ABSTRACT
The purpose of this study was to evaluate the magnetic resonance imaging (MRI) appearance of the hip capsule in patients with femoroacetabular impingement (FAI) undergoing hip arthroscopy with capsular repair versus non-repair. A multicenter clinical trial was performed with 31 patients (49 hips) undergoing hip arthroscopy for treatment of FAI. A small- to moderate-sized interportal capsulotomy was performed. Each hip was randomized to capsular repair versus non-repair of the interportal capsulotomy. MRI was performed at 6 and 24 weeks postoperatively and was analyzed by two musculoskeletal radiologists. Patients and the radiologists were blinded to the treatment applied. Capsular defect size and capsule thickness were recorded on each scan. Mean patient age was 31.4 years. Capsular repair was performed in 23 (46.9%) hips. Mean capsulotomy length was 35 mm at Center X and 23 mm at Center Y. At 6 weeks postoperatively, a healed hip capsule (with no apparent capsulotomy defect) was observed in 10 (43.4%) hips that underwent capsular repair and 4 (15.4%) hips that did not undergo capsular repair (P = 0.13). At 24 weeks postoperatively, 25/30 hips (83.3%) achieved complete closure of the capsulotomy defect, with no significant difference between treatment groups. Repair of an interportal capsulotomy following hip arthroscopy for FAI results in a non-significantly higher percentage of healed hip capsules at 6 weeks postoperatively compared with leaving the capsule unrepaired, though the difference normalizes by 24-week follow-up. Repair of a small- to moderate-sized interportal capsulotomy does not provide a radiographic advantage following hip arthroscopy for FAI.
INTRODUCTION
As the incidence of hip arthroscopy has increased significantly in recent years [1], so too has controversy surrounding the role of the capsulotomy defect created to perform this procedure [2–4]. While arthroscopic repair of the incised capsule is technically demanding, may add significant time to the surgical procedure, and is generally lacking in high-quality evidence supporting its benefits, several case reports have been published of iatrogenic instability following hip arthroscopy [5–11]. Many anatomic, biomechanical and retrospective clinical studies have implicated the unrepaired capsule as one potential factor contributing to postoperative instability [12–24]. For these reasons, some authors have suggested that routine capsular closure be performed at the conclusion of hip arthroscopy cases [4, 25].
Recently, Strickland et al. [26] published the magnetic resonance imaging (MRI) results of 15 patients (30 hips) who underwent bilateral, simultaneous hip arthroscopy for femoroacetabular impingement (FAI) with a small interportal capsulotomy and were randomized to capsular repair versus non-repair. The authors found that, regardless of capsular treatment, all hip capsules progressed to contiguous healing by 24 weeks postoperatively. This study includes nearly double the number of hips, both unilateral and bilateral hip arthroscopy patients, and a multicenter study design to make the results more generalizable. The purpose of this study was to evaluate the MRI appearance of the hip capsule in patients with femoroacetabular impingement (FAI) undergoing hip arthroscopy with capsular repair versus non-repair in a multicenter study design.
MATERIALS AND METHODS
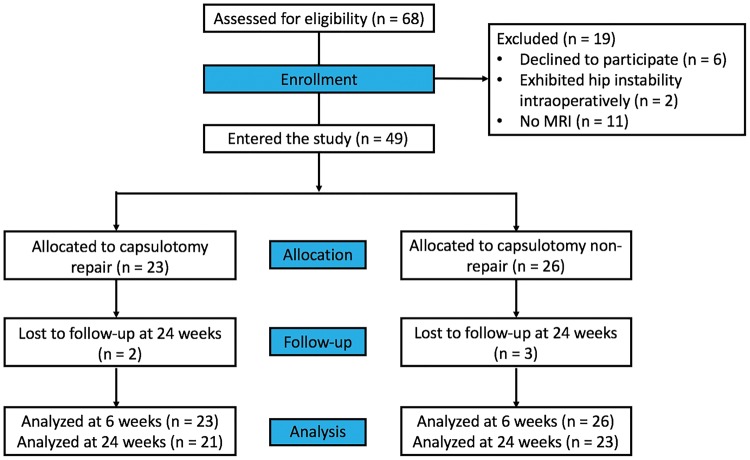
After Institutional Review Board approval was obtained, the authors performed a multicenter, randomized, double-blind clinical trial (clinicaltrials.gov #NCT02990234) on a consecutive cohort of adult patients undergoing hip arthroscopy for treatment of femoroacetabular impingement between 1 January and 31 December 2014 (Fig. 1). One surgeon at each of two sites enrolled patients. Written informed consent was obtained from each patient enrolled in the study. The results of patients included at Center Y were previously reported in [26]. Inclusion criteria for patients selected for this study were as follows: (i) persistent hip pain and mechanical symptoms refractory to nonoperative management lasting at least 3 months, (ii) reproducible clinical examination findings suggestive of impingement and (iii) joint space width more than 3 mm on all views of plain radiography and three-dimensional (3D) computed tomography (CT). Exclusion criteria included patients with hip instability (hip dysplasia or hyperlaxity) [27], as the authors always perform repair of the interportal capsulotomy in these patients; patients who required microfracture or postoperative non-weight bearing precautions and patients undergoing additional surgical treatment for diagnoses of slipped capital femoral epiphysis (SCFE), Legg–Calvé–Perthes disease, osteochondromatosis or post-dislocation syndrome.
Fig. 1.
CONSORT diagram. Sample sizes (n) refer to the number of hips included at each stage.
Surgical technique
Center X performed hip arthroscopy in the lateral decubitus position while Center Y performed surgeries in the supine position as previously described in [26, 28]. After proper distraction was achieved and both arthroscopic portals were established, interportal capsulotomy was carried out using an arthroscopic blade connecting the two portals. If extensive cam work was expected, the anterior limb of the capsulotomy was extended distally in an oblique fashion. Once surgical treatment in the central and peripheral compartments was completed, a randomization envelope was opened, indicating the arm to which the hip was randomized (capsule repair versus non-repair). In cases of simultaneous bilateral surgery, the first hip capsule was treated per the randomization envelope while the second side was treated with the opposite treatment option.
The SpeedStitch (SS, ArthroCare, Austin, TX, USA) was used in all cases by the surgeons at both institutions. While the hip is placed in 20–30 degrees of flexion with the knee fully extended, the SS is loaded with a suture cartridge containing ultra-strength suture (MagnumWire, ArthroCare, Austin, TX, USA; Center X) or with Vicryl No. 2 suture (Ethicon, Inc., Somerville, NJ, USA; Center Y) and inserted through the anterolateral portal while an arthroscopic view was obtained from the midtrochanteric or parallel posterior portal. The SS is used to grab the proximal (medial) far anterior capsular stump with a sufficient bite of tissue to allow strong knot-tying and to avoid possible suture cut-through. The SS is then pulled out of the joint, and a knot pusher and ‘suture clip’ are placed on the ‘needle-retrieved’ (short) side of the suture. The free side of the suture is then reloaded on the SS [29], and the device is reinserted into the joint through the same portal, using a slotted or circumferential cannula, to avoid soft-tissue entrapment. The SS is then used to grab the corresponding side of the capsular stump, just opposite of the previously applied suture end. After penetrating this distal (lateral) side of the capsule, the SS is pulled out and the side-to-side stitches are retrieved and tied in a standard fashion. Both authors use an arthroscopic Weston sliding knot with two locking half hitches. The process is repeated until adequate capsular closure and tension have been obtained to the surgeon’s satisfaction. Common capsular repair includes 2–4 knots resulting in closure of the anterior 70% of the capsulotomy. The authors intentionally leave the posterolateral (non-iliofemoral ligament) portion of the capsule open to enable evacuation of the joint’s postoperative hematoma.
Rehabilitation
Patients were instructed to avoid external rotation of the hip for 4 weeks postoperatively. Rehabilitation involving stationary bicycling was commenced at postoperative days 1–2. Patients were restricted to non-weight bearing (in cases of concomitant microfracture) or full weight bearing with crutches for 6 weeks. If microfracture was not performed and no large cam resection was necessary, patients were allowed to discontinue crutches after 3 weeks as long as they were able to walk with no limp.
MR imaging
MRI scans performed at Center X were on a General Electric (GE Medical Systems, Waukesha, WI, USA) 3-Tesla scanner and those at Center Y on a Siemens (Siemens Healthcare, Erlangen, Germany) 3-Tesla scanner.
The MRI protocol used has been previously described in [26]. MRI scans were acquired using a torso array coil with the following parameters: field of view, 180 × 180 mm; matrix, 320 × 224; flip angle, 90°; repetition time, 3200–4300 ms; echo time, 68 ms; section thickness, 3.5 mm; slice spacing, 0.3 mm. Proton density (PD) sequences were acquired in the axial, sagittal and coronal planes. An additional sagittal PD sequence with fat-saturation was also performed. All scans were non-contrast.
Hip capsule assessment
Interpretation of all MRI findings and hip capsule measurements was made by two musculoskeletal fellowship-trained radiologists (CDS, MKJ). The radiologists were blinded to clinical and operative information of the patient to prevent potential bias during interpretation of MRI studies.
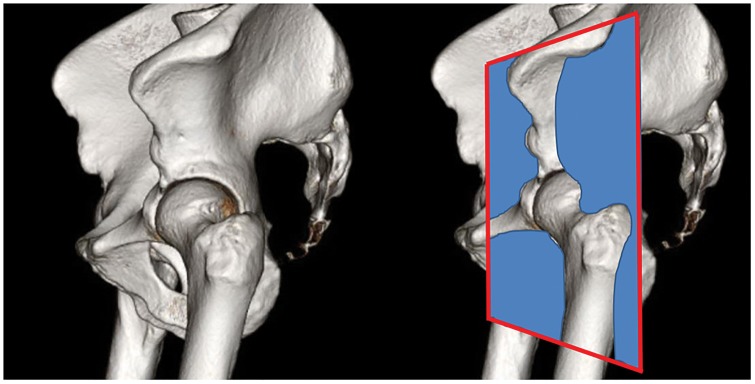
Assessment of the hip capsule has been previously described in [26]. Hip capsule thickness was measured in the mid-coronal plane to the femoral head on the coronal PD sequence (Fig. 2). Capsule thickness measurements were made at the level of the femoral head–neck junction (mid-capsule thickness), at a point midway between the mid-capsule and the labrum (proximal capsule thickness) and at a point equidistant toward the greater trochanter (distal capsule thickness) (Fig. 3). An equivalent set of measurements was also made in a coronal plane at the junction of the anterior and middle third of the femoral head and again at the junction of the middle and posterior thirds of the femoral head in the coronal plane. The anterior coronal plane demonstrated the most consistent depiction of the defect and was therefore chosen for comparison of capsule defect size between the MRI studies performed at 6 and 24 weeks.
Fig. 2.
Pelvis 3D reconstruction showing the anterior-coronal plane used for assessment of hip capsule morphology.
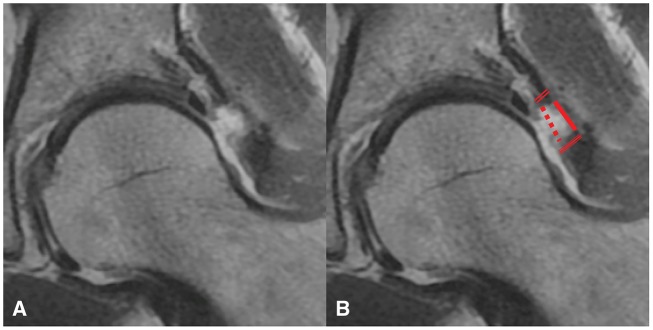
Fig. 3.
Coronal proton density image of a hip capsule defect (A) and the same defect with measurements (B). Articular side gap (dotted line), muscular side gap (solid line), proximal capsule thickness (double line) and distal capsule thickness (triple line) measurements were made.
Capsule thickness was assessed by measuring the low signal intensity substance of the capsule from the articular side to the muscular side. If a gap in the capsule was encountered, the capsule thickness at the site of measurement was reported as 0 mm. For any capsular gap encountered, the distance of separation between capsule fibers at the articular and muscular surfaces was reported.
The only plane that allowed adequate cross-sectional imaging of the capsule in the region of surgical intervention was the coronal plane. The axial and sagittal planes did not show the needed structures with adequate clarity. These planes were used, however, to assess for the presence of cartilage damage, subchondral edema and other secondary signs as well as for localization to find the anterior, middle and posterior coronal planes.
Statistical analysis
All variables were evaluated for distribution of normality using a combination of histograms, quantile–quantile (Q–Q) plots and Shapiro–Wilk tests. Descriptive statistics were summarized as means and standard deviations for quantitative variables and as counts and frequencies for categorical variables. The significance of mean differences in capsular thickness as a function of time, location of measurement, repair status and interaction terms was evaluated using multiple linear regression following the generalized estimating equation (GEE) approach (with an unstructured correlation matrix). Incidence of postoperative subchondral edema and capsular healing were evaluated using chi-square or Fisher’s exact tests. Statistical significance for all comparisons was set at P < 0.05. Analyses were conducted using IBM SPSS Statistics Version 23.0 (Statistical Package for the Social Sciences, Chicago, IL, USA) and SAS Statistical Software Version 9.3 (SAS Institute, Inc., Cary, NC, USA).
In a previous study [26], two fellowship-trained musculoskeletal radiologists performed blinded measurements along five aspects of the hip capsule to evaluate interrater reliability. Interrater reliability was evaluated using a two-way, mixed, absolute-agreement, single-measures intraclass correlation coefficient (ICC). ICC values of >0.80 indicate excellent reliability; 0.61–0.80, substantial reliability; 0.41–0.60, moderate reliability; 0.21–0.40, fair reliability; ≤0.20, poor reliability [30]. Accordingly, the ICC (0.787; 95% confidence interval, 0.733–0.830) demonstrated substantial reliability for the MRI measurements of the hip capsule.
RESULTS
Forty-three patients (68 hips) were identified as being eligible for inclusion in this study. Among them, 12 patients (19 hips) were excluded. Three patients (6 hips) opted out of randomization prior to surgery, asking that capsular repair be performed. One patient (2 hips) exhibited instability characteristics during surgery and the surgeon decided to repair the capsule in both hips. Eight patients (11 hips) were consented and included in the study but did not undergo postoperative MRIs. The remaining 31 patients (49 hips) comprised the final study cohort. Capsular repair was performed in 23 (46.9%) hips. Baseline demographics, including mean age, height, weight and BMI did not vary significantly between treatment groups (Table I).
Table I.
Baseline demographics of each treatment group (N = 49 hips)
| Patient variables | Group |
P-value | |
|---|---|---|---|
| Repaired capsule | Unrepaired capsule | ||
| No. of hips, n (%) | 23 (46.9) | 26 (53.1) | |
| Age, mean (SD), y | 30.6 (9.3) | 32.2 (11.8) | 0.603 |
| Female Gender, n (%) | 16 (69.5) | 15 (57.7) | 0.762 |
| Height, mean (SD), cm | 172.5 (8.75) | 169.3 (9.8) | 0.322 |
| Weight, mean (SD), kg | 71.0 (16.2) | 63.9 (11.8) | 0.157 |
| *BMI, mean (SD), kg/m2 | 23.8 (4.1) | 22.3 (3.0) | 0.240 |
*For this study, BMI (kg/m2) was categorized as follows: normal weight, 18.00–24.99; overweight, 25.00–29.99 or obese, 30.00 or greater.
The average interportal capsulotomy length was 35 mm at Center X and 23 mm at Center Y. Three patients (1 unilateral, 2 bilateral) presented for 6-week, but not 24-week, postoperative follow-up. At 6 weeks postoperatively, a healed hip capsule (with no apparent capsulotomy defect) was observed in 10 (43.4%) hips that underwent capsular repair and 4 (15.4%) hips that did not undergo capsular repair, a difference that was near-significant [χ2(1) = 2.29, P = 0.13]. At 24 weeks postoperatively, 25 of 30 hips (83.3%) achieved complete closure of the capsulotomy defect, with no significant difference between treatment groups (Fig. 4). Of the 5 hips with a persistent defect at the 24-week follow-up, 2 had undergone capsular repair and 3 were left unrepaired.
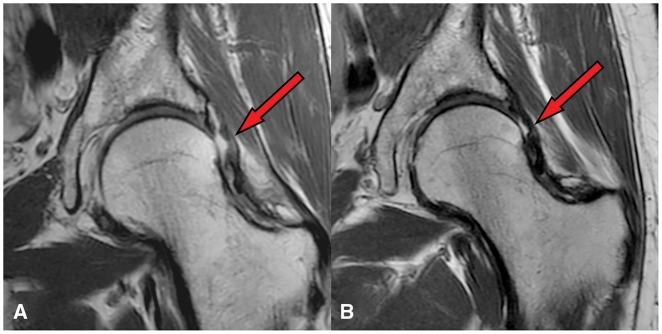
Fig. 4.
Coronal proton density imaging showing hip capsule following hip arthroscopy without capsular repair in a 53-year old male at 6 weeks (A) and 24 weeks (B) postoperatively. Note the irregular but intact capsule (red arrow) at 24 weeks postoperatively.
The capsular defect could be identified in all cases on MRI. When the defect was still open, measurements could be made easily. When healed, a region of thinning could be identified at the surgical site. Among hips with a capsulotomy defect, the distance of separation across attenuated capsular fibers at the articular surface was significantly greater compared with that at the muscular surface (P < 0.001). Both measurements of the capsular defect significantly decreased by 24 weeks postoperatively (P < 0.001; Table II). Along the longitudinal axis of the capsulotomy defect, mean capsular thickness was maximal at the distal portion and minimal at the middle portion of the hip capsule [χ2(2) = 166.08, P < 0.001; Table II] and was statistically equivalent at 6 and 24 weeks postoperatively. Capsular thickness did not vary significantly between treatment groups.
Table II.
Mean capsular thickness in patients undergoing hip arthroscopy with and without capsular repair
| Variable | Hip capsule location | No repair (6 weeks) | Repair (6 weeks) | No repair (24 weeks) | Repair (24 weeks) |
|---|---|---|---|---|---|
| Size of capsular defect | Articular surface | 6.05 (4.64) | 6.23 (4.14) | 2.00 (3.32) | 2.83 (3.33) |
| Muscular surface | 3.95 (3.93) | 3.53 (4.15) | 0.60 (1.88) | 0.83 (2.40) | |
| Capsule thickness | Proximal | 5.52 (1.69) | 6.27 (1.99) | 4.60 (1.73) | 5.17 (1.81) |
| Middle | 1.33 (2.39) | 2.34 (2.72) | 3.65 (2.23) | 3.25 (2.09) | |
| Distal | 8.23 (1.86) | 8.19 (2.75) | 6.90 (2.25) | 7.00 (2.67) |
Capsule thickness was measured as the width of low signal intensity between the joint fluid and overlying muscle. If there was no tissue, a thickness of 0 mm was reported.
All values represented as mean (SD), in mm.
Postoperative hip capsule thickness and the surrounding soft tissue appearance, including gluteus muscle or extra-capsular edema, were unassociated with capsular repair status. The incidence of subchondral edema did not vary significantly between treatment groups. Within the entire cohort, the incidence of subchondral edema decreased significantly from 6 to 24 weeks postoperatively (61.3% versus 18.2%, respectively; P = 0.016).
In all cases, capsular healing status did not vary significantly between genders. There were also no significant differences in capsular healing at 6 or 24 weeks postoperatively between Center X and Center Y, or between bilateral patients only versus the combined bilateral/unilateral cohort.
DISCUSSION
This randomized, double-blind clinical trial is the first multicenter study to compare the postoperative MRI findings of the hip capsule in patients undergoing hip arthroscopy with capsular repair of an interportal capsulotomy versus non-repair. The most significant findings from this study are a non-significantly higher proportion of healed hip capsules at 6-week follow-up in patients undergoing capsular repair (P = 0.13), with progressive capsular healing in both groups that normalizes this proportion by 24-week follow-up. Both postoperative hip capsule thickness and the surrounding soft tissue appearance were unassociated with capsular repair status.
As the incidence of hip arthroscopy has increased significantly in recent years [1], so too has controversy surrounding the role of the capsulotomy defect created to perform this procedure [2–4]. Recently, Ortiz-Declet et al. [4] performed a systematic review of the literature to determine the clinical and biomechanical evidence of instability following capsular repair or plication in patients undergoing hip arthroscopy for FAI or instability. The authors concluded that, based on short-term outcome studies, capsular closure in non-arthritic patients is effective and may yield superior outcomes compared with the unrepaired capsulotomy. Furthermore, the authors stated that current evidence supports routine capsular closure in most cases. However, this was a level IV systematic review with no level I studies and only one level II study included. Furthermore, this review did not distinguish among interportal, T-type and H-type capsulotomies. In one of these studies (level IV evidence), Domb et al. [18] retrospectively reviewed the outcomes of 403 patients who underwent hip arthroscopy with capsular repair versus non-repair, with capsular management decided on an individual basis depending on a number of factors including patient age, preoperative joint stiffness/adhesive capsulitis, and borderline or frank hip dysplasia. The authors concluded that the use of capsular repair did not show clinically relevant superiority over the use of unrepaired capsulotomy.
In a recent, single-center study, Strickland et al. [26] evaluated the postoperative MRI appearance of a repaired versus unrepaired, small (<3 cm) interportal capsulotomy in 15 patients undergoing simultaneous bilateral hip arthroscopy for FAI. All patients had one hip randomized to capsular repair/non-repair and the contralateral hip capsule treated with the opposite treatment. The authors found that, at 24 weeks postoperatively, 100% of hips demonstrated progression to healing, with a contiguous MRI appearance without defects and no difference in capsular dimensions between groups.
This study provides strong evidence that the defect in a non-repaired hip capsule following an interportal capsulotomy, without a ‘T’ extension, will decrease in size and approach that of a repaired capsule, with a small subset of capsules which may stay quite distinct. The reason for these distinct differences in MRI findings may be related to patient age, activity level or increased laxity (though patients with hyperlaxity were excluded from this study). Overall, though, the proportion of healed hip capsules in patients with unrepaired hip capsules normalized by 24 weeks postoperatively to an extent not significantly different from those who underwent capsular repair.
Furthermore, the presence of subchondral edema did not differ significantly between groups, though collectively was more common at 6 weeks compared with 24 weeks postoperatively, correlating it to labral repair anchor drilling and placement. This is to be expected given normal postoperative physiologic changes which reside over time. Although clinical outcomes are necessary to confirm the significance of these findings, the results of this study suggest that, in patients lacking hip instability, repair of a small- to moderate-sized interportal capsulotomy after arthroscopic treatment of FAI may not provide biological advantages compared with leaving the capsulotomy unrepaired.
Although some authors have advocated for routine capsular closure following hip arthroscopy [4, 25], patient characteristics as well as capsulotomy type must be taken into account when deciding on the appropriate management of a capsulotomy defect. Arthroscopic repair of the incised capsule is technically demanding and may add significant time to the surgical procedure, and therefore surgeons must strongly consider whether routine capsular closure is necessary in all cases. In our study, only patients with FAI and a lack of hip instability symptoms were included. In addition, all capsulotomies in this study were interportal and small to moderate in size and the surgeons involved were careful not to trim down excessive capsule tissue from both sides of the incised capsule stumps.
The results of this study are strengthened by several methodological factors in addition to the double-blind, randomized study design. The majority of the patients in this study underwent simultaneous bilateral hip arthroscopy, in which one hip capsule was repaired while the other side was left unrepaired. This methodology eliminates bias resulting from gender, surgeon, hip pathology, surgical technique, age and rehabilitation protocol. The only difference seen between both operated hips was related to capsular repair status. Furthermore, no differences were found between Center X and Center Y, in which surgeons at these two sites used different surgical techniques (lateral decubitus versus supine), different capsulotomy size (23 mm versus 35 mm on average), the number of sutures used to repair the capsulotomy (3–4 versus 2–3) and different types of suture used for capsular repair (ultra-strength versus a resorbable suture).
The limitations of this study should also be noted. In particular, the results of this study are limited to radiographic outcomes and therefore further studies are necessary to relate these to clinical outcomes. Non-contrast MRI scans were used for this study, whereas the use of contrast may result in capsular distention thereby improving visualization of capsular defects postoperatively. Related to this, it is possible that MRI assessment of the hip capsule may be limited in cases in which there is not a joint effusion. In addition, small- to moderate-sized interportal capsulotomies were performed in this study rather than a T-capsulotomy or large (6–8 cm) interportal capsulotomies, and therefore the results of this study are limited to the described techniques. Finally, patients with hip dysplasia and/or hyperlaxity were excluded, as capsular repair is always performed in these patients by the surgeons in this study.
In patients undergoing hip arthroscopy for the treatment of femoroacetabular impingement, capsular repair of an interportal capsulotomy results in a non-significantly higher proportion of healed hip capsules at 6-week follow-up compared with non-repair of the hip capsule. However, this proportion can be expected to normalize over time in patients with repaired and non-repaired hip capsules by 24-week follow-up.
FUNDING
This study was funded with the assistance of a research grant from ArthroCare.
CONFLICT OF INTEREST STATEMENT
Dr. Brick is a paid speaker for Stryker and receives IP royalties from Arthrex, Inc. Dr. Garabekyan is a paid consultant for Stryker. Dr. Mei-Dan is a paid consultant for and receives IP royalties and research support from Stryker, and receives stock or stock options from MITA.
REFERENCES
- 1. Truntzer JN, Shapiro LM, Hoppe DJ et al. Hip arthroscopy in the United States: an update following coding changes in 2011. J Hip Preserv Surg 2017; 4: 250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ekhtiari S, de Sa D, Haldane CE. Hip arthroscopic capsulotomy techniques and capsular management strategies: a systematic review. Knee Surg Sports Traumatol Arthrosc 2017; 25: 9–23. [DOI] [PubMed] [Google Scholar]
- 3. Matsuda DK. Editorial commentary: hip capsule: to repair or not? Arthroscopy 2017; 33: 116–7. [DOI] [PubMed] [Google Scholar]
- 4. Ortiz-Declet V, Mu B, Chen AW et al. Should the capsule be repaired or plicated after hip arthroscopy for labral tears associated with femoroacetabular impingement or instability? A systematic review. Arthroscopy 2018; 34: 303–18. [DOI] [PubMed] [Google Scholar]
- 5. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy 2009; 25: 405–7. [DOI] [PubMed] [Google Scholar]
- 6. Dierckman BD, Guanche CA. Anterior hip capsuloligamentous reconstruction for recurrent instability after hip arthroscopy. Am J Orthop (Belle Mead NJ) 2014; 43: E319–E23. [PubMed] [Google Scholar]
- 7. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy 2009; 25: 400–4. [DOI] [PubMed] [Google Scholar]
- 8. Mei-Dan O, McConkey MO, Brick M. Catastrophic failure of hip arthroscopy due to iatrogenic instability: can partial division of the ligamentum teres and iliofemoral ligament cause subluxation? Arthroscopy 2012; 28: 440–5. [DOI] [PubMed] [Google Scholar]
- 9. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Jt Surg Am 2009; 91: 192–7. [DOI] [PubMed] [Google Scholar]
- 10. Rosenbaum A, Roberts T, Flaherty M et al. Posterior dislocation of the hip following arthroscopy—a case report and discussion. Bull Hosp Jt Dis (2013) 2014; 72: 181–4. [PubMed] [Google Scholar]
- 11. Sansone M, Ahldén M, Jónasson P et al. Total dislocation of the hip joint after arthroscopy and iliopsoas tenotomy. Knee Surg Sports Traumatol Arthrosc 2013; 21: 420–3. [DOI] [PubMed] [Google Scholar]
- 12. Abrams GD, Hart MA, Takami K et al. Biomechanical evaluation of capsulotomy, capsulectomy, and capsular repair on hip rotation. Arthroscopy 2015; 31: 1511–7. [DOI] [PubMed] [Google Scholar]
- 13. Bayne CO, Stanley R, Simon P et al. Effect of capsulotomy on hip stability—a consideration during hip arthroscopy. Am J Orthop (Belle Mead NJ) 2014; 43: 160–5. [PubMed] [Google Scholar]
- 14. Bedi A, Galano G, Walsh C et al. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy 2011; 27: 1720–31. [DOI] [PubMed] [Google Scholar]
- 15. Bowman KF Jr, Fox J, Sekiya JK. A clinically relevant review of hip biomechanics. Arthroscopy 2010; 26: 1118–29. [DOI] [PubMed] [Google Scholar]
- 16. Chivas DJ, Smith K, Tanzer M. Role of capsular repair on dislocation in revision total hip arthroplasty. Clin Orthop Relat Res 2006; 453: 147–52. [DOI] [PubMed] [Google Scholar]
- 17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy 2013; 29: 162–73. [DOI] [PubMed] [Google Scholar]
- 18. Domb BG, Stake CE, Finley ZJ et al. Influence of capsular repair versus unrepaired capsulotomy on 2-year clinical outcomes after arthroscopic hip preservation surgery. Arthroscopy 2015; 31: 643–50. [DOI] [PubMed] [Google Scholar]
- 19. Frank RM, Lee S, Bush-Joseph CA et al. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med 2014; 42: 2634–42. [DOI] [PubMed] [Google Scholar]
- 20. Martin HD, Savage A, Braly BA et al. The function of the hip capsular ligaments: a quantitative report. Arthroscopy 2008; 24: 188–95. [DOI] [PubMed] [Google Scholar]
- 21. Myers CA, Register BC, Lertwanich P et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med 2011; 39: 85. [DOI] [PubMed] [Google Scholar]
- 22. Telleria JJ, Lindsey DP, Giori NJ, Safran MR. An anatomic arthroscopic description of the hip capsular ligaments for the hip arthroscopist. Arthroscopy 2011; 27: 628–36. [DOI] [PubMed] [Google Scholar]
- 23. Wagner FV, Negrão JR, Campos J et al. Capsular ligaments of the hip: anatomic, histologic, and positional study in cadaveric specimens with MR arthrography. Radiology 2012; 263: 189–98. [DOI] [PubMed] [Google Scholar]
- 24. Weidner J, Büchler L, Beck M. Hip capsule dimensions in patients with femoroacetabular impingement: a pilot study. Clin Orthop Relat Res 2012; 470: 3306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harris JD, Slikker W 3rd, Gupta AK et al. Routine complete capsular closure during hip arthroscopy. Arthrosc Tech 2013; 2: e89–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strickland CD, Kraeutler MJ, Brick MJ et al. MRI evaluation of repaired versus unrepaired interportal capsulotomy in simultaneous bilateral hip arthroscopy: a double-blind, randomized controlled trial. J Bone Jt Surg Am 2018; 100: 91–8. [DOI] [PubMed] [Google Scholar]
- 27. Kraeutler MJ, Garabekyan T, Pascual-Garrido C et al. Hip instability: a review of hip dysplasia and other contributing factors. Muscles Ligaments Tendons J 2016; 6: 343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mei-Dan O, Kraeutler MJ, Garabekyan T et al. Hip distraction without a perineal post: a prospective study of 1000 hip arthroscopy cases. Am J Sports Med 2018; 46: 632–41. [DOI] [PubMed] [Google Scholar]
- 29. Mei-Dan O, Young DA. A novel technique for capsular repair and labrum refixation in hip arthroscopy using the SpeedStitch. Arthrosc Tech 2012; 1: e107–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. [PubMed] [Google Scholar]