Abstract
Palladium-catalyzed cross-coupling reactions are one of the most powerful and versatile methods to synthesize a wide range of complex functionalized molecules. However, the development of solid-state cross-coupling reactions remains extremely limited. Here, we report a rational strategy that provides a general entry to palladium-catalyzed Buchwald-Hartwig cross-coupling reactions in the solid state. The key finding of this study is that olefin additives can act as efficient molecular dispersants for the palladium-based catalyst in solid-state media to facilitate the challenging solid-state cross-coupling. Beyond the immediate utility of this protocol, our strategy could inspire the development of industrially attractive solvent-free palladium-catalyzed cross-coupling processes for other valuable synthetic targets.
Cross-coupling reactions have been achieved in solution, yet tend to be inefficient in a solid state. Here, the authors report a solid-state palladium-catalyzed Buchwald-Hartwig cross-coupling using olefins as molecular dispersants, enabling reduction of solvent waste.
Introduction
Historically, most organic transformations have been carried out in solution. Such solution-based organic syntheses generally require liquid organic solvents to dissolve reactants or catalysts in a reaction flask. Accordingly, the pharmaceutical industry and the fine chemicals industry strongly depend on solvent-based organic synthesis, which has led to serious problems with regard to solvent waste, as organic solvents usually account for ~80–90% of the total mass used in any organic reaction1–3. Although solvent recycling is a very effective way to reduce solvent waste, organic chemists should focus on (re)designing organic syntheses to use less or no solvent. In this context, solid-state organic transformations have attracted considerable attention as cleaner and sustainable synthetic alternatives4–7. In addition, these methods would be exciting opportunities to access large areas of hitherto unexplored chemical space that exhibit different reactivity and selectivity compared to conventional solution-based reactions8–20.
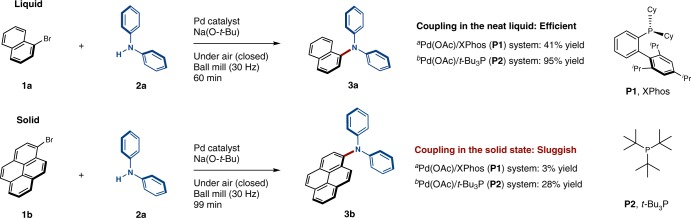
Palladium-catalyzed cross-coupling reactions have long been used as arguably the most powerful, versatile, and well-established organic transformations with broad applications ranging from natural product synthesis and medicinal chemistry to polymer and materials science21–24. Despite recent significant progress, the exploration of new strategies, reaction media, and concepts for the improvement of the sustainability of cross-coupling reactions still remains an important and challenging research subject. Conventionally, palladium-catalyzed cross-coupling reactions of liquid and solid substrates are conducted in organic solvents (Fig. 1a)21,22. When using liquid substrates, the cross-coupling reactions can in some cases be carried out under neat conditions, where liquid substrates serve as reactants and reaction solvent (Fig. 1a)25–32. Whereas the benefits of these solution-based reactions are well-established, the value of palladium-catalyzed cross-coupling processes becomes even more apparent when solid-state cross-coupling reactions are considered, especially in the context of solvent-waste prevention and environmental protection4–7. In addition, solid-state coupling reactions should be particularly useful for poorly soluble substrate classes such as large polycyclic aromatics due to strong intermolecular interactions (e.g., π–π interactions). Nevertheless, the solid state has remained extremely limited as a reaction medium for palladium-catalyzed cross-coupling processes (Fig. 1a)21,22. Thus, we sought to re-design palladium-based catalyst systems for the solid state, which could potentially unlock versatile applications for solid-state synthesis.
Fig. 1.
Overview of the olefin-accelerated solid-state couplings using mechanochemistry. a Current application range of palladium-catalyzed cross-coupling reactions. b General and scalable solid-state C–N cross-coupling reactions using olefin additives as molecular dispersants. c A proposed acceleration mechanism, wherein olefin additives could act as dispersants for catalysts in solid-state media and facilitate the solid-state cross-coupling
Mechanochemical solvent-free reactions using ball milling or milling with a catalytic amount of liquid, the so-called liquid-assisted grinding (LAG), have emerged as powerful alternatives to synthesis in solution33–40, and mechanochemical palladium-catalyzed cross-coupling reactions have already been reported41–53. However, these methods focus mostly on neat liquids41–46,48–53. For solid-state substrates, the scope is significantly more restricted and low conversion rates are common42,47,49. Here, we report the development of a potentially general and scalable solvent-free method for solid-state palladium-catalyzed C–N cross-coupling reactions using mechanochemistry (Fig. 1b)54. The key finding in this study is that the addition of a small amount of olefin dramatically accelerates these challenging solid-state cross-coupling reactions. Based on a transmission electron microscopy (TEM) analysis, we discovered that some such olefin additives can act as efficient molecular dispersants for palladium catalysts in solid-state media to inhibit undesired aggregation of the catalyst that may lead to catalyst deactivation (Fig. 1c). The protocol should be particularly useful for the rapid access to structurally complex triarylamines, which can be found in a wide range of organic materials including solar cells and light-emitting diodes (Fig. 1b)55–57. In fact, we will demonstrate (vide infra) that when using the solid-state cross-coupling reaction presented herein, arylamine-based hole-transporting materials can be prepared faster and in better yield relative to conventional methods57. Thus, we anticipate that the present solvent-free solid-state palladium-catalyzed cross-coupling reactions may potentially find broad applications in industrially relevant syntheses.
Results
Development of solid-state C–N cross-coupling
All reactions were conducted in a Retsch MM400 mill (stainless-steel milling jar; 30 Hz; stainless-steel balls). Initially, we compared the reactivity of liquid 1-bromonaphthalene (1a) and solid 1-bromopyrene (1b) in the palladium-catalyzed C–N cross-coupling reaction with diphenylamine 2a under solvent-free mechanochemical conditions (Fig. 2). Very recently, Su and co-workers have reported the mechanochemical palladium-catalyzed C–N cross-coupling of aryl chloride using NaCl as a mechanochemical auxiliary51. Even though this development is indisputably remarkable, the substrate scope mostly focused on liquid substrates. Thus, we anticipated that the reaction of liquid substrate 1a should proceed readily using the Pd(OAc)2/XPhos (P1) catalyst system developed by Su51. Indeed, the corresponding coupling product (3a) was obtained in moderate yield (41% yield; Fig. 2). When we used the Pd(OAc)2/t-Bu3P (P2) catalyst system, which is a high-performance catalyst for C–N coupling that has been reported by Hartwig and co-workers58, 3a was obtained quantitatively (95% yield; Fig. 2). Other liquid aryl halides could also be coupled with diphenylamine 2a in the presence of Pd(OAc)2/t-Bu3P (P2) in high yield (see Supplementary Figure 3). Then, we proceeded to investigate the C–N cross-coupling behavior of solid 1-bromopyrene (1b) (Fig. 2). We found that the solid-state cross-coupling reactions involving 1b were sluggish using either the Pd(OAc)2/XPhos (P1) or the Pd(OAc)2/t-Bu3P (P2) catalyst systems (3% and 28% yield, respectively; Fig. 2). These results suggest the presence of a considerable reactivity gap between liquid and solid substrates, even under mechanochemical conditions.
Fig. 2.
Comparison of the reactivity of liquid and solid aryl bromides. aThe following reaction conditions51 were used: 0.6 mmol of 1; 0.5 mmol of 2a; 0.01 mmol of Pd(OAc)2; 0.02 mmol of XPhos (P1); 1.0 mmol of Na(O-t-Bu); 2.0 g of NaCl; in a stainless-steel ball-milling jar (25 mL) with two stainless-steel balls (15 mm); 30 Hz; 99 min. bThe following reaction conditions were used: 0.5 mmol of 1; 0.5 mmol of 2a; 0.025 mmol of Pd(OAc)2; 0.025 mmol of ligand; 0.75 mmol of Na(O-t-Bu); in a stainless-steel ball-milling jar (1.5 mL) with a stainless-steel ball (3 mm); 30 Hz; 99 min
We therefore decided to focus on commonly used phosphine ligands in solvent-based systems in order to potentially facilitate the solid-state C–N cross-coupling under mechanochemical conditions (entries 1–10, Fig. 3a)54. Experiments involving catalyst systems consisting of 5 mol% Pd(OAc)2 and 5 mol% phosphine ligand revealed that the use of bulky and highly electron-donating monophosphines such as Ad3P (P3) provides the desired coupling product in low yield (18% yield; entry 1). In contrast, the use of Cy3P (P4) did not afford the targeted coupling product (entry 2). The reaction did not proceed when commonly used Buchwald-type ligands such as P5–P9 were employed (entries 3–7)54. While P5 is a highly effective ligand for C–N cross-coupling reactions in solvents at room temperature, the desired product was not formed under solid-state conditions (entry 3)59. Diphosphine ligands such as rac-Binap (P10) and Xantphos (P11) were also examined, but a reaction was not observed (entries 8 and 9). Increasing the catalyst loading did not improve the product yield when P2 was used as a ligand (33% yield; entry 10). Next, we attempted LAG, which uses substoichiometric liquid additives, to improve the reactivity (entries 11–20)33–40. Unless otherwise noted, the following reactions with liquid additives are all characterized by a 0.20 ratio of μL of liquid olefin added per mg of reactant. Although small amounts of toluene, benzene, and tetrahydrofuran (THF), which are commonly used organic solvents in a palladium-catalyzed C–N cross-coupling reactions54, slightly improved the yield of 3b, the yields remained moderate (20–55% yield; entries 11–15). Other commonly used solvents such as dimethylsulfoxide (DMSO), acetonitrile (MeCN). and hexane did not or poorly promote the solid-state cross-coupling (0%, 10% and 16% yield, respectively; entries 16–18). Lastly, we attempted cycloalkanes such as cyclohexane and cyclooctane, which resulted in moderate yields (54% and 46% yield, respectively; entries 19 and 20).
Fig. 3.
Development of olefin-accelerated solid-state C–N cross-coupling reactions. a Comparison of phosphine ligands and LAG additives in solid-state C–N cross-coupling reactions. b Discovery of olefins as molecular dispersants for palladium catalysts. Unless otherwise noted, the following reaction conditions were used: 0.5 mmol of 1b; 0.5 mmol of 2a; 0.025 mmol of Pd(OAc)2; 0.025 mmol of ligand; 0.75 mmol of Na(O-t-Bu); additive (0.20 μL mg–1); in a stainless-steel ball-milling jar (1.5 mL) with a stainless-steel ball (3 mm); 30 Hz; 99 min. Yields were determined by 1H NMR analysis using an internal standard. a10 mol% Pd(OAc)2 and t-Bu3P (P2) were used. bToluene (0.13 μL mg–1) was used
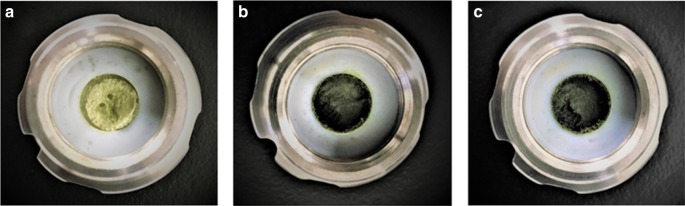
We speculated that one possible reason for the observed reactivity difference between liquid and solid substrates may be facile aggregation of the palladium catalysts in the solid-state reaction mixtures that may lead to catalyst deactivation. Thus, we wondered whether olefin additives could be used as molecular dispersants for the palladium catalyst, i.e., the olefins could coordinate toward any off-cycle palladium species and suppress higher aggregation of catalysts in the solid-state medium (Fig. 3b)60. The following reactions with olefins are all characterized by a 0.20 ratio of microliters of liquid olefin added per milligram of reactant. Initially, 1,5-cyclooctadiene (1,5-cod) was tested, given that 1,5-cod is frequently employed as a weak coordination ligand for low-valent metal complexes. Pleasingly, we found that the reaction in the presence of Pd(OAc)2/t-Bu3P was dramatically accelerated to form the corresponding coupling product (3b) in 99% yield. The use of cyclooctene as an additive also effectively promoted the reaction (96% yield). In sharp contrast, the reaction with cyclooctane did not dramatically affect the reactivity (46% yield), suggesting that the presence of an olefin functional group should be important for the observed acceleration. We noted that the colors of the solid-state reaction mixture after the reaction changed depending on the conditions applied (Fig. 4). For example, the reaction mixtures containing 1,5-cod or cyclooctane appeared as dark green waxy solids, while the reaction mixture without additives appeared as a light-yellow solid. Other olefins such as 1-hexene and (E)-hex-3-ene could also be used to facilitate the solid-state cross-coupling reaction (98% and 89% yield, respectively). In contrast, the use of hexane did not influence on the reactivity (16% yield). Cyclohexene also provided a better yield (92% yield) than cyclohexane (54% yield). Interestingly, the use of norbornadiene decreased the catalytic activity of this reaction (12% yield). This might be due to the irreversible coordination of norbornadiene to active Pd(0) species. Alkynes such as oct-4-yne also showed a moderate acceleration effect (66% yield). It should furthermore be noted that the amount of olefin used in these reactions is comparable to that of the reactant (0.20 μL mg–1), which stands in sharp contrast to conventional solution-based reactions, where often a 10- to 100-fold excess of bulk solvent is used.
Fig. 4.
Reaction mixtures after grinding in a ball mill. Aggregation on the milling ball a after 99 min without additive, b after 99 min with 1,5-cod, and c after 99 min with cyclooctane
Transmission electron microscopy
To gain mechanistic insight into the observed acceleration effect upon adding olefins, we used transmission electron microscopy (TEM) to characterize the palladium nanoparticles generated in situ in the crude reaction mixture of 1b with 2a (Fig. 5). The observed image clearly shows the formation of palladium nanoparticles (approximate size: 3–5 nm) in the reaction mixture in the presence of 1,5-cod (Fig. 5a). Notably, higher aggregation of the palladium particles was not observed. On the other hand, the image obtained for the palladium species derived from the reaction mixture in the presence of cyclooctane (Fig. 5b) and in the absence of any additives (Fig. 5c) showed that the palladium species significantly aggregate into dense particles (Fig 5b, c). Although further mechanistic investigations on how the catalyst diffuses in the solid-state media should provide a deep understanding on the reaction mechanism, such a study would be extremely challenging, i.e., the mechanochemical reaction setup is unlike to be compatible with an in situ extended X-ray absorption fine structure (EXAFS) analysis. Based on the TEM analysis, we would like to propose two possible roles for the olefin additives in these solid-state cross-coupling reactions: (1) olefins could act as dispersants for the palladium catalysts to suppress higher aggregation of the nanoparticles that may lead to catalyst deactivation;60 (2) the leaching rates for active monomeric Pd(0) specie from the palladium nanoparticles would be accelerated by coordination from the olefins, and the dissociated Pd(0) species could be coordinated by t-Bu3P and release the olefin ligands to form [(t-Bu3P)Pd(0)], which could subsequently activate the C–X bond of aryl halides61–63.
Fig. 5.
TEM images of palladium nanoparticles in the crude reaction mixtures. a Crude mixture after 99 min with 1,5-cod, b crude mixture after 99 min with cyclooctane, and c crude mixture after 99 min without additive. Scale bars in the TEM images (bottom left): 20 nm. These results clearly show that 1,5-cod can act as a molecular dispersant for the palladium catalyst in the solid-state reaction mixture, thus facilitating the solid-state C–N cross-coupling reaction
Substrate scope of the solid-state C–N cross-coupling reaction
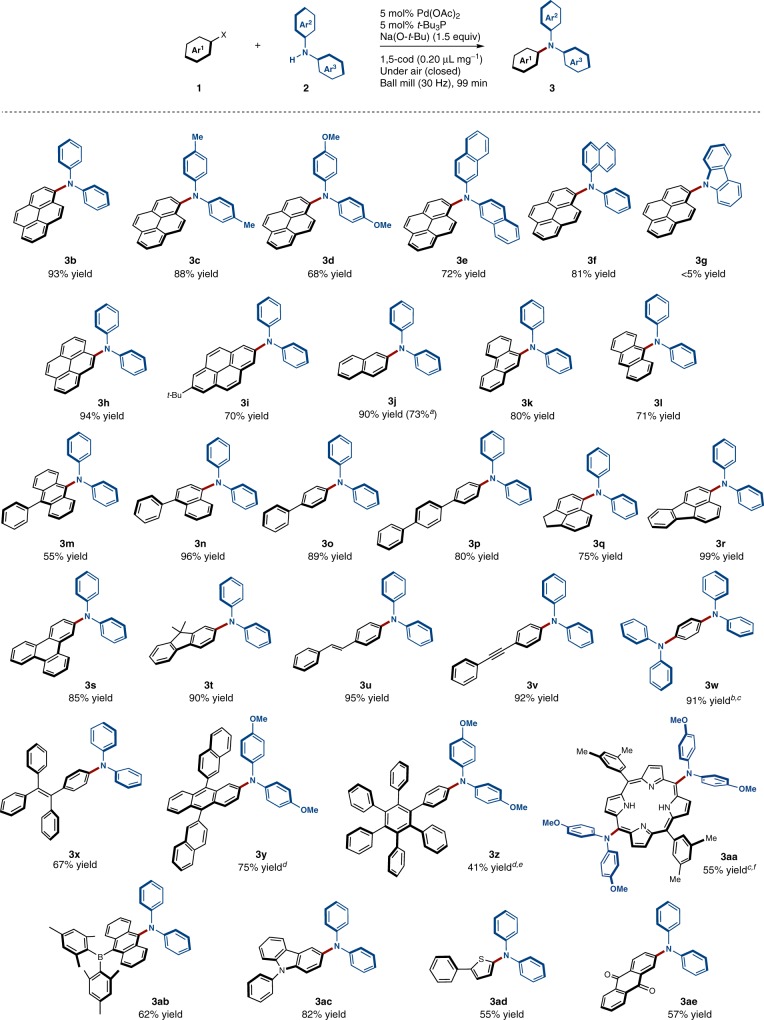
To explore the scope of the present solid-state coupling reaction, various amine nucleophiles were tested (Fig. 6). Both bis(4-methylphenyl) amine (2c) and bis(4-methoxyphenyl)amine (2d) were coupled in high yield (88% and 68%, respectively) under the optimized reaction conditions. Diarylamines containing a naphthyl group (2e and 2f) are also compatible with the applied solid-state conditions (72% and 81%, respectively). Conversely, the reaction did not proceed for carbazole (2g). As solvent-free solid-state reactions can be regarded as reactions that proceed under extremely-high-concentration conditions, the reagents and catalysts in the solid-state interact much more strongly with each other than those in solution. This could tentatively explain the observed low reactivity of 2g, which could potentially coordinate strongly to any off-cycle palladium species, which would lead to catalyst deactivation.
Fig. 6.
Substrate scope. Unless otherwise noted, the following reaction conditions were used: 0.5 mmol of 1; 0.5 mmol of 2; 0.025 mmol of Pd(OAc)2; 0.025 mmol of t-Bu3P; 0.75 mmol of Na(O-t-Bu); 1,5-cod (0.20 μL mg–1); stainless-steel ball-milling jar (1.5 mL) with a stainless-steel ball (3 mm); 30 Hz; 99 min. Isolated yields are shown. aThe aryl chloride was used as a substrate. b0.3 mmol scale. c10 mol% of catalyst and 3.0 equiv of Na(O-t-Bu) were used. d10 mol% of catalyst was used. eA larger stainless-steel ball-milling jar (25 mL) was used with four stainless-steel balls (10 mm). f0.2 mmol scale
Subsequently, we turned our attention to the scope of the aryl halides (Fig. 6). This reaction is characterized by a broad substrate scope and permits constructing a wide range of functionalized triarylamines containing large polycyclic hydrocarbon cores. For example, pyrene derivatives (1b, 1h, 1i) were cleanly coupled with diphenylamine nucleophile 2a to provide 3b, 3h, and 3i in good to excellent yield (93%, 94%, and 70%, respectively). Other aromatic cores such as naphthalene (1j and 1n), phenanthrene (1k), anthracene (1l and 1m), biphenyl (1o), terphenyl (1p), acenaphthene (1q and 1r), triphenylene (1s), and fluorene (1t) efficiently formed the corresponding triarylamines in 71–99%. Notably, 3j was also obtained from the corresponding 2-chloronaphthalene (73%). Triarylamines containing stilbene (3u) and internal alkyne (3v) moieties were generated in high yield (95% and 92%) under the optimized solvent-free conditions. Double aminations also proceeded smoothly to give the corresponding product (3w) in 91%. The reaction of 1x afforded triarylamine-containing tetraphenylethylene 3x (67%), which is a mechanochromic luminescent material64. The present strategy can also be applied to large aromatic substrates (1y and 1z; 75% and 41%, respectively), which are usually poorly soluble. Finally, this method was applied to heteroatom-substituted aromatic substrates (3aa–3ae). Aminoporphyrins, for example, are promising and important core structures in organic materials, especially in the context of porphyrin-sensitized solar cells55,56. Even though palladium-catalyzed cross-coupling reactions between diarylamine nucleophiles and porphyrin electrophiles are widely used to access aminoporphyrin derivatives, such reactions commonly require considerable amounts of organic solvents, which often hampers carrying out this process in large-scale syntheses55,56. Pleasingly, the reaction of bromo-substituted porphyrin 1aa proceeded efficiently under the solid-state conditions to provide aminoporphyrin 3aa in good yield (55%). Dimesitylboryl-containing triarylamine 3ab, which is widely known as a donor-acceptor-type charge-transfer luminescent material65,66, can also be prepared via this solid-state cross-coupling reaction (62%). Carbazole-, thiophene-, and 1,4-benzoquinone-containing triarylamines (3ac–3ae) were also obtained in good yield (82%, 55%, and 57%, respectively).
Solid-state coupling reactions on the gram-scale
To demonstrate the practical utility of this protocol, we investigated the gram-scale synthesis of triarylamines under solvent-free mechanochemical conditions (Fig. 7a). The solid-state cross-coupling of 1b with 2c was carried out on a 7.0 mmol scale using 2 mol% palladium catalyst in a stainless-steel ball-milling jar (25 mL) with four stainless-steel balls (diameter: 10 mm), which afforded 3c in excellent yield (92%). The product can be isolated by simple re-precipitation from CH2Cl2/MeOH. This result clearly demonstrates the potential utility of the present solvent-free protocol for large-scale preparations.
Fig. 7.
Synthetic utility of the solid-state C–N cross-coupling. a Solid-state gram-scale synthesis of 3c. The following conditions were used: 7.0 mmol of 1b; 7.0 mmol of 2c; 0.14 mmol of Pd(OAc)2; 0.14 mmol of t-Bu3P; 10.5 mmol of Na(O-t-Bu); 1,5-cod (0.20 μL mg–1); stainless-steel ball-milling jar (25 mL) with four stainless-steel balls (10 mm); 30 Hz; 99 min. Isolated yield is shown. b Efficient solid-state synthesis of the arylamine-based hole-transporting material 3af. The following conditions were used: 0.5 mmol of 1af; 2.6 mmol of 2d; 0.075 mmol of Pd(OAc)2; 0.075 mmol of t-Bu3P; 3.0 mmol of Na(O-t-Bu); 1,5-cod (0.20 μL mg–1); stainless-steel ball-milling jar (25 mL) with four stainless-steel balls (10 mm); 30 Hz; 99 min. Isolated yield is shown
Synthesis of hole-transporting materials
Triarylamine derivatives have been extensively studied as potential organic materials for perovskite-based solar cells55–57. Recently, Seok and co-workers have reported that arylamine derivatives with pyrene cores could potentially be used as high-performance hole-transporting materials for perovskite-based solar cells57. These authors obtained tetra-substituted pyrene 3af in moderate yield (60%) from a solution-based Buchwald–Hartwig amination between 1af and 2d in toluene that was carried out at high temperature (110 °C) and required prolonged reaction times (2 days) (Fig. 7b, B). Our developed solid-state conditions, in contrast, afford 3af in better yield (89%) after a significantly reduced reaction time (99 min) in the absence of potentially harmful organic solvents (Fig. 7b, A). This reaction illustrates that the present solid-state coupling protocol could potentially be a powerful alternative to solution-based synthetic routes to materials-science-oriented nitrogen-containing polyaromatic compounds.
Monitoring the reaction progress
The reaction progress of the cross-coupling reaction between 1b and 2a in the presence of 1,5-cod was monitored by powder X-ray diffraction (PXRD) analysis (Fig. 8). After 20 min, new diffraction peaks derived from coupling product 3b and NaBr appeared, while the peaks associated with the starting materials remained. After 60 min, the diffraction peaks derived from the starting materials were completely disappeared, and only those of coupling product 3b and NaBr were observed, demonstrating a clean solid-to-solid conversion without melting during this transformation. It should also be noted that the temperature inside the milling jar after the grinding at 30 Hz for 60 min was about 30 °C, which was confirmed by thermography, indicating that this reaction proceeded at around room temperature (Supplementary Figure 6). The possibility to monitor the progress of this solid-state palladium-catalyzed synthesis in situ by PXRD is another potential advantage, given that the generation of inorganic salts can be detected, which might hold key information to understanding the underlying reaction mechanism.
Fig. 8.

Monitoring the reaction progress by PXRD analysis. After 60 min, the diffraction peaks derived from the starting materials completely disappear, while those associated with coupling product 3b and NaBr emerge, which suggests a clean solid-to-solid conversion without melting during the reaction
Kinetic study
Recently, James and co-workers discovered unusual sigmoidal kinetics in the mechanochemical Knoevenagel condensation of vanillin and barbituric acid, in which the physical form of the reaction mixture dramatically changes from a free-flowing powder to a plastic-like appearance during the reaction67. Based on the several control experiments, they conclude that this dramatical change in rheology results in a rapid increase in the reaction rate. To address whether the rate increase is due to the olefin or the change in rheology, the kinetics of the reactions under the different conditions were measured (Fig. 9a). As periodic sampling of the reaction runs requires stopping the mill and opening the jar, each data point was obtained from an individual reaction. The kinetics of the reaction between 1b and 2a in the presence of 1,5-cod were found to be relatively straightforward, i.e., they could be satisfactorily modeled by simple first-order kinetics (Fig. 9a). This result suggests that the observed acceleration upon adding 1,5-cod should not stem from a change in rheology. We also confirmed that dramatic changes in the physical form of the reaction mixtures did not occur during the reaction (Fig. 9b). We also noted that the conversion rate of the reactions that contained cyclooctane or that were free of additives significantly decreased at ~30 min. These results are consistent with the TEM analysis, which revealed the formation of higher aggregates of dense palladium particles after grinding for 30 min (Supplementary Figures 10 and 11).
Fig. 9.
The kinetic study. a The kinetics of the reaction in the presence of 1,5-cod were found to be relatively straightforward (modeled as simple first order). This result suggests that the observed acceleration effect should not stem from changes in the rheology. b Dramatical changes in the physical form of the reaction mixtures containing 1,5-cod were not observed as the reaction progressed
Discussion
We have developed a rational strategy for a potentially general and scalable solid-state palladium-catalyzed cross-coupling reaction using mechanochemistry. Whereas the palladium-catalyzed cross-coupling of neat liquids proceeds readily in ball mills, similar reactions using solid reactants remain challenging. However, we discovered that the addition of small amounts of olefins dramatically accelerates the C–N cross-coupling of such solid substrates. The examination of palladium nanoparticles, which were obtained from these reaction mixtures, by transmission electron microscopy (TEM) suggested that the olefin additives can act as efficient molecular dispersants for the palladium catalysts in solid-state media and thus facilitate this challenging solid-state cross-coupling. We anticipate that the strategy developed in this study could unlock broad areas of chemical space for palladium-catalyzed solid-state syntheses of valuable synthetic targets in various scientific fields.
Methods
Representative procedure for the solid-state coupling
Aryl halide 1 (0.5 mmol), diarylamine 2 (0.5 mmol), and Pd(OAc)2 (0.025 mmol) were placed in a ball-milling jar (1.5 mL) that contained a grinding ball (stainless steel; diameter: 0.3 cm). After the jar had been placed in a glovebox, t-Bu3P (0.025 mmol) and Na(O-t-Bu) (0.75 mmol) were added. The jar was then removed from the glovebox, and 1,5-cod (0.20 μL mg–1) was added in air. The jar was then placed in the ball mill, and after grinding (30 Hz, 99 min), the reaction mixture was passed through a short silica gel column (eluent: EtOAc). The crude product was subsequently purified by flash column chromatography (SiO2; CH2Cl2/hexane, typically 0:100→15:90) to give the corresponding amination product 3.
Supplementary information
Acknowledgements
This work was supported by MEXT/JSPS KAKENHI: Grant Numbers JP17H06370 and JP18H03907 and Institute for Chemical Reaction Design and Discovery (ICReDD) which was established by World Premier International Research Initiative (WPI), MEXT, Japan.
Author contributions
K.K. and H.I. conceived and designed the study and co-wrote the paper. K.K. and S.T. performed the chemical experiments and analyzed the data. K.K. and Y.H. performed the transmission electron microscopy analysis. All authors discussed the results and commented on the manuscript.
Data availability
For full characterization data including NMR spectra of new compounds and experimental details, see the Supplemental Information. All relevant data underlying the results of this study are available from the corresponding authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Koji Kubota, Email: kbt@eng.hokudai.ac.jp.
Hajime Ito, Email: hajito@eng.hokudai.ac.jp.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-08017-9.
References
- 1.Constable DJC, Jimenez-Gonzalez C, Henderson RK. Perspective on solvent use in the pharmaceutical industry. Org. Process Res. Dev. 2007;11:133–137. doi: 10.1021/op060170h. [DOI] [Google Scholar]
- 2.Clark JH, Tavener SJ. Alternative solvents: shades of green. Org. Process Res. Dev. 2007;11:149–155. doi: 10.1021/op060160g. [DOI] [Google Scholar]
- 3.DeSimone JM. Practical approaches to green solvents. Science. 2002;297:799–803. doi: 10.1126/science.1069622. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka, K. Solvent-Free Organic Synthesis, 2nd revised edn (Wiley-VCH, Weinheim, 2009).
- 5.Toda, F. Organic Solid-State Reactions (Springer, Berlin Heidelberg, 2004).
- 6.Toda F. Solid-state organic chemistry: efficient reactions, remarkable yields, and stereoselectivity. Acc. Chem. Res. 1995;28:480–486. doi: 10.1021/ar00060a003. [DOI] [Google Scholar]
- 7.Tanaka K, Toda F. Solvent-free organic synthesis. Chem. Rev. 2000;100:1025–1074. doi: 10.1021/cr940089p. [DOI] [PubMed] [Google Scholar]
- 8.Hernández J, Macdonald NA, Mottillo C, Butler IS, Friščić T. A mechanochemical strategy for oxidative addition: remarkable yields and stereoselectivity in the halogenation of organometallic Re(I) complexes. Green. Chem. 2014;16:1087–1092. doi: 10.1039/C3GC42104J. [DOI] [Google Scholar]
- 9.Belenguer AM, Friščić T, Day GM, Sanders JMK. Solid-state dynamic combinatorial chemistry: reversibility and thermodynamic product selection in covalent mechanosynthesis. Chem. Sci. 2011;2:696–700. doi: 10.1039/c0sc00533a. [DOI] [Google Scholar]
- 10.Shi YX, Xu K, Clegg JK, Ganguly R, Hirao H, Friščić T. The first synthesis of the sterically encumbered adamantoid phosphazane P4(NtBu)6: enabled by mechanochemistry. Angew. Chem., Int. Ed. 2016;55:12736–12740. doi: 10.1002/anie.201605936. [DOI] [PubMed] [Google Scholar]
- 11.Haley RA, Zeller AR, Krause JA, Guan H, Friščić T. Nickel catalysis in a high speed ball mill: a recyclable mechanochemical method for producing substituted cyclooctatetracene compounds. ACS Sustain. Chem. Eng. 2016;4:2464–2469. doi: 10.1021/acssuschemeng.6b00363. [DOI] [Google Scholar]
- 12.Jia KY, Yu JB, Jiang ZJ, Su WK. Mechanochemically activated oxidative coupling of indoles with acylates through C–H activation: Synthesis of 3-vinylindoles and β,β-diindolyl propionates and study of the mechanism. J. Org. Chem. 2016;81:6049–6055. doi: 10.1021/acs.joc.6b01138. [DOI] [PubMed] [Google Scholar]
- 13.Strukil V, Bracin D, Magdysyuk OV, Dinnebier RE, Friščić T. Trapping reactive intermediates by mechanochemistry: Elusive aryl N-thiocarbomoylbenzotriazoles as bench-stable reagents. Angew. Chem., Int. Ed. 2015;54:8440–8443. doi: 10.1002/anie.201502026. [DOI] [PubMed] [Google Scholar]
- 14.Wang GW, Komatsu K, Murata Y, Shiro M. Synthesis and X-ray structure of dumb-bell-shaped C120. Nature. 1997;387:583–586. doi: 10.1038/42439. [DOI] [Google Scholar]
- 15.Komatsu K, Wang GW, Murata Y, Tanaka T, Fujikawa K. Mechanochemical synthesis and characterization of the fullerene dimer C120. J. Org. Chem. 1998;63:9358–9366. doi: 10.1021/jo981319t. [DOI] [Google Scholar]
- 16.Su YT, Wang GW. FeCl3-mediated cyclization of [60]fullerene with N-benzhydrylsulfonamides under high speed vibration milling conditions. Org. Lett. 2013;15:3408–3411. doi: 10.1021/ol4014602. [DOI] [PubMed] [Google Scholar]
- 17.Tan D, Mottillo C, Katsenis AD, Strukil V, Friščić T. Development of C–N coupling using mechanochemistry: catalytic coupling of arylsulfonamides and carbodiimides. Angew. Chem., Int. Ed. 2014;53:9321–9324. doi: 10.1002/anie.201404120. [DOI] [PubMed] [Google Scholar]
- 18.Yu JB, Zhang Y, Jiang ZJ, Su WK. Mechanochemically induced Fe(III) catalysis at room temperature: Solvent-free cross-dehydrogenative coupling of 3-benzylic indoles with methylenes/indoles. J. Org. Chem. 2016;81:11514–11520. doi: 10.1021/acs.joc.6b02197. [DOI] [PubMed] [Google Scholar]
- 19.Rightmire NR, Hanusa TP, Rheingold AL. Mechanochemical synthesis of [1,3-(Me3Si)2C3H3]3(Al,Sc), a base-free tris(allyl)aluminum complex and its scandium analogue. Organometallics. 2014;33:5952–5955. doi: 10.1021/om5009204. [DOI] [Google Scholar]
- 20.Zhao Y, Rocha SV, Swager TM. Mechanochemical synthesis of extended iptycenes. J. Am. Chem. Soc. 2016;138:13834–13837. doi: 10.1021/jacs.6b09011. [DOI] [PubMed] [Google Scholar]
- 21.Johansson Seehurn CCC, Kitching MO, Colacot TJ, Snieckus V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel prize. Angew. Chem., Int. Ed. 2012;51:5062–5085. doi: 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
- 22.Molnár, Á. Palladium-Catalyzed Coupling Reactions: Practical Aspects and Future Development (Wiley-VCH, Weinheim, 2013).
- 23.Meijere A, Diederich F. Metal-catalyzed Cross-coupling Reactions. 2nd ed. Weinheim: Wiley-VCH; 2008. [Google Scholar]
- 24.Miyaura N, Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. [DOI] [Google Scholar]
- 25.Chartoire A, Boreux A, Martin AR, Nolan SP. Solvent-free aryl amination catalyzed by [Pd(NHC)] complexes. RSC Adv. 2013;3:3840–3843. doi: 10.1039/c3ra40386f. [DOI] [Google Scholar]
- 26.Gajare AS, Toyota K, Yoshifuji M, Ozawa F. Solvent-free amination reactions of aryl bromides at room temperature catalyzed by a (π-allyl)palladium complex bearing a diphosphinidenecyclobutene ligand. J. Org. Chem. 2004;69:6504–6506. doi: 10.1021/jo049087n. [DOI] [PubMed] [Google Scholar]
- 27.Topchiy, M. A., Asachenko, A. F. & Nechaev, M. S. Solvent-free Buchwald-Hartwig reaction of aryl and heteroaryl halides with secondary amines. Eur. J. Org. Chem. 2014, 3319–3322 (2014).
- 28.Melucci MM, Barbarella G, Sotgiu G. Solvent-free, microwave-assisted synthesis of thiophene oligomers via Suzuki coupling. J. Org. Chem. 2002;67:8877–8884. doi: 10.1021/jo026269d. [DOI] [PubMed] [Google Scholar]
- 29.Mandai K, Korenaga T, Ema T, Sakai T, Furutani M, Hashimoto H, Takada J. Biogenous iron oxide-immobilized palladium catalyst for the solvent-free Suzuki-Miyaura coupling reaction. Tetrahedron Let. 2012;53:329–332. doi: 10.1016/j.tetlet.2011.11.044. [DOI] [Google Scholar]
- 30.Bandgar BP, Patil AV. A rapid, solvent-free, ligandless and mild method for preparing aromatic ketones from acyl chlorides and arylboronic acids via a Suzuki-Miyaura type of coupling reaction. Tetrahedron Let. 2005;46:7627–7630. doi: 10.1016/j.tetlet.2005.08.111. [DOI] [Google Scholar]
- 31.Basu B, Das P, Bhuiyan MH, Jha S. Microwave-assisted Suzuki coupling on a KF-alumina surface: synthesis of polyaryls. Tetrahedron Lett. 2003;44:3817–3820. doi: 10.1016/S0040-4039(03)00731-7. [DOI] [Google Scholar]
- 32.Liang Y, Xie YX, Li JH. Modified palladium-catalyzed Sonogashira cross-coupling reactions under copper-, amine-, and solvent-free conditions. J. Org. Chem. 2006;71:379–381. doi: 10.1021/jo051882t. [DOI] [PubMed] [Google Scholar]
- 33.Stolle A, Szuppa T, Leonhardt ES, Ondruschka B. Ball milling in organic synthesis: solutions and challenges. Chem. Soc. Rev. 2011;40:2317–2329. doi: 10.1039/c0cs00195c. [DOI] [PubMed] [Google Scholar]
- 34.Hernández JG, Bolm C. Altering product selectivity by mechanochemistry. J. Org. Chem. 2017;82:4007–4019. doi: 10.1021/acs.joc.6b02887. [DOI] [PubMed] [Google Scholar]
- 35.Do JL, Friščić T. Mechanochemistry: a force of synthesis. ACS Cent. Sci. 2017;3:13–19. doi: 10.1021/acscentsci.6b00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James SL, et al. Mechanochemistry: opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012;41:413–447. doi: 10.1039/C1CS15171A. [DOI] [PubMed] [Google Scholar]
- 37.Bowmaker GA. Solvent-assisted mechanochemistry. Chem. Commun. 2013;49:334–348. doi: 10.1039/C2CC35694E. [DOI] [PubMed] [Google Scholar]
- 38.Wang GW. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013;42:7668–7700. doi: 10.1039/c3cs35526h. [DOI] [PubMed] [Google Scholar]
- 39.Howard JL, Cao Q, Browne DL. Mechanochemistry as an emerging tool for molecular synthesis: What can it offer? Chem. Sci. 2018;9:3080–3094. doi: 10.1039/C7SC05371A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodríguez B, Bruckmann A, Rantanen T, Bolm C. Solvent-free carbon-carbon bond formations in ball mills. Adv. Synth. Catal. 2007;349:2213–2233. doi: 10.1002/adsc.200700252. [DOI] [Google Scholar]
- 41.Chen L, Regan M, Mack J. The choice is yours: using liquid-assisted grinding to choose between products in the palladium-catalyzed dimerization of terminal alkynes. ACS Catal. 2016;6:868–872. doi: 10.1021/acscatal.5b02001. [DOI] [Google Scholar]
- 42.Cravotto G, Garella D, Tagliapietra S, Stolle A, Schüßler S, Leonhardt SES, Ondruschka B. Suzuki cross-coupling of (hetero)aryl chlorides in the solid-state. New J. Chem. 2012;36:1304–1307. doi: 10.1039/c2nj40064b. [DOI] [Google Scholar]
- 43.Thorwirth R, Stolle A, Ondruschka B. Fast copper-, ligand- and solvent-free Sonogashira coupling in a ball mill. Green. Chem. 2010;12:985–991. doi: 10.1039/c000674b. [DOI] [Google Scholar]
- 44.Fulmer DA, Shearouse WC, Medonza ST, Mack J. Solvent-free Sonogashira coupling reaction via high speed ball milling. Green. Chem. 2009;11:1821–1825. doi: 10.1039/b915669k. [DOI] [Google Scholar]
- 45.Declerck V, Colacino E, Bantreil X, Martinez J, Lamaty F. Poly(ethylene glycol) as reaction medium for mild Mizoroki-Heck reaction in a ball mill. Chem. Commun. 2012;48:11778–11780. doi: 10.1039/c2cc36286d. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X, Liu J, Chen T, Su W. Mechanically activated synthesis of (E)-stilbene derivatives by high-speed ball milling. Appl. Organo. Chem. 2012;26:145–147. doi: 10.1002/aoc.2827. [DOI] [Google Scholar]
- 47.Tullberg E, Schacher F, Peters D, Frejd T. Solvent-free Heck-Jeffery reactions under ball-milling conditions applied to the synthesis of unnatural amino acids precursors and indoles. Synthesis. 2006;7:1183–1189. [Google Scholar]
- 48.Tulberg E, Peters D, Frejd T. The Heck reaction under ball-milling conditions. J. Organomet. Chem. 2004;689:3778–3781. doi: 10.1016/j.jorganchem.2004.06.045. [DOI] [Google Scholar]
- 49.Schneider F, Ondruschka B. Mechanochemical solid-state Suzuki reactions using an in situ generated base. ChemSusChem. 2008;1:622–625. doi: 10.1002/cssc.200800086. [DOI] [PubMed] [Google Scholar]
- 50.Schneider F, Stolle A, Ondruschka B, Hopf H. The Suzuki-Miyaura reaction under mechanochemical conditions. Org. Process Res. Dev. 2009;13:44–48. doi: 10.1021/op800148y. [DOI] [Google Scholar]
- 51.Shao QL, Jiang ZJ, Su WK. Solvent-free mechanochemical Buchwald-Hartwig amination of aryl chlorides without inert gas protection. Tetrahedron Lett. 2018;59:2277–2280. doi: 10.1016/j.tetlet.2018.04.078. [DOI] [Google Scholar]
- 52.Klingensmith LM, Leadbeater NE. Ligand-free palladium catalysis of aryl coupling reactions facilitated by grinding. Tetrahedron Lett. 2003;44:765–768. doi: 10.1016/S0040-4039(02)02645-X. [DOI] [Google Scholar]
- 53.Jiang ZJ, Li ZH, Yu JB, Su WK. Liquid-assisted grinding accelerating: Suzuki-Miyaura reaction of aryl chlorides under high-speed ball milling conditions. J. Org. Chem. 2016;81:10049–10055. doi: 10.1021/acs.joc.6b01938. [DOI] [PubMed] [Google Scholar]
- 54.Ruiz-Castillo P, Buchwald SL. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 2016;116:12564–12649. doi: 10.1021/acs.chemrev.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ning, Z. & Tian, H. Triarylamine: a promising core unit for efficient photovoltaic materials. Chem. Commun. 5483–5495 (2009). [DOI] [PubMed]
- 56.Li LndashL, Diau EWG. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 2013;42:291–304. doi: 10.1039/C2CS35257E. [DOI] [PubMed] [Google Scholar]
- 57.Jeon NJ, Lee J, Noh JH, Nazeeruddin MK, Grätzel M, Seok S. Efficient inorganic-organic hybrid perovskite solar cells based on pyrene arylamine derivatives as hole-transporting materials. J. Am. Chem. Soc. 2013;135:19087–19090. doi: 10.1021/ja410659k. [DOI] [PubMed] [Google Scholar]
- 58.Hartwig JF, Kawatsura M, Hauck SI, Shaughnessy KH, Alcazar-Roman LM. Room-temperature palladium-catalyzed amination of aryl bromides and chlorides and extended scope of aromatic C–N bond formation with a commercial ligand. J. Org. Chem. 1999;64:5575–5580. doi: 10.1021/jo990408i. [DOI] [PubMed] [Google Scholar]
- 59.Old DW, Wolfe JP, Buchwald SL. A highly active catalyst for palladium-catalyzed cross-coupling reactions: room-temperature Suzuki couplings and amination of unactivated aryl chlorides. J. Am. Chem. Soc. 1998;120:9722–9723. doi: 10.1021/ja982250+. [DOI] [Google Scholar]
- 60.Fairlamb IJS. π-Acidic alkene ligand effects in Pd-catalyzed cross-coupling processes: exploiting the interaction of dibenzylidene acetone (dba) and related ligands with Pd(0) and Pd(II) Org. Biomol. Chem. 2008;6:3645–3656. doi: 10.1039/b811772a. [DOI] [PubMed] [Google Scholar]
- 61.Hu J, Liu Y. Pd Nanoparticle aging and its implications in the Suzuki cross-coupling reaction. Langmuir. 2005;21:2121–2123. doi: 10.1021/la0471902. [DOI] [PubMed] [Google Scholar]
- 62.Yurino T, Ueda Y, Shimizu Y, Tanaka S, Nishiyama H, Tsurugi H, Sato K, Mashima K. Salt-free reduction of nonprecious transition-metal compounds: generation of amorphous Ni nanoparticles for catalytic C–C bond formation. Angew. Chem., Int. Ed. 2015;127:14645–14649. doi: 10.1002/ange.201507902. [DOI] [PubMed] [Google Scholar]
- 63.Yamashita M, Hartwig JF. Synthesis, structure, and reductive elimination chemistry of three-coordinate arylpalladium amido complexes. J. Am. Chem. Soc. 2004;126:5344–5345. doi: 10.1021/ja0315107. [DOI] [PubMed] [Google Scholar]
- 64.Chan CYK, Lam JWY, Zhao Z, Chen S, Lu P, Sung HHY, Kwok HS, Ma Y, Williams ID, Tang BZ. Aggregation-induced emission, mechanochromism and blue electroluminescence of carbazole and triphenylamine-substituted ethenes. J. Mater. Chem. 2014;2:4320–4327. [Google Scholar]
- 65.Li SY, Sun ZB, Zhao CH. Charge-transfer emitting triarylborane π-electron systems. Inorg. Chem. 2017;56:8705–8717. doi: 10.1021/acs.inorgchem.6b02847. [DOI] [PubMed] [Google Scholar]
- 66.Turkoglu G, Cinar ME, Ozturk T. Triarylborane-based materials for OLED applications. Molecules. 2017;22:1522–1546. doi: 10.3390/molecules22091522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hutchings BP, Crawford DE, Gao L, Hu P, James SL. Feedback kinetics in mechanochemistry: the importance of cohesive states. Angew. Chem. Int. Ed. 2017;129:15454–15458. doi: 10.1002/ange.201706723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For full characterization data including NMR spectra of new compounds and experimental details, see the Supplemental Information. All relevant data underlying the results of this study are available from the corresponding authors upon reasonable request.