Abstract
Background and Aims: Intoxications by aliphatic halogenated hydrocarbons (AHH), used as effective solvents, are rare and may cause life-threatening liver injury. Patients with acute intoxications by AHH received an innovative treatment.
Methods: Analyzed were data of 60 patients intoxicated by AHH, such as dichloromethane (n = 3), chloroform (n = 2), carbon tetrachloride (n = 12), 1,2-dichloroethane (n = 18), 1,1,2-trichloroethane (n = 2), trichloroethylene (n = 2), tetrachloroethylene (n = 13) or mixed AHH chemicals (n = 8), who received a new treatment consisting of CO2-induced hyperventilation to accelerate toxin removal via the lungs.
Results: Added to the inspiration air at a flow rate of 2–3 Liter min−1, CO2 increased the respiratory volume up to 25–30 Liter min−1, ensuring forced AHH exhalation. This CO2-induced hyperventilation therapy was commonly well tolerated by the 60 patients and lasted for 106.0±10.5 hours. In most cases, initially increased liver test results of aminotransferases normalized quickly under the therapy, and liver histology obtained at completion of the therapy revealed, in the majority of patients, normal findings or fatty changes, and rarely severe single cell necrosis but no confluent liver cell necrosis. Despite therapy, clinical outcome was unfavorable for 4/60 patients (6.7%) of the study cohort, due to single or combined risk factors. These included late initiation of the CO2-induced hyperventilation therapy, intentional intoxication, uptake of high amounts of AHH, concomitant ingestion of overdosed drugs, consumption of high amounts of alcohol, and history of alcohol abuse.
Conclusions: For intoxications by AHH, effective therapy approaches including forced hyperventilation to increase toxin removal via the lungs are available and require prompt initiation.
Keywords: Aliphatic halogenated hydrocarbons; Dichloromethane; Chloroform; Carbon tetrachloride; 1,2-dichloroethane
Introduction
Applied in animal models, a variety of potentially hepatotoxic chemicals are known, such as ethionine, gallactosamine, nitrosamines, phosphorous and thioacetamide, and are considered as appropriate examples for experimental liver injury studies.1 However, results obtained with these substrates are rarely transferrable from animal liver injury to human liver injury cases or to general human liver diseases of alternative etiologies, such as those caused by alcohol, drugs, herbs, obesity and hepatitis B or C. Other chemicals like those of the aliphatic halogenated hydrocarbons (AHH) group with compounds presenting a broad spectrum of different structures are much more specific in producing liver injury1–3 and have the advantage of causing liver injury in both animals1–13 and humans.14–16 Mimicking human disease, this allows for the transfer of experimental results to patients with intoxications by AHH.14–16 Consequently, such data transfer helps elucidate pathogenetic steps of liver injury hits and develop new therapy strategies.15,16 This will assist clinicians caring for patients with liver injury caused by AHH.
This article describes and analyzes the clinical experience among 60 patients with acute intoxications by seven different AHH, including dichloromethane, chloroform, carbon tetrachloride (CCl4), 1,2-dichloroethane, 1,1,2-trichloroethane, trichloroethylene, and tetrachloroethylene. The focus is on new therapy approaches based on results of experimental and clinical studies, which include increased pulmonary removal of the toxin via the innovative CO2-induced hyperventilation therapy and decreased production of toxic intermediates generated from the parent chemical.
Methods
Patients
The study cohort consisted of 60 patients with acute intoxications by seven different AHH, who were treated at the Intensive Care Unit affiliated to the Medical Departments of the Heinrich Heine University Hospital in Düsseldorf, Germany. The diagnosis of AHH intoxication was verified in the expiration air by the Draeger-tube® system15,16 or in the blood by head space gas liquid chromatography,8,9,14 whereby venous blood was taken with a gas-tight syringe from the cubital vein of the patient.14 In the Intensive Care Unit, electrocardiogram was performed at admission and during the clinical course; also, regularly, all essential cardiorespiratory parameters, diagnostic techniques, and laboratory tests were performed. All patients received the therapy as systematically described in earlier reports.15,16
Treatment modalities
Patients intoxicated by AHH through ingestion or inhalation were treated as outlined in detail in Table 1.
Table 1. Therapy of patients with acute intoxications by AHH.
| Therapy approaches | Ingestion | Inhalation |
| 1. Qualitative and quantitative analysis of AHH as the suspected toxin, associated with evaluation of individual toxicity risk | + | + |
| 2. Endotracheal intubation after evaluation for individual risk of aspiration due to AHH ingestion | + | − |
| 3. Primary toxin elimination by gastro-intestinal lavage in the intubated patient | + | − |
| 4. Forced CO2-induced ventilation | + | + |
| 5. Central venous access | + | + |
| 6. Intravenous cimetidine as bolus (200 mg), then 1600 mg for the initial 24 hours via infusion pump and for the subsequent days | + | + |
| 7. Intravenous 400 g glucose/24 hours at admission and on subsequent days | + | + |
| 8. Intravenous electrolytes and forced diuresis | + | + |
| 9. Liquemin 15,000 IU/24 hours at admission and on subsequent days | + | + |
Hyperventilation for increasing AHH elimination via lungs
To get the body rid of the toxin, an increased pulmonary removal is recommended, best achieved by artificial hyperventilation through CO2 at a flow rate of 2–3 Liter min−1 applied via a conventional nose tube or a conventional sealed nasal oxygen mask, with general details and potentially improved mask devices published recently.15,16 This is certainly the preferred and most innovative approach in adult and adolescent patients with sustained spontaneous respiration. However, in patients with insufficient spontaneous breathing and especially in children, who are quickly exhausted if treated with the nasal tube or the mask device, endotracheal intubation is recommended, whereby CO2 at a flow rate of 2–3 Liter min−1 is added to the inspiration air.15,16 Independent from the mode of CO2 treatment, a respiratory volume of 25–30 Liter min−1 should be achieved, to be verified by regular measurement of the minute respiratory volume in order to achieve early recognition of problems requiring technical adjustments of the CO2 flow or eventually stopping the therapy.
Chronic obstructive pulmonary disease is commonly a contraindication for a CO2-induced therapy or requires a case by case decision whether a cautious therapy can be initiated under strict surveillance. All patients with acute AHH intoxication requiring therapy should be treated in a special setting, such as an intensive care unit that provides experienced pulmonologists and gastroenterologists, as this is a critical therapy in need of a careful 24-hour surveillance of the patient with a regular medical and technical control management.
Cimetidine for inhibiting microsomal AHH metabolism
Liver injury of AHH, including CCl4, depends on its microsomal conversion to toxic intermediates through cytochrome P450 (CYP), especially its isoenzyme CYP 2E1.3,16 Reducing the microsomal metabolism of AHH can be another therapy strategy, using chemicals or drugs that compete with AHH at the site of CYP. For instance, such a competing drug is cimetidine, which reduced liver injury and lethality rate in the CCl4 animal model.13 Based on these experimental results, cimetidine has been introduced early as an essential part in the therapy management of humans intoxicated by AHH (Table 1).15,16 Cimetidine may also be protective in kidneys which exhibit CYP450 2E1,16,17 possibly responsible for renal injury associated with liver injury found in some patients intoxicated by AHH.15,16,18
Glucose for down-regulating microsomal functions
High-dose glucose, daily 400 g for a few days, is advised as part of the infusion program in patients intoxicated by AHH (Table 1),15,16 in analogy to the high amounts of glucose given to patients with acute intermittent porphyria.19 This allows down-regulation of the hepatic δ-aminolevulinic acid (ALA) synthase activity, helping to reduce ALA synthesis,20,21 and impairs the synthesis of heme that is essential for the hemoprotein CYP.21 This ALA-dependent pathway has been established by studies through incorporation of radioactive ALA into microsomal CYP.22
Results
Intoxicating AHH chemicals and affected patients
The overall study cohort consisted of 60 patients, with 52 patients intoxicated by single AHH and 8 patients with intoxication by AHH chemical mixtures classified as mixed AHH chemical intoxications. The 52 patients had been intoxicated by seven single AHH, with preference of 1,2-dichloroethane intoxicating 18 patients, trichloroethylene affecting 13 patients, and CCl4 injuring 12 patients (Supplemental Table 1). The remaining AHH injured 2–3 patients.
Demographic data of the cohort and mode of intoxication
The 60 patients were 41.3±3.9 years-old, with a first peak occurring between 15 and 24 years of age and a second peak between 35 and 54 years of age (Fig. 1A). Patients with an accidental intoxication were significantly older compared to those with an intentional intoxication (49.8±6.5 vs. 31.6±2.8 years; p<0.005). Age was also variable among the different subgroups of intoxicating AHH chemicals (Supplemental Table 1).
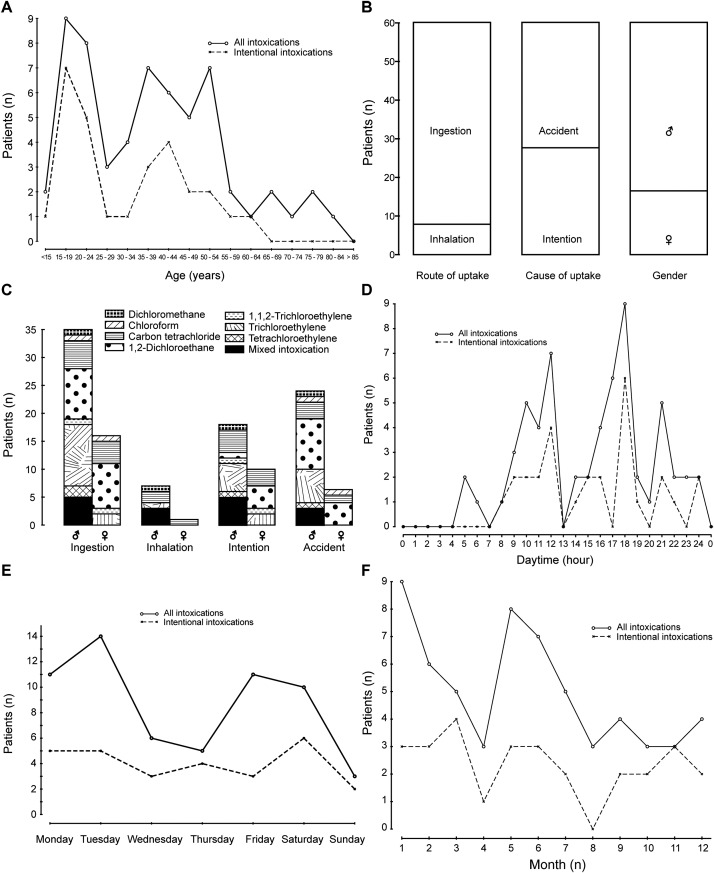
Fig. 1. (A) Age distribution of the 60 patients with intoxication by AHH. (B) Route of AHH uptake, cause of intoxication, and sex distribution among the 60 patients of the study cohort. (C) Sex distribution in relation of the criteria ingestion, inhalation, and background of intoxication among the 60 patients included in the study cohort. (D) Day time of AHH intoxication among the 60 patients of the study cohort. (E) Weekday of intoxication among the study cohort comprising 60 patients with AHH intoxication. (F) Month of intoxication in the study cohort of 60 patients.
Abbreviation: AHH, aliphatic halogenated hydrocarbons.
Intoxications by AHH occurred mostly by ingestion and rarely by inhalation. There were slightly more intoxications with an unintentional than an intentional background, and male patients constituted the majority among the study cohort (Fig. 1B). More specifically, in the cohort, the ratio of males:females was 2.5:1, based on 43 male patients and 17 female patients. Gender differences are also evident for the criteria of ingestion, inhalation, intentional use, and unintentional uptake (Fig. 1C). During the day, a high peak of intoxication was at 4:00 p.m., and another but lower peak occurred at 11:00 a.m. (Fig. 1D). For the week days, most of the intoxications occurred on Tuesday, followed by Friday and Saturday (Fig. 1E). For time of year, intoxications by AHH occurred most frequently in January, followed by June (Fig. 1F).
Hyperventilation details
Duration of the hyperventilation therapy was 106.0±10.5 hours among the 60 patients. Data were variable among the single chemical intoxicants and the mixed chemical intoxication subgroup and were also stratified according to whether the intoxication had an intentional or an unintentional background (Supplemental Table 2). Discharge was after 14.5±1.5 days, calculated from the day of intoxication.
Liver injury
Upon assessing maximum serum activities of alanine transaminase (AST) and alanine transaminase (ALT) in the study cohort of 60 patients during the hyperventilation treatment, variable results were evident among the AHH (Fig. 2A and 2B). In patients intoxicated by CCl4, extremely high values were found for AST (Fig. 2A) and ALT (Fig. 2B).
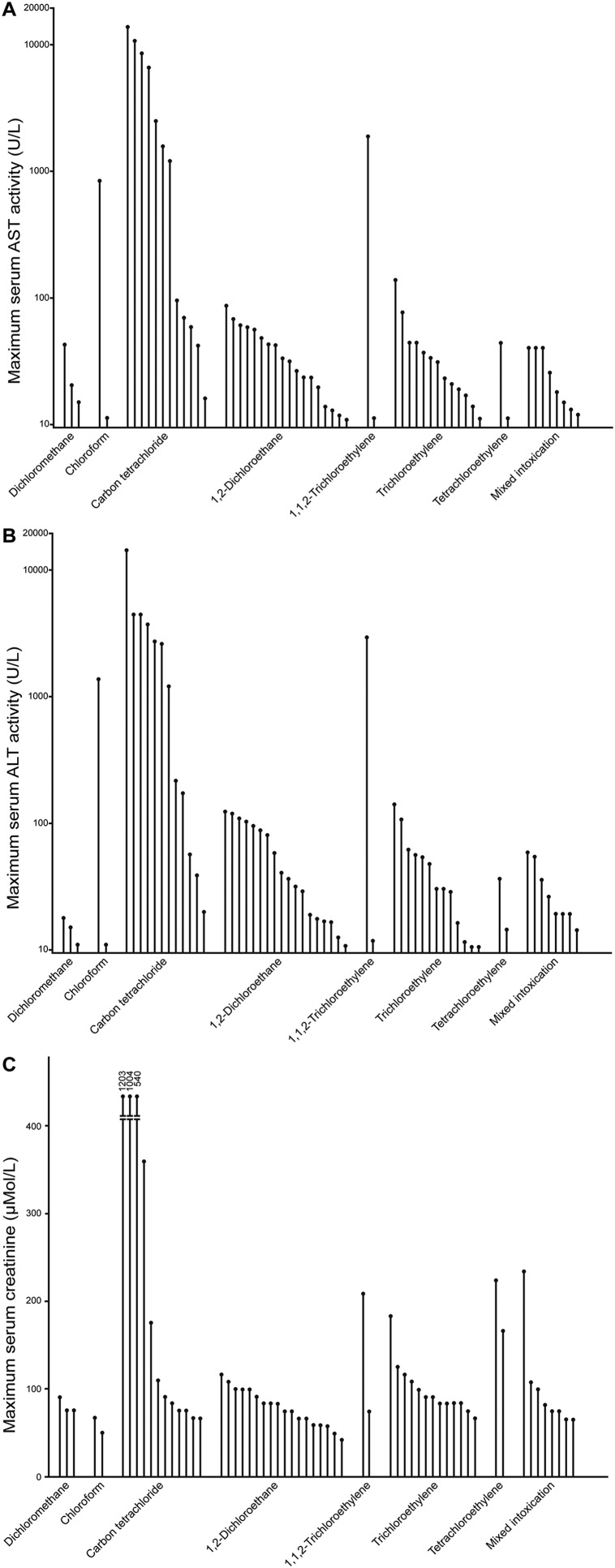
Fig. 2. (A) Maximum serum activities of AST of the study cohort comprising 60 patients with AHH intoxication.
Normal range of AST was <35 U/L. (B) Maximum serum activities of ALT of the study cohort comprising 60 patients with AHH intoxication. Normal range of ALT was <45 U/L. (C) Maximum serum creatinine values of the study cohort comprising 60 patients with AHH intoxication. Normal range of creatinine was 45–90 μM/L.
Abbreviations: AHH, aliphatic halogenated hydrocarbons; ALT, alanine transaminase; AST, aspartate transaminase.
In the initial phase of the clinical study, patients underwent liver biopsy or laparoscopy to obtain liver specimens. Among the 17 patients studied for liver changes, the liver of 12 patients appeared normal upon staining by hematoxylin & eosin or showed steatosis (Supplemental Table 3). However, 2 of the remaining patients tolerated only short-term hyperventilation therapy and their liver disease had to be classified as severe hepatocellular, whereas in 3 other patients, severe liver cell necrosis was found, triggered likely by preexisting alcohol abuse or caused by ingestion of high amounts of AHH. More importantly, among patients with normal or near normal liver histology by light microscopy, electron microscopy data were available for a few patients, showing hepatocellular changes such as mitochondrial injury. As expected, liver injurious effects, though at a low level, were still evident at the time of discontinuation of the therapeutic hyperventilation, but this is not of clinical relevance and does not require a prolongation of the hyperventilation therapy. In the further course after discharge, complete restoration of the previous liver injury is expected but it may take some time, until the AHH stored in various organs, including the fat tissue, has left the body. Considering these aspects, all patients were advised at discharge to abstain from alcohol use for at least 3 months.
Renal injury
Maximum serum creatinine values are illustrated for all AAH during the hyperventilation therapy, with the highest values found in patients intoxicated by CCl4 (Fig. 2C). Therefore, all patients intoxicated by AHH require continuous analysis of serum creatinine values and early decision to introduce forced diuresis (Table 1), to help prevent or ameliorate renal insufficiency. Despite these preventive measures, a few patients of the study cohort required dialysis.
Clinical course and outcome
A brief overview of the 52 patients with intoxications by a single AHH is presented in Table 2 and of the 8 patients with mixed chemical intoxications by AHH in Table 3.
Table 2. Selected case details of 52 patients with acute single chemical intoxication by seven aliphatic halogenated hydrocarbons treated with the CO2-induced hyperventilation.
| Case | AHH intoxication; Route (amount) | Selected case details of 52 patients under CO2-induced hyperventilation |
| 1. Male, 46 years | Dichloromethane; Ingestion (45 mL) | Intentional ingestion with subsequent vomiting. Awake at admission. Clinically lacking signs of toxicity with good outcome. |
| 2. Male, 49 years | Dichloromethane; Inhalation (20 mL) | Accidental inhalation. No narcosis. No apparent clinical toxicity. |
| 3. Female, 50 years | Dichloromethane; Ingestion (40 mL) | Unintentional ingestion with subsequent self-induced vomiting. Dizziness. Subsequent uneventful clinical course. |
| 4. Male, 21 years | Chloroform; Ingestion (50 mL) | Following consumption of beer (1.5 L), unintentional ingestion of chloroform. Seizures. Narcosis requiring endotracheal intubation for gastrointestinal lavage and short-term artificial ventilation. |
| 5. Female, 51 years | Chloroform; Ingestion (10 mL) | Unintentional ingestion of chloroform. Lack of clinical toxicity. |
| 6. Male, 15 years | CCl4; Ingestion (30 mL) | Patient swallowed CCl4 intentionally and experienced vomiting twice. On day 3 after intoxication, serum activities of liver enzymes slightly increased, reached a maximum on day 4, and normalized until day 14 after intoxication. Liver biopsy on day 13 showed no abnormalities. |
| 7. Female, 14 years | CCl4; Ingestion (10–20 mL) | Intentional ingestion of CCl4 accessed from a dry cleaning business where her mother was employed. She was fully oriented. Liver histology at termination of the hyperventilation therapy showed no overt liver cell necrosis but severe microvesicular fatty liver in 80–90% of the liver cells. By electron microscopy, cristae of mitochondria were reduced and disorganized. Within the mitochondria, crystalline inclusion bodies were found. At discharge all laboratory values were in the normal range. |
| 8. Male, 31 years | CCl4; Ingestion (50 mL) | Patient intentionally swallowed CCl4 and was found by his mother. After initial treatment in a local hospital and recurrent vomiting, his clinical course was uneventful, except for a short increase of serum AST and ALT activities during days 3 and 5 after ingestion. |
| 9. Male, 70 years | CCl4; Ingestion (∼50 mL) | Patient unintentionally ingested CCl4 contained in a bottle labeled erroneously as lemonade. He was sleepy and experienced diarrhea with black-colored stools, with later recurrent vomiting also. Peak serum activities for AST and ALT were observed on day 7 after ingestion. |
| 10. Male, 40 years | CCl4; Ingestion (100 mL) | Following intentional ingestion of CCl4, the somnolent patient received short-term endotracheal intubation for initiating CO2-induced hyperventilation during transport via plane. Maximum values for serum activities of AST and ALT were observed on day 4 after ingestion, associated with an increase of serum creatinine. Liver histology on day 14 after ingestion revealed moderate centrilobular microvesicular fatty liver with low liver cell necrosis. Electron microscopy showed a striking proliferation and pronounced dilatation of the smooth endoplasmic reticulum of the hepatocytes. These dilated cisterns contained small membrane vortexes, similar to myelin. In the cytoplasm, vacuoles predominated. Mitochondria were enlarged and showed degeneration and vacuoles. Microvilli presented with injurious irregularities. Abnormal laboratory results returned to normal values rapidly. |
| 11. Female, 50 years | CCl4; Inhalation (∼10 mL) | Unintentional intoxication was by inhalation of CCl4, which the patient used for cleaning of a spot on her carpet. She experienced nausea for a day and had a blood ethanol level of 1.77. Under hyperventilation therapy, serum activities of AST and ALT remained virtually unchanged. |
| 12. Female, 50 years | CCl4; Ingestion (∼50 mL) | Patient unintentionally swallowed CCl4 contained in a mineral water bottle and vomited intentionally. Symptoms included severe headaches and nausea. Peak enzyme activities were determined for serum AST and ALT 4 days after ingestion, with retarded decline and normalization. |
| 13. Male, 36 years | CCl4; Ingestion (50 mL) | Patient intentionally ingested CCl4 and consumed beer (∼2.5 Liter) and hard liquor (∼0.5 Liter). At admission, his blood ethanol was 2.5–3.0. On day 3 after ingestion, a peak activity was observed for AST and AST, with subsequent decline. Serum total bilirubin and creatinine increased steadily. Endotracheal intubation, dialysis, and two reanimations were required. Complications included acute liver failure, pneumonia, and respiratory insufficiency, leading to a fatal outcome. |
| 14. Female, 22 years | CCl4; Inhalation (∼50 mL) | Intentional inhalation of CCl4 led to nausea, stomach cramps, tachycardia, and intermittent somnolence. Prompted decline of initially increased serum activities of ALT and AST. |
| 15. Male, 33 years | CCl4; Inhalation (amount?) | As a conservator and owner of a business for restoring oil paintings and art work, the patient unintentionally inhaled CCl4 for several days, a typical occupational intoxication. Symptoms included loss of appetite, nausea, vomiting, headaches and pains in the neck. Throughout the clinical course, values of serum total bilirubin and creatinine remained in the normal range. Previous alcohol use of 0.7 Liter wine and 0.2 Liter beer daily was considered as risk factor of the liver toxicity by CCl4. On day 5 after admission, liver histology showed a moderate steatosis with small and large fat droplets as well as some inflammation but no necrosis. By electron microscopy, the mitochondria were swollen and their cristae were reduced. Abundant bile pigments were seen between the nucleus and bile canaliculus. Initially increased serum activities for AST and ALT normalized rapidly. |
| 16. Female, 29 years | CCl4; Inhalation (amount?) | Patient unintentionally inhaled CCl4 for 4–5 weeks under similar working conditions as described for Patient 15 above, in whose business she was employed as conservator. Symptoms included malaise, loss of appetite, fever, dark urine, flu-like joint pains and back pains. Her alcohol use was quantified as 2 Liter wine per week. Liver histology obtained 3 days after cessation of the hyperventilation therapy showed in zone 3 a mild steatosis involving 20–30% of the hepatocytes, severe single cell necrosis, and a moderate activation of hepatic stellate cells. The clinical course was complicated by oliguric renal insufficiency, treated with forced diuresis. The initially increased serum activities of AST and ALT normalized until day 11. |
| 17. Male, 31 years | CCl4; Inhalation (amount?) | Patient unintentionally inhaled CCl4 and had worked together with Patients 15 and 16 above in the business of Patient 15 above and under similar working conditions. Symptoms included nausea, headaches, joint pains, lower back pains, sore throat, dark urine, vomiting and diarrhea. Via a university hospital, he was transferred by helicopter, together with Patient 11, to our Intensive Care Unit. He showed beginning withdrawal symptoms, likely related to his alcohol use which was reported as 2.5 Liter beer daily and occasionally more. Initially increased serum activities of AST and ALT normalized within 17 days. Liver histology obtained 39 days after the last CCl4 exposure showed a low-grade fatty liver and residues of a toxic event. Complications included basal pneumonia, respiratory insufficiency requiring O2 application, and renal insufficiency requiring intermittent hemodialysis on 12 days. Discharge was possible after 42 days in fairly good condition. This case, as well as the 2 cases above, had been reported to the respective trade association. |
| 18. Male, 37 years | 1,2-Dichloroethane; Ingestion (15 mL) | Accidental ingestion, vomiting. During weekends, 1.0 – 1.5 Liter beer. Marginally increased serum activities of AST and ALT. |
| 19. Male, 40 years | 1,2-Dichloroethane; Ingestion (15–20 mL) | Unintentional uptake. No symptoms at admission. Minimally increased serum activities of AST and ALT. |
| 20. Male, 16 years | 1,2-Dichloroethane; Ingestion (∼50 mL) | Intentional ingestion. Gastrointestinal lavage in the local hospital. Serum activities of AST and ALT remained in the normal range. |
| 21. Female, 24 years | 1,2-Dichloroethane; Ingestion (7 mL) | Accidental ingestion. Normal serum AST and ALT. |
| 22. Male, 48 years | 1,2-Dichloroethane; Ingestion (5 mL) | Unintentional ingestion. Vomiting, subsequent nausea. Serum activities of AST and ALT were slightly increased. |
| 23. Male, 44 years | 1,2-Dichloroethane; Ingestion (25 mL) | Accidental uptake, self-induced vomiting followed by spontaneous vomiting, nausea. Superficial ulceration of the tongue. Slightly increased serum values of AST and ALT. |
| 24. Female, 17 years | 1,2-Dichloroethane; Ingestion (40 mL) | Intentional ingestion, with 100 mg medazepam and 1.0 L beer. Recurrent vomiting. Transfer by helicopter. Small increases of serum AST and ALT activities. |
| 25. Male, 46 years | 1,2-Dichloroethane; Ingestion (5 mL) | Accidental ingestion, vomiting. Uneventful clinical course with normal serum activities of AST and ALT. |
| 26. Male, 78 years | 1,2-Dichloroethane; Ingestion (35 mL) | Unintentional ingestion after alcohol use. Vomiting and nausea. Cautious CO2-induced hyperventilation for 5 days, premature cessation due to COPD. Slight increases of AST and ALT activities. |
| 27. Male, 37 years | 1,2-Dichloroethane; Ingestion (35 mL) | Unintentional ingestion, vomiting. Gastrointestinal lavage. Virtually normal serum AST and ALT activities. |
| 28. Male, 34 years | 1,2-Dichloroethane; Ingestion (15 mL) | Accidental ingestion. Gastrointestinal lavage in local hospital. Slightly increased serum activities of AST and ALT. |
| 29. Male, 35 years | 1,2-Dichloroethane; Ingestion (25 mL) | Unintentional ingestion, vomiting several times. Transfer by helicopter. Dizziness and nausea. Slightly increased serum activities of AST and ALT. |
| 30. Female, 18 years | 1,2-Dichloroethane; Ingestion (80 mL) | Intentional ingestion, vomiting at home. Minimally increased serum activities of AST and ALT. |
| 31. Female, 57 years | 1,2-Dichloroethane; Ingestion (50 mL) | Intentional ingestion, then vomiting, and gastrointestinal lavage. Moderate alcohol use. Under hyperventilation therapy, normal AST and ALT. |
| 32. Female, 79 years | 1,2-Dichloroethane; Inhalation (∼8 mL) | Unintentional inhalation with usual treatment thereafter. Marginally increased serum activities of AST and ALT. |
| 33. Male, 32 years | 1,2-Dichlorethane; Inhalation (∼6 mL) | Unintentional inhalation for 2 hours. Vomiting and nausea. Minimally increased serum activities of AST and ALT. |
| 34. Female, 81 years | 1,2-Dichloroethane Ingestion (20 mL) |
Unintentional ingestion, immediate vomiting and later short somnolence. Gastrointestinal lavage. Single defibrillation for cardiac ventricular flutter. Under therapy, virtually normal serum activities of AST and ALT. |
| 35. Female, 21 year | 1,2-Dichloroethane; Ingestion (35 mL) | Intentional intake, with prior ingestion of 20 mL hard liquor. Vomiting. Gastrointestinal lavage. Serum activities of AST and ALT remained normal. |
| 36. Female, 20 years | 1,1,2-Trichloroethane; Ingestion (100 mL) | Intentional ingestion, together with oxaxepam (20 tablets). Narcosis. Systemic seizures. Vomiting and nausea. ECG: significant extrasystoles. Acrocyanosis. Rapid start of CO2-induced hyperventilation. Serum activities of AST and ALT remained normal. |
| 37. Male, 19 years | 1,1,2-Trichloroethane; Ingestion (unknown amount) | Intentional ingestion, also of temazepam (340 mg). Found unconscious. For 4 days, initial treatment in a local hospital, gastrointestinal lavage. At transfer, intubated, moribund, and death the other day. Forced diuresis. Artificial ventilation. Before death, maximum serum activities: AST 1870 U/L, ALT 2950 U/L. |
| 38. Male, 49 years | Trichloroethylene; Ingestion (40 mL) | Unintentional ingestion. Vomiting, diarrhea. Dizziness. Transfer by airplane and start, on board, with CO2-induced hyperventilation, which was continued for 4 days and caused undetectable blood levels of the AHH and virtually normal serum activities of AST and ALT. |
| 39. Male, 50 years | Trichloroethylene; Ingestion (100 mL) | Unintentional ingestion. Narcosis. Transport by airplane, narcosis receded. Incipient pneumonia. Serum activities of AST and ALT remained in the normal range. |
| 40. Male, 55 years | Trichloroethylene; Ingestion (50 mL) | Accidental ingestion. Preexisting AFLD, presently reduced alcohol use. Dizziness. Slightly increased serum activities of AST and ALT. |
| 41. Male, 16 years | Trichloroethylene; Ingestion (300 mL) | Intentional ingestion. Narcosis. Marginally increased serum activities of AST and ALT. |
| 42. Male, 18 years | Trichloroethylene; Ingestion (>10 mL) | Unintentional ingestion after drinking 1.0 L beer. Nausea, narcosis. Slightly increased serum activities of AST and ALT. |
| 43. Male, 67 years | Trichloroethylene; Ingestion (30 mL) | Accidental ingestion. Following gastrointestinal lavage and under CO2-induced hyperventilation, serum activities of AST and ALT remained normal. |
| 44. Male, 49 years | Trichloroethylene; Ingestion (90 mL) | Intentional ingestion together with Stroh rum. Known alcohol abuse. Vomiting, narcosis, endotracheal intubation, subsequent gastrointestinal lavage. Suspected shock in lungs, artificial ventilation combined with CO2-induced hyperventilation, whereby CO2 was added to the inspiration air. Serum activities of AST and ALT moderately increased at admission, thereafter rapid normalization. |
| 45. Male, 17 years | Trichloroethylene; Ingestion (70 mL) | Unintentional ingestion. Somnolence. Vomiting. Normal serum AST and ALT activities. |
| 46. Male, 26 years | Trichloroethylene; Ingestion (100 mL) | Intentional ingestion. Alcohol abuse. Vomiting. Narcosis. Transfer by plane. Serum activities of AST and ALT remained in normal range. |
| 47. Female, 20 years | Trichloroethylene; Ingestion (100 mL) | Intentional ingestion, together with diclofenac (1.0 g), hydrochlorothiazide (1.5 g), and acetyl salicylic acid (5.6 g). Narcosis, endotracheal intubation. After transfer, start with hyperventilation by means of a respirator and adding CO2 to the inspiration mixture, which reduced serum trichloroethylene levels from >1 mg/mL down to 1 μg/mL. Serum activities of AST and ALT remained normal. The clinical course was fatal due to respiratory insufficiency and complications, ascertained by autopsy: Purulent trachea-bronchitis, pneumonia, and congestion of the liver. |
| 48. Male, 28 years | Trichloroethylene; Ingestion (>500 mL) | Intentional ingestion. Spontaneous and later self-induced vomiting. Narcosis. Serum activities of AST and ALT remained in the normal range. |
| 49. Male, 49 years | Trichloroethylene; Ingestion (700 mL) | Intentional ingestion. Somnolence. Became wet, fecal leakage. Known alcohol abuse. Marginally increased serum activities of AST and ALT. |
| 50. Female, 40 years | Trichloroethylene; Ingestion (200 mL) | Intentional ingestion. Narcosis, fecal leakage. Minimally increased activities of AST and ALT. |
| 51. Male, 51 years | Tetrachloroethylene; Ingestion (160 mL) | Accidental ingestion. Dizziness. Self-induced vomiting. Transfer by helicopter. Serum activities of AST and ALT remained normal. |
| 52. Male, 61 years | Tetrachloroethylene; Ingestion (500 mL) | Intentional ingestion. Narcosis. Complicated clinical course with aspiration pneumonia. Minimally increased serum activities of AST and ALT. |
Case data are presented in condensed form. For listed patients with intoxications by CCl4, many additional data of interest are available, though not included in this table; the data had been reported previously in a recent publication.16 For all patients with intoxication by ingestion of AHH, treatment was commonly initiated in the local hospital by gastrointestinal lavage, sometimes already. Intoxicated patients by ingestion or inhalation all received the CO2-induced hyperventilation therapy, whereby CO2 was provided via nasal tube and rarely via an oxygen mask. A few patients required endotracheal intubation and received CO2 together with the inspiration air. Treatment was provided as outlined (Table 1).
Abbreviations: AFLD, alcoholic fatty liver disease: ALT, alanine transaminase; AST, aspartate transaminase; CCl4, carbon tetrachloride; COPD, chronic obstructive pulmonary disease.
Table 3. Summary of case details of 8 patients with acute mixed chemical intoxication by aliphatic halogenated hydrocarbons and other chemicals.
| Patient | Intoxicating chemicals | Amounts | Route of uptake | Outcome |
| 1. | Dichloromethane Methylethylketone Toluene-2,4-diisocyanate Ethanol (absolute) |
30 mL 25 mL 30 mL 25 mL |
All by ingestion | Favorable |
| 2. | Dichloromethane Trichloroethane Tetrachloroethylene Benzene |
25 mL 25 mL 50 mL 25 mL |
All by ingestion | Favorable |
| 3. | Dichloromethane Toluene |
100 mL 20 mL |
All by ingestion | Favorable |
| 4. | CCl4 Diethylether Clenbuterol Oxeladin |
Unknown Unknown ∼30 mL Unknown |
All by ingestion | Favorable |
| 5. | CCl4 Trichloroethylene Tetrachloroethylene Diethylether |
Unknown for all chemicals | All by inhalation | Favorable |
| 6. | CCl4 Tetrachloroethylene |
Unknown for all chemicals | All by inhalation | Favorable |
| 7. | 1,2-Dichloroethane 1,1,2-Trichloroethane |
60 mL 90 mL |
All by ingestion | Fatal |
| 8. | Trichloroethylene Tetrachloroethylene Benzene Toluol Xylol |
Unknown for all chemicals | All by inhalation | Favorable |
Only patients intoxicated by ingestion received a gastrointestinal lavage, all patients were treated with the CO2-induced hyperventilation according to described details (Table 1).
Abbreviation: CCl4, carbon tetrachloride.
Clinical course was favorable among 56/60 patients, but outcome was fatal in 4/60 patients, corresponding to a lethality rate of 6.7%. In those patients with a fatal outcome, the ratio of males:females was 3:1. All patients with a fatal outcome had used AHH intentionally (cases 13, 37 and 47 in Table 2; case 7 in Table 3). In more detail, a 22 year-old male patient (case 7 in Table 3) ingested 60 mL 1,2-dichloroethane plus 90 mL1,1,2-trichloroethane and was treated by CO2-induced hyperventilation, initiated 5 hours after intoxication; death occurred 25 hours after the intoxication (case 7 in Table 3). The second patient (case 37 in Table 2), a 19 year-old man, died 122 hours after ingestion of 1,1,2-trichloroethane in unknown amounts and of temazepam after transfer in moribund conditions for hyperventilation therapy at 111 hours following ingestion. The third patient (case 47 in Table 2) was a 20 year-old woman, who had ingested 100 mL trichloroethylene combined with diclofenac, hydrochlorothiazide, and acetyl salicylic acid. The fourth patient (case 13 in Table 2) was a 36 year-old male, who had ingested 50 mL CCl4, 0.5 Liter hard liquor, and 1–2 Liter beer, resulting in a blood alcohol level of 3‰ at admission.
Various factors may influence the clinical outcome. These include early start and extent of primary toxin elimination through vomiting, gastrointestinal lavage, and CO2-induced hyperventilation, apart from effectivity of the intravenously applied cimetidine and glucose in high amounts. Risk factors are also intentional intoxication, uptake of high amounts of AHH, concomitant ingestion of overdosed drugs with high amounts of alcohol, and a history of alcohol abuse.
Individual cases with liver injury caused by specific AHH
Dichloromethane: Intoxications caused only small changes in laboratory values (Fig. 2A–2C). The clinical course was without peculiarities (cases 1–3 in Table 2).
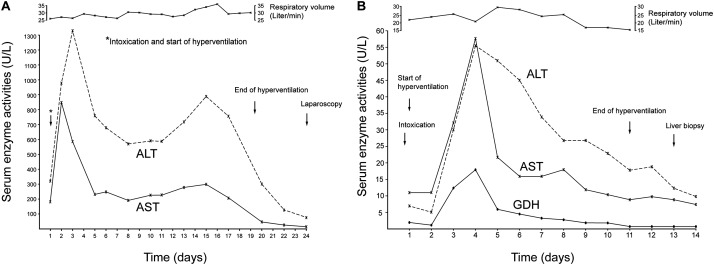
Chloroform: Intoxications occurred in 2 patients (cases 4 and 5 in Table 2), leading to variably increased serum activities of AST and ALT (Fig. 2A and 2B) but leaving serum creatinine levels unchanged (Fig. 2C). As an example, the data for 1 of the cases are presented with all details in Fig. 3A.
Fig. 3. (A) Serum activities of AST and ALT after intoxication by ingested chloroform.
The patient (case 4 in Table 2) was a house painter and had stored chloroform in a beer bottle, from which he erroneously ingested 50 mL chloroform. Prior to intoxication, he had drank 1.5 L beer contained in other beer bottles. Narcosis requiring endotracheal intubation for gastrointestinal lavage and short-term artificial ventilation. The CO2-induced hyperventilation was started 6 hours after ingestion, whereby a respiratory volume between 24 and 34 Liter min-1 was achieved as shown at the top of the figure. Via a nasal tube, CO2 was provided at a flow rate of 3 to 6 Liter min−1. Blood showed pH that was between 7.36 and 7.46, PO2 between 83 and 110 mmHg, PCO2 between 35 and 55, and HCO3 between 21 and 31, and respiratory frequency between 15 and 30 min−1. The therapy using hyperventilation was well tolerated by the cooperative patient and had to be extended for 19 days because serum activities of AST and ALT declined slowly. Serum activities of liver enzymes showed two peaks. On day 2, AST was 850 U/L and ALT was 1330 U/L on day 3. Both enzymes declined and had a second peak on day 15, with AST of 300 U/L and ALT of 890 U/L. Total bilirubin was in a range between 0.8 and 2.1 mg/dL. Hemoglobin was between 11.8 and 15.5, erythrocytes between 3800 and 4800, leucocytes between 5.100 and 11500. Serum creatinine remained in the normal range, considering that forced diuresis was provided for prophylactic reasons. Liver biopsy obtained at laparoscopy on day 24 revealed a striking proliferation of stellate cells, no fatty changes of the hepatocytes, and no necrosis by light microscopy. (B) Serum activities of AST, ALT, and GDH after intoxication by ingested CCl4. The patient (case 6 in Table 2) intentionally swallowed 30 mL CCl4, vomited twice, and received a gastrointestinal lavage. CO2-induced hyperventilation was started 9 hours after intoxication and continued for 11 days. CO2 was applied via a nasal tube at a flow rate of 2–4 Liter min−1 and resulted in a respiratory volume of up to 30 Liter min−1, as shown on the top of the figure. CO2 was given at a flow rate between 3.5 and 5.0 Liter min−1 that resulted in a respiratory frequency ranging from 28 to 36 per minute. Blood showed pH of 7.31 to 7.33, PO2 of around 111, HCO3 of around 21.3, and PCO2 of around 43.9. On day 3 after intoxication, serum activities of liver enzymes increased and reached a maximum on day 4 (AST of 59 U/L, ALT of 56 U/L, GDH of 18 U/L) and normalized during the next days until day 14 after intoxication. Other laboratory test were in the normal range, except leucocytes (at 14400). Liver biopsy on day 13 showed no abnormalities. The patient was discharged on day 15.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CCl4, carbon tetrachloride; GDH, glutamate dehydrogenase.
CCl4: For some of the 12 patients experiencing intoxications (cases 6–17 in Table 2), high values were found for serum activities of AST (Fig. 2A) and ALT (Fig. 2B). Although treatment included forced diuresis, patients were also at risk for renal injury, as evidenced by increased serum levels of creatinine (Fig. 2C). The data for 1 patient (case 6 in Table 2) with an uneventful outcome are illustrated in Fig. 3B.
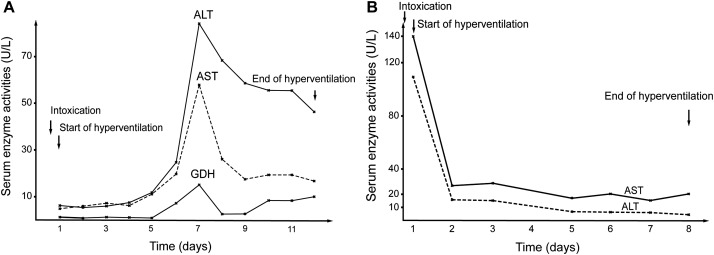
1,2-Dichloroethane: For the overall 18 patients (cases 18–35 in Table 2), laboratory values of serum AST, ALT and creatinine were only moderately elevated or remained in the normal range (Fig. 2A–2C). The data for a typical case are presented with all details in Fig. 4A.
Fig. 4. (A) Serum activities of ALT, AST, and GDH after intoxication by ingested 1,2-dichloroethane.
The patient (case 30 in Table 2) intentionally ingested 80 mL dichloroethane. After vomiting and gastrointestinal lavage 4 hours after ingestion, the usual CO2-induced hyperventilation was started using for CO2 application by the nasal tube approach to achieve a respiratory volume between 20 and 33 Liter min−1 and a respiratory frequency between 20 and 31 per minute. Treatment was for 12 days, prolonged due to slow decline of the liver values. At day 7 after intoxication, maximum serum activities were as follows: AST of 58 U/L; ALT of 85 U/L; and, GDH of 15 U/L. (B) Serum activities of ALT and AST in a patient with ingested trichloroethylene. Presented are data beginning at time of intoxication and during the subsequent clinical course. The patient (case 44 in Table 2) intentionally ingested 70 mL trichloroethylene together with Stroh rum. Known alcohol abuse. Vomiting, narcosis, endotracheal intubation prior to gastrointestinal lavage. Suspected shock in lungs, artificial ventilation combined with CO2-induced hyperventilation for 8 days, whereby CO2 was added to the inspiration air. Already at admission, the serum activities of AST (140 U/L) and of ALT (110 U/L) were slightly increased, followed by rapid normalization. At laparoscopy 1 month after intoxication, suspicion of fatty liver, confirmed by histology showing severe macrovesicular fatty liver without necrosis or infiltration considered compatible with alcoholic fatty liver but not with alcoholic steatohepatitis or alcoholic hepatitis. Shortly after admission, the patient experienced withdrawal symptoms requiring specific drug treatment. The initially increased AST and ALT activities were likely due to the preexisting alcohol abuse associated with alcoholic fatty liver, considering that AST values were higher than ALT values, providing a ratio of AST/ ALT of >1.0 that is highly suggestive for alcohol as cause. Such a ratio was not observed in any other patients intoxicated by AHH, who all had higher values of ALT than of AST.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; GDH, glutamate dehydrogenase.
1,1,2-Trichloroethane: Considered were 2 patients (cases 36 and 37 in Table 2). Only 1 of the patients presented with high laboratory values (Fig. 2A–2C).
Trichloroethylene: For all 13 patients (cases 38–50 in Table 2), laboratory values were mostly unchanged or rarely moderately increased (Fig. 2A–2C). The data for 1 of these patients are given with all details in Fig. 4B.
Tetrachloroethylene: Among the 2 patients (cases 51 and 52 in Table 2), serum activities of aminotransferases were either unchanged or slightly increased (Fig. 2A and 2B), whereas serum creatinine presented with moderately increased levels despite forced diuresis (Fig. 2C).
Mixed AHH chemical intoxications: Overall, 8 patients experienced intoxications with variable combinations of AHH chemicals (cases 1–8 in Table 3). Serum activities remained unchanged or were slightly increased for AST (Fig. 2A) and ALT (Fig. 2B), while serum levels of creatinine were virtually normal except for in 1 patient (Fig. 2C).
Discussion
This report provides highlights and a comprehensive overview of an innovative therapy approach applied in 60 patients with acute intoxications by AHH, focusing on the CO2-induced hyperventilation (Table 1). The basic idea to treat patients intoxicated by AHH using forced ventilation originated from preliminary results of a single case published by Pebay-Peyroula and Nicaise.23 Subsequently, this new form of therapy was clinically introduced and refined at the Heinrich Heine University hospital of Düsseldorf in Germany, first in children18,24 and later in adults, with the aim to present practical and specific recommendations (Table 1).15,16
Several experimental and clinical studies have since provided additional data in support of the CO2-induced hyperventilation therapy. For instance, intoxicating AHH, such as CCl4, taken up by animals are mostly eliminated (99%) through the lungs,25 while up to 44% of the AHH taken up by humans are eliminated via the lungs at a rate of up to 44% within the first hour.26 Furthermore, addition of CO2 to the inspiration air of patients with AHH intoxication increased the minute respiration volume via an increased respiration frequency, and pulmonary exhalation of CCl4 was in parallel with the minute respiratory expiration volume.16 Reduced respiration due to pneumonia caused increased blood levels of CCl4 in intoxicated patients.14,16 Conditions similar to intermittent interruption of the hyperventilation therapy that also led to temporarily increased blood levels of CCl4 until reintroduction of the CO2-induced hyperventilation therapy again caused a decline of blood levels.16 The beneficial effect of hyperventilation had been ascertained in an experimental model of liver injury by CCl4.11,12,14 For instance, experimental CO2-induced hyperventilation applied in CCl4 intoxication attenuated the extent of liver injury,11 significantly reduced the LD50,11,16,27 and diminished CCl4 levels in blood, liver and fat tissue.12,16 The combination of these facts led to the refinement of the hyperventilation therapy15,16 that was applied in the 60 patients of this study (Supplemental Tables 1 and 2).
In the clinical setting of the studied 60 patients with AHH intoxication, the individual contribution of hyperventilation on reducing liver injury is difficult to assess, due to existing confounders. For instance, the study cohort was not homogenous with respect to variability of AHH as intoxication chemicals; most patients experienced vomiting which contributed to toxin elimination, and all patients with intoxication by ingestion received a gastrointestinal lavage for primary toxin elimination. Finally, patients intoxicated by ingestion or inhalation received a therapy that included high glucose infusion and intravenously given cimetidine. Considering the liver test results for AST and ALT, in a few patients the serum activities of AST and ALT remained in the normal range during the observation period under therapy. Other patients had high serum activities of AST (Fig. 2A) and ALT (Fig. 2B), suggesting that the therapy approaches taken together will not be able to prevent liver injury completely. Nevertheless, clinical experience strongly suggests that therapy for intoxicated patients should be initiated as soon as possible, starting with gastrointestinal lavage in the intubated patient with intoxication by ingestion as the first measure in the local hospital that first takes care of the patient.
Intoxications by AHH and related liver toxicity is a worldwide issue, with partially high lethality rates as shown by abundant publications; as examples, a few of these are selectively referenced.28–49 The low lethality rate of 6.7% in the present study is encouraging but cannot be fully appreciated due to the lack of an appropriate control group of patients who did not receive the therapy outlined in this study (Table 1). Despite these shortcomings, it is hoped that more patients with acute AHH intoxications will receive this therapy and experience their benefits rather than being confronted with a risky wait-and-see approach by their physicians.
Conclusions
With the establishment of modern, active therapeutic approaches, including CO2-induced hyperventilation, the prognosis is now substantially better for patients acutely intoxicated by AHH, provided treatment is initiated without delay and replacing a wait-and-see approach conflicted by poor prognosis. In the near future, progress can be expected after successful search for additional drugs or compounds that provide inhibition of AHH metabolism, which may be better than the cimetidine that is used as the preferred drug presently. Studying such inhibition at the level of CYP 2E1 is facilitated using experimental CCl4 models, because their result can easily be transferred to patients intoxicated by CCl4 or other AHH. Inhibitor candidates may come from drugs already on the market or from Herbal & Traditional Chinese Medicine.
Supplementary information
Acknowledgement
The author is in debt to previous members of the working group at the Heinrich Heine University in Düsseldorf (Germany): H. Frenzel, J. Gellert, L. Goldermann, K.H. Hauptmeier, T. Heidenreich and W. Vierke, who were authors of own published work or co-authors of publications all referenced in this article.
Abbreviations
- AHH
aliphatic halogenated hydrocarbons
- ALA
aminolevulinic acid
- ALT
alanine transaminase
- AST
aspartate transaminase
- CCl4
carbon tetrachloride
- CYP450
cytochrome P450
- CYP450 2E1
cytochrome P450 2E1
- DILI
drug-induced liver injury
- GDH
glutamate dehydrogenase
- ROS
reactive oxygen species
References
- 1.Zimmerman HJ. Hepatotoxicity. Philadelphia: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 2.Helmenstine AM. Aliphatic hydrocarbon definition. Available from: https://www.thoughtco.com/definition-of-aliphatic-hydrocarbon-604763 .
- 3.Raucy JL, Kraner JC, Lasker JM. Bioactivation of halogenated hydrocarbons by cytochrome P4502E1. Crit Rev Toxicol. 1993;23:1–20. doi: 10.3109/10408449309104072. [DOI] [PubMed] [Google Scholar]
- 4.Plaa GL. Chlorinated methanes and liver injury: highlights of the past 50 years. Annu Rev Pharmacol Toxicol. 2000;40:42–65. doi: 10.1146/annurev.pharmtox.40.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Recknagel RO, Glende EA, Jr, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 6.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 7.Hasumura Y, Teschke R, Lieber CS. Increased carbon tetrachloride hepatotoxicity, and its mechanism, after chronic ethanol consumption. Gastroenterology. 1974;66:415–422. doi: 10.1016/S0016-5085(74)80142-3. [DOI] [PubMed] [Google Scholar]
- 8.Teschke R, Vierke W, Goldermann L. Carbon tetrachloride (CCl4) levels and serum activities of liver enzymes following acute CCl4 intoxication. Toxicol Lett. 1983;17:175–180. doi: 10.1016/0378-4274(83)90054-1. [DOI] [PubMed] [Google Scholar]
- 9.Vierke W, Gellert J, Teschke R. Head-space gas chromatographic analysis for rapid quantitative determination of carbon tetrachloride in blood and liver of rats. Arch Toxicol. 1982;51:91–99. doi: 10.1007/bf00279324. [DOI] [Google Scholar]
- 10.Teschke R, Hauptmeier KH, Frenzel H. Effect of an acute dose of ethanol on the hepatotoxicity due to carbon tetrachloride. Liver. 1983;3:100–109. doi: 10.1111/j.1600-0676.1983.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 11.Frenzel H, Heidenreich T, Gellert J, Teschke R. Protective effect of CO2-induced hyperventilation on the hepatotoxicity elicited by carbon tetrachloride. Liver. 1982;2:376–384. doi: 10.1111/j.1600-0676.1982.tb00837.x. [DOI] [PubMed] [Google Scholar]
- 12.Gellert J, Goldermann L, Teschke R. Effect of CO2-induced hyperventilation on carbon tetrachloride (CCl4) levels following acute CCl4 poisoning. Intensive Care Med. 1983;9:333–337. doi: 10.1007/bf01692552. [DOI] [PubMed] [Google Scholar]
- 13.Homann J, Rotter S, Schneider S, Röttger P, Kratz F, Kroker R, et al. Influence of cimetidine on ccl4 -induced liver injury and survival in rats. Biochem Pharmacol. 1985;34:415–416. doi: 10.1016/0006-2952(85)90066-8. [DOI] [Google Scholar]
- 14.Goldermann L, Gellert J, Teschke R. Quantitative assessment of carbon tetrachloride levels in human blood by head-space gas chromatography: application in a case of suicidal carbon tetrachloride intoxication. Intensive Care Med. 1983;9:131–135. doi: 10.1007/bf01772580. [DOI] [PubMed] [Google Scholar]
- 15.Teschke R. Review: Intoxications by aliphatic halogenated hydrocarbons: hepatotoxic risks for patients and clinical issues including role of CO2-induced hyperventilation as therapy option. J Clin Exp Tox. 2018;2:20–24. [Google Scholar]
- 16.Teschke R. Liver injury by carbon tetrachloride intoxication in 16 patients treated with forced ventilation to accelerate toxin removal via the lungs: A clinical report. Toxics. 2018;6:25. doi: 10.3390/toxics6020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teschke R. Alcoholic steatohepatitis (ASH) and alcoholic hepatitis (AH): cascade of events, clinical aspects, and pharmacotherapy options. Expert Opin Pharmacother. 2018;19:779–793. doi: 10.1080/14656566.2018.1465929. [DOI] [PubMed] [Google Scholar]
- 18.Pothmann R, Lemburg P, Sprock I, Göbel U. Hyperventilationstherapie bei oraler Vergiftung mit halogenierten Kohlenwasserstoffen. In: Lemburg P, editor. Pädiatrische Intensivemedizin II. Thieme Stuttgart. 1981. pp. 229–233. [Google Scholar]
- 19.Pischik E, Kauppinen R. An update of clinical management of acute intermittent porphyria. Appl Clin Genet. 2015;8:201–214. doi: 10.2147/TACG.S48605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tschudy DP, Welland FH, Collins A, Hunter G., Jr The effect of carbohydrate feeding on the induction of delta-aminolevulinic acid synthetase. Metabolism. 1964;13:396–406. doi: 10.1016/0026-0495(64)90113-1. [DOI] [PubMed] [Google Scholar]
- 21.Puy H, Deybach JC. Haem biosynthesis and excretion of porphyrins. Chapter 2.3.10. In: Rodés J, Benhamou JP, Blei A, Reichen J, Rizzetto M, editors. Textbook of Hepatology, third edition. eBooks, GastroHep.com; 2018. pp. 207–214. Available from: http://www.gastrohep.com/ebooks/rodes/Rodes_2_3_10.pdf . Accessed July 28 2018. [Google Scholar]
- 22.Levin W, Jacobson M, Kutzman R. Incorporation of radioactive-delta-aminolevulinic acid into microsomal cytochrome P450. Arch Biochem Biophys. 1972;148:262–269. doi: 10.1016/0003-9861(72)90140-3. [DOI] [PubMed] [Google Scholar]
- 23.Pebay-Peyroula F. Pulmonary elimination of poisons. Measurement. Toxicological applications. Eur J Toxicol. 1970;3:300–308. [PubMed] [Google Scholar]
- 24.Lemburg P, Sprock I, Bretschneider A, Storm W, Göbel U. A new concept of therapy in accidental intoxications with halogenated hydrocarbons. Vet Hum Toxicol. 1979;21(Suppl):37–40. [PubMed] [Google Scholar]
- 25.McLean AEM. Drugs, diet and liver injury. In: Gerok W, Sickinger K, editors. Drugs and the Liver. Schattauer Stuttgart; 1975. pp. 143–148. [Google Scholar]
- 26.Morgan A, Black A, Belcher DR. The excretion in breath of some aliphatic halogenated hydrocarbons following administration by inhalation. Ann Occup Hyg. 1970;13:219–233. doi: 10.1093/annhyg/13.4.219. [DOI] [PubMed] [Google Scholar]
- 27.Gellert J, Frenzel H, Heidenreich T, Goldermann L, Vierke W, Teschke R. Effektivität der CO2-induzierten Hyperventilationstherapie durch halogenierte Kohlenwasserstoffe. Intensivmed. 1982;19:293–297. [Google Scholar]
- 28.Schlosser PM, Bale AS, Gibbons CF, Wilkins A, Cooper GS. Human health effects of dichloromethane: key findings and scientific issues. Environ Health Perspect. 2015;123:114–119. doi: 10.1289/ehp.1308030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Environmental Protection Agency (EPA) Toxicological review of dichloromethane (methylene chloride) Available from: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0070tr.pdf . Accessed November 2011.
- 30.Kim NY, Park SW, Suh JK. Two fatal cases of dichloromethane or chloroform poisoning. J Forensic Sci. 1996;41:527–529. doi: 10.1520/jfs13951j. [DOI] [PubMed] [Google Scholar]
- 31.TOXNET Dichloromethane. Available from: https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+66 .
- 32.Bomski H, Sobolewska A, Strakowski A. Toxic damage of the liver by chloroform in chemical industry workers. Int Arch Arbeitsmed. 1967;24:127–134. [PubMed] [Google Scholar]
- 33.Lee DG, Lee CH, Jang KH, Chae HJ, Moon JD. A suspicious case of chloroform induced acute toxic hepatitis in laboratory worker. Korean J Occup Environ Med. 2012;24:304–310. [Google Scholar]
- 34.Docherty JF, Burgess E. The action of carbon tetrachloride on the liver. Br Med J. 1922;2:907–908. doi: 10.1136/bmj.2.3228.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGuire LW. Carbon tetrachloride poisoning. JAMA. 1932;99:988–989. doi: 10.1001/jama.1932.02740640030008. [DOI] [Google Scholar]
- 36.Lehnherr ER. Acute carbon tetrachloride poisoning: Report of a case. Arch Int Med. 1935;56:98–104. doi: 10.1001/archinte.1935.03920010106007. [DOI] [Google Scholar]
- 37.Moon HD. The pathology of fatal carbon tetrachloride poisoning with special reference to the histogenesis of the hepatic and renal lesions. Am J Pathol. 1950;26:1041–1057. [PMC free article] [PubMed] [Google Scholar]
- 38.Jennings RB. Fatal fulminant acute carbon tetrachloride poisoning. AMA Arch Pathol. 1955;59:269–284. [PubMed] [Google Scholar]
- 39.Guild WR, Young JV, Merrill JP. Anuria due to carbon tetrachloride intoxication. Ann Intern Med. 1958;48:1221–1227. doi: 10.7326/0003-4819-48-6-1221. [DOI] [PubMed] [Google Scholar]
- 40.Truss CD, Killenberg PG. Treatment of carbon tetrachloride poisoning with hyperbaric oxygen. Gastroenterology. 1982;82:767–769. [PubMed] [Google Scholar]
- 41.Ruprah M, Mant TG, Flanagan RJ. Acute carbon tetrachloride poisoning in 19 patients: implications for diagnosis and treatment. Lancet. 1985;1:1027–1029. doi: 10.1016/S0140-6736(85)91624-1. [DOI] [PubMed] [Google Scholar]
- 42.Mydlík M, Derzsiová K, Frank K. Renal replacement therapy in acute poisonings–one center experience. Przegl Lek. 2013;70:381–385. [PubMed] [Google Scholar]
- 43.Martin G, Knorpp K, Huth K, Heinrich F, Mittermayer C. Zur Klinik, Pathogenese und Therapie der Dichloräthan-vergiftung. Dtsch Med Wschr. 1968;93:2002–2010. doi: 10.1055/s-0028-1110869. [DOI] [PubMed] [Google Scholar]
- 44.1,1,2-Trichloroethane Available from: https://pubchem.ncbi.nlm.nih.gov/compound/6574 .
- 45.Agency for Toxic Substances and Disease Registry U.S. Public Health Service. Toxicological profile for 1,1,2-Trichloroethane. Available from: https://www.atsdr.cdc.gov/toxprofiles/tp148.pdf . Accessed December 1989. [PubMed]
- 46.TOXNET Toxicology Data Network. U.S. National Library of Medicine. Trichloroethylene. Available from: https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/∼xveGMT:1 .
- 47.U.S. Department of Health and Human Services, Public Health Services Agency for Toxic Substances and Disease Registry (ATSDR). Draft toxicological profile for tetrachloroethylene. Available from: https://www.atsdr.cdc.gov/toxprofiles/tp18.pdf . Accessed October 2014. [PubMed]
- 48.Gold LS, De Roos AJ, Waters M, Stewart P. Systematic literature review of uses and levels of occupational exposure to tetrachloroethylene. J Occup Environ Hyg. 2008;5:807–839. doi: 10.1080/15459620802510866. [DOI] [PubMed] [Google Scholar]
- 49.Shen C, Zhao CY, Liu F, Wang YD, Wang W. Acute liver failure associated with occupational exposure to tetrachloroethylene. J Korean Med Sci. 2011;26:138–142. doi: 10.3346/jkms.2011.26.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.