerations, as well as current understanding of pathogenesis of these disorders within the context of the recent findings related to pachychoroid disease.
Subject terms: Macular degeneration, Outcomes research
摘要
肥厚型脉络膜疾病是近年来提出的新概念, 其描述了一类特征为脉络膜毛细血管层的静脉扩张, 并且与进行性视网膜色素上皮功能障碍和新生血管的形成有关的一类疾病。定义肥厚型脉络膜相关疾病的重点已经从简单的脉络膜厚度异常 (脉络膜肥厚) 转向影响功能的病理状态 (“肥厚型脉络膜疾病”) 的详细形态学定义, 这将在本综述中详细讨论。属于肥厚型脉络膜疾病谱的疾病, 包括中心性浆液性脉络膜视网膜病变, 肥厚型脉络膜色素上皮病变, 肥厚型脉络膜新生血管病变, 息肉状脉络膜血管病变/1型动脉瘤新生血管, 局灶性脉络膜凹陷, 视盘周围毛细脉络膜肥厚综合症。这些病症都表现出特征性的脉络膜改变, 并且被认为代表了常见致病过程的不同临床表现。 本综述基于目前已发表的文献和作者自身的临床经验以及最近在肥厚型脉络疾病的相关研究结果, 重点强调了临床表现、影像学特征、治疗要点以及目前对这些疾病发病机制的探讨。
Method
This comprehensive literature review was performed based on a search of peer-reviewed published papers relevant to the pachychoroid disease spectrum according to our current knowledge, up to January 2018, available on the PubMed database. This review will highlight clinical and imaging features, pathogenesis, and management options. Remaining areas of controversy will be discussed and how future research may clarify these.
Advances in imaging technology over the past decade have led to new insights and understanding of changes within the choroid in diseases previously identified predominantly by their retinal manifestations [1, 2]. Hyperpermeable and dilated choroidal vessels have been observed using indocyanine green angiography (ICGA) in eyes with central serous chorioretinopathy (CSC) and polypoidal choroidal vasculopathy (PCV) [3]. With the advent of enhanced-depth imaging optical coherence tomography (EDI-OCT) and subsequently swept-source optical coherence tomography (SS-OCT) [4, 5], increased choroidal thickness was also described. The terms pachychoroid and, subsequently, pachychoroid disease were introduced to describe a phenotype characterized by focal or diffuse increase in choroidal thickness, accounted for by dilated choroidal vessels in Haller’s layer (pachyvessels), accompanied by thinning of the choriocapillaris and Sattler’s layer with/without retinal pigment epithelium (RPE) abnormalities overlying the pachyvessels [6–10]. While a thick choroid is frequently seen, choroidal thickness per se is not the most important criterion for defining the pachychoroid disease phenotype. Instead, the presence of characteristic morphologic changes which implicate structural and functional choroidal alteration as key pathophysiologic mechanism is essential to diagnose pachychoroid disease [9]. These features will be discussed in detail in this review. Although the etiology of pachychoroid disease remains controversial, these choroidal changes are believed to play an important pathogenic role in the development of the following clinical manifestations which reside within the pachychoroid disease spectrum [6–10]:
-
i.
Central serous chorioretinopathy (CSC).
-
ii.
Pachychoroid pigment epitheliopathy (PPE).
-
iii.
Pachychoroid neovasculopathy (PNV).
-
iv.
Polypoidal choroidal vasculopathy (PCV)/aneurysmal type 1 neovascularization (AT1).
-
v.
Focal choroidal excavation (FCE).
-
vi.
Peripapillary pachychoroid syndrome (PPS).
These conditions are believed to represent different manifestations of a common pathogenic process, as overlapping features have been observed, and progression from one to another has been well described [11, 12].
Choroidal features common to pachychoroid disease entities
Focal or diffuse increase in choroidal thickness
Using EDI-OCT or SS-OCT, the choroid–scleral interface (CSI) can be delineated in most eyes, thus facilitating quantitative analysis of choroidal thickness (Fig. 1) [1]. Subfoveal choroidal thickness in normal subjects has been reported to be between 191–350 µm in previously studies [13], but choroidal thickness can be influenced by a variety of factors, including age, axial length, refractive error, blood pressure, as well as time of the day. Thus, a wide range of values have been reported even in normal subjects. Nonetheless, increased choroidal thickness was subsequently described in patients with CSC (345–505 µm) [13–15] and PCV (223–590 µm) [16–19]. In these patients, increased choroidal thickness in the contralateral eye was also commonly found [15]. In view of the wide range of choroidal thickness and the multiple factors which may influence this parameter, there is no definitive quantitative threshold for defining an eye as having abnormally thick choroid. However, many investigators may consider subfoveal choroidal thickness >300 µm as pathological [4, 20]. Regional variation of choroidal thickness has also been described, with studies showing the thickest region beneath the fovea and thinnest areas nasally [21]. Regional variations in choroidal profile can be evaluated in detail using SS-OCT, as the shorter acquisition time allows for dense scans which can produce choroidal volume maps. Eyes with pachychoroid disease may have normal subfoveal choroidal thickness, but exhibit an extrafoveal focus of increased choroidal thickness (defined as exceeding subfoveal choroidal thickness by 50 µm) [4]. The area of maximal choroidal thickness is likely to be of significance if it correlates spatially with the distribution of pachyvessels and with the disease focus [7].
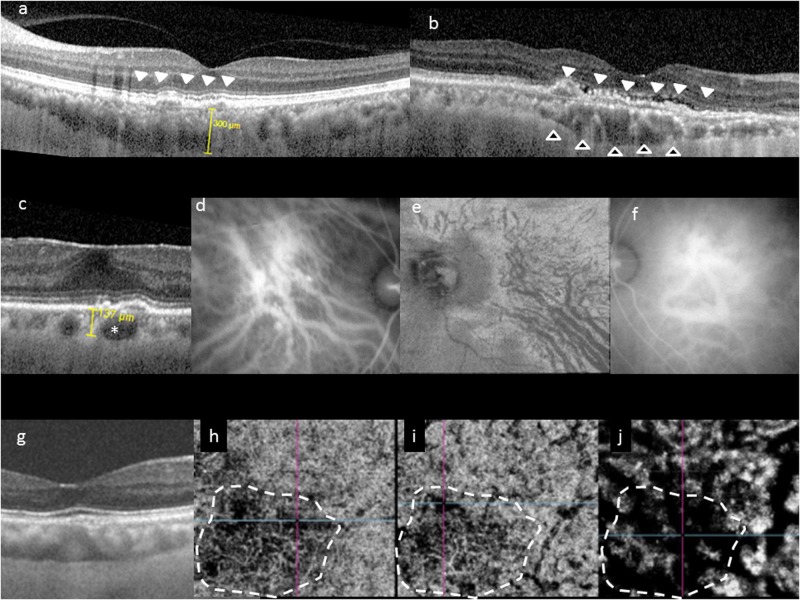
Fig. 1.
Choroidal features common to pachychoroid phenotype. Typical choroidal features in the pachychoroid disease phenotype include diffuse or focal increase in choroidal thickness, which is typically associated with abnormally dilated Haller layer vessels (pachyvessels) and attenuation of the inner choroid (Sattler’s layer and choriocapillaris). Choroidal thickness can be readily evaluated in cross-sectional optical coherence tomography (OCT), which may show diffuse thickening and increased subfoveal choroidal thickness (a), or focal thickening (b, hollow arrowheads). In some eyes, an irregular elevation of the retinal pigment epithelium (RPE) can be seen to overlie these choroidal abnormalities (white arrowheads). Pachyvessels can be identified as a choroidal vessel with enlarged caliber (c, *) which can occupy almost the entire thickness of the choroid. Pachyvessels can also be seen as dilated submacular vessels which do not taper toward the posterior pole on ICGA (d) or on en face OCT (e). These pachyvessels may be distributed in a diffuse (d) or patchy manner (e). Pachyvessels usually exhibit choroidal vascular hyperpermeability with indocyanine green angiography (ICGA) (f) which may suggest choriocapillaris ischemia. Using OCT angiography (OCTA), the spatial correlation between choriocapillaris blood flow and pachyvessels can be evaluated in a depth-resolved manner. In g–j, choroidal thickening and choriocapillaris attenuation seen in cross-sectional OCT (g) can be correlated with OCTA findings showing attenuation of flow signal (dash white outline) within the choriocapillaris (h) and inner choroid (i) which directly overlie areas with dilated outer choroidal vessels (j)
Pachyvessels
Increased choroidal thickness is thought to result predominantly from dilatation of choroidal vessels in Haller’s layer. Increased diameter of choroidal vessels has been observed as larger hyporeflective lumen in cross-sectional OCT in eyes with CSC, PCV, focal choroidal excavation (FCE), and PPS [22, 23]. In histological sections in eyes with PCV, dilated choroidal vessels with diameter of up to 300 µm have been observed. With en face OCT, detailed morphological changes within the intermediate and large choroidal vessels can be evaluated in a depth-specific manner. Pathologically dilated vessels in eyes with pachychoroid disease can be detected within the deep choroid [5]. In addition to increase in caliber, pachyvessels can also be distinguished from normal choroidal vessels as they do not taper toward the posterior pole, but retain their large caliber and terminate abruptly. This feature is best appreciated using en face OCT or ICGA. Pachyvessels may be present diffusely or focally (e.g., in 1 or 2 quadrants) [4]. When localized, they correlate spatially with the areas of maximal choroidal thickening, as well as disease focus within the RPE or retina [4]. Choriocapillaris thinning may be observed in areas overlying pachyvessels, as evidenced by inward displacement of large dilated choroidal vessels which become visible in a more superficial en face plane [4].
Attenuation of inner choroid
Thickened choroid per se may not necessarily have any pathologic consequences. Healthy eyes with abnormally thick choroids may be considered to have “pachychoroid” or “uncomplicated pachychoroid”. Regardless of choroidal thickness, presence of morphological features of pathologic sequelae resulting from abnormally dilated choroid may be a more significant finding for diagnosing “pachychoroid disease” [9, 10]. A key feature is inner choroidal attenuation, characterized by focal or diffuse attenuation of the choriocapillaris and intermediate caliber vessels within Sattler’s layer in areas overlying abnormally dilated Haller’s layer vessel [4]. In severe cases, the Haller’s layer vessel may occupy the full extent of the choroidal thickness. If the luminal volume increase in the outer choroidal vessel is offset by a reduction in inner choroidal volume resulting from atrophy of the latter, it is possible for an eye to have normal or even subnormal choroidal thickness but still exhibit the pachychoroid disease phenotype [9]. Therefore, in addition to evaluating choroidal thickness, detailed examination of the morphology of the choroid is also required to determine if any eye exhibits pachychoroid disease. To facilitate this, automated software based on binarized OCT or ICGA images have been developed to quantify the ratio between choroidal vascular luminal area to total choroidal area [24], and some have reported increased choroidal vascularity in eyes with pachychoroid disease [24–26]. However, challenges remain in optimizing such algorithms as signal strength within the choroid may be affected by blood, subretinal fluid (SRF), and pigment epithelial detachment (PED), particularly in eyes with PCV, CSC, and pachychoroid neovasculopathy (PNV) [19]. In addition to attenuation of the inner choroid, thinning of outer nuclear layer (ONL) has also been observed in eyes with pachychoroid disease. Interestingly, the ONL was thinner in eyes with pachychoroid pigment epitheliopathy (PPE) than in eyes with uncomplicated pachychoroid in one study, suggesting that degeneration of photoreceptors and/or RPE may also occur, even in the absence of SRF [20].
Choroidal vascular hyperpermeability
On ICGA, pachyvessels appear as a cluster of relatively straight and dilated choroidal vessels. In addition to choroidal venous dilatation, choroidal filling defects, delayed arterial filling in the early phase, and focal or punctate hyperfluorescence have been observed in eyes with CSC, PCV, and FCE, suggestive of possible choroidal ischemia [27–30]. In the mid to late phase, patchy areas of ICGA hyperfluorescence can be seen corresponding to the leakage and staining sites observed on fluorescein angiography (FA) [27–30]. Choroidal hyperpermeability is believed to result from increased extravasation of fluid and lipoprotein-bound ICGA from the choriocapillaris or the larger choroidal vessels into the surrounding choroidal stroma. Previous studies have reported choroidal hyperpermeability to be present in >90% of eyes with PPE and CSC [27], and in 10–50% of eyes with PCV [2, 18]. Punctate hyperfluorescence spots have also been observed in the mid to late phase of ICGA in eyes with CSC and PCV [18, 31]. Both diffuse and punctate hyperfluorescent spots have been frequently observed in contralateral eyes of patients with CSC and PCV without overt pathology [27, 30]. These ICGA findings characteristically persist in eyes with CSC even after resolution of SRF [30]. These observations support the hypothesis that the choroidal changes are likely the primary etiology in these disease entities. Eyes with choroidal hyperpermeability usually have increased choroidal thickness, but not all eyes with thick choroids exhibit choroidal hyperpermeability [32]. Interestingly, choroidal hyperpermeability was reported to be more common in eyes with CSC and PPE than in eyes with uncomplicated pachychoroid [27]. As choroidal hyperpermeability may indicate structural choriocapillaris damage producing relative ischemia, this finding suggests functional implications of the pachychoroid phenotype beyond increased choroidal thickness.
Clinical and imaging features of specific conditions
Central serous chorioretinopathy
CSC is a common disorder characterized by serous retinal detachment with or without serous PED (Table 1, Fig. 2). CSC occurs mainly in young to middle-aged men with emmetropic or hyperopic eyes, but this gender predilection decreases with age [33]. Maumenee was the first to describe leaks at the level of the RPE seen with FA in eyes with CSC [34], and later ICGA confirmed choroidal vascular congestion as being involved in the pathogenesis of CSC [28, 30, 35]. Although the precise etiology and pathogenesis of CSC remain unknown, systemic use of corticosteroids and sympathomimetics are well-known major risk factors. Patients with CSC usually complain of decreased or distorted vision, relative scotoma, and micropsia, and manifest a small hyperopic shift. Younger patients usually present with unilateral involvement, while older patients are more likely to show bilateral disease.
Table 1.
Clinical and multi-modal imaging features of pachychoroid spectrum of disorders
| Pachychoroid spectrum | ||||||
|---|---|---|---|---|---|---|
| Central serous chorioretinopathy (CSC) | Pachychoroid pigment epitheliopathy (PPE) | Pachychoroid neovasculopathy (PVN) | Polypoidal choroidal vasculopathy/ aneurysmal type 1 neovascularization (PCV/AT1) | Focal choroidal excavation (FCE) | Peripapillary pachychoroid syndrome (PPS) | |
| Common choroidal features | ||||||
| Fundus | Reduced fundus tessellation in areas of increased choroidal thickness | |||||
| EDI-OCT or SS-OCT |
1 Focal or diffuse choroidal thickening a. Subfoveal choroid may be normal but extrafoveal area of increase thickness (>50 µm more than subfoveal measurement) may be detected 2 Dilated choroidal vessels (pachyvessels) a. Increased diameter of choroidal vessel lumen on cross-sectional OCT b. Large caliber vessels in Haller’s layer on en face OCT, but inward displacement of pachyvessels often leads to their visualization in a more superficial en face plane 3 Thinning/absence of choriocapillaris and Sattler’s layer overlying pachyvessels. Pachyvessels may occupy the full extent of the choroidal thickness If luminal volume increase in pachyvessels is offset by reduction in inner choroidal volume resulting from inner choroid atrophy, it is possible for an eye to display pachychoroid phenotype without increased choroidal thickness |
|||||
| ICGA |
• Dilated choroidal vessels • Choroidal filling defects, delayed arterial filling in early phase, focal or punctate hyperfluorescence areas suggestive of possible choroidal ischemia • Choroidal vascular hyperpermeability, best observed in mid to late phase. CVH may also be observed in contralateral eye without active disease, and/or persist after resolution of active disease CVH is usually correlated with increased choroidal thickness. However, eyes with increased choroidal thickness may not display CVH |
|||||
|
Retinal features specific to individual condition Note: These changes are observed to correlate spatially with choroidal changes described above |
||||||
| OCT |
• Well-defined serous retinal detachment in acute CSC • PED • Shallow, broad retinal detachment in chronic CSC • Elongated photoreceptor outer segments • Outer retinal atrophy (disruption to EZ, thinning of ONL) • Cystoid macular degeneration in chronic CSC |
• Focal RPE abnormalities overlying pachychoroid disease changes • Absent SRF |
• Type 1 NV overlying pachychoroid disease changes appearing as shallow irregular RPE detachment (double-layer sign) |
• Type 1 NV with aneurysmal lesions are located between RPE and the inner collagenous layer of BM • Exudative changes tend to originate from aneurysms |
• Abrupt changes of choroidal thickness beneath the FCE lesion • Increased choroidal thinning with highly reflective choroidal tissue and poorly defined CSI beneath area of FCE • Normal, smooth inner scleral surface suggesting absence of scleral excavation or ectasia • Conforming no separation between photoreceptor tips and RPE • Non-conforming photoreceptor tips appear detached from underlying RPE, with a hyporeflective intervening space |
• Maximal choroidal thickness occurs close to the optic nerve rather than subfoveally • Nasal macular intraretinal and/or subretinal fluid |
| FAF |
• May show signs as seen in PPE • Vertical, gravitational tracts of RPE hypopigmentation • Geographic areas of speckled hyper-AF |
• Granular hypo-AF • Mixed stippled hyper-AF and hypo-AF |
• Granular hypo-AF • Ring-shaped abnormalities with hypo-AF center and hyper-AF surrounding may be seen |
• Hyper-AF or hypo-AF appearances |
• Mottled hypoautofluorescence in peripapillary area • Gravitational tracks may be seen |
|
| OCTA | No evidence of choroidal neovascularization | No evidence of choroidal neovascularization | • “Tangled network” of flow signal corresponding to type 1 neovascularization |
• Flow signal representing type 1 neovascularization is usually well visualized • Variable flow signal within aneurysms has been described |
NA | NA |
| FA |
• Leaks at the level of RPE • Ink-blot or smoke-stack leakage pattern common in acute CSC • Multiple indistinct leaks inside granular window defect in chronic CSC |
• Exudation from aneurysms/BVN typically leads to occult pattern of leakage and staining • Occasionally classic pattern of leakage may be seen in the presence of significant RPE erosion from underlying aneurysms or secondary type 2 neovascularization |
• Hyperfluorescence corresponding to transmission defects associated with RPE attenuation in mid or late phase FA • Absence of leakage unless complicated by CNV or CSC • Hypofluorescence related to RPE alterations |
• Speckled hyperfluorescent window defects and staining typically in area between optic disc and fovea • May be associated with disc leakage |
||
| ICGA |
• Choroidal hyperpermeability • Choroidal venous dilation and vascular congestion • Choroidal filling delay |
• Choroidal hyperpermeability | • Choroidal hyperpermeability |
• Choroidal hyperpermeability • Branching vascular network with terminal aneurysmal dilatations |
• Early hypofluorescence suggesting filling detects • Late phase: punctate, patchy, or diffuse hyperfluorescence around FCE |
• Large choroidal vessels are more prominent nasal than temporal to the fovea • May show peripapillary choroidal hyperpermeability |
| Other features | • Bullous variant may be seen |
• RPE mottling on funduscopic examination, frequently extrafoveal in location and may be confused with pigmentary AMD or pattern dystrophy • Drusenoid RPE lesions (pachydrusen) • Reduced fundus tessellation • Patients are relatively younger than those with AMD |
• Lack of other identifiable risk factors for CNV such as soft drusen, myopic degeneration, inflammation, or angioid streaks |
• Chronic multiple recurrent exudative changes • Hemorrhage from aneurysms may occur • Lack of soft drusen, reticular pseudodrusen, intraretinal RPE migration, geographic atrophy |
• Fundus may appear normal or show non-specific pigmentary changes or indistinct yellow-whitish spot(s) • May be associated with moderate myopic refractive error |
• May be associated with choroidal folds • Usually associated with crowded disc • Hyperopic refractive error is common |
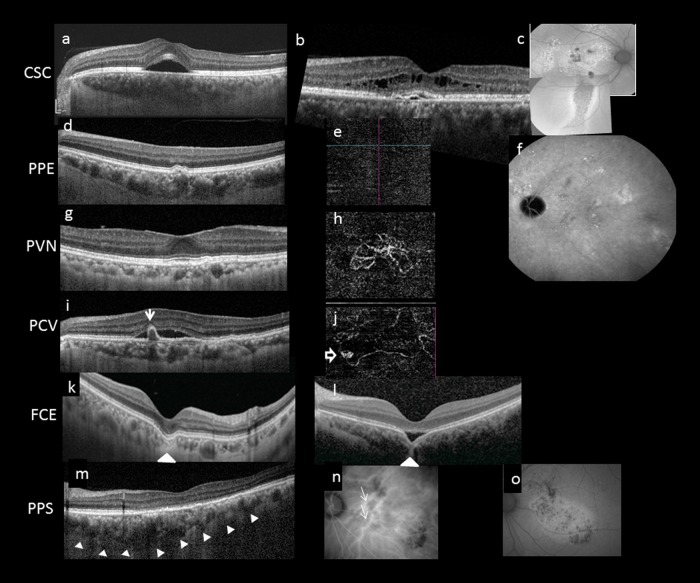
Fig. 2.
Multimodal imaging features of pachychoroid disease. Several clinical manifestations have been described to reside within the pachychoroid disease spectrum, including central serous chorioretinopathy (CSC) (a–c), pachychoroid pigment epitheliopathy (PPE) (d–f), pachychoroid neovasculopathy (PVN) (g, h), polypoidal choroidal vasculopathy (PCV)/aneurysmal type 1 neovascularization (AT1) (i, j), focal choroidal excavation (FCE) (k, l), and peripapillary pachchoroid syndrome (PPS) (m–o). Note that the choroidal features described in Fig. 1 can be seen in all cases in Fig. 2. In acute CSC (a), a solitary neurosensory detachment is commonly seen. In contrast, in chronic CSC (b), the neurosensory detachment tends to be shallower and broader. A shallow pigment epithelial detachment (PED), shallow subretinal fluid (SRF), and cystic intraretinal fluid can also be seen in this case. The RPE changes are best evaluated using fundus autofluorescence (FAF). In chronic CSC (c), multiple areas showing mixed hyperautofluorescence and hypoautofluorescence can be seen in the posterior pole. A downward gravitational tract of hypoautofluorescent can be seen. PPE is thought to be a forme fruste of CSC. On irregular RPE elevation overlying pachychoroid disease features without subretinal fluid can be seen in d. OCTA confirmed absence of neovascularization (e) and FAF showed milder granular autofluorescence disturbance than in c, without a gravitational tract. Cross-sectional OCT findings in PCN may be similar to those in chronic CSC and PPE, characterized by irregular RPE elevation with or without SRF. OCTA can readily detect the presence of neovascularization (h). Features of PCV/AT1 overlap significantly with PCN, with the additional feature of aneurysms. Although indocyanine green angiography (ICGA) is considered the gold standard for diagnosing PCV/AT1, common features on OCT include irregular RPE elevation overlying pachychoroid disease changes and narrow-peaked PEDs (arrow). In the corresponding OCTA (j), an aneurysm (hollow arrow) can be seen to arise from a branching vascular network. FCE is characterized by a localized area of choroidal excavation on OCT. In the conforming type (k), the photoreceptor tips are not separated from the underlying RPE, whereas in the non-conforming type (l) a hyporeflective space can be observed between the photoreceptor tips and RPE. Unusual hyperreflective choroidal tissue (arrowhead) can be seen to bridge the space between the bottom of the excavation and the outer choroidal boundary. Dilated choroidal vessel and thickened choroid can be seen on either side of the excavation. In PPS, the choroid is thicker on the nasal side to the fovea compared to the temporal side in the cross-sectional OCT (m). More dilated choroidal vessels (white arrows) are seen on the nasal side compared to the temporal side of the fovea on ICGA (n). Fundus autofluorescence illustrates mottled autofluorescence temporal to the disc and extending downwards (o)
The most common type is acute CSC, mainly seen in younger patients. Typical manifestations include solitary and localized neurosensory retinal detachment and serous PEDs. FA demonstrates one or multiple focal leaks at the level of the RPE in “ink-blot” or “smoke-stack” patterns. ICGA shows multifocal areas of choroidal hyperpermeability as hyperfluorescent patches in the mid phase study [28, 30, 35]. Other ICGA findings of CSC include areas of delayed choroidal filling and prominent venous dilation. Most acute cases of CSC resolve withing 4–6 months. When serous retinal detachment persists over several months, small yellowish dots appear beneath the detachment. These dots probably represent shed outer segments of photoreceptor, with some phagocytized by macrophages [36]. These dots appear hyperautofluorescent with fundus autofluorescence (FAF) [36]. In chronic CSC, which may be defined by duration >6 months, or more importantly by presence of RPE damage, more diffuse RPE abnormalities with flat and broad areas of serous retinal detachment are commonly observed. These findings often extend inferiorly, in the form of a descending tract, most readily visualized with FAF as granular and sometimes confluent hypoautofluorescence with a hyperautofluorescent margin. FA shows multiple indistinct leaks inside granular window defect. Multifocal areas of choroidal hyperpermeability are seen on ICGA, which may become widespread. A rare variant of chronic CSC is bullous retinal detachment.
OCT is an essential tool to evaluate the areas of SRF and PEDs in both acute and chronic CSC. In acute CSC, well-defined serous retinal detachment with or without serous PEDs is typically confined to the macula; however, when SRF persists, elongated photoreceptor outer segments are often observed together with subretinal fibrin, intraretinal lipid deposition, and subretinal yellowish dots [36]. In chronic CSC, the retinal detachment are usually shallow and broad, with attenuation of outer retinal layers related to chronic serous detachment. Intraretinal fluid, sometimes referred to as “cystoid macular degeneration”, may develop in some cases when there are defects in the external limiting membrane allowing fluid to enter the retina [37]. Recent research using EDI-OCT and SS-OCT has revolutionized our understanding of CSC. Many reports have demonstrated a pathologically thickened choroid in CSC eyes [14, 38]. In addition, the mean subfoveal choroidal thickness in symptomatic eyes is usually greater than that in asymptomatic fellow eyes [39]. Yang and associates [22] demonstrated that CSC eyes had significantly larger hyporeflective vascular lumen than that seen in the choroid of normal control eyes. The dilation of choroidal vessels in the Haller layer appears to account for thickened choroid seen in CSC [40]. Using binarization method to determine the sizes of the hyporeflective lumen and hyperreflective stroma, larger hyperreflective stroma in the inner choroid has been found and is thought to be related to the inflammation and edema occurring during the acute stage of CSC, in addition to dilation in the larger vessels in the outer choroid [41]. The application of OCT angiography (OCTA) is very helpful for detecting choroidal neovascularization (CNV) secondary to chronic CSC (see section “Pachychoroid neovasculopathy”) [42]. However, for CSC itself the clinical utility of OCTA is still being explored.
Pachychoroid pigment epitheliopathy
The term PPE was first introduced by Warrow and colleagues to refer to a condition characterized by RPE changes which occurred in the posterior pole over regions of choroidal thickening [6]. While these changes had been observed in uninvolved fellow eyes of patients with unilateral CSC, the authors noted that a significant number of patients who lacked a history neurosensory detachment presented with a similar pigment epitheliopathy in one or both eyes. These patients were often misdiagnosed with pigmentary age-related macular degeneration (AMD), and sometimes with pattern dystrophy or “retinal pigment epitheliitis” [7]. However, PPE is usually asymptomatic. The clinical appearance of the pigment epitheliopathy included mottling of the RPE, irregular areas of RPE elevation termed “drusenoid RPE lesions”, and an absence of soft drusen seen in eyes with AMD. FAF showed similarly mottled hypoautofluorescence but also revealed hyperautofluorescent features which correlated with foci of apparent RPE thickening or hyperplasia seen on cross-sectional OCT [4]. The choroids of patients with these findings exhibited hyperpermeability with ICGA in the distribution of the pigment epitheliopathy, as well as pathologically dilated vessels in Haller’s layer [4]. Reduced fundus tessellation was also noted and, together with the frequently extrafoveal location of the pigment epitheliopathy and the relatively young age of the patients, helped to distinguish this condition from non-neovascular AMD.
Since none of the eyes had manifested neurosensory detachment, PPE was considered a forme fruste of CSC. Moreover, it was subsequently observed that patients with PPE could go on to develop type 1 neovascularization, with or without aneurysmal (polypoidal) lesions, without necessarily developing CSC [4, 43, 44].
Pachychoroid neovasculopathy
Although development of secondary CNV has been described in CSC [33, 45], the incidence has not been well established. Differentiating chronic CSC from AMD can be challenging as the two conditions may have very similar features on FA and ICGA, characterized by RPE atrophy and diffuse leakage. With advances in choroidal imaging, differences in choroidal features have been noted among patients presenting with type 1 neovascularization. Fung described a subgroup of patients presenting with type 1 neovascularization with clinical and imaging findings more consistent with long-standing CSC than with AMD [12]. Increased choroidal thickness, absent or minimal soft drusen, and younger age were among the key features which differentiated this group of patients from neovascular AMD. Some of these eyes also had aneurysmal (polypoidal) structures within their type 1 neovascular network. Importantly, Fung’s study established a clear temporal sequence of CSC which predated the development of type 1 neovascularization (mean interval of 139 months), and thus support a pathogenic sequence. The authors also emphasized that this type of neovascularization should be differentiated from that occurring in typical neovascular AMD [12].
Subsequently, the occurrence of type 1 neovascularization was described in eyes with other pachychoroid disease entities. Pang and Freund described development of type 1 neovascularization in three eyes over background changes consistent with PPE, and introduced the term “PNV” to describe this condition [7]. The authors proposed that PNV resides within the pachychoroid disease spectrum and occurs due to a pachychoroid-driven process involving choroidal congestion and hyperpermeability. Characteristic features of PNV include presence of type 1 neovascularization which appears on OCT as a shallow irregular separation of the RPE from Bruch’s membrane which appears as “double layer sign” overlying pachyvessels [46]. The presence of heterogeneously hyperreflective material in the sub-RPE space further suggests the presence of sub-RPE neovascularization. Small peaked PEDs may develop the margin of these lesions within which aneurysmal (polypoidal) lesions may be identified with ICGA or OCTA. Eyes with PNV display background features common to the pachychoroid disease spectrum, including an absence of soft drusen and reduced fundus tessellation indicative of a thickened choroid in the area of the type 1 neovascular lesion. Characteristic choroidal findings of the pachychoroid phenotype described in section “Choroidal features common to pachychoroid disease entities” can be seen on OCT. Importantly the areas of type 1 neovascularization are correlated spatially to areas displaying pachychoroid features [4]. RPE changes overlying areas of pachyvessels can be seen as abnormal FAF. Presence of neovascularization can be confirmed by detection of leakage on FA, typically in the form of late leakage with undetermined origin, and a corresponding late staining “plaque” on ICGA. Although similar angiographic signs may be seen in chronic CSC as a result of diffuse RPE disturbance and choroidal hyperpermeability, eyes with PNV do not exhibit the classic serous macular detachment or the characteristic changes on FAF such as descending tracts seen in CSC [43]. These differences might be helpful to determine whether SRF in an eye is the consequence of PNV or CSC.
With the advent of OCTA, the diagnosis and confirmation of neovascularization has become easier in cases of suspected PCN. Neovascularization can be identified non-invasively as a tangled network of flow signal between the RPE and Bruch’s membrane corresponding to the flat-irregular PED identified on structural OCT (Fig. 3) [42, 44, 47]. In a series of 88 patients with chronic CSC, neovascularization was detected in 35.6% of eyes with shallow irregular PEDs using OCTA [47]. Eyes with pachychoroid features and a shallow irregular PED on SD-OCT should therefore be evaluated in more detail with OCTA, as these eyes frequently harbor neovascular tissue.
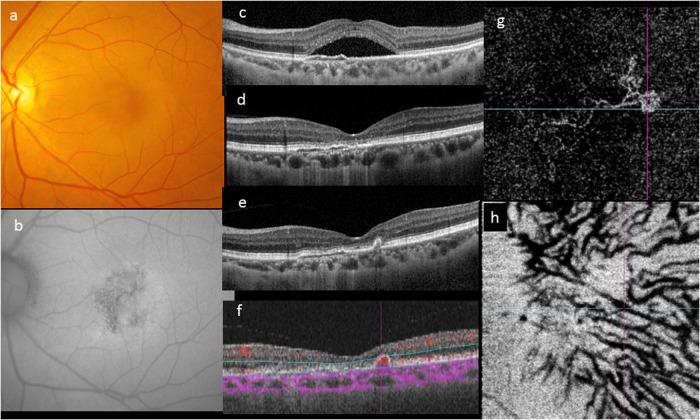
Fig. 3.
Evolution of a case over 4 years. A 46-year-old woman first presented with a solitary neurosensory detachment and mottled fundus autofluorescence (a–c). A shallow elevation of the RPE was noted. The SRF resolved spontaneously within 3 months and the eye remained unchanged for the following 4 years (d). During routine follow-up, development of a narrow-peaked PED without SRF was noted on OCT (e). OCTA showed a localized area of abnormal flow signal within the narrow-peaked PED (f). En face OCTA showed a neovascular network with aneurysms its temporal margin (g). The outline of pachyvessels can be seen as dark silhouettes in the OCTA segmented through Haller’s layer (h)
Polypoidal choroidal vasculopathy/aneurysmal type 1 neovascularization (AT1)
Idiopathic PCV was first described by Yannuzzi et al. [48] in 1990 as a novel clinical entity in which hemorrhagic and exudative neurosensory detachments occurred in the peripapillary region and macula of a predominantly black cohort of middle aged women. In 1995, Spaide and colleagues described the characteristic ICGA findings of this disorder which included a branching vascular network (BVN) with terminal aneurysmal (polypoidal) dilatations [49]. Lacking the benefit of depth-resolved structural OCT, these authors localized these structures to the inner choroid. A recent editorial highlighted the results from newer multimodal imaging demonstrating that PCV is in fact a variant of type 1 (sub-RPE) neovascularization, as both the vascular dilations and their feeding vascular network are consistently found in a potential space bounded anteriorly by the RPE and its basal lamina and posteriorly by the inner collagenous layer of Bruch’s membrane [50]. These authors reasoned that there was ample evidence from imaging to support that the “polypoidal” lesions are in fact vascular structures with the potential to rupture and bleed, as opposed to fleshy masses of tissue arising from epithelial surfaces, as the term “polypoidal” suggests. They noted that following its original description, the term “PCV” had been used in reports spanning a wide range of different clinical presentations, thus creating confusion as to whether PCV was a distinct disease or a neovascular growth pattern occurring in multiple different pathologic settings leading to sub-RPE angiogenesis. The authorship, which included Lawrence Yannuzzi, who coined the term “PCV”, suggested renaming this form of sub-RPE vascular proliferation “AT1” in an effort to clarifying its true nature. In the rest of this review, we shall refer to this entity as PCV/AT1.
PCV/AT1 is a common occurrence in Asian patients with neovascular AMD, in whom it primarily originates within the macula rather than in the peripapillary region [51]. While sharing many of the same systemic and genetic risk factors with white AMD patients, Asian AMD patients with PCV/AT1 are less likely to manifest certain non-neovascular AMD features such as soft drusen, cuticular drusen, reticular pseudodrusen (subretinal drusenoid deposits), and geographic atrophy. The reported prevalence of PCV/AT1 among Asian neovascular AMD patients ranges from 20 to 60% [51]. This wide range of reported prevalence may arise from inconsistent criteria used for identifying the aneurysms occurring in these patients [52]. The prevalence of PCV/AT1 in white patients with neovascular AMD has been reported to be much lower at 4–12%, but this may be an underestimation as ICGA is not routinely performed by most ophthalmologists in the United States [51]. The propensity to form aneurysms suggests there may be specific pathophysiologic mechanisms driving the formation of aneurysms in PCV/AT1 eyes including atherosclerosis and other vascular wall alterations, and changes in intravascular flow dynamics. These mechanisms are supported by several clinicopathologic studies, demonstrating hyalinization within vessel walls from tissue obtained from eyes of Asian AMD patients with PCV/AT1 [53].
The advent of SD-OCT, EDI OCT, and SS-OCT has provided valuable information on the pathophysiology of PCV/AT1. As mentioned above, both the BVN and the aneurysms occurring in PCV/AT1 are consistently identified below RPE and its basal lamina and anterior to the inner collagenous layer of Bruch’s membrane, confirming an origin as type 1 (sub-RPE) neovascularization [46].
In PCV/AT1 patients with visual symptoms of recent onset, the exudative changes typically originate from the aneurysms, not from the neovascular complex (BVN) feeding these lesions. This observation suggests that in many cases, PCV/AT1 may originate from non-exudative type 1 lesion in eyes with long-standing pachychoroid neovascularization. Once exudation begins, it can sometimes resolve spontaneously, presumably due to thrombosis of leaking aneurysm. However, the remaining type 1 lesion forming the BVN will usually show continued growth, forming new aneurysms over time which typically occur at the margins of the neovascular lesion. Growth of the BVN may continue for years, or even decades, with a remitting-relapsing course that is clinically associated with chronic, multiple, recurrent exudative changes. During this process, the aneurysms may hemorrhage and the associated type 1 lesion (BVN) may evolve into a more active form of neovascular proliferation producing additional exudation [54]. It is generally accepted that PCV/AT1 has a better visual prognosis than typical neovascular AMD, as progression is slow and subretinal fibrous proliferation is unusual in the absence of large subretinal hemorrhage. However, the visual prognosis is variable over its natural course and with long-term follow-up, many patients eventually lose central vision [54, 55].
Recent studies using EDI-OCT and SS-OCT have demonstrated that patients with PCV/AT1 frequently have thick choroids in contrast to choroidal thinning that is often observed in eyes with typical neovascular AMD [16]. The presence of choroidal thickening and choroidal hyperpermeability in patients with PCV/AT1 suggests a link between this entity and the pachychoroid disease spectrum, in particular PNV. A number of studies have reported the occurrence of aneurysms originating from type 1 lesions in eyes with PVN and other pachychoroid disease entities [11, 12]. Although the mean choroidal thickness is greater in PCV/AT1 eyes than in eyes with typical neovascular AMD, there is a pronounced inter-individual variability with values distributed over a wide range. In one study including over 300 eyes, the mean subfoveal choroidal thickness was ~270 μm, with a wide range (40–650 μm) [10]. The subfoveal choroidal thickness exhibited a bimodal distribution, with peaks at 170 and 390 μm, suggesting that PCV/AT1 cohorts may in fact contain two overlapping phenotypes of which only one exhibits markedly increased subfoveal choroidal thickness. In that cohort, subfoveal choroidal thickness was >200 μm in 61%, and <200 μm in 39% (Fig. 4). However, in over 90% of both groups, pachyvessels were observed below the presumed origin of the BVN feeding the aneurysms, these pachyvessels occupied nearly the full thickness of the choroid and demonstrated overlying attenuation of the inner choroidal layers. The area of maximal choroidal thickness correlated spatially with the distribution of pachyvessels and with the disease focus, even in eyes in which the absolute choroidal thickness was not particularly high [10]. Quantitative values of total choroidal thickness at the disease focus were significantly greater than those measured at the fovea and these disease sites had significantly lower inner choroidal (choriocapillaris/Sattler layer) to total choroidal thickness ratios compared with the fovea. In eyes with peripapillary PCV/AT1, in which the disease focus is located around optic nerve, away from the fovea, similar morphologic changes within the choroid have been observed. The normal choroidal thickness profile is U-shaped with the thickest area located beneath the fovea, correlating with the need for a robust choroidal circulation in the macula due to a high metabolic demand and need for heat dispersion. In contrast, in eyes with peripapillary PCV/AT1, the thickest point in the choroidal U-shape curvature is shifted nasally. In these eyes, the choroid is thicker on the nasal side of the fovea than on its temporal side [10, 56].
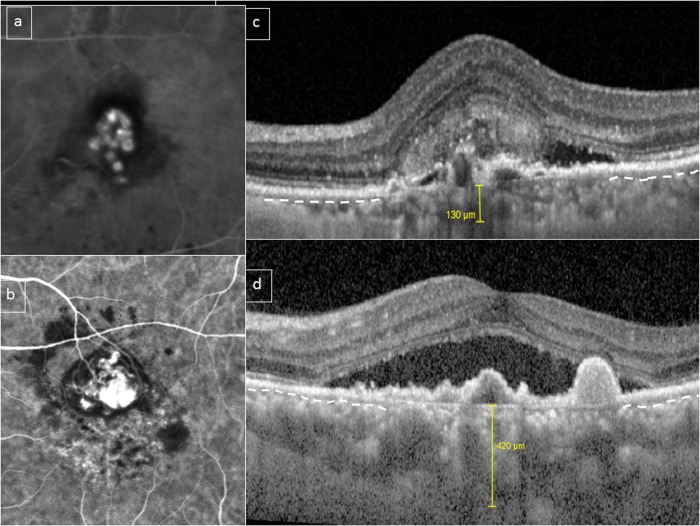
Fig. 4.
Choroidal features in polypoidal choroidal vasculopathy (PCV)/aneurysmal type 1 neovascularization (AT1). A bimodal distribution of choroidal thickness has been described in PCV/AT1. This figure shows two eyes with PCV/AT1 confirmed with indocyanine green angiography (ICGA) (a, b). Subfoveal choroidal thickness was 130 and 420 μm, respectively (c, d). However, in both cases, choriocapillaris/inner choroid (outline by dashed white line in preserved areas) appears to be compressed and attenuated by underlying outer choroidal vessels in the subfoveal area (c, d)
Focal choroidal excavation
FCE is characterized by localized area(s) of choroidal excavation without evidence of posterior staphyloma or scleral ectasia in patients who typically lack a history of disease known to result in choroidal thinning [8, 57]. Most patients with FCE have been diagnosed in their fourth to fifth decade, but no gender predilection has been found. Most cases described have moderate myopia. Patients tend to be asymptomatic or report mild blurring of vision or metamorphopsia. Fundus examination may appear normal or show non-specific pigmentary changes or indistinct yellow-whitish spots in an area of reduced fundus tessellation. OCT is the preferred diagnostic imaging modality. Two patterns of excavation have been described. In conforming FCE, the photoreceptor tips are in direct contact with the RPE, whereas in non-conforming FCE the photoreceptor tips appeared to be detached from the underlying RPE, and a hyporeflective cleft, presumably representing SRF, can be observed in the intervening space [8, 58]. Unusual hyperreflective choroidal tissue is observed to bridge between the bottom of the exaction and the outer choroidal boundary in some eyes [59, 60]. A smooth CSI is observed [59, 60]. Findings on FA include atrophic RPE window defect leading to varying degrees of hyperfluorescence and hypofluorescence. Hypofluorescence on ICGA and abnormal staining in the late phase suggest choriocapillaris loss [61]. Pachychoroid features have been described in eyes with FCE, including increased subfoveal choroidal thickness and choroidal vascular hyperpermeability in ICGA [59, 61]. FCEs were located within or adjacent to areas of fluorescein leakage and choroidal hyperpermeability, supporting the pachychoroid features likely have etiologic significance [59].
Following initial reports of sporadic cases describing association of FCE with CSC and PCV, the association of FCE with other conditions within the pachychoroid disease spectrum was described by several investigators [59, 62–64]. Lim et al. reported the prevalence of FCE in the presenting and contralateral eyes of patients with CSC, CNV, or PCV. FCE was found in 18 (2.3%) of 598 eyes evaluated. FCE was detected in 6.0% of eyes presenting with PCV and 1.0% of eyes presenting with CNV. In addition, FCE was detected in 2.9% of contralateral eyes of patients presenting in PCV and in 1.2% of patients with CSC [62]. Ellabban et al. reported prevalence of FCE in 7.8% of eyes with CSC [59]. CNV associated with FCE may be type 1 or type 2 [63]. Luk et al. also reported that FCE was found in 7 (6.0%) of 116 patients with CSC and 4 patients had bilateral FCE [65]. These patients tend to be much younger than those presenting with neovascular AMD. The CNV lesions in these cases tends to be located within the boundary of the FCE, suggesting that the CNV growth pattern and extent may be determined by the degree of damage to the RPE/BM complex resulting from the FCE, as well as age [63, 64].
There are limited reports regarding the long-term course of eyes with FCE, but most studies to-date have reported relatively stationary lesions [57, 59, 60, 62].
Peripapillary pachychoroid syndrome
Peripapillary pachychoroid syndrome was recently described by Phasukkijwatana and colleagues as distinct variant of the pachychoroid disease spectrum in which maximal choroidal thickness occurs close to the optic nerve rather than subfovealy [23]. These patients typically present with nasal macular intraretinal and/or SRF and occasional optic nerve edema. Other sequelae of the pachychoroid disease phenotype are often present, including pigment epitheliopathy, serous PEDs, and gravitating autofluorescence tracks can occur and tend to be localized to the peripapillary region. In their initial series, Phasukkijwatana et al. reported that of 31 study eyes of 16 patients, 77% had choroidal folds, 39% had axial lengths <23 mm, and 80% had hyperopic refractive errors. None of the subjects had inflammatory eye disease, and the range of findings was felt to be distinct from the uveal effusion syndrome.
Current understanding of pathogenesis
The apparent complexity of the pathogenesis of the pachychoroid disease spectrum testifies to our incomplete understanding of the subject. This is due in part to the relatively recent introduction of pachychoroid terminology and the evolving nature of its definition. Nevertheless, there are chains of thought, which have been articulated consistently through much of the literature on CSC and which continue to inform our efforts in delineating pachychoroid disease and its boundaries.
CSC is recognized primarily by the development of neurosensory detachments at the posterior pole, but the manifestations occurring at the level of the RPE and outer retina appear to be secondary not merely to chronic SRF but to a disease which is primarily choroidal [66]. Just as a phenotypic spectrum is unraveled by extending the catalog of features backwards and forwards in time, so too might pathogenesis be elucidated.
Time-resolved multimodal imaging supports this approach in pachychoroid disorders as well, showing, in the earliest stages of disease, that choroidal hyperpermeability to ICG dye precedes clinically detectable features [35]. The earliest clinical features are those of PPE, in which RPE disease occurs at locations coinciding with regional choroidal hyperpermeability and focal choroidal thickening. Similarly, in both CSC and PNV, the defining clinical features of each occur in association with regional hyperpermeability and focal choroidal thickening. These observations raise the question as to how choroidal changes, manifesting as hyperpermeability in the first instance, might lead to RPE sequelae.
It is well documented in the ICGA literature that choroidal hyperpermeability is a function of the choriocapillaris. Indeed, scrutiny of ICGA images from eyes with pachychoroid disease shows that while hyperpermeability is seen regionally, it is absent at foci within those regions where the choriocapillaris is atrophic [4]. Moreover, the clinical complications of PPE, chronic CSC, and PNV at the tissue level occur at locations where the choriocapillaris appears structurally attenuated on OCT. This observation has been explored by Lee’s group in two recent studies of PCV/AT1, the first of which explored the validity of the ratio of choriocapillaris thickness to total choroidal thickness, as a quantitative representation for choriocapillaris attenuation and as a possible outcome measure for defining the pachychoroid disease phenotype [10]. The authors found choroidal thickness to be distributed bimodally in a cohort of patients with macular PCV/AT1. They also demonstrated a reduced ratio of choriocapillaris to total choroidal thickness, even among the eyes with thinner choroids. The second study included eyes with peripapillary PCV/AT1 and found that maximal choroidal thickness occurred at the site of peripapillary disease, and not at the fovea [56]. Maximal choroidal thickness was attributable to dilated Haller’s layer vessels with a reduced ratio of choriocapillaris to total choroidal thickness.
Taken together, these findings suggest a sequence wherein choriocapillaris hyperpermeability (choroidal dysfunction) is followed by structural choriocapillaris attenuation, RPE complications, and neovascularization with or without the occurrence of aneurysms. The emphasis in defining pachychoroid disease has therefore shifted away from absolute choroidal thickness thresholds toward a morphological definition which forms a better foundation for formulating mechanistic hypotheses. For example, it has been suggested that choriocapillaris attenuation produces an ischemic mileu which promotes VEGF expression supporting neovascularization [43, 44, 50].
The notion that choriocapillaris dysfunction and structural attenuation should lead to RPE dysfunction is acceptable intuitively, but the relative specificity of certain patterns of retinal pigment epitheliopathy for pachychoroid disease is more difficult to explain. For example, patients with PPE or chronic CSC often exhibit reticular or stellate pigment epithelial figures, which may be scattered in the posterior pole but may also be seen in the nasal retina. Unlike the reticular pigmentary changes seen in AMD, those of pachychoroid disease often feature hyperautofluorescent foci at which the RPE appears thickened or hyperplastic on OCT. The basis for these differences remains unresolved but clues may lie in a deeper understanding of apolipoprotein E and lipofuscin metabolism to explain differences in pigment epitheliopathy between pachychoroid and AMD. A lesion resembling drusen seen in AMD can occur in some eyes with pachychoroid disease. These “drusenoid RPE lesions” described by Warrow et al. [6] were later renamed “pachydrusen” by Spaide, who described a morphology, distribution, and grouping pattern which differed from the soft drusen occurring in typical AMD [67]. Spaide proposed that choroidal characteristics may act as a modulator that alters the manifestation of AMD, whether in the phenotype of drusen or neovascular subtype. Eyes with thick choroids have a propensity to have pachydrusen and type 1 neovascularization with or without aneurysms [67, 68].
The pathogenesis of pachychoroid disease itself remains unknown. There is a volume of evidence that points to aberrant steroid metabolism as an upstream factor, which in the older literature, helped to distinguish CSC from inflammatory disorders that are associated with serous or exudative retinal detachment. This altered response to steroids is thought to explain why patients with CSC often report having experienced a period of psychological stress or sleep deprivation at the time of peak symptoms, even when they have not been exposed to exogenous steroids. It has been shown recently that mineralocorticoid receptors are expressed in the choroid [69]. Stimulation of these receptors increases choroidal thickness and congestion and the effect is reversed by mineralocorticoid receptor antagonists [70]. The effects of spironolactone and eplerenone have been studied in humans but with limited or unpredictable benefit which might be due to variations in oral bioavailability [71]. In addition to environmental factors, there is also evidence that suggests choroidal thickness may be heritable [72]. The observation of significant variation of choroidal thickness among different ethnicities further supports this hypothesis [73].
The mechanisms that underlie the formation of serous PEDs and serofibrinous retinal detachments also remain elusive. It has been suggested that choroidal thickening might be associated with increased hydrostatic pressure which challenges the Bruch’s membrane–RPE complex and eventually overcomes the tight junctions between RPE cells resulting in focal and diffuse leakage and occasionally an RPE “blowout” phenomena.
Neovascularization in PNV may have a different etiology compared to typical neovascular AMD, which may have important implications in management. Some authors speculate that the neovascular process may be triggered by focal RPE disturbances and inner choroid attenuation overlying pachyvessels in CSC and PPE [45]. Others have proposed that chronic inflammation involving the choriocapillaris may also play a role in angiogenesis [29]. A study evaluating the frequencies of 12 known AMD risk alleles reported nearly identical genetic profiles in patients developing neovascularization in the context of either typical AMD or pachychoroid disease phenotypes [74]. However, the frequencies of these risk allelles were low and not significantly different between non-neovascular pachychoroid disease patients and normal subjects, suggesting that these AMD risk alleles influence neovascularization but do not determine the pachychoroid disease phenotype itself [74]. In a study evaluating Japanese patients, an association was found between PNV and ARMS2 rs10490924 and CFH, but with a smaller effect size compared to typical neovascular AMD [75].
Given the currently available data, pachyvessels and attenuation of inner choroid seem to be key features of pachychoroid changes in eyes with PCV. Loss of the choriocapillaris may produce a relatively ischemic environment, leading to overexpression of angiogenic factors. Changes of overlying RPE/Bruch complex, such as irregularities, thinning, convex elevation, and disruption are frequently seen on OCT. Expansion of Haller vessel volume within a limited space and the absence of a buffer choriocapillaris might induce damage to focal areas of overlying tissues mechanically, inducing atrophic changes of RPE and a focal break in Bruch’s membrane. Whether the occurrence of dilated Haller vessels (pachyvessels) is a primary event or secondary to the inner choroidal attenuation is still under investigation. It is possible that ischemic, inflammatory, or involutional insults to the inner choroidal circulation result in loss of inner choroid, inducing arteriovenous shunting with resultant venous dilation.
The etiology of FCE is also unclear. Some investigators proposed that FCE may be a congenital malformation [8]. The observation of abnormal choroidal tissue beneath the excavation suggests that FCE may have formed from RPE retraction caused by focal scarring of choroidal connective tissue from previous inflammatory processes [59]. FCE has been likened to an inverse PED, which may compress choriocapillaris and further exacerbate choroidal ischemia already present as a result of pachyvessels, and lead to further local damage of the RPE/Bruch membrane complex and predispose to the development of NV or CSC [57, 58].
A putative hypothesis linking the spectrum of pachychoroid disease proposed that the pachychoroid disease phenotype may be an inherited trait. If the RPE is able to overcome the chronic fluid overload, patients will continue to manifest the uncomplicated pachychoroid phenotype. However, in some eyes, features of PPE may develop along with progressive dysfunction of RPE. With further RPE damage, increasing breakdown of the RPE barrier and concomitant choriocapillaris loss may lead to the development of CSC. Neovascularization (PPN) may ensue with further damage to Bruch’s membrane and outer retinal ischemia. Recent OCTA studies have detected non-exudative type 1 neovascularization in the contralateral eyes of patients with CNV or PCV/AT1 [76, 77]. The presence of PPE has been found to be a significant risk factor for such “silent” lesions [77]. With longitudinal follow-up, the risk of developing exudation was significantly higher in eyes harboring these lesions [76, 77].
Finally, aneurysms may develop in some long-standing neovascularization, particularly in lesions with high blood pressure and/or neovascular flow [78].
Management considerations of pachychoroid disorders
In general, most pachychoroid disease in asymptomatic patients can be observed and monitored without treatment. Observed cases might include eyes with PPE, FCE, eyes with CSC and extrafoveal SRF and/or PED, and PCV/AT1 with inactive non-leaking aneurysms. However, in patients experiencing vision loss due to pachychoroid disease, treatment should be considered to improve or stabilize visual function. These cases might include CSC eyes with persistent central SRF and/or large PEDs, PCN associated with macular exudation, and PCV/AT1 macular exudation originating from either the aneurysm or the associated BVN.
Treatment of symptomatic CSC
In view of the strong association between exogenous steroids and CSC, a careful history should be carried out in patients with CSC to inquire about the use of all forms of exogenous steroids. In CSC patients who are taking corticosteroid, cessation or tapering of steroid therapy should be the first-line of management of CSC if the underlying medical condition allows. In an observational case series, it was reported that 88% of eyes with severe CSC had resolution of SRF and reattachment of neurosensory retina after discontinuation of corticosteroids [79]. Non-prescription items such as traditional herbal medicine like ginseng and cordyceps may also act on steroid receptors and might also need to be stopped.
In most cases of acute CSC, SRF spontaneously resolves. Therefore, treatment for acute CSC may be deferred for up to 4–6 months depending on the degree of symptoms and amount of subfoveal fluid [80]. Early treatment may be considered in patient highly symptomatic patients, those demanding rapid recovery of vision, or patients with poor vision in the fellow eye. However, up to 50% of cases experience recurrences after complete resolution of SRF [81]. For acute CSC, conventional thermal laser photocoagulation to extrafoveal leaking points identified on FA has been used as a treatment option since the 1980s, and has been shown to reduce the duration of CSC by 2 months [82]. The mechanism of action is thought to be restoration of RPE barrier function by sealing the RPE defect and preventing further accumulation of SRF. However, a long-term follow-up study of CSC patients treated with focal thermal laser photocoagulation showed little improvement in visual acuity after treatment [83]. In addition, focal thermal laser photocoagulation can be associated with a risk of iatrogenic CNV especially when a laser spot size of <100 µm is used. Therefore, many clinicians have shifter to newer treatment modalities for CSC associated with persistent macular fluid.
Recently, subthreshold micropulse laser photocoagulation producing less thermal injury to the RPE and the neurosensory retina has been used to treat CSC with persistent fluid. Like thermal laser, subthreshold micropulse laser appears to be most effective in eyes with focal leaks detected with FA. However, since subthreshold micropulse laser does not produce a visible laser burn during treatment, it has been used to treat RPE leaks closer to the fovea than those felt amenable to convention thermal laser. A randomized controlled trial compared the use of subthreshold micropulse laser or half-dose verteporfin photodynamic therapy (vPDT) to untreated control eyes and showed a significant improvement in visual acuity and central macular thickness following either subthreshold micropulse laser or vPDT treatment compared to controls [84]. Subthreshold micropulse using a yellow wavelength (577 nm) has been used to treat CSC and a short-term study showed a significantly higher proportion of eyes with resolution of SRF 6 weeks after treatment compared to eyes receiving half-dose vPDT [85]. However, subthreshold micropulse laser appears less effective in CSC eyes with diffuse RPE leakage and rescue vPDT is often needed in these cases [86]. One reason for the limited efficacy laser photocoagulation in CSC eyes with diffuse RPE leakage may be its limited effect on reducing choroidal thickness. One study showed that laser photocoagulation resulted in no significant change in choroidal thickness up to 4 weeks after treatment despite resolution of SRF [38].
Verteporfin PDT is generally recommended for chronic CSC. The rationale for using vPDT to treat CSC is to target the primary pathology in CSC by reducing choroidal hyperperfusion and hyperpermeability. In the past, vPDT using the standard drug dosage and laser fluence was used in the treatment of CSC and treatment resulted in a high proportion of patients with complete resolution of SRF and reduction in the dilated choroidal vasculature [87]. However, potential adverse events with conventional full-dose PDT have been reported including transient visual loss, transient reduction in multifocal electroretinography response amplitude, RPE atrophy, secondary CNV formation, and even more serious complications such as choroidal ischemia and infarction. As CSC eyes usually have better visual acuity than eyes with other macular diseases considered for treatment with vPDT, a high safety margin is required when treating these cases. Therefore, half-fluence as well as half-dose verteporfin PDT has been performed in patients with CSC to enhance the safety of the treatment [80, 88]. The main therapeutic effects of vPDT in CSC are its impact on the choroidal circulation as vPDT has been shown to reduce the choroidal thickness in eyes with CSC [38]. In a study evaluating changes in choroidal structures after half-dose vPDT for CSC, vPDT not only decreased the choroidal thickness but also altered the intrachoroidal structures [89]. More specifically, the thickness of Haller layer significantly decreased after vPDT, while the thickness of choriocapillaris/Sattler layer remained unchanged. The hyporeflective lumen was also decreased, while the hyperreflective stroma did not change. Accordingly, vPDT may produce a treatment effect on the dilated choroidal vessels in Haller layer to return to a more “normal” choroidal structure.
A large number of studies have demonstrated good safety and efficacy in the use of half-dose vPDT in treating CSC patients [90, 91]. A randomized, double-blinded, placebo-controlled trial on the use of half-dose vPDT for acute CSC has demonstrated that patients treated with half-dose vPDT had significantly better visual acuity at 3, 6, 9, and 12 months compared with placebo [92]. Half-dose vPDT-treated group also had significantly lower OCT central foveal thickness at 1, 3, 6, 9, and 12 months [92]. In a long-term study, 75 eyes treated with half-dose vPDT were compared with 117 untreated control eyes with a minimum follow-up of 3 years [93]. Eyes treated with half-dose vPDT showed significantly better visual acuity at the last visit compared with untreated control. A survival analysis demonstrated that eyes treated with half-dose vPDT were significantly less likely to develop recurrent fluid compared to untreated controls. One study exploring the lowest effective verteporfin dose that can be used to treat acute CSC concluded that this was 30% of the standard full dose [94].
A number of systemic agents have been suggested to be useful in the treatment of symptomatic CSC. These include finasteride (an inhibitor of dihydrotestosterone synthesis) [95], mifepristone (a glucocorticoid receptor antagonist) [96], and mineralocorticoid antagonist such as spironolactone and eplerenone [97, 98]. A randomized, double-blinded, placebo-controlled cross-over study of 16 eyes of 16 patients with CSC showed significant reduction in SRF and subfoveal choroidal thickness in eyes of spironolactone-treated patients compared to those receiving placebo but no significant change in BCVA [97]. Another retrospective observational case series used spironolactone, eplerenone, or both consecutively over 12 months in 23 eyes of 14 CSC patients. These investigators found improvement in vision in all eyes but no significant reduction in choroidal or macular thickness [98]. However, a recent prospective, double-blind, randomized placebo-controlled study showed no benefits for the use of eplerenone for chronic CSC compared with placebo after 3 months of treatment [99]. Since many studies exploring the use of systemic agents to treat CSC with persistent fluid do not include a control group, it is often difficult to conclude whether anatomic and/or visual improvements observed in these reports are the result of the treatment or due to the natural history of a disease known to be associated with a highly variable course.
It has been postulated that CSC may be caused by choroidal lobular ischemia with an associated increase in local VEGF production. Therefore, reducing VEGF with the use of intravitreal anti-VEGF therapy might be a potential treatment for CSC. Although several studies have shown some positive effects from using intravitreal anti-VEGF therapy for CSC, a meta-analysis failed to demonstrate a true beneficial effect for the use of intravitreal bevacizumab in CSC [100]. No significant reduction in subfoveal choroidal thickness was observed in bevacizumab-treated eyes despite resolution of SRF. In the PROMETHEUS trial, patients with macular edema due to uncommon causes (i.e., causes other than diabetic retinopathy, neovascular AMD, or retinal vein occlusion) were randomized to receive intravitreal ranibizumab or sham. In this study, no significant visual benefit was observed in eyes with macular edema secondary to CSC [101]. Therefore, based on existing studies, there does not appear to be sufficient evidence to support intravitreal anti-VEGF therapy for treating persistent fluid in CSC eyes lacking evidence of macular neovascularization.
Treatment of neovascularization secondary to CSC and PNV
Although anti-VEGF therapy does not appear useful for treating CSC patients, these agents appear to have an important role in the treatment of pachychoroid disease associated with CNV such as CSC with secondary CNV, FCE with secondary CNV, PCN, and PCV/AT1. A retrospective study of 46 eyes with CNV associated with CSC treated with intravitreal anti-VEGF therapy demonstrated a 1.16 line improvement in visual acuity after a mean follow-up of 38.3 months [102]. In the MINERVA study, eyes with CNV due to uncommon causes were randomized to receive intravitreal ranibizumab vs. sham injections [103]. Twenty-three eyes with CNV due to CSC treated with ranibizumab experienced a +6.6 letter gain in visual acuity at the primary endpoint of 2 months, compared to a +1.6 letter gain in the sham group. In cases with recurrent or persistent fluid, the addition of vPDT has been reported to result in variable levels of control [43].
Some investigators have observed that certain eyes with PNV respond favorably to intravitreal anti-VEGF therapy with a significantly longer retreatment-free interval than those seen in more typical neovascular AMD following an initial loading series injection [75]. The apparent lower dependence on repeated injections in these cases may relate to lower intraocular VEGF concentrations in PNV eyes compared to eyes with more typical neovascular AMD [104]. However, some eyes with PCN appear refractory to intravitreal anti-VEGF monotherapy. Anti-VEGF therapy in combination with vPDT appears useful in these cases, with some eyes achieving complete fluid absorption and visual improvement following combined therapy [105].
Intravitreal anti-VEGF therapy has been used to treat CNV associated with FCE. In a case report of extrafoveal CNV with hemorrhage occurring in an eye with pachychoroid disease and FCE, the patient experienced subjective improvement in vision following two monthly injections of intravitreal Aflibercept. EDI-OCT and OCT-A demonstrated resolution of macular exudation and near-complete regression of a CNV lesion which originated from within the FCE.
Treatment of symptomatic PCV/AT1
vPDT monotherapy is the treatment modality most commonly used for PCV/AT1 associated with pachychoroid disease, with most studies reporting favorable 1 year visual outcomes and regression of aneurysms [106, 107]. However, long-term recurrences following vPDT monotherapy for PCV/AT1 is common with up to 50–60% of eyes developing recurrent exudation after the first year post-vPDT [108]. It has been suggested that lesions in which the aneurysms appear to form grape-like clusters often evolve into more typical CNV lesions associated with persistent or recurrent exudation despite disappearance of the visible aneurysms following treatment [109]. Therefore, following treatment, PCV/AT1 patients should continue to be monitored indefinitely.
Intravitreal anti-VEGF therapy with or without adjunctive vPDT is now commonly used as the standard treatment of symptomatic macular PCV/AT1 related to pachychoroid diseases [110, 111]. The use of anti-VEGF agents for this purpose is supported by findings of elevated intraocular levels of VEGF in eyes with PCV/AT1 [110]. Recently, two large randomized controlled trials, EVEREST II and PLANET, provided level 1 evidence for the use of intravitreal anti-VEGF therapy with or without vPDT for the treatment of PCV/AT1 [112, 113]. In the EVEREST II study, 322 patients were randomized to combination therapy with ranibizumab and vPDT vs. ranibizumab monotherapy [112]. Results showed that at month 12, the mean visual acuity gain of 8.3 letters in combined therapy group was significantly higher than the mean gain of 5.1 letters seen in the ranibizumab monotherapy group. The proportion of eyes with complete polyp regression at 12 months was also significantly higher in eyes treated with combination therapy compared with ranibizumab monotherapy (69.3 vs. 34.7%). Combination therapy was also able to reduce the number of ranibizumab treatments over 12 months, with a median of 4.0 injections in the combination group and a median of 7.0 injections in the monotherapy group. Change in central choroidal thickness was evaluated as one of the secondary outcomes in the EVEREST II study. The combination therapy cohort had a higher reduction (55.2 µm) in central choroidal thickness compared with the ranibizumab monotherapy cohort (30 µm) [114].
In the PLANET study, eyes with PCV/AT1 received three initial loading doses of aflibercept at 4 weekly intervals followed by regular fixed dosing of aflibercept with or without subsequent rescue vPDT [113]. The mean visual acuity improvements at 52 weeks were similar between eyes with or without rescue vPDT, with 10.9 letters and 10.7 letters, respectively. The rate of polyp closure was also similar between aflibercept-treated eyes with or without rescue vPDT, with rates of 44.8 and 38.9%, respectively. The changes in choroidal thickness for eyes in the PLANET study have not been reported. In another study evaluating the use of combined therapy with either ranibizumab or aflibercept followed by vPDT, choroidal thickness appeared to be an important factor with respect to visual outcomes [115]. At 12 months, better visual acuity and better visual acuity improvement were significantly associated with a greater subfoveal choroidal thickness at baseline in eyes treated with photodynamic therapy combined with intravitreal ranibizumab or Aflibercept. Other studies suggest that eyes with thicker and/or hyperpermeable choroids are more likely to respond poorly to intravitreal anti-VEGF monotherapy [2, 18].
Future research directions
Significant advances have occurred in our understanding of the pachychoroid disease spectrum. However, further research is needed to clarify several areas of uncertainty. For instance, it remains unclear why some eyes with thick choroids with large vessels show no evidence of RPE alterations or SRF (uncomplicated pachychoroid). Similarly, elucidating the pathophysiology leading to a “switch” from the non-neovascular pachychoroid disease entities PPE, CSC, FCE, and PPS to the neovascular variants (PCN and PCV/AT1) will be important in developing future therapies. The mechanisms which lead to the characteristic choroidal changes in the pachychoroid disease phenotype, as well as genetic and/or environmental factors remain to be clarified. To evaluate whether venous congestion may indeed be responsible for the dilated choroidal vessels, future imaging studies which enable in vivo determination of flow direction and velocity will need to be developed. Similarly, improved resolution of various imaging techniques will be necessary to allow more accurate quantification of choriocapillaris thickness and blood flow. If these questions can be answered, therapies aimed at modulating the pathologic changes within the choroid may be developed as a common therapeutic platform for all the conditions within the pachychoroid disease spectrum.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Mrejen S, Spaide RF. Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol. 2013;58:387–429. doi: 10.1016/j.survophthal.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Yanagi Y, Ting DSW, Ng WY, et al. Choroidal vascular hyperpermeability as a predictor of treatment response for polypoidal choroidal vasculopathy. Retina. 2017 Jul 12. Epub ahead of print. [DOI] [PubMed]
- 3.Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Hope-Ross M, Orlock DR. Digital indocyanine-green videoangiography of occult choroidal neovascularization. Ophthalmology. 1994;101:1727–35. doi: 10.1016/s0161-6420(13)31433-x. [DOI] [PubMed] [Google Scholar]
- 4.Dansingani KK, Balaratnasingam C, Naysan J, Freund KB. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina. 2016;36:499–516. doi: 10.1097/IAE.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara D, Mohler KJ, Waheed N, et al. En face enhanced-depth swept-source optical coherence tomography features of chronic central serous chorioretinopathy. Ophthalmology. 2014;121:719–26. doi: 10.1016/j.ophtha.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013;33:1659–72. doi: 10.1097/IAE.0b013e3182953df4. [DOI] [PubMed] [Google Scholar]
- 7.Pang CE, Freund KB. Pachychoroid pigment epitheliopathy may masquerade as acute retinal pigment epitheliitis. Invest Ophthalmol Vis Sci. 2014;55:5252. doi: 10.1167/iovs.14-14959. [DOI] [PubMed] [Google Scholar]
- 8.Margolis R, Mukkamala SK, Jampol LM, et al. The expanded spectrum of focal choroidal excavation. Arch Ophthalmol. 2011;129:1320–5. doi: 10.1001/archophthalmol.2011.148. [DOI] [PubMed] [Google Scholar]
- 9.Balaratnasingam C, Lee WK, Koizumi H, Dansingani K, Inoue M, Freund KB. Polypoidal choroidal vasculopathy: a distinct disease or manifestation of many? Retina. 2016;36:1–8. doi: 10.1097/IAE.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 10.Lee WK, Baek J, Dansingani KK, Lee JH, Freund KB. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or subnormal subfoveal choroidal thickness. Retina. 2016;36:S73–82. doi: 10.1097/IAE.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 11.Yannuzzi LA, Freund KB, Goldbaum M, et al. Polypoidal choroidal vasculopathy masquerading as central serous chorioretinopathy. Ophthalmology. 2000;107:767–77. doi: 10.1016/s0161-6420(99)00173-6. [DOI] [PubMed] [Google Scholar]
- 12.Fung AT, Yannuzzi LA, Freund KB. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina. 2012;32:1829–37. doi: 10.1097/IAE.0b013e3182680a66. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann M, Bousquet E, Beydoun T, Behar-Cohen F. PACHYCHOROID: an inherited condition? Retina. 2015;35:10–6. doi: 10.1097/IAE.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 14.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–73. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445–50. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011;118:840–5. doi: 10.1016/j.ophtha.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Ting DS, Ng WY, Ng SR, et al. Choroidal thickness changes in age-related macular degeneration and polypoidal choroidal vasculopathy: a 12-month prospective study. Am J Ophthalmol. 2016;164:128–36 e121. doi: 10.1016/j.ajo.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S. Relationship between clinical characteristics of polypoidal choroidal vasculopathy and choroidal vascular hyperpermeability. Am J Ophthalmol. 2013;155:305–13 e301. doi: 10.1016/j.ajo.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Gupta P, Ting DSW, Thakku SG, et al. Detailed characterization of choroidal morphologic and vascular features in age-related macular degeneration and polypoidal choroidal vasculopathy. Retina. 2017;37:2269–80. doi: 10.1097/IAE.0000000000001481. [DOI] [PubMed] [Google Scholar]
- 20.Ersoz M. Giray, Karacorlu Murat, Arf Serra, Hocaoglu Mumin, Sayman Muslubas Isil. OUTER NUCLEAR LAYER THINNING IN PACHYCHOROID PIGMENT EPITHELIOPATHY. Retina. 2018;38(5):957–961. doi: 10.1097/IAE.0000000000001655. [DOI] [PubMed] [Google Scholar]
- 21.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–5. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Jonas JB, Wei W. Choroidal vessel diameter in central serous chorioretinopathy. Acta Ophthalmol. 2013;91:e358–62. doi: 10.1111/aos.12059. [DOI] [PubMed] [Google Scholar]
- 23.Phasukkijwatana N, Freund KB, Dolz-Marco R, et al. Peripapillary pachychoroid syndrome. Retina. 2017 Nov 10. Epub ahead of print. [DOI] [PubMed]
- 24.Agrawal R, Gupta P, Tan KA, Cheung CM, Wong TY, Cheng CY. Choroidal vascularity index as a measure of vascular status of the choroid: measurements in healthy eyes from a population-based study. Sci Rep. 2016;6:21090. doi: 10.1038/srep21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ting DSW, Yanagi Y, Agrawal R, et al. Choroidal remodeling in age-related macular degeneration and polypoidal choroidal vasculopathy: a 12-month prospective study. Sci Rep. 2017;7:7868. doi: 10.1038/s41598-017-08276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agrawal R, Chhablani J, Tan KA, Shah S, Sarvaiya C, Banker A. Choroidal vascularity index in central serous chorioretinopathy. Retina. 2016;36:1646–51. doi: 10.1097/IAE.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 27.Ersoz MG, Arf S, Hocaoglu M, Sayman Muslubas I, Karacorlu M. Indocyanine green angiography of pachychoroid pigment epitheliopathy. Retina. 2017 Jul 18. Epub ahead of print. [DOI] [PubMed]
- 28.Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26–34. doi: 10.1016/s0002-9394(14)70531-8. [DOI] [PubMed] [Google Scholar]
- 29.Kitaya N, Nagaoka T, Hikichi T, et al. Features of abnormal choroidal circulation in central serous chorioretinopathy. Br J Ophthalmol. 2003;87:709–12. doi: 10.1136/bjo.87.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iida T, Kishi S, Hagimura N, Shimizu K. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina. 1999;19:508–12. doi: 10.1097/00006982-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Tsujikawa A, Ojima Y, Yamashiro K, et al. Punctate hyperfluorescent spots associated with central serous chorioretinopathy as seen on indocyanine green angiography. Retina. 2010;30:801–9. doi: 10.1097/IAE.0b013e3181c72068. [DOI] [PubMed] [Google Scholar]
- 32.Hata M, Oishi A, Shimozono M, Mandai M, Nishida A, Kurimoto Y. Early changes in foveal thickness in eyes with central serous chorioretinopathy. Retina. 2013;33:296–301. doi: 10.1097/IAE.0b013e31826710a0. [DOI] [PubMed] [Google Scholar]
- 33.Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103:2070–9. doi: 10.1016/s0161-6420(96)30386-2. [DOI] [PubMed] [Google Scholar]
- 34.Maumenee AE. Macular diseases: clinical manifestations. Trans Am Acad Ophthalmol Otolaryngol. 1965;69:605–13. [PubMed] [Google Scholar]
- 35.Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A, Orlock D. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057–62. doi: 10.1001/archopht.1994.01090200063023. [DOI] [PubMed] [Google Scholar]
- 36.Spaide RF, Klancnik JM., Jr. Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology. 2005;112:825–33. doi: 10.1016/j.ophtha.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Iida T, Yannuzzi LA, Spaide RF, Borodoker N, Carvalho CA, Negrao S. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003;23:1–7. doi: 10.1097/00006982-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, Spaide RF. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010;117:1792–9. doi: 10.1016/j.ophtha.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 39.Maruko I, Iida T, Sugano Y, Ojima A, Sekiryu T. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011;31:1603–8. doi: 10.1097/IAE.0b013e31820f4b39. [DOI] [PubMed] [Google Scholar]
- 40.Chung YR, Kim JW, Kim SW, Lee K. Choroidal thickness in patients with central serous chorioretinopathy: assessment of haller and sattler layers. Retina. 2016;36:1652–7. doi: 10.1097/IAE.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 41.Sonoda S, Sakamoto T, Kuroiwa N, et al. Structural changes of inner and outer choroid in central serous chorioretinopathy determined by optical coherence tomography. PLoS ONE. 2016;11:e0157190. doi: 10.1371/journal.pone.0157190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonini Filho MA, de Carlo TE, Ferrara D, et al. Association of choroidal neovascularization and central serous chorioretinopathy with optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:899–906. doi: 10.1001/jamaophthalmol.2015.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015;35:1–9. doi: 10.1097/IAE.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 44.Dansingani KK, Balaratnasingam C, Klufas MA, Sarraf D, Freund KB. Optical coherence tomography angiography of shallow irregular pigment epithelial detachments in pachychoroid spectrum disease. Am J Ophthalmol. 2015;160:1243–54 e1242. doi: 10.1016/j.ajo.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 45.Peiretti E, Ferrara DC, Caminiti G, Mura M, Hughes J. Choroidal neovascularization in caucasian patients with longstanding central serous chorioretinopathy. Retina. 2015;35:1360–7. doi: 10.1097/IAE.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 46.Sato T, Kishi S, Watanabe G, Matsumoto H, Mukai R. Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina. 2007;27:589–94. doi: 10.1097/01.iae.0000249386.63482.05. [DOI] [PubMed] [Google Scholar]
- 47.Bousquet Elodie, Bonnin Sophie, Mrejen Sarah, Krivosic Valérie, Tadayoni Ramin, Gaudric Alain. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY OF FLAT IRREGULAR PIGMENT EPITHELIUM DETACHMENT IN CHRONIC CENTRAL SEROUS CHORIORETINOPATHY. Retina. 2018;38(3):629–638. doi: 10.1097/IAE.0000000000001580. [DOI] [PubMed] [Google Scholar]
- 48.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV) Retina. 1990;10:1–8. [PubMed] [Google Scholar]
- 49.Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995;15:100–10. doi: 10.1097/00006982-199515020-00003. [DOI] [PubMed] [Google Scholar]
- 50.Dansingani Kunal K, Gal-Or Orly, Sadda Srinivas R, Yannuzzi Lawrence A, Freund K Bailey. Understanding aneurysmal type 1 neovascularization (polypoidal choroidal vasculopathy): a lesson in the taxonomy of ‘expanded spectra’ - a review. Clinical & Experimental Ophthalmology. 2017;46(2):189–200. doi: 10.1111/ceo.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong CW, Yanagi Y, Lee WK, et al. Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res. 2016;53:107–39. doi: 10.1016/j.preteyeres.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–16. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 53.Nakashizuka H, Yuzawa M. Hyalinization of choroidal vessels in polypoidal choroidal vasculopathy. Surv Ophthalmol. 2011;56:278–9. doi: 10.1016/j.survophthal.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol. 2002;133:639–48. doi: 10.1016/s0002-9394(02)01404-6. [DOI] [PubMed] [Google Scholar]
- 55.Huang LZ, Li YJ, Xie XF, et al. Whole-exome sequencing implicates UBE3D in age-related macular degeneration in East Asian populations. Nat Commun. 2015;6:6687. doi: 10.1038/ncomms7687. [DOI] [PubMed] [Google Scholar]
- 56.Baek JDK, Lee WK, Freund KB. Choroidal morphology in eyes with peripapillary polypoidal choroidal vasculopathy. Retina. 2018 May 8. Epub ahead of print. [DOI] [PubMed]
- 57.Obata R, Takahashi H, Ueta T, Yuda K, Kure K, Yanagi Y. Tomographic and angiographic characteristics of eyes with macular focal choroidal excavation. Retina. 2013;33:1201–10. doi: 10.1097/IAE.0b013e31827b6452. [DOI] [PubMed] [Google Scholar]
- 58.Chung H, Byeon SH, Freund KB. Focal choroidal excavation and its association with pachychoroid spectrum disorders: a review of the literature and multimodal imaging findings. Retina. 2017;37:199–221. doi: 10.1097/IAE.0000000000001345. [DOI] [PubMed] [Google Scholar]
- 59.Ellabban AA, Tsujikawa A, Ooto S, et al. Focal choroidal excavation in eyes with central serous chorioretinopathy. Am J Ophthalmol. 2013;156:673–83. doi: 10.1016/j.ajo.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Lim FP, Loh BK, Cheung CM, Lim LS, Chan CM, Wong DW. Evaluation of focal choroidal excavation in the macula using swept-source optical coherence tomography. Eye. 2014;28:1088–94. doi: 10.1038/eye.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shinojima A, Kawamura A, Mori R, Yuzawa M. Morphologic features of focal choroidal excavation on spectral domain optical coherence tomography with simultaneous angiography. Retina. 2014;34:1407–14. doi: 10.1097/IAE.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 62.Lim FP, Wong CW, Loh BK, et al. Prevalence and clinical correlates of focal choroidal excavation in eyes with age-related macular degeneration, polypoidal choroidal vasculopathy and central serous chorioretinopathy. Br J Ophthalmol. 2016;100:918–23. doi: 10.1136/bjophthalmol-2015-307055. [DOI] [PubMed] [Google Scholar]
- 63.Lee JH, Lee WK. Choroidal neovascularization associated with focal choroidal excavation. Am J Ophthalmol. 2014;157:710–8 e711. doi: 10.1016/j.ajo.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 64.Xu H, Zeng F, Shi D, Sun X, Chen X, Bai Y. Focal choroidal excavation complicated by choroidal neovascularization. Ophthalmology. 2014;121:246–50. doi: 10.1016/j.ophtha.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 65.Luk FO, Fok AC, Lee A, Liu AT, Lai TY , Medscape. Focal choroidal excavation in patients with central serous chorioretinopathy. Eye. 2015;29:453–9. doi: 10.1038/eye.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984;91:1554–72. doi: 10.1016/s0161-6420(84)34117-3. [DOI] [PubMed] [Google Scholar]
- 67.Spaide Richard F. DISEASE EXPRESSION IN NONEXUDATIVE AGE-RELATED MACULAR DEGENERATION VARIES WITH CHOROIDAL THICKNESS. Retina. 2018;38(4):708–716. doi: 10.1097/IAE.0000000000001689. [DOI] [PubMed] [Google Scholar]
- 68.Spaide Richard F. IMPROVING THE AGE-RELATED MACULAR DEGENERATION CONSTRUCT. Retina. 2018;38(5):891–899. doi: 10.1097/IAE.0000000000001732. [DOI] [PubMed] [Google Scholar]
- 69.Zhao M, Valamanesh F, Celerier I, et al. The neuroretina is a novel mineralocorticoid target: aldosterone up-regulates ion and water channels in Muller glial cells. FASEB J. 2010;24:3405–15. doi: 10.1096/fj.09-154344. [DOI] [PubMed] [Google Scholar]
- 70.Zhao M, Celerier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122:2672–9. doi: 10.1172/JCI61427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Behar-Cohen F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2013;33:2096–102. doi: 10.1097/IAE.0b013e318297a07a. [DOI] [PubMed] [Google Scholar]
- 72.Sardell RJ, Nittala MG, Adams LD, et al. Heritability of choroidal thickness in the amish. Ophthalmology. 2016;123:2537–44. doi: 10.1016/j.ophtha.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Switzer DW, Jr., Mendonca LS, Saito M, Zweifel SA, Spaide RF. Segregation of ophthalmoscopic characteristics according to choroidal thickness in patients with early age-related macular degeneration. Retina. 2012;32:1265–71. doi: 10.1097/IAE.0b013e31824453ac. [DOI] [PubMed] [Google Scholar]
- 74.Dansingani KK, Perlee LT, Hamon S, et al. Risk alleles associated with neovascularization in a pachychoroid phenotype. Ophthalmology. 2016;123:2628–30. doi: 10.1016/j.ophtha.2016.06.060. [DOI] [PubMed] [Google Scholar]
- 75.Cheng CY, Yamashiro K, Chen LJ, et al. New loci and coding variants confer risk for age-related macular degeneration in East Asians. Nat Commun. 2015;6:6063. doi: 10.1038/ncomms7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roisman L, Zhang Q, Wang RK, et al. Optical coherence tomography angiography of asymptomatic neovascularization in intermediate age-related macular degeneration. Ophthalmology. 2016;123:1309–19. doi: 10.1016/j.ophtha.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yanagi Y, Mohla A, Lee WK, et al. Prevalence and risk factors for nonexudative neovascularization in fellow eyes of patients with unilateral age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2017;58:3488–95. doi: 10.1167/iovs.16-21167. [DOI] [PubMed] [Google Scholar]
- 78.Manayath George J., Shah Vanee Sheth, Saravanan Veerappan R., Narendran Venkatapathy. POLYPOIDAL CHOROIDAL VASCULOPATHY ASSOCIATED WITH CENTRAL SEROUS CHORIORETINOPATHY. Retina. 2018;38(6):1195–1204. doi: 10.1097/IAE.0000000000001665. [DOI] [PubMed] [Google Scholar]
- 79.Sharma T, Shah N, Rao M, et al. Visual outcome after discontinuation of corticosteroids in atypical severe central serous chorioretinopathy. Ophthalmology. 2004;111:1708–14. doi: 10.1016/j.ophtha.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 80.Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi: 10.1016/j.preteyeres.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013;58:103–26. doi: 10.1016/j.survophthal.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robertson DM, Ilstrup D. Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy. Am J Ophthalmol. 1983;95:457–66. doi: 10.1016/0002-9394(83)90265-9. [DOI] [PubMed] [Google Scholar]
- 83.Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984;68:815–20. doi: 10.1136/bjo.68.11.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kretz FT, Beger I, Koch F, Nowomiejska K, Auffarth GU, Koss MJ. Randomized clinical trial to compare micropulse photocoagulation versus half-dose verteporfin photodynamic therapy in the treatment of central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging Retin. 2015;46:837–43. doi: 10.3928/23258160-20150909-08. [DOI] [PubMed] [Google Scholar]
- 85.Scholz P, Altay L, Fauser S. Comparison of subthreshold micropulse laser (577 nm) treatment and half-dose photodynamic therapy in patients with chronic central serous chorioretinopathy. Eye. 2016;30:1371–7. doi: 10.1038/eye.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen SN, Hwang JF, Tseng LF, Lin CJ. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology. 2008;115:2229–34. doi: 10.1016/j.ophtha.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 87.Yannuzzi LA, Slakter JS, Gross NE, et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003;23:288–98. doi: 10.1097/00006982-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 88.Ma J, Meng N, Xu X, Zhou F, Qu Y. System review and meta-analysis on photodynamic therapy in central serous chorioretinopathy. Acta Ophthalmol. 2014;92:e594–601. doi: 10.1111/aos.12482. [DOI] [PubMed] [Google Scholar]
- 89.Izumi T, Koizumi H, Maruko I, et al. Structural analyses of choroid after half-dose verteporfin photodynamic therapy for central serous chorioretinopathy. Br J Ophthalmol. 2017;101:433–7. doi: 10.1136/bjophthalmol-2016-308921. [DOI] [PubMed] [Google Scholar]
- 90.Chan WM, Lai TY, Lai RY, Tang EW, Liu DT, Lam DS. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina. 2008;28:85–93. doi: 10.1097/IAE.0b013e318156777f. [DOI] [PubMed] [Google Scholar]
- 91.Lim JI, Glassman AR, Aiello LP, et al. Collaborative retrospective macula society study of photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology. 2014;121:1073–8. doi: 10.1016/j.ophtha.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 92.Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008;115:1756–65. doi: 10.1016/j.ophtha.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 93.Lai TY, Wong RL, Chan WM. Long-term outcome of half-dose verteporfin photodynamic therapy for the treatment of central serous chorioretinopathy (an American ophthalmological society thesis) Trans Am Ophthalmol Soc. 2015;113:T8. [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao MW, Zhou P, Xiao HX, et al. Photodynamic therapy for acute central serous chorioretinopathy: the safe effective lowest dose of verteporfin. Retina. 2009;29:1155–61. doi: 10.1097/IAE.0b013e3181a6c028. [DOI] [PubMed] [Google Scholar]
- 95.Forooghian F, Meleth AD, Cukras C, Chew EY, Wong WT, Meyerle CB. Finasteride for chronic central serous chorioretinopathy. Retina. 2011;31:766–71. doi: 10.1097/IAE.0b013e3181f04a35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nielsen JS, Jampol LM. Oral mifepristone for chronic central serous chorioretinopathy. Retina. 2011;31:1928–36. doi: 10.1097/IAE.0b013e31821c3ef6. [DOI] [PubMed] [Google Scholar]
- 97.Bousquet E, Beydoun T, Rothschild PR, et al. Spironolactone for nonresolving central serous chorioretinopathy: a randomized controlled crossover study. Retina. 2015;35:2505–15. doi: 10.1097/IAE.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghadiali Q, Jung JJ, Yu S, Patel SN, Yannuzzi LA. Central serous chorioretinopathy treated with mineralocorticoid antagonists: a one-year pilot study. Retina. 2016;36:611–8. doi: 10.1097/IAE.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz R, Habot-Wilner Z, Martinez MR, et al. Eplerenone for chronic central serous chorioretinopathy-a randomized controlled prospective study. Acta Ophthalmol. 2017;95:e610–8. doi: 10.1111/aos.13491. [DOI] [PubMed] [Google Scholar]
- 100.Chung YR, Seo EJ, Lew HM, Lee KH. Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review. Eye. 2013;27:1339–46. doi: 10.1038/eye.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Staurenghi Giovanni, Lai Timothy Y.Y., Mitchell Paul, Wolf Sebastian, Wenzel Andreas, Li Jun, Bhaumik Amitabha, Hykin Philip G. Efficacy and Safety of Ranibizumab 0.5 mg for the Treatment of Macular Edema Resulting from Uncommon Causes. Ophthalmology. 2018;125(6):850–862. doi: 10.1016/j.ophtha.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 102.Chhablani J, Kozak I, Pichi F, et al. Outcomes of treatment of choroidal neovascularization associated with central serous chorioretinopathy with intravitreal antiangiogenic agents. Retina. 2015;35:2489–97. doi: 10.1097/IAE.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 103.Lai TYY, Staurenghi G, Lanzetta P, et al. Efficacy and safety of ranibizumab for the treatment of choroidal neovascularization due to uncommon cause: twelve-month results of the MINERVA study. Retina. 2017 Jul 12. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 104.Hata M, Yamashiro K, Ooto S, et al. Intraocular vascular endothelial growth factor levels in pachychoroid neovasculopathy and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:292–8. doi: 10.1167/iovs.16-20967. [DOI] [PubMed] [Google Scholar]
- 105.Lee JH, Lee WK. One-year results of adjunctive photodynamic therapy for type 1 neovascularization associated with thickened choroid. Retina. 2016;36:889–95. doi: 10.1097/IAE.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 106.Chan WM, Lam DS, Lai TY, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology. 2004;111:1576–84. doi: 10.1016/j.ophtha.2003.12.056. [DOI] [PubMed] [Google Scholar]
- 107.Gomi F, Ohji M, Sayanagi K, et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008;115:141–6. doi: 10.1016/j.ophtha.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 108.Silva RM, Figueira J, Cachulo ML, Duarte L, Faria de Abreu JR, Cunha-Vaz JG. Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefes Arch Clin Exp Ophthalmol. 2005;243:973–9. doi: 10.1007/s00417-005-1139-4. [DOI] [PubMed] [Google Scholar]
- 109.Lee WK, Kim KS, Kim W, Lee SB, Jeon S. Responses to photodynamic therapy in patients with polypoidal choroidal vasculopathy consisting of polyps resembling grape clusters. Am J Ophthalmol. 2012;154:355–65 e351. doi: 10.1016/j.ajo.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 110.Tong JP, Chan WM, Liu DT, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006;141:456–62. doi: 10.1016/j.ajo.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 111.Cheung Chui Ming Gemmy, Lai Timothy Y.Y., Ruamviboonsuk Paisan, Chen Shih-Jen, Chen Youxin, Freund K. Bailey, Gomi Fomi, Koh Adrian H., Lee Won-Ki, Wong Tien Yin. Polypoidal Choroidal Vasculopathy. Ophthalmology. 2018;125(5):708–724. doi: 10.1016/j.ophtha.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 112.Koh A, Lai TYY, Takahashi K, et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol. 2017;135:1206–13. doi: 10.1001/jamaophthalmol.2017.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tomohiro I. Results of the PLANET study. 10th Asia-Pacific Vitreo-Retina Society Congress. Bangkok; 2016.
- 114.Lai TYGT, Margaron P, Tan C. Anatomical outcomes of ranibizumab 0.5 mg combined with verteporfin photodynamic therapy versus ranibizumab monotherapy in patients with polypoidal choroidal vasculopathy: 12-month results from the EVEREST II study. 17th EURETINA Congress; Barcelona; 2017.
- 115.Sakurada Y, Sugiyama A, Tanabe N, Kikushima W, Kume A, Iijima H. Choroidal thickness as a prognostic factor of photodynamic therapy with aflibercept or ranibizumab for polypoidal choroidal vasculopathy. Retina. 2017;37:1866–72. doi: 10.1097/IAE.0000000000001427. [DOI] [PubMed] [Google Scholar]