Understanding the mechanisms of bacterial community assembly across environmental gradients is one of the major goals of marine microbial ecology. Thermal effluents from two nuclear power plants have been present in the subtropical Daya Bay for more than 20 years and have generated a comparatively stable and long thermal gradient (a temperature increase from 0 to 10°C). The environmental patches across thermal gradients are heterogeneous and very strongly interconnected on a microbial scale; thus, this is a useful model for the study of the metacommunity processes (i.e., patch dynamics, species sorting, mass effects, and neutral processes) that underlie marine bacterioplankton assembly. The significance of our research is to reveal how environmental conditions and dispersal-related processes interact to influence bacterioplankton metacommunity processes and their seasonal differences across thermal gradients. Our results may advance the understanding of marine microbial ecology under future conditions of global warming.
KEYWORDS: marine ecosystem, metacommunity processes, microbial ecology, subtropical bay, thermal impacts
ABSTRACT
Thermal effluents from nuclear power plants greatly change the environmental and ecological conditions of the receiving marine water body, but knowledge about their impact on microbial ecology is limited. Here we used high-throughput sequencing of the 16S rRNA gene to examine marine bacterioplankton metacommunity assembly across thermal gradients in two representative seasons (i.e., winter and summer) in a subtropical bay located on the northern coast of the South China Sea. We found high heterogeneity in bacterioplankton community compositions (BCCs) across thermal gradients and between seasons. The spatially structured temperature gradient created by thermal effluents was the key determinant of BCCs, but its influence differed by season. Using a metacommunity approach, we found that in the thermal discharge area, i.e., where water is frequently exchanged with surrounding seawater and thermal effluent water, the BCC spatial patterns were shaped by species sorting rather than by mass effects from surrounding seawater or by dilution of thermal effluent water by surrounding seawater. However, this effect of species sorting was weaker in summer than in winter seawater. In both seasons, the bacterioplankton community structure was predominately determined by niche sharing; however, the relative importance of niche segregation was enhanced in summer seawater. Our findings suggest that for the seasonal differences in metacommunity processes, the BCCs of subtropical summer seawater were more sensitive to temperature and were more difficult to predict than those of winter seawater in the face of different intensities of thermal impacts.
IMPORTANCE Understanding the mechanisms of bacterial community assembly across environmental gradients is one of the major goals of marine microbial ecology. Thermal effluents from two nuclear power plants have been present in the subtropical Daya Bay for more than 20 years and have generated a comparatively stable and long thermal gradient (a temperature increase from 0 to 10°C). The environmental patches across thermal gradients are heterogeneous and very strongly interconnected on a microbial scale; thus, this is a useful model for the study of the metacommunity processes (i.e., patch dynamics, species sorting, mass effects, and neutral processes) that underlie marine bacterioplankton assembly. The significance of our research is to reveal how environmental conditions and dispersal-related processes interact to influence bacterioplankton metacommunity processes and their seasonal differences across thermal gradients. Our results may advance the understanding of marine microbial ecology under future conditions of global warming.
INTRODUCTION
The increasing number of marine nuclear power plants has given rise to concerns about the undesirable thermal effects of power plant cooling systems on marine environments and ecology (1–3). Bacterioplankton play important roles in the ecological processes of marine ecosystems (4, 5). Despite their ecological importance, the response of bacterioplankton communities to thermal effects is not as well understood as those of phytoplankton (1, 6–9), protozoa (10), zooplankton (11), and fish (12). Current studies about thermal effects on marine bacterioplankton communities have focused mainly on production (13), respiration (14), and the growth rate (15). Studies suggest that thermal effluents have profound impacts on bacterioplankton metabolism abilities and that the effects are dependent on the season (13–15). Bacterioplankton community compositions (BCCs) subject to thermal effects, however, have rarely been examined by use of high-throughput sequencing, except for a recent study showing that thermal effluents from a coal power plant (water temperature, 15.0 to 18.6°C) had significant effects on marine spring BCCs (16). The habitats with more thermal effluents had lower relative abundances of Alphaproteobacteria and Gammaproteobacteria but higher relative abundances of Cyanobacteria (16). Our knowledge about the thermal impact of nuclear power plants on BCCs is thus inadequate. We know little about the seasonal differences in BCC responses to thermal effluents and the underlying ecological mechanisms.
Exploring the mechanisms of bacterioplankton assembly is one of the major goals of marine microbial ecology (17–19). Generally, aquatic bacterioplankton community structure is shaped by environmental or dispersal-related processes (see, e.g., references 20 to 22). Metacommunity theory incorporates the interplay between environmental conditions and dispersal-related processes into four main categories: patch dynamics, species sorting, mass effects, and neutral processes (23). Dispersal-related processes include mass effects and dispersal limitation (20, 23). Very high dispersal rates can result in mass effects, with taxa existing in less suitable habitats due to continuous supply (23, 24), whereas very low dispersal rates can result in dispersal limitation, leading to purely spatial biogeography patterns (20). Neutral processes imply a lack of differences in bacterial fitness and niche, so that community structure depends on demographic stochasticity (23, 24). Species sorting in metacommunity concepts is similar to niche separation and sharing (25, 26), emphasizing niche roles above and beyond spatial dynamics (18, 23).
Thermal effluents from nuclear power plants generate thermal gradients in the discharge area (2, 8, 27). The environmental patches across thermal gradients are heterogeneous and very strongly interconnected on a microbial scale (2, 8); thus, this is a useful model with which to study the environmental and dispersal-related processes that underlie marine bacterioplankton assembly (18). Bacterioplankton are easily dispersed through both flowing thermal effluent water and water flows and tides from the surrounding seawater. High dispersal rates in the thermal effluent area may induce mass effects with strong source-sink relations among heterogeneous habitats (23). Across thermal gradients, mass effects probably swamp the species-sorting roles of thermal effects and result in greater difficulty in predicting bacterioplankton communities on the basis of local environmental characteristics (23). The BCC patterns across thermal gradients may be determined by mass effects from surrounding seawater or spatial factors due to the continuous dilution effect of surrounding seawater on thermal effluent water. In addition, due to seasonal differences in hydrologic and environmental conditions, bacterioplankton metacommunity processes across thermal gradients may differ by season. However, thus far, research on the potential ecological mechanisms of bacterioplankton assembly subject to thermal effects is scarce, and we know little about the way in which the environmental conditions and dispersal-related processes interact to influence bacterioplankton community structure and their seasonal differences in response to thermal effects.
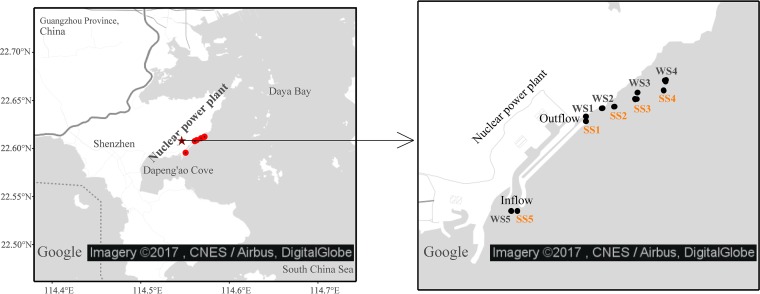
To determine the spatial distribution of bacterioplankton communities across thermal gradients, their seasonal differences, and the underlying ecological mechanisms, we used high-throughput sequencing of the 16S rRNA gene to study marine BCCs across thermal gradients in two representative seasons (i.e., winter and summer) in a subtropical bay (Daya Bay) located on the northern coast of the South China Sea (Fig. 1). In the area studied, thermal effluents from the cooling systems of two nuclear power stations have been present for more than 20 years and have generated a comparatively stable and long thermal gradient (a temperature increase from 0 to 10°C [2, 8]). There are strong seasonal differences in both hydrologic and environmental conditions in the area studied. For instance, compared to summer, winter is usually characterized by hydrologic conditions of a higher tide level and higher current velocity but lower wind speed (6, 28, 29) and has the environmental traits of lower temperatures but higher concentrations of dissolved oxygen (DO) (2, 3). We used a metacommunity approach (17, 18, 22) as a logical framework for studying the impact of the environmental conditions and dispersal-related processes underlying bacterioplankton assembly (30, 31). The potential species-sorting processes (e.g., niche separation and sharing) were further examined by a co-occurrence network analysis of bacterioplankton communities (32, 33). We hypothesized that (i) in the thermal discharge area, i.e., where water is frequently exchanged with surrounding seawater and thermal effluent water, the thermal effluents may have nonsignificant species-sorting effects on marine bacterioplankton assembly; (ii) the BCC pattern across the thermal gradient may depend on spatial factors because of the continuous dilution effect of surrounding seawater on thermal effluent water; and (iii) due to seasonal differences in hydrologic and environmental conditions, bacterioplankton metacommunity structure and processes under thermal impacts may differ by season.
FIG 1.
Sampling sites across the thermal gradients in subtropical Daya Bay on the northern coast of the South China Sea. Five sites were set for sampling in each season. Four sites were located along the thermal effluent (WS1, WS2, WS3, and WS4 for the winter and SS1, SS2, SS3, and SS4 for the summer), and the remaining site (WS5 for the winter and SS5 for the summer) was set as a control and was located in the inflow area of the cooling system upstream of the thermal effluents. The maps were generated based on an open-access Google satellite map using the ggmap package (https://github.com/dkahle/ggmap) in the R statistical computing environment (https://www.R-project.org).
RESULTS
Environmental characteristics.
In each season studied, the thermal effluents from the two nuclear power plants had profound influences on the water temperature (see Fig. S1 and S2 in the supplemental material). In the winter, the water temperature increased from 21°C to 31°C, and in the summer, the temperature increased from 31°C to 39°C. In both seasons, the water temperature decreased significantly along our sampling sites (from site 1 to site 5) (P, <0.05 by one-way analysis of variance [ANOVA] in both cases) (Fig. S1 and S2), whereas the DO content of the water increased significantly (P, <0.05 by one-way ANOVA in both cases) (Fig. S1 and S2). In both seasons studied, the total organic carbon (TOC), NH4+-N, PO43–-P, and SiO32– contents had no obvious differences across the thermal gradient (P, >0.05 by one-way ANOVA in all cases) (Fig. S1 and S2). Between the two seasons studied, the environmental characteristics temperature, pH, and PO43–-P and NO3–-N concentrations were significantly higher in summer than in winter seawater (P, <0.05 by the t test in all cases) (Fig. S1 and S2), whereas DO and SiO32– concentrations were significantly lower in summer than in winter seawater (P, <0.05 by the t test in both cases) (Fig. S1 and S2).
Shifts in community structure.
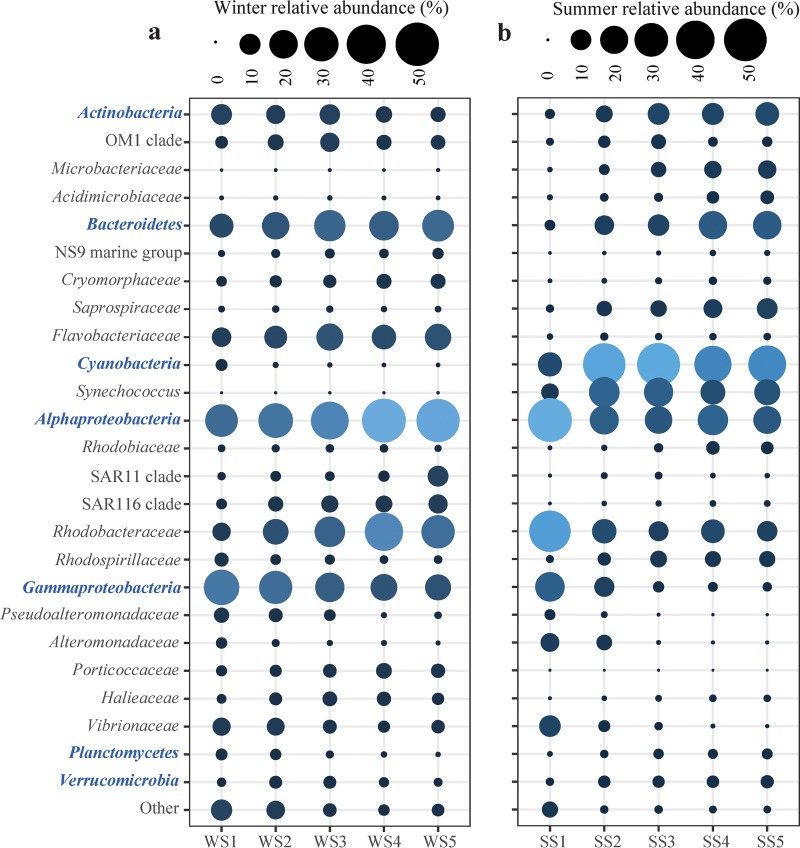
The main bacterioplankton in seawater in both summer and winter were Actinobacteria, Bacteroidetes, Cyanobacteria, Alphaproteobacteria, Gammaproteobacteria, Planctomycetes, and Verrucomicrobia. The relative abundances of these bacterioplankton groups were significantly different for the two seasons (P, <0.05 by the t test in all cases) (Fig. 2), and for each season, the relative abundances were significantly different across the thermal gradient (P, <0.05 by one-way ANOVA in all cases) (Fig. 2). In the winter, the thermal impact increased the relative abundances of Actinobacteria, Cyanobacteria, Gammaproteobacteria, Planctomycetes, and other rare phyla (relative abundance of total sequences, <1%) and decreased the relative abundances of Bacteroidetes and Alphaproteobacteria (Fig. 2a). However, in the summer, the sites with more effluents had higher relative abundances of Alphaproteobacteria and Gammaproteobacteria and lower relative abundances of Actinobacteria, Bacteroidetes, Planctomycetes, and Verrucomicrobia (Fig. 2b). The relative abundance of Cyanobacteria (primarily Synechococcus) was significantly higher in seawater in the summer than in the winter (Fig. 2a and b), and the relative abundance increased as the thermal effluents intensified but decreased when the water temperature was approximately 39°C (Fig. 2a and b).
FIG 2.
Differences in the spatial distributions of the relative abundances of bacterioplankton clades across the thermal gradients in winter (a) and summer (b) seawater. The phyla and subphyla are shown in blue boldface. Following the abundant phyla and subphyla (Actinobacteria, Bacteroidetes, Cyanobacteria, Alphaproteobacteria, and Gammaproteobacteria), their abundant families are listed.
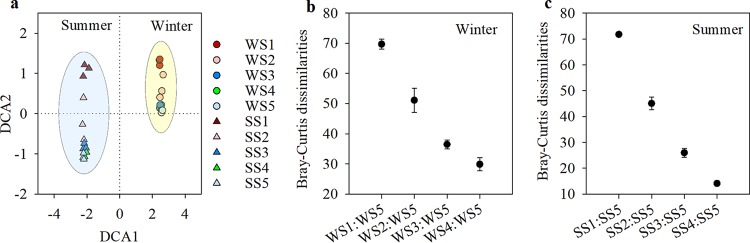
At the level of operational taxonomic units (OTUs), our results showed that winter and summer had significantly different BCCs across the thermal gradients, as revealed by detrended correspondence analysis (DCA) and permutational multivariate analysis of variance using distance matrices (PERMANOVA) (F = 39.687; P < 0.01) (Fig. 3a; see also Table S1 in the supplemental material). In each season, significant differences in community structure were detected among the five sampling sites by PERMANOVA (F = 5.919, P < 0.01 for winter; F = 9.284, P < 0.01 for summer) (Table S1), and the sites with more thermal effluents had BCCs that differed more dramatically from those of the control site (Fig. 3b and c). Pairwise comparisons by PERMANOVA showed that in the winter, the BCCs were significantly different between any two thermal effluent sites (P, <0.05 in all cases), except for the comparisons of WS2 and WS3 and the comparisons of WS3 and WS4 (Table S1). However, in the summer, the BCC differences in all pairwise comparisons of the thermal effluent sites (from site 1 to site 4) were significant (P, <0.05 in all cases) (Table S1).
FIG 3.
(a) Detrended correspondence analysis (DCA) of bacterioplankton community structures in winter and summer seawater. (b and c) BCC dissimilarities between sites along the thermal effluent (winter: WS1, WS2, WS3, and WS4; summer: SS1, SS2, SS3, and SS4) and control sites (winter, WS5; summer, SS5) located in the inflow area in winter (b) and summer (c) seawater.
Relating community structure to environmental factors and geographic distances.
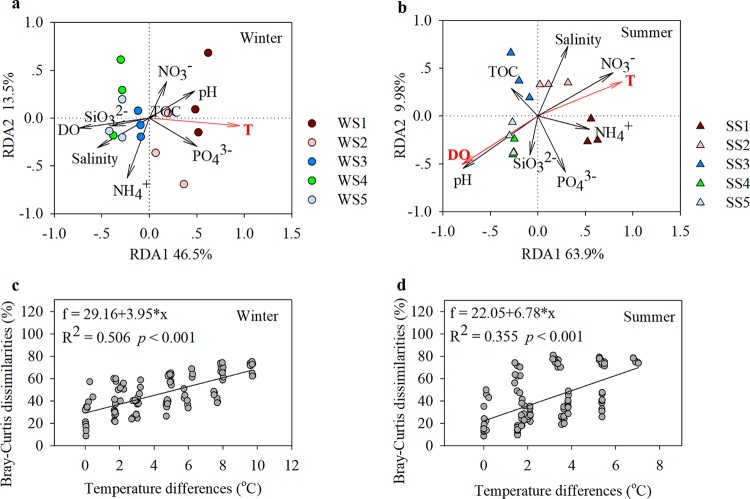
Variance partitioning analysis (VPA) for each season revealed that the subset of pure environmental variables explained a larger portion of the BCC variation in winter (32.7%) than in summer (13.9%) seawater (Table 1). The geographic distances between sampling sites also had an effect on bacterioplankton community structure and, by themselves, explained 7.8% of winter community structure and 2.3% of summer community structure (Table 1). The VPA results were further tested by redundancy analysis (RDA)-based partial permutation tests, and the results showed that in both seasons, bacterioplankton community structures across the thermal gradient were significantly explained by purely environmental factors (F = 7.192, P < 0.001 for winter; F = 9.495, P < 0.001 for summer) rather than by geographic distances only (F = 1.024, P > 0.05 for winter; F = 1.152, P > 0.05 for summer) (Table 1). A larger portion of community structure could not be explained by the selected environmental factors and geographic distances in summer (61.6%) than in winter (34.0%) seawater (Table 1). In addition, strong mixed effects of the selected environmental factors and geographic distances (25.5% for winter; 22.2% for summer) were detected (Table 1).
TABLE 1.
Variance partitioning analysis for BCCsa
| Factor(s) | % of variation in BCCb |
|
|---|---|---|
| Winter | Summer | |
| Pure E | 32.7 | 13.9 |
| Pure G | 7.8 | 2.3 |
| Mixed effect | 25.5 | 22.2 |
| Residuals | 34.0 | 61.6 |
E, environmental variables; G, geographic distance between sampling sites; mixed effect, mixed effects of geographic distance and environmental variables; residuals, unexplained components. The PCNM (principal coordinates of neighborhood matrix) method was performed to transform geographic distances between any pair of sites within S1, S2, S3, and S4 into 7 principal coordinates (from pcnm1 to pcnm7) in each season.
The best subset of environmental parameters (winter: temperature; summer: temperature and dissolved oxygen) and geographic distance (winter: pcnm1 and pcnm4; summer: pcnm1 and pcnm7) in the VPA was chosen by the BIOENV procedure.
We found that in each season, the seawater temperature was a key environmental factor in determining bacterioplankton community structure across the thermal gradient (Fig. 4a and b) and that in any pairwise comparison of sampling sites, the Bray-Curtis dissimilarities in BCC correlated positively with temperature differences (R2 = 0.506, P < 0.001 for winter; R2 = 0.355, P < 0.001 for summer [Fig. 4c and d]). However, the regression coefficient for the correlation between the BCC dissimilarities and the temperature differences was significantly higher for seawater in summer than in winter (P < 0.01) (Fig. 4c and d).
FIG 4.
(a and b) Redundancy analysis (RDA) of bacterioplankton community compositions (BCCs) and the environmental factors examined in winter (a) and summer (b) seawater. The environmental factors that correlated significantly with BCCs are shown in red. (c and d) Correlations between Bray-Curtis dissimilarities in BCC and temperature differences, determined by pairwise comparisons of sampling sites, in winter (c) and summer (d) seawater.
Shifts in bacterioplankton network structure.
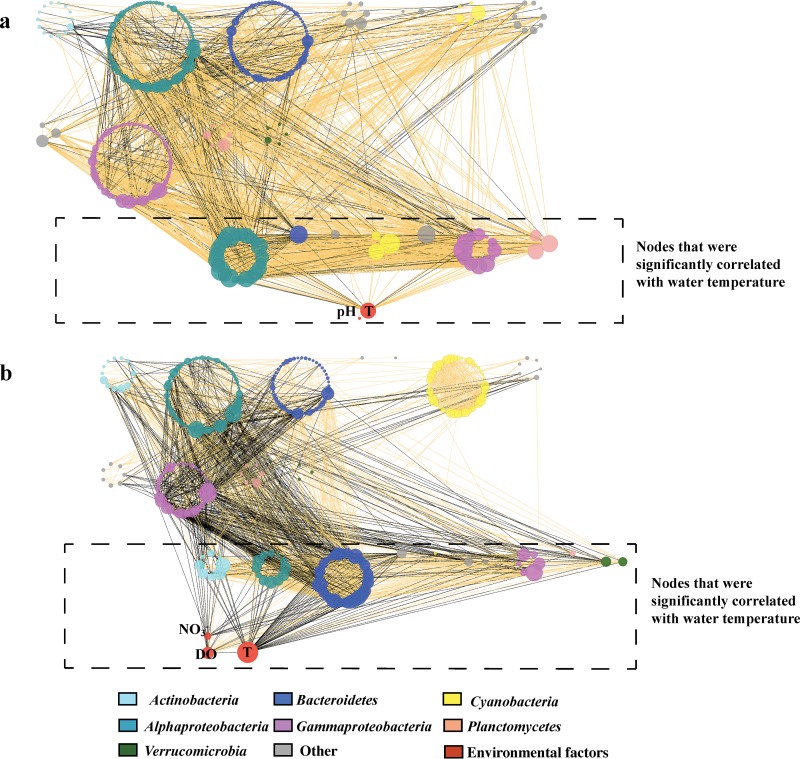
OTU co-occurrence patterns and their relationships with environmental factors were investigated by co-occurrence network analysis (Fig. 5). A network of 332 nodes and 2,183 links was obtained for winter BCCs (Fig. 5a), and the network was fragmented into 8 components (Table 2). The summer BCCs resulted in a network of 314 nodes and 2,226 links that was fragmented into 4 components (Fig. 5b; Table 2). The observed networks of both the winter and summer bacterioplankton communities were significantly different from the corresponding random networks in the topological properties of average shortest path length, clustering coefficient, and topological coefficient (P, <0.05 in all cases). The observed-versus-random network clustering coefficient ratio (log response ratios, 0.99 for the winter network and 0.94 for the summer network) showed that the connectivity of the two networks fitted the power law model well and that the two networks were scale free. In addition, the observed clustering coefficients and characteristic path lengths were both greater than those of the corresponding random networks with the same network nodes and edges (Table 2), indicating that the networks of both the winter and summer BCCs showed small-world characteristics. In both seasons, the bacterioplankton communities tended to be copresent more than would be expected by chance; however, fewer copresence links and more exclusion links were observed in the co-occurrence network for the summer than in that for the winter (Table 2). The keystone species OTUs (depicted as nodes with a high degree in the network) differed greatly between the winter and the summer networks (see Fig. S3 in the supplemental material). The top 50 keystone OTUs belonged mainly to the phyla Alphaproteobacteria and Gammaproteobacteria in the winter network and were affiliated with the phyla Bacteroidetes and Alphaproteobacteria in the summer network (Fig. S3).
FIG 5.
Co-occurrence network structures in winter (a) and summer (b) seawater. The networks are visualized with group attribute layouts based on phylum or subphylum. Nodes that were significantly correlated with water temperature are shown in dashed boxes. The colors of the nodes indicate different phylum or subphylum OTUs, as shown in the key at the bottom. An orange line indicates a positive interaction between two OTUs (nodes), while a black line indicates a negative interaction.
TABLE 2.
Major topological properties of the empirical bacterioplankton co-occurrence networks in seawater in winter and in summer and their associated random networks
| Parameter | Winter |
Summer |
||
|---|---|---|---|---|
| Observed | Random | Observed | Random | |
| No. of original OTUs | 500 | 500 | ||
| No. of nodes | 332 | 332 | 314 | 314 |
| No. of edges | 2,183 | 2,183 | 2,226 | 2,226 |
| Avg no. of neighbors | 13.15 | 13.15 | 14.18 | 14.18 |
| Clustering coefficient | 0.407 | 0.041 | 0.384 | 0.044 |
| Betweenness centrality | 0.0087 | 0.0047 | 0.016 | 0.0047 |
| Closeness centrality | 0.308 | 0.394 | 0.254 | 0.407 |
| Network diam | 11 | 4 | 14 | 4 |
| Network radius | 1 | 3 | 1 | 3 |
| Network centralization | 0.155 | 0.039 | 0.183 | 0.038 |
| Network density | 0.04 | 0.04 | 0.045 | 0.045 |
| Characteristic path length | 3.86 | 2.539 | 4.784 | 2.461 |
| Network heterogeneity | 1.122 | 0.271 | 0.952 | 0.264 |
| Connected components | 8 | 1 | 4 | 1 |
| Log response ratio | 0.99 | 0.94 | ||
| Copresence links | 1,696 | 1,429 | ||
| Exclusion links | 487 | 797 | ||
| Exclusion links/total links | 0.22 | 0.36 | ||
For both the summer and winter networks, temperature was among the most connected nodes. The subnetwork built around temperature showed that more OTUs correlated significantly with the temperature in seawater in the summer than in the winter (Fig. 5; see also Fig. S4 in the supplemental material). In the winter subnetwork, temperature tended to have positive correlations with OTUs (37 positive links; 46 total links), including the classified clades belonging to the phyla Alphaproteobacteria (12 positive links; 20 total links), Gammaproteobacteria (9 positive links; 11 total links), and Planctomycetes (3 positive links; 3 total links) (Fig. S4). However, in the summer subnetwork, temperature tended to have negative correlations with OTUs (61 negative links; 68 total links), including the classified clades belonging to the phyla Actinobacteria (8 negative links; 8 total links), Bacteroidetes (26 negative links; 27 total links), Alphaproteobacteria (8 negative links; 11 total links), and Gammaproteobacteria (4 negative links; 6 total links) (Fig. S4). In the subnetworks that included only temperature-related and unrelated nodes, fewer copresence links and more exclusion links were observed in seawater in the summer than in the winter (Fig. 5; see also Table S2 in the supplemental material). By removing the OTUs that had significant correlations with temperature from the networks, subnetworks were generated (see Fig. S5 in the supplemental material), and these showed fewer copresence links and more exclusion links in seawater in the summer than in the winter (Fig. S5 and Table S2).
DISCUSSION
The thermal effluents from two nuclear power plants in Daya Bay have been present for more than 20 years and have generated comparatively stable and long thermal gradients (a temperature increase from 0 to 10°C) in the discharge areas. The environmental patches across thermal gradients are heterogeneous and very strongly interconnected on a microbial scale; thus, this is a useful model for the study of the metacommunity structure and processes of bacterioplankton. Our results showed that the bacterioplankton community structure was highly heterogeneous across the thermal gradients and between winter and summer. Using a metacommunity approach, we found that the spatial BCC patterns in the thermal discharge area were significantly explained by purely environmental factors rather than by purely spatial factors, suggesting that species sorting had a strong effect on bacterioplankton assembly. However, this species-sorting effect was weaker in summer than in winter seawater. Co-occurrence network analysis revealed that bacterioplankton communities coexisted more (copresence links) than expected by chance in both seasons; however, the exclusion links/total links ratio was higher in summer seawater than in winter seawater.
Thermal effluents had significant impact on bacterioplankton community structure; however, the effect differed by season.
High heterogeneity of bacterioplankton community structure was detected across the thermal gradients and between seasons, even at coarse levels of taxonomic resolution, reflecting the different environmental optima of bacterioplankton taxa across the thermal gradients and between seasons (34–37) or differences in the intensity of water warming (16). In findings similar to our winter results, decreased relative abundances of Alphaproteobacteria and Gammaproteobacteria in warmer waters were also observed by Xiong et al. (16) and Von Scheibner et al. (38) in investigations of water temperatures from about 20°C to 30°C. In addition, we showed that high temperatures of <38°C promoted the relative abundance of Cyanobacteria (primarily Synechococcus), supporting previous observations that temperature was an important factor influencing Synechococcus distribution and was positively correlated with the abundance of Synechococcus when the temperature was in an adaptive range (39, 40). In summer seawater, we found lower percentages of Actinobacteria at sites with more thermal effluents, which was probably related to the obviously increased relative abundance of Cyanobacteria. This finding is consistent with previous reports showing negative relationships between the relative abundances of Cyanobacteria and Actinobacteria in aquatic ecosystems (19, 41). Furthermore, Bacteroidetes have been found to be likely to attach to marine eukaryotic algae or animals (42, 43). The decreased abundances of eukaryotic algae and zooplankton under thermal impacts in our study area (2, 3, 8) might therefore be one of the reasons for the lower relative abundances of Bacteroidetes at sites with higher levels of effluents in summer seawater.
Among all the environmental factors examined, the spatially structured temperature created by thermal effluents played the strongest role in determining bacterioplankton metacommunity structure across thermal gradients in both seasons studied. Differences in traits cause different bacterial populations to have different sensitivities to temperature during their growth processes (44–47); thus, temperature is one of the most important factors in shaping bacterioplankton community structure (19, 35, 48). This finding was further confirmed by the positive linear correlations between BCC dissimilarities and temperature differences in any pairwise comparison of sampling sites. A higher regression coefficient was also observed for summer seawater than for winter seawater, indicating that summer bacterioplankton communities showed higher turnover in response to temperature and thus were more sensitive to thermal impacts than winter bacterioplankton communities. This finding might be related to the fact that to keep up with the increased rates of biological activity (for example, colonization, extinction, reproduction, and dispersal) created by faster metabolic kinetics in warmer environments (i.e., summer versus winter coastal ecosystems), aquatic organisms must maintain higher turnover at higher temperatures (11, 49).
In a network analysis, we found that temperature had more positive correlations with OTUs in winter seawater and showed more negative correlations with OTUs in summer seawater. In Daya Bay, the surface water temperature is >25°C during most of the year, reaching a minimum of 14°C in January and a maximum of 32.8°C in July (50). Because of the adaptation of local bacterioplankton communities to the ambient temperature (51), most bacterioplankton may have an optimum temperature near the average water temperature of Daya Bay. In the winter, the background sea temperature in the bay was 21°C, and increased water temperatures caused by thermal effluents might promote the growth of most bacterioplankton taxa. In the summer, the ambient seawater temperature was 31°C, which might be higher than the optimum temperature of many bacterioplankton taxa; thus, the increased water temperatures caused by thermal effluents might stress and inhibit the growth of more bacterioplankton taxa (52).
BCC spatial patterns were predominantly shaped by species sorting, but this effect was weaker in summer than in winter seawater.
We found significant heterogeneity in the BCCs across the thermal gradients in both seasons, suggesting that in the thermal discharge area, i.e., where water is frequently exchanged with the surrounding seawater (current velocity, 0.05 to 0.10 m/s [28, 29]), the high dispersal rates of bacterioplankton communities between specific habitats and the surrounding seawater did not induce mass effects with BCC homogenization across thermal gradients. The VPA revealed that in each season, the BCC variations across the thermal gradient were significantly explained by purely environmental factors rather than by geographic distances only, suggesting that species sorting played a more important role than the spatially dependent dilution role of surrounding seawater on the thermal effluent water in generating BCC spatial patterns across thermal gradients. Previous studies suggest that the strong effect of species sorting is most likely due to the extremely high population growth rates of bacteria (21, 30). Studies of currents that focused on the dynamics of bacterioplankton community structure have confirmed that BCCs can rapidly track changes in the environment (53–55). Therefore, the persistence of newly arrived bacterioplankton populations that migrated via flowing water was more likely to be determined by species sorting of heterogeneous patches than by mass effects (56). This result was in accordance with recent studies that revealed that bacterial spatial distributions were more closely linked to local environmental characteristics than to purely spatial factors (30, 57).
Our results showed that across thermal gradients, purely environmental factors explained a smaller portion of bacterioplankton community structure in summer than in winter seawater, suggesting weaker species sorting of bacterioplankton metacommunity processes in summer than in winter seawater. A larger portion of the BCC variation could not be explained by the selected environmental factors and geographic distances for summer than for winter seawater, suggesting that bacterioplankton assembly might be more stochastic and difficult to predict on the basis of the environmental and spatial factors examined in summer than in winter seawater. This result was further confirmed by linear correlations of the BCC Bray-Curtis dissimilarities with temperature differences between any pair of sampling sites, which show a lower correlation coefficient for summer correlations than for winter correlations. Previous studies showed that the exponential increase in the metabolic rate as the temperature increases affects nearly all biological processes (11, 49). Thus, in the two seasons studied, high temperatures in summer seawater may simply increase the stochasticity of the colonization and extinction of bacteria across thermal gradients. Moreover, the higher level of disorder in biological and environmental conditions created by faster metabolic kinetics and irregular molecular movement in summer seawater may also contribute to the enhanced stochasticity of the bacterioplankton assembly (11, 19, 58) and make bacterioplankton assembly more difficult to predict on the basis of environmental and geographic factors in summer than in winter seawater. Our results also revealed strong mixed effects of temperature and geographic distance in both seasons, emphasizing that marine bacterioplankton assembly was determined by spatially structured environmental gradients (16, 59).
Niche sharing dominated the processes of species sorting, but the relative importance of niche segregation was enhanced in summer seawater.
Co-occurrence networks allow a deeper analysis of the ecological processes structuring microbial communities, such as neutral processes and species sorting (e.g., niche sharing and segregation) (32, 33). Our results showed that the observed co-occurrence networks of both the winter and summer bacterioplankton communities were remarkably different from the corresponding random networks, suggesting that species sorting plays a more important role than neutral processes in shaping marine bacterioplankton assembly. This finding was in agreement with our VPA and other evidence in recent studies (19, 33). Our study also revealed that bacterioplankton communities coexisted more (copresence links) than expected by chance in both seasons. Copresence links could be caused by niche sharing because of ecological interactions, such as facilitative or mutual attraction, while exclusion links could result from niche segregation due to competition interactions (32, 60). In the thermal discharge seawater we studied, co-occurrence bacteria tended to show more niche sharing than niche segregation in both winter and summer seawater, suggesting that although co-occurrence taxa were more likely to share nutritional resources and thus compete more, they tended to co-occur frequently, probably because they also shared other traits (e.g., cross-feeding, coaggregation, or cocolonization) that allowed them to survive together.
The summer seawater temperature was higher than the optimum temperature of many bacterioplankton taxa, and the increased temperatures caused by thermal effluents might inhibit the growth of many heat-sensitive taxa (52). Therefore, the roles of niche segregation between heat-sensitive and -insensitive OTUs might be enhanced in summer seawater (52). This inference was further confirmed by the co-occurrence network analysis, which showed a higher ratio of negative correlations between the relative abundances of the temperature-related and non-temperature-related OTUs in the summer network than in the winter network. This finding probably reveals one of the reasons for the higher sensitivity of bacterioplankton communities to temperature in summer seawater than in winter seawater. Moreover, in the subnetworks excluding temperature-related OTUs, a higher ratio of negative correlations between the relative abundances of OTUs was also observed for summer seawater than for winter seawater, suggesting that in addition to temperature, other factors (e.g., resources) might also have had a species-sorting effect on bacterioplankton assembly and resulted in niche segregation between co-occurrence OTUs in summer seawater. In the bay we studied, where there is no shortage of nutrients, since it has been a typical aquaculture area for years (50), bacterioplankton growth may not be constrained by resources in the winter. However, in the summer, with further increases in the water temperature and higher metabolic rates of bacterioplankton communities, resource consumption and demand probably both continued to increase and likely resulted in the insufficiency of certain resources for the growth of some bacterioplankton in higher-temperature habitats (58). Therefore, the roles of niche segregation between resource-sensitive and -insensitive OTUs might be enhanced in summer seawater (61). With the increased roles of niche segregation, co-occurrence bacterioplankton OTUs in summer seawater tended to have fewer copresence links and more exclusion links than those in winter seawater.
Conclusion.
Our results suggest that in the thermal effluent area, i.e., where water is exchanged very frequently with the surrounding seawater and thermal effluent water, the spatial patterns of marine BCCs were significantly shaped by species sorting rather than by mass effects or by the dilution of thermal effluent water by the surrounding seawater. However, this species-sorting effect was weaker in summer than in winter seawater. In addition, bacterioplankton metacommunity structure was predominately shaped by niche sharing in both seasons, but the niche segregation roles were enhanced in summer community assembly. Finally, we propose that with the enhanced niche segregation between heat-sensitive and -insensitive taxa and the increased stochastic assembly processes in summer seawater, bacterioplankton communities were more sensitive to thermal impacts and were more difficult to predict in summer than in winter seawater in the face of different intensities of thermal impacts.
MATERIALS AND METHODS
Study area.
Our study area, Daya Bay, is located in the northern South China Sea (22.5°N to 22.9°N, 114.5°E to 114.9°E) between Shenzhen and Huizhou in Guangdong Province near Hong Kong (Fig. 1). The region of Daya Bay is characterized by a subtropical climate. The area of Daya Bay is 650 km2 at flood tide, and its depth is between 6 and 15 m. The surface water salinity ranges from 22 to 33, and the temperature ranges from 15°C to 32°C (2, 8, 27). The bay is dominated by an irregular semidiurnal tide with a narrow tidal range. The mean wind velocity is 4.4 m/s in winter and 4.8 m/s in summer (8, 27). The two nuclear power stations are located in Dapeng Cove, which is an important maricultural area (27). The 1,800-MW Daya Bay Nuclear Power Station (DNPS) has operated since 1994 and discharges heated water at a rate of 2.9 × 107 m3/year (3). Another nuclear power station, the Lingao Nuclear Power Station (LNPS), which is located near the DNPS, has been in operation since 2002. In the cooling system, cooling water is drawn from intake points in a coastal water body. After going through the cooling systems, heated water is released back into the same water body at a unified outlet site away from the intake points. The velocity of thermal effluents at the outlet site is about 2.0 m/s (3, 28, 29). By the influence of currents, the velocity of effluents is slowed down, with a mean velocity of about 0.08 m/s in the discharge area (3, 28, 29). Thermal effluents elevate the temperature of the receiving water body and significantly affect the ecosystems in Daya Bay (2, 27).
Water sampling and chemical determination.
In our study area, Daya Bay, the warmest months in the year of sampling (i.e., 2017) included June, July, August, and September, with an average high temperature of 31.0°C; the coldest months were January, February, and December, with an average high temperature of 21.0°C. Samples were collected on 7 January and 28 July 2017, which were typical winter and summer days in the subtropical bay with water temperatures of 21.0°C and 31.0°C, respectively. For this study, we set five sampling sites for each season. Four sites were located along the direction of the thermal effluent (winter: WS1, WS2, WS3, and WS4; summer: SS1, SS2, SS3, and SS4), and the remaining site (winter, WS5; summer, SS5) was set as a control and was located in the inflow area of the cooling system upstream of the thermal effluents (Fig. 1). In winter and summer, the site layouts were inconsistent due to the differences in hydrologic conditions (e.g., higher tide level and higher current velocity in winter than in summer seawater [28, 29]). At each sampling site, we obtained three replicates. The maps of the sampling sites were generated based on an open-access Google satellite map using the ggmap package (https://github.com/dkahle/ggmap) in the R statistical computing environment (https://www.R-project.org) (Fig. 1). A 5-liter surface seawater sample was collected at each replicate site and was filtered through 0.2-μm-pore-size Isopore filters (Millipore, Billerica, MA, USA). Before the experiments, the filters were unsealed and UV sterilized in a clean bench. The filters were stored at –70°C until further analyses of the bacterioplankton communities. The water temperature, dissolved oxygen, pH, and salinity were measured in the field. Approximately 500 ml of surface water was collected for the nutrient analysis. Ammonium nitrogen (NH4+), nitrate nitrogen (NO3–), soluble reactive phosphate (PO43–), and silicate (SiO32–) levels (all measured in micromoles per liter) were determined using a UV-visible spectrophotometer (UV2450; Shimadzu, Tokyo, Japan) according to marine monitoring specifications (62). Total organic carbon (TOC) was measured using a TOC analyzer (TOC-VCPH; Shimadzu, Tokyo, Japan) according to a standard procedure (62).
DNA extraction, amplification, sequencing, and data processing.
Genomic DNA was extracted from the biomass collected on the filters using a PowerWater DNA isolation kit (MoBio Laboratories, Carlsbad, CA, USA) and was purified using a PowerClean DNA clean-up kit (MoBio Laboratories, Carlsbad, CA, USA). DNA was quantified, and its quality was determined, using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE). The V3 and V4 hypervariable regions of bacterial 16S rRNA genes were amplified with primers F341 (5′-CCTACGGGAGGCAGCAG-3′) and R806 (3′-GGACTACHVGGGTWTCTAAT-5′). To pool multiple samples in one Illumina sequencing run, a unique 12-mer tag was added to the 5′ end of each primer for each DNA sample. Three replicates of each sample were PCR amplified in a 50-μl reaction mixture, which contained 25 μl 2× PCR Premix Taq, 10 mM each primer, 60 ng of genomic DNA, and 20 μl of nuclease-free water. Cycling conditions were as follows: 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 52°C for 30 s, extension at 72°C for 30 s, and a final extension at 72°C for 10 min. The PCR products were visualized on 1% agarose gels, and the positive amplicons were quantified using the PicoGreen dsDNA assay kit (Invitrogen Corporation, Carlsbad, CA, USA) and were equally combined and purified with Zymo’s Genomic DNA Clean & Concentrator kit (Zymo Research Corporation, Irvine, CA, USA). Finally, amplicons were sequenced using the Illumina HiSeq 2500 platform.
Raw reads of the 16S rRNA gene sequences were processed using the mothur software package (version 1.30.0, 2013) (http://www.mothur.org) according to the MiSeq standard operating procedure (63). In brief, the raw reads were combined, denoised, trimmed, quality-filtered, and aligned to the SILVA databases (64) using mothur. After initial processing, the high-quality sequences were clustered into OTUs at a 97% similarity level. Each of the representative OTU sequences was classified using the SILVA databases at the recommended bootstrap threshold of 80% (65). To minimize bias caused by sequencing depth, all singletons and OTUs occurring in only one sample were excluded from the OTU table. To correct for differences in sequencing depth, the minimum number of sequences in the whole sample (i.e., 40,851 sequences per sample) was randomly subsampled for analyses of the following metrics described below.
Network analysis.
In each season studied, we selected the 500 most abundant OTUs, which accounted for 88.2% of the total winter sequences and 83.9% of the total summer sequences, for the performance of co-occurrence network analyses. We removed OTUs present in less than one-third of the samples, because they could cause artifactual associations (32). By using the Cytoscape plugin CONET (http://psbweb05.psb.ugent.be/conet/), significantly positive and negative interactions were identified based on an ensemble approach that combined five different measures: Bray-Curtis and Kullback-Leibler dissimilarities, Pearson and Spearman correlations, and mutual information (32). These five similarity measures were computed to cover a wide range of relationships (e.g., linear or nonlinear), to address noise and outliers, and to reduce the impact of choosing a single measure (32). For each measure, we requested the 1,000 top positive and negative edges in the “threshold-setting menu” (32). P values were further computed from method- and edge-specific permutations and bootstrap score distributions with 1,000 iterations each. Measure-specific P values were merged using Brown’s method and were corrected for multiple testing by the Benjamini-Hochberg method. The unstable edges with scores outside the 0.95 range of their bootstrap distribution were removed. Co-occurrence networks were further visualized by Cytoscape, version 3.5.1, with a group attribute layout based on the phylum or subphylum (66). The subnetworks of nodes (OTUs) that had nonsignificant correlations with the seawater temperature were also generated. The topology of the resulting undirected network was investigated using the implemented tool network analyzer of Cytoscape (67) and was compared to an Erdös–Rényi random network of similar size, which was calculated by the implemented tool Network Randomizer, version 1.1.3.
Statistical analyses.
To assess the significant differences in the environmental characteristics and the relative abundances of phyla and of lineages and clades across the thermal gradients and between seasons, we conducted one-way ANOVA followed by post hoc comparisons and Student t tests using the stats package in R. A detrended correspondence analysis (DCA) and permutational multivariate analysis of variance using distance matrices (PERMANOVA) were performed to test the significant differences in the BCCs across the thermal gradient and between seasons using the vegan package in R. Using the lmPerm package in R, analysis of covariance (ANCOVA) with permutation tests was used to test the differences between the summer and winter regression lines formed between BCC dissimilarities and temperature differences for any pair of sampling sites.
The PCNM (principal coordinates of neighborhood matrix) method was used to transform geographic distances between any pair of sites within S1, S2, S3, and S4 into rectangular data that were suitable for constrained ordination or regression. A partial redundancy analysis (RDA)-based variance partitioning analysis (VPA) was carried out to partition the variations in BCCs across sites S1, S2, S3, and S4 into purely environmental, purely geographic distance-related, mixed geographic distance- and environment-related, and unexplained components (30, 31). The best subset of environmental parameters and geographic distance in the VPA was chosen by the BIOENV procedure. The VPA results were further tested by RDA-based partial permutation tests. To further identify the key environmental factors that significantly explained the BCC pattern across the thermal gradient, RDA was performed. The models in RDA were validated by analysis of variance. PCNM, VPA, BIOENV procedure, RDA, and RDA-based partial permutation tests were all carried out in the vegan package of R. The top 50 keystone OTUs and the OTUs that had significant links to the seawater temperature were visualized by heat maps using the phyloseq package in R.
Accession number(s).
The sequence data were submitted to the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) (https://www.ncbi.nlm.nih.gov/sra) under accession number SRP158638.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Fuwu Xie, Yadong Huang, Jiaxing Liu, Chenhui Xiang, Xin Jiang, Weiwei Liu, Kaizhi Li, and Dajun Qiu for assistance with experimental sampling and data analyses.
This work was supported by the National Key Research and Development Program of China (grant 2017YFC0506302), the National Key Basic Research Program of China (973 program, grant 2015CB452904), the National Science Foundation (grants 416210002, 31730013, 31870445, and 31500376), the “Strategic Priority Research Program” of the Chinese Academy of Sciences (award XDA13020102), the Key Research Program of Frontier Science, CAS (award QYZDJ-SSW-DQC030), and the Science and Technology Planning Project of Guangdong Province, China (grant 2017B030314052).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02088-18.
REFERENCES
- 1.Poornima EH, Rajadurai M, Rao TS, Anupkumar B, Rajamohan R, Narasimhan SV, Rao VNR, Venugopalan VP. 2005. Impact of thermal discharge from a tropical coastal power plant on phytoplankton. J Therm Biol 30:307–316. doi: 10.1016/j.jtherbio.2005.01.004. [DOI] [Google Scholar]
- 2.Wang YS, Lou ZP, Sun CC, Sun S. 2008. Ecological environment changes in Daya Bay, China, from 1982 to 2004. Mar Pollut Bull 56:1871–1879. doi: 10.1016/j.marpolbul.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Hao Y, Tang D, Boicenco L, Wang S. 2016. Environmental ecological response to increasing water temperature in the Daya Bay, Southern China in 1982–2012. Nat Resour 7(4):184–192. doi: 10.4236/nr.2016.74017. [DOI] [Google Scholar]
- 4.Cotner JB, Biddanda BA. 2002. Small players, large role: microbial influence on biogeochemical processes in pelagic aquatic ecosystems. Ecosystems 5:105–121. doi: 10.1007/s10021-001-0059-3. [DOI] [Google Scholar]
- 5.Azam F, Malfatti F. 2007. Microbial structuring of marine ecosystems. Nat Rev Microbiol 5:782–791. doi: 10.1038/nrmicro1747. [DOI] [PubMed] [Google Scholar]
- 6.Li T, Liu S, Huang L, Huang H, Lian J, Yan Y, Lin S. 2011. Diatom to dinoflagellate shift in the summer phytoplankton community in a bay impacted by nuclear power plant thermal effluent. Mar Ecol Prog Ser 424:75–85. doi: 10.3354/meps08974. [DOI] [Google Scholar]
- 7.Li XY, Li B, Sun XL. 2014. Effects of a coastal power plant thermal discharge on phytoplankton community structure in Zhanjiang Bay, China. Mar Pollut Bull 81:210–217. doi: 10.1016/j.marpolbul.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Song S, Qi Y. 2014. A comparative study of phytoneuston and the phytoplankton community structure in Daya Bay, South China Sea. J Sea Res 85:474–482. doi: 10.1016/j.seares.2013.08.002. [DOI] [Google Scholar]
- 9.Lo WT, Hsu PK, Fang TH, Hu JH, Hsieh HY. 2016. Phytoplankton communities impacted by thermal effluents off two coastal nuclear power plants in subtropical areas of northern Taiwan. Terr Atmos Ocean Sci 27:107–120. doi: 10.3319/TAO.2015.09.24.01(Oc). [DOI] [Google Scholar]
- 10.Choi KH, Kim YO, Lee JB, Wang SY, Lee MW, Lee PG, Soh HY. 2012. Thermal impacts of a coal power plant on the plankton in an open coastal water environment. J Mar Sci Technol 20:187–194. [Google Scholar]
- 11.Hillebrand H, Soininen J, Snoeijs P. 2010. Warming leads to higher species turnover in a coastal ecosystem. Glob Chang Biol 16:1181–1193. doi: 10.1111/j.1365-2486.2009.02045.x. [DOI] [Google Scholar]
- 12.Teixeira TP, Neves LM, Araújo FG. 2012. Thermal impact of a nuclear power plant in a coastal area in Southeastern Brazil: effects of heating and physical structure on benthic cover and fish communities. Hydrobiologia 684:161–175. doi: 10.1007/s10750-011-0980-1. [DOI] [Google Scholar]
- 13.Choi DH, Park JS, Hwang CY, Huh SH, Cho BC. 2002. Effects of thermal effluents from a power station on bacteria and heterotrophic nanoflagellates in coastal waters. Mar Ecol Prog Ser 229:1–10. doi: 10.3354/meps229001. [DOI] [Google Scholar]
- 14.Shiah FK, Wu TH, Li KY, Kao SJ, Tseng YF, Chung JL, Jan S. 2006. Thermal effects on heterotrophic processes in a coastal ecosystem adjacent to a nuclear power plant. Mar Ecol Prog Ser 309:55–65. doi: 10.3354/meps309055. [DOI] [Google Scholar]
- 15.Shiah FK, Tu YY, Tsai HS, Kao SJ, Jan S. 2005. A case study of system and planktonic responses in a subtropical warm plume receiving thermal effluents from a power plant. Terr Atmos Ocean Sci 16:513–528. doi: 10.3319/TAO.2005.16.2.513(O). [DOI] [Google Scholar]
- 16.Xiong J, Xiong S, Qian P, Zhang D, Liu L, Fei Y. 2016. Thermal discharge-created increasing temperatures alter the bacterioplankton composition and functional redundancy. AMB Express 6:68. doi: 10.1186/s13568-016-0238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prosser JI, Bohannan BJ, Curtis TP, Ellis RJ, Firestone MK, Freckleton RP, Green JL, Green LE, Killham K, Lennon JJ, Osborn AM, Solan M, van der Gast CJ, Young JPW. 2007. The role of ecological theory in microbial ecology. Nat Rev Microbiol 5:384–392. doi: 10.1038/nrmicro1643. [DOI] [PubMed] [Google Scholar]
- 18.Logue JB, Mouquet N, Peter H, Hillebrand H, Metacommunity Working Group . 2011. Empirical approaches to metacommunities: a review and comparison with theory. Trends Ecol Evolut 26:482–491. doi: 10.1016/j.tree.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Ren L, He D, Chen Z, Jeppesen E, Lauridsen TL, Søndergaard M, Liu Z, Wu QL. 2017. Warming and nutrient enrichment in combination increase stochasticity and beta diversity of bacterioplankton assemblages across freshwater mesocosms. ISME J 11:613–625. doi: 10.1038/ismej.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martiny JBH, Bohannan BJM, Brown JH, Colwell RK, Fuhrman JA, Green JL, Horner-Devine MC, Kane M, Krumins JA, Kuske CR, Morin PJ, Naeem S, Øvreås L, Reysenbach AL, Smith VH, Staley JT. 2006. Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4:102–112. doi: 10.1038/nrmicro1341. [DOI] [PubMed] [Google Scholar]
- 21.Lindström ES, Langenheder S. 2012. Local and regional factors influencing bacterial community assembly. Environ Microbiol Rep 4:1–9. doi: 10.1111/j.1758-2229.2011.00257.x. [DOI] [PubMed] [Google Scholar]
- 22.Adams HE, Crump BC, Kling GW. 2014. Metacommunity dynamics of bacteria in an arctic lake: the impact of species sorting and mass effects on bacterial production and biogeography. Front Microbiol 5:82. doi: 10.3389/fmicb.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A. 2004. The metacommunity concept: a framework for multi‐scale community ecology. Ecol Lett 7:601–613. doi: 10.1111/j.1461-0248.2004.00608.x. [DOI] [Google Scholar]
- 24.Hubbell SP. 2001. The unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton, NJ. [DOI] [PubMed] [Google Scholar]
- 25.MacArthur RH. 1958. Population ecology of some warblers of northeastern coniferous forests. Ecology 39:599–619. doi: 10.2307/1931600. [DOI] [Google Scholar]
- 26.Pianka ER. 1966. Latitudinal gradients in species diversity: a review of concepts. Am Nat 100:33–46. doi: 10.1086/282398. [DOI] [Google Scholar]
- 27.Tang D, Kester DR, Wang Z, Lian J, Kawamura H. 2003. AVHRR satellite remote sensing and shipboard measurements of the thermal plume from the Daya Bay, nuclear power station, China. Remote Sens Environ 84:506–515. doi: 10.1016/S0034-4257(02)00149-9. [DOI] [Google Scholar]
- 28.Wei X, Ni P, Zhan H. 2013. Monitoring cooling water discharge using Lagrangian coherent structures: a case study in Daya Bay, China. Mar Pollut Bull 75:105–113. doi: 10.1016/j.marpolbul.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 29.Lei Y. 2013. Comparative study of IRS and TM image monitoring of Daya Bay warm water thermal pollution. A dissertation submitted to China University of Geosciences for a Master’s degree. China University of Geosciences, Beijing, China. (In Chinese.) [Google Scholar]
- 30.Van der Gucht K, Cottenie K, Muylaert K, Vloemans N, Cousin S, Declerck S, Jeppesen E, Conde-Porcuna JM, Schwenk K, Zwart G, Degans H, Vyverman W, De Meester L. 2007. The power of species sorting: local factors drive bacterial community composition over a wide range of spatial scales. Proc Natl Acad Sci U S A 104:20404–20409. doi: 10.1073/pnas.0707200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramette A, Tiedje JM. 2007. Multiscale responses of microbial life to spatial distance and environmental heterogeneity in a patchy ecosystem. Proc Natl Acad Sci U S A 104:2761–2766. doi: 10.1073/pnas.0610671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faust K, Raes J. 2012. Microbial interactions: from networks to models. Nat Rev Microbiol 10:538–550. doi: 10.1038/nrmicro2832. [DOI] [PubMed] [Google Scholar]
- 33.Layeghifard M, Hwang DM, Guttman DS. 2017. Disentangling interactions in the microbiome: a network perspective. Trends Microbiol 25:217–228. doi: 10.1016/j.tim.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuhrman JA, Hewson I, Schwalbach MS, Steele JA, Brown MV, Naeem S. 2006. Annually reoccurring bacterial communities are predictable from ocean conditions. Proc Natl Acad Sci U S A 103:13104–13109. doi: 10.1073/pnas.0602399103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersson AF, Riemann L, Bertilsson S. 2010. Pyrosequencing reveals contrasting seasonal dynamics of taxa within Baltic Sea bacterioplankton communities. ISME J 4:171–181. doi: 10.1038/ismej.2009.108. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert JA, Steele JA, Caporaso JG, Steinbrück L, Reeder J, Temperton B, Huse S, McHardy AC, Knight R, Joint I, Somerfield P, Fuhrman JA, Field D. 2012. Defining seasonal marine microbial community dynamics. ISME J 6:298–308. doi: 10.1038/ismej.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia X, Vidyarathna NK, Palenik B, Lee P, Liu H. 2015. Comparison of the seasonal variations of Synechococcus assemblage structures in estuarine waters and coastal waters of Hong Kong. Appl Environ Microbiol 81:7644–7655. doi: 10.1128/AEM.01895-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Von Scheibner M, Dörge P, Biermann A, Sommer U, Hoppe HG, Jürgens K. 2014. Impact of warming on phyto‐bacterioplankton coupling and bacterial community composition in experimental mesocosms. Environ Microbiol 16:718–733. doi: 10.1111/1462-2920.12195. [DOI] [PubMed] [Google Scholar]
- 39.Agawin NS, Duarte CM, Agusti S. 1998. Growth and abundance of Synechococcus sp. in a Mediterranean Bay: seasonality and relationship with temperature. Mar Ecol Prog Ser 170:45–53. doi: 10.3354/meps170045. [DOI] [Google Scholar]
- 40.Agawin NS, Duarte CM, Agustí S. 2000. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnol Oceanogr 45:591–600. doi: 10.4319/lo.2000.45.3.0591. [DOI] [Google Scholar]
- 41.Ghai R, Mizuno CM, Picazo A, Camacho A, Rodriguez-Valera F. 2014. Key roles for freshwater Actinobacteria revealed by deep metagenomic sequencing. Mol Ecol 23:6073–6090. doi: 10.1111/mec.12985. [DOI] [PubMed] [Google Scholar]
- 42.Webster NS, Wilson KJ, Blackall LL, Hill RT. 2001. Phylogenetic diversity of bacteria associated with the marine sponge Rhopaloeides odorabile. Appl Environ Microbiol 67:434–444. doi: 10.1128/AEM.67.1.434-444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grossart HP, Levold F, Allgaier M, Simon M, Brinkhoff T. 2005. Marine diatom species harbour distinct bacterial communities. Environ Microbiol 7:860–873. doi: 10.1111/j.1462-2920.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 44.White PA, Kalff J, Rasmussen JB, Gasol JM. 1991. The effect of temperature and algal biomass on bacterial production and specific growth rate in freshwater and marine habitats. Microb Ecol 21:99–118. doi: 10.1007/BF02539147. [DOI] [PubMed] [Google Scholar]
- 45.Rivkin RB, Anderson MR, Lajzerowicz C. 1996. Microbial processes in cold oceans. 1. Relationship between temperature and bacterial growth rate. Aquat Microb Ecol 10:243–254. doi: 10.3354/ame010243. [DOI] [Google Scholar]
- 46.Shurin JB, Clasen JL, Greig HS, Kratina P, Thompson PL. 2012. Warming shifts top-down and bottom-up control of pond food web structure and function. Philos Trans R Soc Lond B Biol Sci 367:3008–3017. doi: 10.1098/rstb.2012.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Özen A, Šorf M, Trochine C, Liboriussen L, Beklioglu M, Søndergaard M, Lauridsen TL, Johansson LS, Jeppesen E. 2013. Long-term effects of warming and nutrients on microbes and other plankton in mesocosms. Freshwater Biol 58:483–493. doi: 10.1111/j.1365-2427.2012.02824.x. [DOI] [Google Scholar]
- 48.Fuhrman JA, Steele JA, Hewson I, Schwalbach MS, Brown MV, Green JL, Brown JH. 2008. A latitudinal diversity gradient in planktonic marine bacteria. Proc Natl Acad Sci U S A 105:7774–7778. doi: 10.1073/pnas.0803070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren L, He D, Zeng J, Wu QL. 2013. Bacterioplankton communities turn unstable and become small under increased temperature and nutrient-enriched conditions. FEMS Microbiol Ecol 84:614–624. doi: 10.1111/1574-6941.12089. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, Zhao J, Zhang Y, Cao Y. 2009. Phytoplankton community structure and environmental parameters in aquaculture areas of Daya Bay, South China Sea. J Environ Sci 21:1268–1275. doi: 10.1016/S1001-0742(08)62414-6. [DOI] [PubMed] [Google Scholar]
- 51.Hall EK, Neuhauser C, Cotner JB. 2008. Toward a mechanistic understanding of how natural bacterial communities respond to changes in temperature in aquatic ecosystems. ISME J 2:471–481. doi: 10.1038/ismej.2008.9. [DOI] [PubMed] [Google Scholar]
- 52.Pomeroy LR, Wiebe WJ. 2001. Temperature and substrates as interactive limiting factors for marine heterotrophic bacteria. Aquat Microb Ecol 23:187–204. doi: 10.3354/ame023187. [DOI] [Google Scholar]
- 53.Van der Gucht K, Sabbe K, De Meester L, Vloemans N, Zwart G, Gillis M, Vyverman W. 2001. Contrasting bacterioplankton community composition and seasonal dynamics in two neighbouring hypertrophic freshwater lakes. Environ Microbiol 3:680–690. doi: 10.1046/j.1462-2920.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- 54.Muylaert K, Van der Gucht K, Vloemans N, De Meester L, Gillis M, Vyverman W. 2002. Relationship between bacterial community composition and bottom-up versus top-down variables in four eutrophic shallow lakes. Appl Environ Microbiol 68:4740–4750. doi: 10.1128/AEM.68.10.4740-4750.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinhassi J, Sala MM, Havskum H, Peters F, Guadayol O, Malits A, Marrasé C. 2004. Changes in bacterioplankton composition under different phytoplankton regimens. Appl Environ Microbiol 70:6753–6766. doi: 10.1128/AEM.70.11.6753-6766.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Logue JB, Lindström ES. 2010. Species sorting affects bacterioplankton community composition as determined by 16S rDNA and 16S rRNA fingerprints. ISME J 4:729–738. doi: 10.1038/ismej.2009.156. [DOI] [PubMed] [Google Scholar]
- 57.Horňák K, Corno G. 2012. Every coin has a back side: invasion by Limnohabitans planktonicus promotes the maintenance of species diversity in bacterial communities. PLoS One 7:e51576. doi: 10.1371/journal.pone.0051576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen AP, Brown JH, Gillooly JF. 2002. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297:1545–1548. doi: 10.1126/science.1072380. [DOI] [PubMed] [Google Scholar]
- 59.Wang K, Ye X, Chen H, Zhao Q, Hu C, He J, Qian Y, Xiong J, Zhu J, Zhang D. 2015. Bacterial biogeography in the coastal waters of northern Zhejiang, East China Sea is highly controlled by spatially structured environmental gradients. Environ Microbiol 17:3898–3913. doi: 10.1111/1462-2920.12884. [DOI] [PubMed] [Google Scholar]
- 60.Pascual-García A, Tamames J, Bastolla U. 2014. Bacteria dialog with Santa Rosalia: are aggregations of cosmopolitan bacteria mainly explained by habitat filtering or by ecological interactions? BMC Microbiol 14:284. doi: 10.1186/s12866-014-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.López-Urrutia Á, Morán XAG. 2007. Resource limitation of bacterial production distorts the temperature dependence of oceanic carbon cycling. Ecology 88:817–822. doi: 10.1890/06-1641. [DOI] [PubMed] [Google Scholar]
- 62.General Administration of Quality Supervision, Inspection and Quarantine (AQSIQ) of the People’s Republic of China. 2007. The specification for marine monitoring of China. Part 4: seawater analysis. GB 173784-2007. Standards Press of China, Beijing, China. (In Chinese.) [Google Scholar]
- 63.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2012. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Assenov Y, Ramírez F, Schelhorn SE, Lengauer T, Albrecht M. 2008. Computing topological parameters of biological networks. Bioinformatics 24:282–284. doi: 10.1093/bioinformatics/btm554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.