Abstract
This study was aimed to investigate the effect of bio-organic phosphate either alone or in combination with phosphorus solubilizing bacteria strain (Bacillus MWT-14) on the growth and productivity of two wheat cultivars (Galaxy-2013 and Punjab-2011) along with recommended (150–100 NP kg ha−1) and half dose (75–50 NP kg ha−1) of fertilizers. The combined application of bio-organic phosphate and the phosphorous solubilizing bacteria strain at either fertilizer level significantly improved the growth, yield parameters and productivity of both wheat cultivars compared to non-inoculated control treatments. The cultivar Punjab-2011 produced the higher chlorophyll contents, crop growth rate, and the straw yield at half dose of NP fertilizer; while Galaxy-2013, with the combined application of bio-organic phosphate and phosphorous solubilizing bacteria under recommended NP fertilizer dose. Combined over both NP fertilizer levels, the combined use of bio-organic phosphate and phosphorous solubilizing bacteria enhanced the grain yield of cultivar Galaxy-2013 by 54.3% and that of cultivar Punjab-2011 by 83.3%. The combined application of bio-organic phosphate and phosphorous solubilizing bacteria also increased the population of phosphorous solubilizing bacteria, the soil organic matter and phosphorous contents in the soil. In conclusion, the combined application of bio-organic phosphate and phosphorous solubilizing bacteria offers an eco-friendly option to harvest the better wheat yield with low fertilizer input under arid climate.
Keywords: Phosphorous, Phosphorous solubilizing bacteria, Bioavailability, Rhizosphere, Low fertilizer input
Introduction
Bread wheat (Triticum aestivum L.) is consumed as staple by the billions across the globe owning to its greater calories and protein contents as compared to the other cereals.1, 2 However, the average yield of bread wheat per unit area is very low than its actual potential in many developing countries of the world including the Pakistan. Many factors such as improper sowing time, poor seed quality, poor irrigation/nutrient management and the low soil organic matter (SOM) contents,1, 2, 3, 4, 5, 6, 7, 8 are responsible for this low productivity of bread wheat.
Among these factors, the SOM is very crucial as it affects the availability of plant nutrients.3 The SOM contents of about 3% are considered essential for the suitable soil physical condition, better cation exchange capacity and the nutrient availability to obtain the higher crop yields.3, 9 However, the SOM contents of the Pakistani soils are >0.5% owing to high temperature.3, 10 which decreases the availability of nitrogen and phosphorous due to reduction in the cation exchange capacity.11, 12, 13
Phosphorous is crucial element required for the plant growth and development.14, 15, 16 The unique source of phosphorous fertilizer is rock phosphate which is a complex of phosphate and calcium.17 However, the production of elemental phosphorous, phosphoric acid, and other phosphorous based fertilizer from the rock phosphate is itself an energy consuming process.17, 18, 19
In this aspect, the conversion of rock phosphate through biochemical means into soluble and readily available state which may directly be fed to plants is a useful technique.17 The best available, ecosystem friendly, and cheaper technique to convert the rock phosphate into plant available phosphate is the use of compost (agriculture waste) and bacteria.20, 21, 22, 23 This biochemical process has low energy input demand and does not require the chemical catalyst. The end product obtained in this process is rich in available phosphorous and SOM contents,17 with optimum carbon (C) and N ratio (C:N).
The rhizospheric soil is dominated by the diverse bacterial communities.24, 25, 26, 27, 28 These bacterial communities are involved in developing the mutual interaction with plant roots and thus improve the growth and development of plants through various mechanisms.19, 29, 30, 31 These mechanisms include biological nitrogen fixation (BNF), production of growth hormones, improvement in the availability of the nutrients particularly phosphorous, and the bio-control of plant pathogens.19, 31, 32
The bacterial strains belonging to genera Azospirillum, Azotobacter, Bacillus, Enterobacter, Pseudomonas and Pantoea have been isolated from the rhizosphere of various crops and are reported to improve the plant growth.28, 31, 33, 34, 35, 36, 37, 38 Among these, Bacillus is well known genus whose multiple strains possess the potential to promote the growth of wheat. 28, 31, 36, 39
As the bio-organic phosphate (BOP) is rich in soluble phosphorous, its addition to soil may improve the plant growth more efficiently as compared with the use of synthetic phosphorous fertilizer sources such as diammonium phosphate and single super phosphate. Furthermore, the use of phosphate solubilizing bacteria (PSB) along with synthetic phosphorous fertilizer may improve the productivity of crops by increasing the solubility of the phosphorous in the soil. As the BOP is rich in the organic matter, it may further improve the availability of other nutrients to the plants and may thus provide the suitable conditions for the survival, multiplication and functioning of the beneficial rhizosphere bacteria. These bacteria in turn increase the soil fertility through the process of bio-mineralization.40 The PSB solubilize the soil phosphorous through releasing the organic acids (e.g. acetic acid, formic acid, lactic acid, glycolic acid and succinic acids, etc.) and the carboxyl and hydroxyl groups of the organic acids produced by PSB, chelate the phosphate bounded cations, thereby converting phosphorous into soluble forms.41
Addition of PSB stimulates the process of phosphorous solubilization both in soil as well as in BOP, as the phosphorous solubilization is major activity of PSB. However, to the best of our knowledge, very limited information is available about the effect of combined application of BOP and PSB on soil fertility as well as on the growth and productivity of contrasting wheat cultivars grown at different fertilizer levels. For this study, we hypothesized that the use of PSB along with BOP and NP fertilizer may enhance the soluble phosphorus within the soil. The specific objective of this study was to check the combined effect of BOP and PSB on soil fertility and productivity of bread wheat under different levels of NP fertilizer.
Materials and methods
Preparation of bioorganic phosphate
Bioorganic phosphate (enriched with available phosphorous and organic matter contents) was prepared using compost, biogas residue and rock phosphate by adopting the procedure as described by Narayanan17 with some modifications. For this purpose, rock phosphate (free from silica with 26% total phosphorous) was pulverized and refined to get a uniform size of 75 microns. The compost was prepared after composting of crop and fruit residues for 15 days. The biogas residue was obtained from a local biogas plant which uses poultry waste and animal waste i.e. cow dung. Thereafter, both the biogas residues and compost were air-dried in tray and grounded in a ball mill (CAM starter Type QS5-15A-500V 15 A, China) to pass through a 1.0 mm sieve. The ground material was mixed with ore of rock phosphate in the ratio of 1:2. The blend was then suspended in a water stirred tank with a water-solid ratio of 7:3. The reaction was carried out in agitated stirred tank (bioreactor, CEM Mk II, Armfield Ltd, UK) using the conditions proposed by Narayanan.17 Bacterial culture of phosphate solubilizing bacterial (PSB) strain Bacillus MWT-14,42 was added to the reactor keeping the inoculum strength of 107 cfu/mL and tank volume of 3–5%.
The whole process was carried out in three steps. At the first step, slurry (mixture of biogas residue, compost and rock phosphate) was added slowly and continuously to the bio-reactor (CEM Mk II, Armfield Ltd, UK) to allow the fermentation by keeping tank on agitation for 7–10 days at temperature, pressure and pH conditions as mentioned by Narayanan.17 After this step, PSB strain Bacillus MWT-14 was inoculated to the bioreactor and kept on continuous agitation for an extra 5 days to allow the diffusion of atmospheric oxygen/air into the reactor. After 5 days, bacterial culture (107 cfu/mL) of nitrogen fixing bacteria Azospirillum (unpublished data) was inoculated to the reactor; and the reactor was kept on agitation for an extra 5 days. After the complete fermentation process, the product was filtered to separate the solid portion. This solid portion was dried, ground and analyzed on spectrophotometer to measure the concentration of soluble phosphorous using the procedure described previously.43
Concentration of organic matter in the solid material was determined by using the procedure proposed by Allison44 with some modifications; 2.0 g weight of the sample was used instead of 1.0 g in 500 mL conical flask and 5.0 mL of K2Cr2O7 was added to avoid the early color change. This final product was named as BOP (16.5% P2O5 and 25% organic matter with 19:1 C:N ratio).
Preparation of inoculum and field experimentation
Bacterial strain Bacillus sp. MWT-14 (accession no. KT933232) with known phosphate solubilization and growth promoting potential,42 was inoculated to nutrient broth (250 mL) and was kept on shaking (150 rpm) for 24 h at a temperature of 30 ± 2 °C. Inoculum strength was determined on spectrophotometer by measuring the optical density of the grown bacterial culture and was maintained at 107 cfu mL–1. Further, we re-determined the strength of inoculum using serial dilution plate count method.31 The reason for selecting the Bacillus sp. MWT-14 was that this strain showed maximum phosphorous solubilization activity and improved the wheat growth in our previous study.42 The BOP @ 125 kg ha−1 and PSB strain were applied as sole treatment as well as in combined form along with recommended (150–100 NP kg ha−1, respectively) and half of the recommended (75–50 NP kg ha−1, respectively) doses in two bread wheat cultivars (Galaxy-2013 and Punjab-2011). Urea (46% N) and diammonium phosphate (46% P, 18% N) were used a source of nitrogen and phosphorous, respectively. Potassium was applied at a uniform rate of 100 K2O kg ha−1 in all experimental plots using sulphate of potassium (K2SO4) as source. The full dose of BOP, and K was soil applied at the time of sowing. However, nitrogen was applied in three equal splits at sowing, tillering and dough stage.
The experiment was conducted during the year 2015–16 at the experimental farm of College of Agriculture, Bahauddin Zakariya University, Bahadur Sub-Campus, Layyah (latitude of 30.9; longitude of 70.9 and altitude of 143 m above sea level). The experiment was laid out in randomized complete block design (RCBD) in factorial arrangement with three replications maintaining a gross plot size of 4 m × 1.8 m.
For bacterial inoculation, the bacterial culture of the strain Bacillus sp. MWT-14 was mixed with sterilized filter-mud and the seeds of both the bread wheat cultivars were pelleted with this mixture containing sterilized filter-mud and inoculla (107 cfu/g of carrier). Inoculated seeds were sown at a rate of 120 kg ha−1 using a manual hand drill.
All the other agronomic practices were kept uniform for all the experimental treatments. Physico-chemical characteristics of experimental soil were determined before conducting and after harvesting the experiment. Silt loam textured soil, with EC 2.8 dsm−1, pH 7.9, organic matter 0.33%, available phosphorous contents 9.0 mg kg−1 of dry soil, and available N contents 0.083%, was used in this study. The magnesium contents of the Pakistani soils are 1000–8500 ppm.
Recording of data
Population of phosphate solubilizing bacteria
The soil samples of each experimental unit were collected in sterilized plastic bags from the rhizosphere of wheat at different growth stages i.e. tillering [at 30–40 days after sowing (DAS)], booting (60–70 DAS) and grain filling stage (100–120 DAS), and were stored at 4 °C for further processing. Population of PSB was measured using plate count serial dilution method.45 For this purpose, 0.1 g of the collected rhizosphere soil (of each experimental unit) was suspended in test tube containing 9.0 mL sterilized saline solution (0.8%, w/v). Serial dilutions were prepared up to 10−5 and 100 μL of each dilution was spread on the plates containing Pikovskaya agar medium,46 supplemented with tri-calcium phosphate (TCP) as insoluble phosphorous source. The plates were incubated at 30 ± 2 °C for 48–72 h (h). The numbers of colony forming units (cfu) showing transparent halo zone formation (indicates solubilization of insoluble phosphorous) were counted and log values were obtained to get the bacterial population (cfu/g dry soil).
Crop allometry
Data on plant growth parameters viz. leaf area index (LAI), crop growth rate (CGR), net assimilation rate (NAR) and the chlorophyll contents were recorded fortnightly starting from 40 DAS of the wheat crop. A homogenous area (0.5 m2) was cut randomly to record the growth parameters. To record the LAI, total area of the separated leaves was measured by using a leaf area meter (DT Area Meter, Model MK2, Delta T Devices, Cambridge, UK). The LAI was calculated by dividing the total area of the leaves to the total ground area which had been harvested for the sampling. Data on CGR, was recorded after harvesting the crop samples from an area of 0.5 m2 at 15 days interval and were weighed freshly and then oven-dried at 70 ± 5 °C for 48 h. Thereafter, CGR and NAR were calculated by following the protocol as described by Hunt.47 The chlorophyll contents were measured using the chlorophyll meter (SPAD 502, Spectrum Technologies, Inc, Aurora, IL) at each 15 days interval.
Morphological and yield parameters
At maturity, spike bearing tillers (fertile or reproductive tillers) from an area of 1 m2 area of each plot were counted. Thereafter, twenty plants from each plot were randomly selected to measure the plant height, average number of spikelets per spike and number of grains per spike. The biological yield of the each treatment was measured by harvesting the area of 3.0 m2 from the each plot. The harvested samples were sun-dried for a week, and were weighed. To measure the grain yield, the dried bundles were threshed and the grains were separated from the straw. The separated grains were weighed and the grain weight was recorded. From seed lot of each plot, three random samples of 1000 grains were collected, weighed and averaged to get the 1000-grain weight. The straw yield was calculated by subtracting the grain yield from the biological yield. The harvest index was calculated as the ratio of grain yield to the biological yield expressed in percentage.
Statistical analysis
The collected data was statistically analyzed using the software “Statistix (8.1version, Tallahassee, Florida)” using the fisher analysis of variance technique. The difference among the treatments were compared using the least significant difference test at 5% probability level.48 The co-relation co-efficients were drawn to observe the relationship of growth parameters of bread wheat cultivars with the grain yield of wheat using the software “Statistix (8.1version, Tallahassee, Florida)
Results
Crop allometry
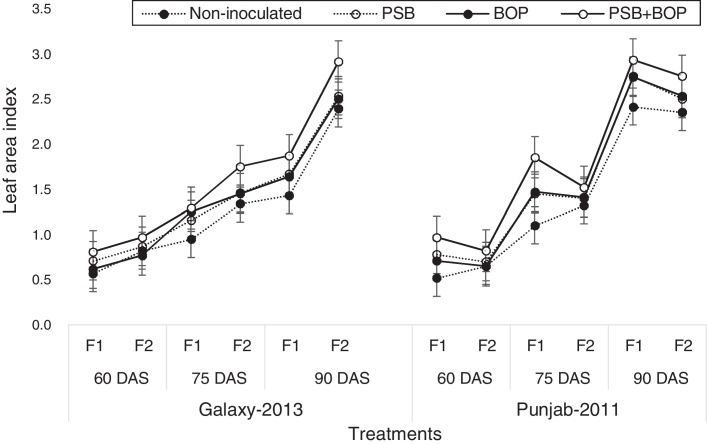
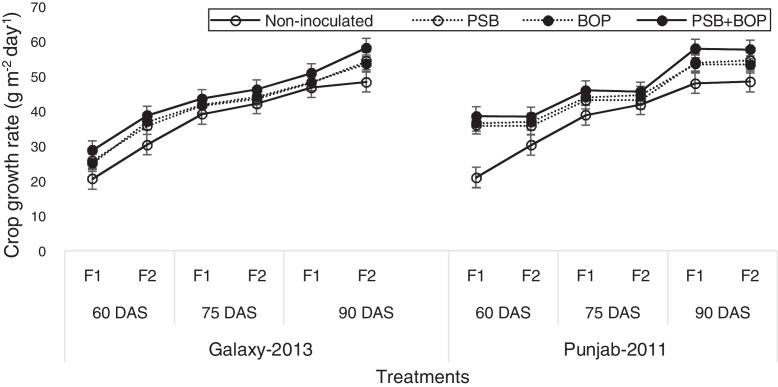
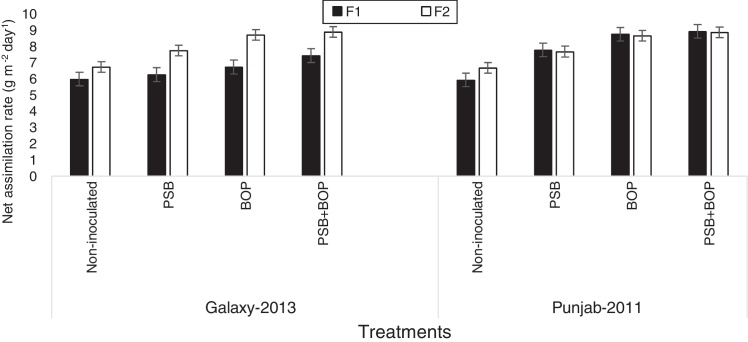
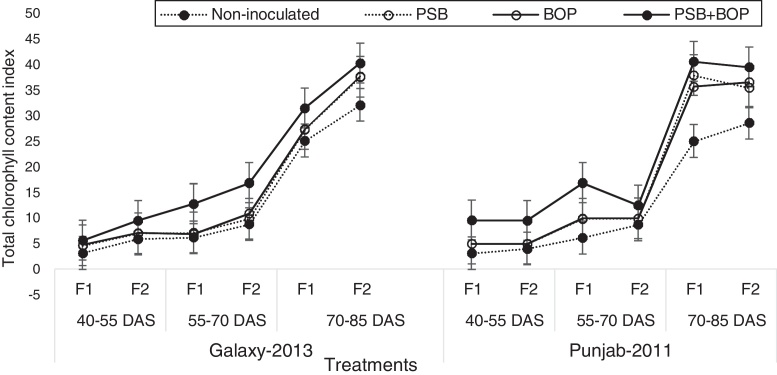
Sole or combined application of BOP or PSB strain Bacillus MWT-14 with recommended dose of NP (150–100 NP kg ha−1), and half of the recommended dose of NP (75–50 NP kg ha−1) fertilizer significantly (p ≤ 0.05) increased the LAI, CGR, NAR and the chlorophyll contents of both bread wheat cultivars than their respective non-inoculated control (Fig. 1, Fig. 2, Fig. 3, Fig. 4).
Fig. 1.
Leaf area index of bread wheat cultivars as influenced by bio-organic phosphate, PSB and different levels of NP chemical fertilizers; F1 = half of the recommended NP fertilizer level (N–P at 75–50 kg ha−1); F2 = recommended NP fertilizer level (N–P at 150–100 kg ha−1); PSB = phosphate solubilizing bacteria; BOP = bio-organic phosphate.
Fig. 2.
Crop growth rate of bread wheat cultivars as influenced by bio-organic phosphate, PSB and different levels of NP chemical fertilizers; F1 = half of the recommended NP fertilizer level (N–P at 75–50 kg ha−1); F2 = recommended NP fertilizer level (N–P at 150–100 kg ha−1); PSB = phosphate solubilizing bacteria; BOP = bio-organic phosphate.
Fig. 3.
Net assimilation rate of bread wheat cultivars as influenced by bio-organic phosphate, PSB and different levels of NP chemical fertilizers; F1 = half of the recommended NP fertilizer level (N–P at 75–50 kg ha−1); F2 = recommended NP fertilizer level (N–P at 150–100 kg ha−1); PSB = phosphate solubilizing bacteria; BOP = bio-organic phosphate.
Fig. 4.
Total chlorophyll contents of bread wheat cultivars as influenced by bio-organic phosphate, PSB and different levels of NP chemical fertilizers; F1 = half of the recommended NP fertilizer level (N–P at 75–50 kg ha−1); F2 = recommended NP fertilizer level (N–P at 150–100 kg ha−1); PSB = phosphate solubilizing bacteria; BOP = bio-organic phosphate.
In Galaxy-2013, the highest LAI was recorded with BOP + PSB treatment along with the recommended NP fertilizer (i.e. NP at 150–100 kg ha−1); in Punjab-2011, the LAI was the maximum with BOP + PSB along with the half of the recommended dose of NP fertilizer (75–50 kg ha−1) at 90 DAS (Fig. 1). In Galaxy-2013, the highest CGR was recorded with BOP + PSB along with recommended NP fertilizer (150–100 kg ha−1); in Punjab-2011, the CGR was the maximum with BOP + PSB along with recommended dose of NP fertilizer (150–100 kg ha−1), and half of the recommended dose of NP fertilizer (75–50 kg ha−1) at 90 DAS (Fig. 2). Likewise, in Galaxy-2013, the highest NAR was recorded with BOP + PSB along with the recommended dose of NP fertilizer (150–100 kg ha−1); in Punjab-2011, the NAR was almost the similar in BOP + PSB along with recommended (NP at 150–100 kg ha−1) and half of the recommended dose of NP fertilizer (75–50 kg ha−1) (Fig. 3). Similarly, in Galaxy-2013, the highest chlorophyll contents were recorded with BOP + PSB along with the recommended dose of the NP fertilizer (150–100 kg ha−1); in Punjab-2011, the chlorophyll contents were almost similar with BOP + PSB along with recommended (150–100 kg ha−1) and half of the recommended dose of the NP fertilizer (75–50 kg ha−1) (Fig. 4). At a given NP level, Galaxy-2013 showed relatively greater LAI, CGR, NAR and chlorophyll contents than Punjab-2011 (Fig. 1, Fig. 2, Fig. 3, Fig. 4).
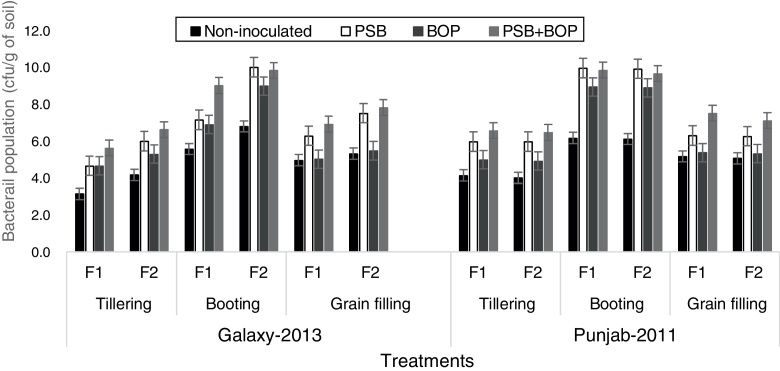
Population of PSB
Population in terms of colony forming units (cfu) of PSB recorded at three different growth stages i.e. tillering, booting and grain filling stage showed an increasing trend from tillering to booting, and decreased at grain filling stage for all the experimental treatments (Fig. 5). In Galaxy-2013, the highest population of PSB (averaged over three sampling stages) was recorded with BOP + PSB along with the recommended dose of NP fertilizer (150–100 kg ha−1); in Punjab-2011, the population of PSB (averaged over three sampling stages) was almost similar with BOP + PSB along with recommended (NP at 150–100 kg ha−1) and half of the recommended dose of NP fertilizer (75–50 kg ha−1). Overall, the population of PSB was the highest at booting stage (8.67 cfu per gram soil) followed by grain filling (6.0 cfu per gram soil) and tillering stage (5.36 cfu per gram soil) (Fig. 5).
Fig. 5.
Population dynamics of phosphate solubilizing bacteria in rhizosphere of bread wheat cultivars as influenced by bio-organic phosphate, PSB and different levels of NP chemical fertilizers; F1 = half of the recommended NP fertilizer level (N–P at 75–50 kg ha−1); F2 = recommended NP fertilizer level (N–P at 150–100 kg ha−1); PSB = phosphate solubilizing bacteria; BOP = bio-organic phosphate.
Morphological and yield parameters
This study indicated that three-way interaction of bread wheat cultivars with fertilizer levels and the PSB was significant for all the morphological/yield parameters, grain yield, harvest index, SOM, soil phosphorous and soil N (Table 1). At a given NP level, sole or combined application of the PSB or BOP to both the wheat cultivars showed an improvement in the morphological/yield parameters, grain yield, harvest index, SOM, and the soil phosphorous with respect to their non-inoculated control (Table 1).
Table 1.
Plant height, number of productive tillers, spikelets per spike, grains per spike, 1000-grain yield, biological yield, grain yield, straw yield, harvest index of two wheat cultivars, and the soil organic matter, phosphorous and nitrogen at wheat harvest as affected by the application of bio-organic phosphate and phosphorous solubilizing bacteria.
| Treatments | Galaxy-2013 |
Punjab-2011 |
Galaxy-2013 |
Punjab-2011 |
Galaxy-2013 |
Punjab-2011 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N–P (kg ha−1) |
N–P (kg ha−1) |
N–P (kg ha−1) |
N–P (kg ha−1) |
N–P (kg ha−1) |
N–P (kg ha−1) |
|||||||
| 75–50 | 150–100 | 75–50 | 150–100 | 75–50 | 150–100 | 75–50 | 150–100 | 75–50 | 150–100 | 75–50 | 150–100 | |
| Plant height (cm) | Number of productive tillers (m−2) | Spikelets per spike | ||||||||||
| Control | 93.3e | 94.6de | 87.8a–c | 85.3bc | 257e | 311c–e | 98b | 89b | 15.3g | 16.5ef | 14.8d | 15.2c |
| PSB | 97.9c–e | 104.1bc | 83.3c | 90.1a–c | 336b–d | 303de | 123b | 116b | 16.1f | 18.1b | 15.9c | 15.9c |
| BOP | 102.6b–d | 104.6bc | 92.5ab | 87.1a–c | 362bc | 373b | 122b | 117b | 16.8de | 17.2cd | 15.7c | 15.5c |
| PSB + BOP | 107.7b | 123.1a | 94.5a | 87.3a-c | 390b | 474a | 174a | 109b | 17.7bc | 20.2a | 18.0a | 17.5b |
| LSD (p ≤ 0.05) | 9.3 | 7.9 | 38.2 | 44.0 | 0.59 | 0.50 | ||||||
| Grains per spike | 1000-grain weight (g) | Biological yield (Mg ha−1) | ||||||||||
| Control | 30.6g | 33.0ef | 29.0b | 29.6b | 44.7e | 47.9c–e | 38.7c | 40.6bc | 5.5d | 6.4cd | 5.1cd | 5.4c |
| PSB | 32.2f | 36.2b | 31.4ab | 31.8ab | 46.9de | 52.7b | 41.2b | 42.2ab | 6.8c | 7.6bc | 5.9bc | 6.4b |
| BOP | 33.6de | 34.4cd | 31.0ab | 31.4ab | 49.1b–e | 50.1b–d | 44.1ab | 42.9ab | 6.9c | 7.6bc | 6.0b | 6.2b |
| PSB + BOP | 35.4bc | 40.4a | 32.0a | 32.0a | 51.6bc | 58.9a | 46.5a | 46.7a | 7.9b | 8.9a | 7.6a | 7.7a |
| LSD (p ≤ 0.05) | 0.59 | 0.50 | 4.57 | 4.00 | 0.43 | 0.41 | ||||||
| Grain yield (Mg ha−1) | Straw yield (Mg ha−1) | Harvest index (%) | ||||||||||
| Control | 2.5bc | 3.0b | 2.0b | 2.2ab | 3.0cd | 3.4c | 3.1bc | 3.2bc | 45.5e | 46.9d | 39.2d | 40.7c |
| PSB | 3.3b | 3.8ab | 2.5ab | 2.8ab | 3.5bc | 3.8b | 3.4ab | 3.6ab | 48.5c | 50.0ab | 42.4a | 43.8.ab |
| BOP | 3.4b | 3.8ab | 2.5ab | 2.7ab | 3.5bc | 3.8b | 3.5ab | 3.5ab | 49.3b | 50.0ab | 41.7b | 43.5b |
| PSB + BOP | 4.0ab | 4.5a | 3.8a | 3.9a | 3.9b | 4.4a | 3.8a | 3.8a | 50ab | 50.6a | 50.0a | 50.6a |
| LSD (p ≤ 0.05) | 0.43 | 0.43 | 0.42 | 0.44 | 0.50 | 0.42 | ||||||
| Soil organic matter (%) | Phosphorous (mg kg−1) | Nitrogen (%) | ||||||||||
| Control | 0.33d | 0.33d | 0.33c | 0.32c | 9.1cd | 9.0cd | 9.2c | 9.1d | 0.083 | 0.083 | 0.081 | 0.082 |
| PSB | 0.38c | 0.39bc | 0.39bc | 0.38bc | 9.3c | 9.5b | 9.5c | 9.3c | 0.085 | 0.086 | 0.085 | 0.084 |
| BOP | 0.40bc | 0.41b | 0.41b | 0.39bc | 9.5b | 9.5b | 9.5c | 9.2c | 0.085 | 0.085 | 0.086 | 0.084 |
| PSB + BOP | 0.45a | 0.48a | 0.46a | 0.47a | 9.8ab | 10.3a | 9.9b | 10.3a | 0.095 | 0.099 | 0.098 | 0.099 |
| LSD (p ≤ 0.05) | 0.03 | 0.02 | 0.2 | 0.3 | NS | NS | ||||||
Figures of main effects and interactions sharing the same case letter do not differ significantly at p ≤ 0.05; BOP, bio-organic phosphorous; PSB, phosphorous solubilizing bacteria; N, nitrogen; P, phosphorous.
The highest plant height, number of productive tillers, spikelets per spike, grains per spike, 1000-grain weight, biological yield, straw yield and the harvest index was recorded with the BOP + PSB, in Galaxy-2013 and Punjab-2011, with the recommended (NP at 150–100 kg ha−1) and half of the recommended dose of NP fertilizer (75–50 kg ha−1) respectively. However this treatment combination was statistically similar with the sole application of BOP along with the half of the recommended dose of NP fertilizer (75–50 kg ha−1) in Punjab-2011 for plant height; combined application of BOP + PSB along with the recommended dose of NP fertilizer (150–100 kg ha−1) in Punjab-2011 for grain per spike, 1000-grain weight, biological yield, grain yield, straw yield and harvest index; and sole application of PSB along with half of the recommended dose of NP fertilizer (75–50 kg ha−1) in Punjab-2011 for the harvest index (Table 1).
Soil properties
This experiment revealed significant (p < 0.05) increase in SOM and soil phosphorous contents due to sole or combined application of BOP and PSB over non-inoculated control plots (Table 1). The highest SOM was recorded with the application of BOP + PSB along with the recommended (NP at 150–100 kg ha−1) and half of the recommended dose of NP fertilizer (75–50 kg ha−1) in both of bread wheat cultivars. In both bread wheat cultivars, the phosphorous contents were the maximum with recommended dose of NP fertilizer (150–100 kg ha−1) and that was statistically similar with the half of the recommended dose of NP fertilizer (75–50 kg ha−1) in Galaxy-2013. Although the differences were non-significant, the highest soil N contents were noted with combined application of BOP and PSB at high NP fertilizer levels in both bread wheat cultivars (Table 1).
The correlation predicted that the LAI, CGR and the chlorophyll contents were strongly positively correlated with the grain yield of wheat at both fertilizer levels in both of the bread wheat cultivars (Table 2).
Table 2.
Correlation co-officiants of leaf area index, chlorophyll contents and crop growth rate with the grain yield of two bread wheat cultivars as affected by application of PGPRs at recommended and half dose of fertilizer.
| Treatments | Galaxy-2013 |
Punjab-2011 |
||
|---|---|---|---|---|
| N–P (kg ha−1) |
N–P (kg ha−1) |
|||
| 75–50 | 150–100 | 75–50 | 150–100 | |
| Leaf area index | 0.99 | 0.82 | 0.99 | 0.82 |
| Chlorophyll contents | 0.86 | 0.97 | 0.88 | 0.95 |
| Crop growth rate | 0.93 | 0.90 | 0.82 | 0.81 |
N, nitrogen; P, phosphorous.
Discussion
In the present study, soil properties were substantially improved with the application of BOP along with the PSB which was visible through improvement in soil OM and soil phosphorous contents. This improvement in soil phosphorous was due to positive influence of PSB which ultimately enhanced the solubility and release of phosphorous from NP fertilizer in the rhizosphere of the both bread wheat cultivars. Several previous studies have reported improvement in SOM contents and nutrient balance due to supplementation of soil with bio-organic compounds,23, 49, 50, 51 and PSB. However, most of the studies are restricted to sole application of PSB or bio-organics for soil reconditioning and crop productivity. In the present study, the combined application of BOP and PSB at a given fertilizer level was a better choice than the sole application of BOP and PSB in both the bread wheat cultivars. This was due to the fact that BOP and PSB showed synergistic behavior, improved the performance and multiplied the effect of each other. Indeed, the PSB improve the efficiency of the BOP by improving the P availability, and BOP provides the optimum conditions for the multiplication of PSB (increased population of PSB 5.61–9.83 cfu/g dry soil; Fig. 5). Moreover, the bio-organics (e.g. compost) acts as the main source of easily available nutrients, substances with growth promoting potential and improves the population of plant growth promoting rhizobacteria like N fixers, phosphorous solubilizer and cellulose decomposers,23, 52 as was observed in this study. The population of PSB was significantly higher with the combined application of BOP and PSB irrespective of the NP fertilizer dose. Indeed, the application of BOP enriched with organic carbon provided the source of energy to bacteria and increased the multiplication rate of rhizobacteria due to which the population of PSB was enhanced in this study.
This study indicated that the chlorophyll contents, LAI, CGR and NAR of both the bread wheat cultivars under an arid climate were significantly improved with the application of BOP and PSB; the combined application being most beneficial. Indeed, the application of BOP to the soil improves the availability and uptake of the nutrients like N, phosphorous, K and Zn to the growing plants which ultimately stimulates the plant growth which was visible through improvement in CGR, LAI and NAR in this study. Moreover, the inoculation of bacterial strain Bacillus MWT-14 (with known phosphorous solubilization and IAA producing activity; Hussain et al.42) also multiplied the effect of BOP by increasing the PSB population (Fig. 5) and phosphorous solubility which contributed to improved performance of both bread wheat cultivars. Several past studies have reported the improved activity and an increase in the population of PGPRs in the soils with good SOM and the pivotal role of PGPRs in improving the phosphorous solubilization,18, 19, 20, 49, 50, 51, 52, 53, 54, 55, 56 which ultimately resulted in an increase in the cholrophyll contents,57, 58 the LAI, CGR and NAR in this study.
At a given NP level, Galaxy-2013 showed relatively greater LAI, CGR, NAR and chlorophyll contents than Punjab-2011. This better performance of Glaxy-2013 than Punjab-2011 in terms of growth parameters might be due to inherited genetic potential of this wheat cultivar to produce more biomass at a given NP levels.
In our study, the variable response of bread wheat cultivars to the BOP and PSB treatments were observed. The cultivar Galaxy-2013 performed better with the combined application of BOP and PSB at recommended NP fertilizer level (150–100 kg ha−1); the performance of Punjab-2011 was almost similar at recommended and half of the recommended dose of NP fertilizer. This indicates that the cultivar Punjab-2011 can be grown at low fertilizer input with the use of BOP and PSB without any compromise on the grain yield.
Improvement in LAI, CGR and NAR finally resulted in better plant height of both bread wheat cultivars. Highest plant height and the more LAI and CGR due to the combined application of BOP and PSB ultimately enhanced the total biomass and straw yield of both bread wheat cultivars. Better growth attributes due to combined application of BOP and PSB also resulted in the more number of productive tillers and better allocation of photosynthates from source to sink which enhanced the grains per spike and 1000-grain weight. More number of productive tillers and improved grains per spike and 1000-grain weight thus produced the highest grain yield. Improvement in growth and yield attributes due to due to inoculation with PGPR are well documented in several previous studies.28, 30, 31, 37, 38, 59, 60
Conclusion
The soil fertility, growth and productivity of bread wheat cultivars was significantly increased with the application of BOP or PSB; the effects being more pronounced with the combined application of the both. Indeed, the application of BOP and PSB enhanced the soil SOM and soil phosphorous which worked as soil conditioner thus improving the growth of both bread wheat cultivars which was visible through improvement in LAI, CGR and NAR. Improved growth of the both bread wheat cultivars due to combined application of BOP and PSB ultimately enhanced the morphological and yield parameters (number of productive tillers, 1000-grain weight, grains per spike) which ultimately enhanced the grain yield at a given fertilizer level. Being a stress tolerant cultivar, Punjab-2011 performed efficiently due to the combined application of BOP and PSB at the half of the recommended level of NP fertilizer. In contrast, Galaxy-2013 showed positive response in terms of plant growth and grain yield at a recommended dose of NP fertilizer level. It is recommended that the BOP should be applied in combination with PSB to maximize the benefits. Thus, the combined use of BOP and PSB at low fertilizer input might be quite useful to increase the soil fertility, the crop growth and the productivity of bread wheat. This will lead toward elimination of the environmental pollution through minimizing the manufacturing and use of the synthetic fertilizers.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The technical assistance of Mr. Ali Ahmad (Lab Assistant) in performing lab work at BZU, Multan is acknowledged. Special thanks to Mr. Wazeer, Zeshan, Asghar and Husnain for their kind help in conducting field experiments and data collection. I am grateful to management team of Hexon Group of Companies, Multan for providing the material to provide the lab facilities for formulating the bio-organics.
Associate Editor: Jerri Zilli
References
- 1.Abouziena H., Shararafaida A., El-Desoki E. Efficacy of cultivar selectivity and weed control treatments on wheat yield and associated weeds in sandy soils. World J Agric Sci. 2008;4:384–389. [Google Scholar]
- 2.Abd-El-Haleem S., Reham M., Mohamed S. Genetic analysis and RAPD polymorphism in some durum wheat genotypes. Global J Biotechnol Biochem. 2009;4:01–09. [Google Scholar]
- 3.Rasmussen P., Rohde C. Long-term tillage and nitrogen fertilization effects on organicnitrogen and carbon in a semiarid soil. Soil Sci Soc Am J. 1988;52:1114–1117. [Google Scholar]
- 4.Mulla D., Bhatti A., Hammond M., Benson J. A comparison of winter wheat yield and quality under uniform versus spatially variable fertilizer management. Agric Ecosyst Environ. 1992;38:301–311. [Google Scholar]
- 5.Akhtar M., Cheema M., Jamil M., Ali L. Effect of time of sowing on some important characters of wheat, Triticum aestivum genotypes. J Agric Res. 2006;44:255–259. [Google Scholar]
- 6.Kibe A., Singh S., Kalra N. Water–nitrogen relationships for wheat growth and productivity in late sown conditions. Agric Water Manage. 2006;84:221–228. [Google Scholar]
- 7.Farooq M., Aziz T., Basra S., Cheema M., Rehman H. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J Agron Crop Sci. 2008;194:161–168. [Google Scholar]
- 8.Sattar A., Cheema M.A., Farooq M., Wahid M.A., Wahid A., Babar B.H. Evaluating the performance of wheat cultivars under late sown conditions. Int J Agric Biol. 2010;4:561–565. [Google Scholar]
- 9.Soane B. The role of soil organic matter in soil compatibility: a review of some practical aspects. Soil Till Res. 1990;16:179–201. [Google Scholar]
- 10.Ismail I., Blevins R., Frye W. Long-term no-tillage effects on soil properties and continuous corn yields. Soil Sci Soc Am J. 1994;58:193–198. [Google Scholar]
- 11.Bot A., Benites J. Food and Agriculture Organization of the United Nations; Viale delle Terme di Caracalla, 00100 Rome, Italy: 2005. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production; pp. 41–46. [Google Scholar]
- 12.Boateng S.A., Zickermann J., Kornahrens M. Poultry manure effect on growth and yield of maize. West Afr J Appl Ecol. 2006;9:12–18. [Google Scholar]
- 13.DeLuca T.H., MacKenzie M.D., Gundale M.J. Biochar effects on soil nutrient transformations. In: Lehmann J., Joseph S., editors. Biochar for Environmental Management: Science and Technology. Earthscan; London, UK: 2009. pp. 251–270. [Google Scholar]
- 14.Richardson A.E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct Plant Biol. 2001;28:897–906. [Google Scholar]
- 15.Khan A.A., Jilani G., Akhtar M.S., Naqvi S.M.S., Rasheed M. Phosphorus solubilizing bacteria: occurrence, mechanisms and their role in crop production. J Agric Biol Sci. 2009;1:48–58. [Google Scholar]
- 16.Richardson A.E., Barea J.M., McNeill A.M., Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil. 2009;321:305–339. [Google Scholar]
- 17.Narayanan C. Production of phosphate-rich biofertiliser using vermicompost and anaerobic digestor sludge – a case study. Adv Chem Eng Sci. 2012;2:187–191. [Google Scholar]
- 18.Castagno L., Estrella M., Sannazzaro A., Grassano A., Ruiz O. Phosphate solubilization mechanism and in vitro plant growth promotion activity mediated by Pantoea eucalypti isolated from Lotus tenuis rhizosphere in the Salado River Basin (Argentina) J Appl Microbiol. 2011;110:1151–1165. doi: 10.1111/j.1365-2672.2011.04968.x. [DOI] [PubMed] [Google Scholar]
- 19.Bhardwaj D., Ansari M.W., Sahoo R.K., Tuteja N. Biofertilizers function as keyplayer in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Fact. 2014;13:66–76. doi: 10.1186/1475-2859-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badr M., Taalab A. Release of phosphorus from rock phosphate through composting using organic materials and its effect on corn growth. Bull Natl Res Centre (Cairo) 2005;30:629–638. [Google Scholar]
- 21.Malboobi M.A., Owlia P., Behbahani M. Solubilization of organic and inorganic phosphates by three highly efficient soil bacterial isolates. World J Microbiol Biotechnol. 2009;25:1471–1477. [Google Scholar]
- 22.Novak J.M., Busscher W.J., Laird D.L., Ahmedna M., Watts D.W., Niandou M.A. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009;174:105–112. [Google Scholar]
- 23.Kumar R., Sharma S., Sood S., Prasad R., Dubey Y. Bioorganic nutrient source effect on growth, biomass, and quality of natural sweetener plant stevia and soil fertility in the Western Himalayas. Comm Soil Sci Plant Anal. 2015;46:1170–1186. [Google Scholar]
- 24.Buee M., De Boer W., Martin F., Overbeek L.V., Jurkevitch E. The rhizospherezoo: an overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors. Plant Soil. 2009;321:189–212. [Google Scholar]
- 25.Farina R., Beneduzi A., Ambrosini A. Diversity of plant growth-promoting rhizobacteria communities associated with the stages of canola growth. Appl Soil Ecol. 2012;55:44–52. [Google Scholar]
- 26.Bouizgarne B., Aouamar A.A.B. In: Diversity of Plant Associated Actinobacteria, Bacterial Diversity in Sustainable Agriculture. Maheshwari D.K., editor. Springer International Publishing; Switzerland: 2014. pp. 41–99. [Google Scholar]
- 27.Mirza B.S., Potisap C., Nusslein K., Bohannan B.J., Rodrigues J.L. Response of free-living nitrogen-fixing microorganisms to land use change in the Amazon rainforest. Appl Environ Microbiol. 2014;80:281–288. doi: 10.1128/AEM.02362-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tahir M., Mirza M.S., Hameed S., Dimitrov M.R., Smidt H. Cultivation-based and molecular assessment of bacterial diversity in the rhizosheath of wheat under different crop rotations. PLoS ONE. 2015;10:e0130030. doi: 10.1371/journal.pone.0130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tien T., Gaskins M., Hubbell D. Plant growth substances produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.) Appl Environ Microb. 1979;37:1016–1024. doi: 10.1128/aem.37.5.1016-1024.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vessey J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. [Google Scholar]
- 31.Tahir M., Mirza M.S., Zaheer A., Dimitrov M.R., Smidt H., Hameed S. Isolation and identification of phosphate solubilizer Azospirillum, Bacillus, and Enterobacter strains by16SrRNA sequence analysis and their effect on growth of wheat (Triticum aestivum L.) Aust J Crop Sci. 2013;7:1284–1292. [Google Scholar]
- 32.Bhattacharyya P., Jha D. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- 33.Yasmin S., Bakar M.A.R., Malik K.A., Hafeez F.Y. Isolation, characterization and beneficial effects of rice associated plant growth promoting bacteria from Zanzibar soils. J Basic Microbiol. 2004;44:241–252. doi: 10.1002/jobm.200310344. [DOI] [PubMed] [Google Scholar]
- 34.Mirza M.S., Mehnaz S., Normand P. Molecular characterization and PCR detection of a nitrogen-fixing Pseudomonas strain promoting rice growth. Biol Fertil Soil. 2006;43:163–170. [Google Scholar]
- 35.Mishra A., Chauhan P.S., Chaudhry V., Tripathi M., Nautiyal C.S. Rhizosphere competent Pantoea agglomerans enhances maize (Zea mays) and chickpea (Cicer arietinum L.) growth, without altering the rhizosphere functional diversity. Antonie Van Leeuwenhoek. 2011;100:405–413. doi: 10.1007/s10482-011-9596-8. [DOI] [PubMed] [Google Scholar]
- 36.Kumar P., Dubey D., Maheshwari D. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res. 2012;167:493–499. doi: 10.1016/j.micres.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Shahid M., Hameed S., Tariq M., Zafar M., Ali A., Ahmad N. Characterization of mineral phosphate-solubilizing bacteria for enhanced sunflower growth and yield-attributing traits. Ann Microbiol. 2014;65:1525–1536. [Google Scholar]
- 38.Mehnaz S., Mirza M.S., Haurat J. Isolation and 16S rRNA sequence analysis of the beneficial bacteria from the rhizosphere of rice. Can J Microbiol. 2001;47:110–117. doi: 10.1139/w00-132. [DOI] [PubMed] [Google Scholar]
- 39.Abbasdokht H., Gholami A. The effect of seed inoculation (Pseudomonas putida + Bacillus lentus) and different levels of fertilizers on yield and yield components of wheat (Triticum aestivum L.) cultivars. World Aced Sci Eng Technol. 2010;68:979–983. [Google Scholar]
- 40.Rashid M.I., Mujawar L.H., Shahzad T., Almeelbi T., Ismail I.M., Oves M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol Res. 2016;183:26–41. doi: 10.1016/j.micres.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Lee K.K., Mok I.K., Yoon M.H., Kim H.J., Chung D.Y. Mechanisms of phosphate solubilization by PSB (Phosphate-solubilizing Bacteria) in soil. Kor J Soil Sci Fertil. 2012;45:169–176. [Google Scholar]
- 42.Hussain M., Asgher Z., Tahir M. Bacteria in combination with fertilizers improve growth, productivity and net returns of wheat (Triticum aestivum L.) Pak J Agric Sci. 2016;53:633–645. [Google Scholar]
- 43.Olsen S.R., Cole C.V., Watanabe F.S., Dean L.A. Vol. 939. US Dept Agr Cir; 1954. p. 19. (Estimation of Available Phosphorous in Soils by Extraction with Sodium Bicarbonate). [Google Scholar]
- 44.Allison L. Wet-combustion apparatus and procedure for organic and inorganic carbon in soil. Soil Sci Soc Am J. 1960;24:36–40. [Google Scholar]
- 45.Sanders E.R. Aseptic laboratory techniques: plating methods. J Vis Exp. 2012;63:e3064. doi: 10.3791/3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pikovskaya R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiol. 1948;17:362–370. [Google Scholar]
- 47.Hunt . The Institute Biology's Studies in Biology. Edward Arnold (Pub.) Ltd; 1978. Plant Growth Analysis; pp. 8–38. [Google Scholar]
- 48.Steel R.G.D., Torrie J.H., Dicky D.A. 3rd ed. McGraw Hill, Inc. Book Co.; N.Y., USA: 1997. Principles and Procedures of Statistics, A Biometrical Approach; pp. 352–358. [Google Scholar]
- 49.Aira M., Monroy F., Domínguez J., Mato S. How earthworm density affects microbial biomass and activity in pig manure. Eur J Soil Biol. 2002;38:7–10. [Google Scholar]
- 50.Tao R., Liang Y., Wakelin S.A., Chu G. Supplementing chemical fertilizer with an organic component increases soil biological function and quality. Appl Soil Ecol. 2015;96:42–51. [Google Scholar]
- 51.Trisha Roy D.R., Biswas S.C., Datta B.S. Solubilization of purulia rock phosphate through organic acid loaded nanoclay polymer composite and phosphate solubilizing bacteria and its effectiveness as p-fertilizer to wheat. J Indian Soc Soil Sci. 2015;63:327–338. [Google Scholar]
- 52.Kolton M., Harel Y.M., Pasternak Z., Graber E.R., Elad Y., Cytryn E. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl Environ Microbiol. 2011;77:4924–4930. doi: 10.1128/AEM.00148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y., Rekha P., Arun A., Shen F., Lai W.A., Young C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol. 2006;34:33–41. [Google Scholar]
- 54.Asai H., Samson B.K., Stephan H.M. Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res. 2009;111:81–84. [Google Scholar]
- 55.Gupta S., Meena M.K., Datta S. Isolation, characterization of plant growth promoting bacteria from the plant Chlorophytum borivilianum and in-vitro screening for activity of nitrogen fixation, phosphate solubilization and IAA production. Int J Curr Microbiol Appl Sci. 2014;3:1082–1090. [Google Scholar]
- 56.Ahmad M., Zahir Z.A., Jamil M., Nazli F., Latif M., Akhtar M.F. Integrated use of plant growth promoting rhizobacteria, biogas slurry and chemical nitrogen for sustainable production of maize under salt-affected conditions. Pak J Bot. 2014;46:375–382. [Google Scholar]
- 57.Manzoor M., Abbasi M.K., Sultan T. Isolation of phosphate solubilizing bacteria from maize rhizosphere and their potential for rock phosphate solubilization–mineralization and plant growth promotion. Geomicrobiol J. 2017;34:81–95. [Google Scholar]
- 58.Elkoca E., Kantar F., Sahin F. Influence of nitrogen fixing and phosphorus solubilizing bacteria on the nodulation, plant growth, and yield of chickpea. J Plant Nutr. 2007;31:157–171. [Google Scholar]
- 59.Mathivanan S., Chidambaram A., Sundramoorthy P., Baskaran L., Kalaikandhan R. Effect of combined inoculations of plant growth promoting rhizobacteria (PGPR) on the growth and yield of groundnut (Arachis hypogaea L.) Int J Curr Microbiol Appl Sci. 2014;3:1010–1020. [Google Scholar]
- 60.Tahir M., Nadeem A., Nazim H. Plant growth promoting rhizobacteria improve growth of nursery transplanted rice under agro ecological conditions of Southern Punjab. Environ Plant Syst. 2015;1:22–27. [Google Scholar]