As an alternative to fossil fuel, H2 is a promising renewable energy source. Although photofermentative H2 production from acetate is key to developing an efficient process of biohydrogen production from biomass-derived sugars, H2 yields from acetate and l-glutamate by R. sphaeroides have been reported to be low. In this study, we observed that in addition to the endogenous EMC pathway, heterologous expression of the glyoxylate bypass in R. sphaeroides markedly increased H2 yields from acetate and l-glutamate. Therefore, this study provides a novel strategy for improving H2 yields from acetate in the presence of l-glutamate and contributes to a clear understanding of acetate metabolism in R. sphaeroides during photofermentative H2 production.
KEYWORDS: Rhodobacter sphaeroides, acetate, ethylmalonyl-CoA, glyoxylate, hydrogen production, nitrogenase, polyhydroxyalkanoate synthesis
ABSTRACT
Rhodobacter sphaeroides produces hydrogen gas (H2) from organic compounds via nitrogenase under anaerobic-light conditions in the presence of poor nitrogen sources, such as l-glutamate. R. sphaeroides utilizes the ethylmalonyl-coenzyme A (EMC) pathway for acetate assimilation, but its H2 yield from acetate in the presence of l-glutamate has been reported to be low. In this study, the deletion of ccr encoding crotonyl-coenzyme A (crotonyl-CoA) carboxylase/reductase, a key enzyme for the EMC pathway in R. sphaeroides, revealed that the EMC pathway is essential for H2 production from acetate and l-glutamate but not for growth and acetate consumption in the presence of l-glutamate. We introduced a plasmid expressing aceBA from Rhodobacter capsulatus encoding two key enzymes for the glyoxylate bypass into R. sphaeroides, which resulted in a 64% increase in H2 production. However, compared with the wild-type strain expressing heterologous aceBA genes, the strain with aceBA introduced in the genetic background of an EMC pathway-disrupted mutant showed a lower H2 yield. These results indicate that a combination of the endogenous EMC pathway and a heterologously expressed glyoxylate bypass is beneficial for H2 production. In addition, introduction of the glyoxylate bypass into a polyhydroxybutyrate (PHB) biosynthesis-disrupted mutant resulted in a delay in growth along with H2 production, although its H2 yield was comparable to that of the wild-type strain expressing heterologous aceBA genes. These results suggest that PHB production is important for fitness to the culture during H2 production from acetate and l-glutamate when both acetate-assimilating pathways are present.
IMPORTANCE As an alternative to fossil fuel, H2 is a promising renewable energy source. Although photofermentative H2 production from acetate is key to developing an efficient process of biohydrogen production from biomass-derived sugars, H2 yields from acetate and l-glutamate by R. sphaeroides have been reported to be low. In this study, we observed that in addition to the endogenous EMC pathway, heterologous expression of the glyoxylate bypass in R. sphaeroides markedly increased H2 yields from acetate and l-glutamate. Therefore, this study provides a novel strategy for improving H2 yields from acetate in the presence of l-glutamate and contributes to a clear understanding of acetate metabolism in R. sphaeroides during photofermentative H2 production.
INTRODUCTION
Purple nonsulfur photosynthetic bacteria (PNSB) are capable of producing hydrogen gas (H2) using light energy and organic compounds (1–3). In photofermentative H2 production, proton reduction is catalyzed by nitrogenase with ferredoxin carrying electrons generated via carbon metabolism and ATP derived from cyclic photophosphorylation. Nitrogenase plays a physiological role in dinitrogen fixation by catalyzing the reduction of dinitrogen to ammonia with concomitant release of H2 as a by-product, whereas it produces only H2 in the absence of dinitrogen (4). Since nitrogenase is repressed in the presence of ammonia at both transcriptional and posttranslational levels, poor nitrogen sources, such as l-glutamate instead of ammonium salts, have been used as the nitrogen source for efficient H2 production (1). Unlike in dark fermentative H2 production, that is, H2 fermentation via hydrogenase by using nonphotosynthetic bacteria or archaea (5, 6), PNSB can utilize short-chain organic acids as the substrate for H2 production (1). Hence, various combinations of dark fermentation and photofermentation, for example, cocultures or two-stage processes, have been studied to develop a high-yield biohydrogen production system to secure a steady supply of H2 as a renewable fuel alternative to fossil fuels (7–10). As acetate is a fermentation product by many organisms from a variety of substrates, it is also a major by-product of dark fermentation (11). Thus, it is important to improve the yields of photofermentative H2 production from acetate to improve the overall H2 yields from biomass-derived sugars.
Rhodobacter sphaeroides has been extensively studied regarding its metabolic versatility, as it can grow aerobically, anaerobically with dimethyl sulfoxide (12), and photoheterotrophically under anaerobic conditions, and it is capable of fixing dinitrogen in the absence of a fixed nitrogen source. R. sphaeroides also has been recognized as a model for studying photofermentative H2 production (13). In this context, transcriptome and metabolic flux analyses have been performed for this bacterium under H2 production conditions (14, 15). However, H2 yields from acetate in the presence of l-glutamate have been reported to be lower than H2 yields from other organic acids, such as lactate, malate, succinate, and fumarate (16, 17).
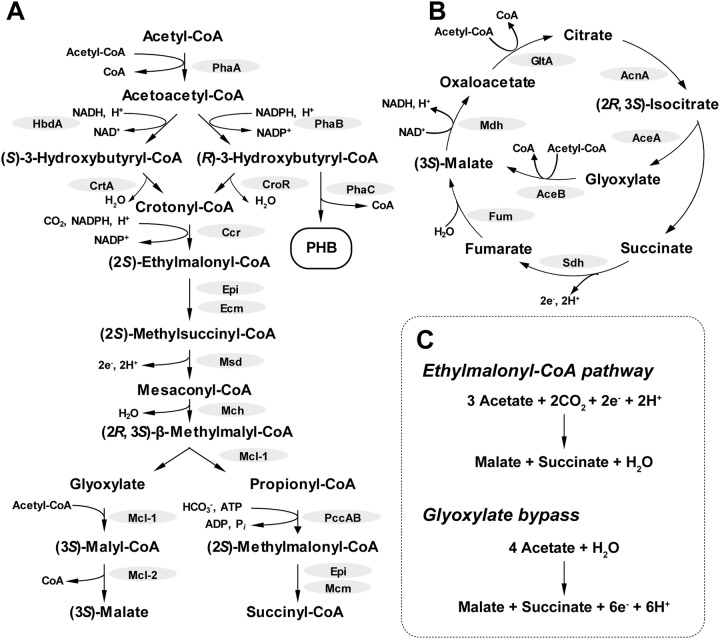
Two major acetate-assimilating pathways have been identified in PNSB. Rhodopseudomonas palustris, another PNSB representative, uses the glyoxylate bypass for acetate assimilation (18); however, R. sphaeroides lacks isocitrate lyase, a key enzyme for the glyoxylate bypass, and instead uses the ethylmalonyl-coenzyme A (EMC) pathway (19–22). As shown in Fig. 1, the EMC pathway requires reducing equivalents for the reduction of acetoacetyl-coenzyme A (acetoacetyl-CoA) and crotonyl-CoA with the incorporation of CO2, while the glyoxylate bypass releases six reducing equivalents to convert four acetyl-CoA into malate and succinate. Thus, the EMC pathway has been suggested to balance the excessive reducing equivalents formed during photoheterotrophic growth with acetate (23, 24). It should be noted that this redox-balancing function of the EMC pathway may detract from the electron flow that is primarily generated in the tricarboxylic acid (TCA) cycle to multiple electron sinks, including nitrogenase, thereby lowering H2 yields (25, 26). Nevertheless, to date, there has been no attempt thus far to examine the effects of the two acetate-assimilating pathways, the EMC pathway and the glyoxylate bypass, on photofermentative H2 production from acetate.
FIG 1.
The ethylmalonyl-CoA pathway and the glyoxylate bypass for acetate assimilation. (A) Ethylmalonyl-CoA pathway. (B) Glyoxylate bypass. (C) Balanced equation for ethylmalonyl-CoA pathway and glyoxylate bypass. Enzymes catalyzing the reactions are abbreviated as follows: PhaA, ketothiolase; PhaB, acetoacetyl-CoA reductase; PhaC, polyhydroxyalkanoate synthase; CroR, crotonase (R-specific); HbdA, (S)-3-hydroxybutyryl-CoA dehydrogenase; CrtA, crotonase (S-specific); Ccr, crotonyl-CoA carboxylase/reductase; Epi, ethylmalonyl-CoA/methylmalonyl-CoA epimerase; Ecm, ethylmalonyl-CoA mutase; Msd, methylsuccinyl-CoA dehydrogenase; Mch, mesaconyl-CoA hydratase; Mcl-1, (3S)-malyl-CoA/β-methylmalyl-CoA lyase; Mcl2, (3S)-malyl-CoA thioesterase; PccAB, propionyl-CoA carboxylase; Mcm, methylmalonyl-CoA mutase; GltA, citrate synthase; AcnA, aconitase; AceA, isocitrate lyase; AceB, malate synthase; Sdh, succinate dehydrogenase; Fum, fumarase; Mdh, malate dehydrogenase. Pi, inorganic phosphate.
In this study, we constructed a plasmid for establishing a heterologous glyoxylate bypass in R. sphaeroides. In addition to the wild-type strain, the heterologous glyoxylate bypass was introduced into an EMC pathway-disrupted mutant and a polyhydroxybutyrate (PHB) biosynthesis-disrupted mutant, the upstream biosynthetic pathway of which is shared with the EMC pathway (Fig. 1), and their H2 production from acetate and l-glutamate was evaluated. Through the comparisons made among these recombinant strains of R. sphaeroides, the role of acetate metabolism in photofermentative H2 production from acetate and l-glutamate is discussed.
RESULTS
Effects of EMC pathway disruption on H2 production from acetate and l-glutamate in R. sphaeroides.
We constructed a deletion mutant of the ccr (RSP_0960) gene encoding crotonyl-CoA carboxylase/reductase in a genetic background of R. sphaeroides wild-type strain 2.4.1 to confirm the role of the EMC pathway in H2 production from acetate and l-glutamate. First, the resulting strain (Δccr mutant) and the wild-type (WT) strain were grown photoheterotrophically in mineral medium containing 18 mM sodium bicarbonate for pH maintenance (16) supplemented with 25 mM acetate and 10 mM NH4Cl under an N2 atmosphere. Under those conditions, no H2 production was observed for WT and the Δccr mutant because of the repression of nitrogenase by ammonia (data not shown). As reported previously (27), the Δccr mutant barely grew (Fig. 2A), and there was no apparent consumption of acetate (Fig. 2B). When a plasmid carrying the ccr gene was introduced into the Δccr mutant, growth and acetate consumption were restored, as observed for the resulting Δccr(pMGccr) mutant strain with acetate only. These results confirmed that the EMC pathway is required for proper growth with acetate.
FIG 2.
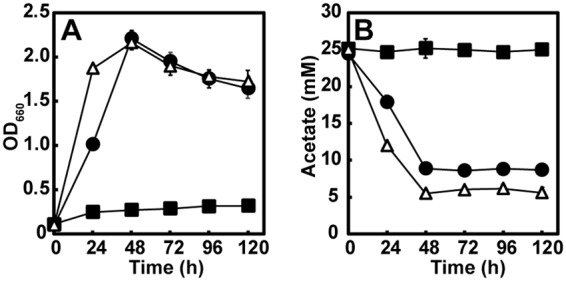
Photoheterotrophic growth of Rhodobacter sphaeroides wild-type strain 2.4.1 (WT), the EMC pathway-disrupted strain (Δccr mutant), and the ccr-complemented strain [Δccr(pMGccr) mutant] with acetate and NH4Cl under an N2 atmosphere. (A) Growth. (B) Acetate consumption. Each value represents the mean ± standard deviation of the results from at least three independent cultures; error bars that are not visible are smaller than the symbol. Black circle, WT; black square, Δccr mutant; open triangle, Δccr(pMGccr) mutant.
Next, to evaluate the effects of l-glutamate as the carbon source besides its effect as a poor nitrogen source for the derepression of nitrogenase, these strains were grown photoheterotrophically with 10 mM l-glutamate in the absence of acetate and NH4Cl under an Ar atmosphere. All the strains exhibited growth with a concomitant decrease in l-glutamate in the culture medium (see Fig. S1 in the supplemental material); however, no H2 production was observed during the 240-h culturing period (data not shown). These results indicate that R. sphaeroides photoheterotrophically utilizes l-glutamate as the carbon and the nitrogen sources, and l-glutamate alone is not sufficient for H2 production.
Next, the WT, Δccr mutant, and Δccr(pMGccr) mutant were grown photoheterotrophically with 25 mM acetate and 10 mM l-glutamate in the absence of NH4Cl, which represents nitrogenase-derepressing conditions. H2 production, optical density, acetate consumption, and l-glutamate consumption during WT, Δccr mutant, and Δccr(pMGccr) mutant culturing are shown in Fig. 3. The WT strain produced 0.94 ± 0.04 ml H2 per ml of culture after culturing for 240 h (Fig. 3A), and the strain completely consumed acetate after culturing for 144 h (Fig. 3C), whereas 28% of l-glutamate remained in the culture supernatant after 240 h (Fig. 3D). It should be noted that under photoheterotrophic growth conditions with acetate and NH4Cl (Fig. 2A), acetate was not completely consumed by the WT, although the maximal optical density was comparable to that under the conditions with acetate and l-glutamate in the absence of NH4Cl (Fig. 3C). These results suggest that additionally consumed acetate and l-glutamate were used for H2 production with the concurrent release of CO2. Despite the inability to grow photoheterotrophically with acetate and NH4Cl as described above, the Δccr mutant grew efficiently, with a concomitant decrease in acetate in the culture medium when l-glutamate was added (Fig. 3B and C). These results indicate that the Δccr mutant can assimilate acetate in the presence of l-glutamate independent of the EMC pathway; however, no H2 production was detected during the culturing of this mutant strain for 240 h, indicating that the EMC pathway is essential for H2 production of R. sphaeroides during photoheterotrophic growth with acetate and l-glutamate in the absence of NH4Cl. Although the optical density may not reflect the accurate cell number, the maximal optical density of the Δccr mutant was higher than that of the WT, despite the decreased consumption of acetate and l-glutamate by the EMC pathway-disrupted mutant; 41% of acetate and 83% of l-glutamate remained in the culture supernatant after culturing for 240 h. Since the Δccr mutant did not produce H2, the differences in substrate consumption between the WT and the Δccr mutant may reflect the cost for H2 production. However, the maximal optical density of the WT strain was higher than that in the case of growth with l-glutamate only (Fig. S1), suggesting that acetate is also consumed for growth in the WT strain during photoheterotrophic growth with acetate and l-glutamate. In the case of the Δccr(pMGccr) mutant, H2 production, acetate consumption, and l-glutamate consumption were similar to those in the case of WT, confirming that the EMC pathway is essential for H2 production from acetate and l-glutamate; however, the maximal optical density of the Δccr(pMGccr) mutant was lower than that of the WT.
FIG 3.
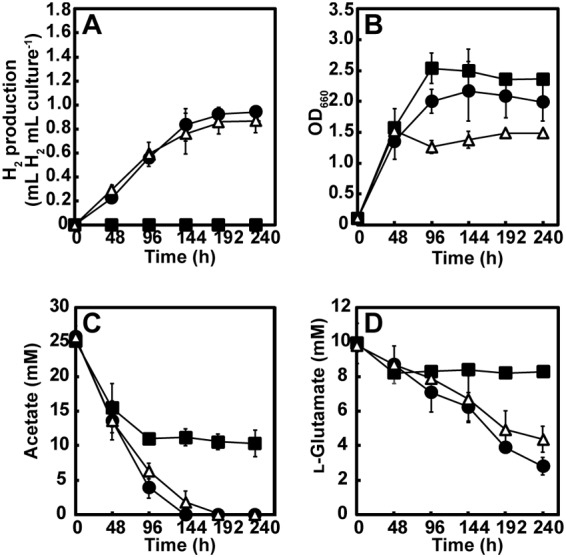
Photoheterotrophic growth of the R. sphaeroides wild-type strain 2.4.1 (WT), the EMC pathway-disrupted strain (Δccr mutant), and the ccr-complemented strain [Δccr(pMGccr) mutant] with acetate and l-glutamate in the absence of NH4Cl under an Ar atmosphere. (A) H2 production. (B) Growth. (C) Acetate consumption. (D) l-Glutamate consumption. Each value represents the mean ± standard deviation of the results from at least three independent cultures; error bars that are not visible are smaller than the symbol. Black circle, WT; black square, Δccr mutant; open triangle, Δccr(pMGccr) mutant.
Heterologous expression of the genes involved in the glyoxylate bypass derived from Rhodobacter capsulatus in R. sphaeroides.
To introduce the genes involved in the glyoxylate bypass into R. sphaeroides, we focused on aceA (RCAP_rcc03338) and aceB (RCAP_rcc03337), which encode isocitrate lyase and malate synthase, respectively, in the genome of R. capsulatus, which is phylogenetically related to R. sphaeroides (Fig. 4A). It should be noted that glcB (RSP_1980), a homolog of aceB, is present in the genome of R. sphaeroides. In addition, Mcl-1 and Mcl-2, involved in the lower part of the EMC pathway (Fig. 1A), catalyze apparent malate synthase activity; however, we chose to introduce both aceA and aceB because the introduction of aceA alone could possibly cause an accumulation of glyoxylate, which has been reported to be toxic to several bacteria (28, 29). Although R. capsulatus aceA is not expressed during growth with acetate (19), the isocitrate lyase activity of its gene product has been confirmed previously (30). Since the malate synthase activity of the R. capsulatus aceB (RcAceB) gene product has not been confirmed yet, we prepared the C-terminal His6-tagged RcAceB protein produced in Escherichia coli (Fig. S2) and analyzed its malate synthase activity. We observed that the C-terminal His6-tagged RcAceB protein exhibited malate synthase activity; the apparent KM and kcat for glyoxylate were 27 ± 6 μM and 9.1 ± 0.4 s−1, respectively, and the apparent KM and kcat for acetyl-CoA were 17 ± 2 μM and 8.6 ± 0.2 s−1, respectively. These results show that RcAceB is a functional malate synthase. Subsequently, we constructed a plasmid, pMGPaceBA, containing the R. capsulatus aceBA operon fused to a light-inducible puc promoter from R. capsulatus (Fig. 4B). The plasmid expressing glyoxylate bypass genes derived from R. capsulatus was introduced into R. sphaeroides to generate the WT(pMGPaceBA) strain. In the cell extracts of WT(pMGPaceBA) cultured in van Niel’s yeast medium under anaerobic-light conditions, isocitrate lyase and malate synthase activities were detected as 0.36 ± 0.04 μmol min−1 mg−1 and 0.61 ± 0.06 μmol min−1 mg−1, respectively. In contrast, these enzymatic activities were lower than 0.01 μmol min−1 mg−1 in cell extracts prepared from WT or the WT harboring pMGP as a plasmid control [WT(pMGP) mutant]. These results indicate that genes encoding key enzymes for the glyoxylate bypass derived from R. capsulatus were successfully expressed in R. sphaeroides. In addition, when WT(pMGPaceBA) was grown under aerobic-dark conditions, isocitrate lyase and malate synthase activities in the cell extract were 0.04 ± 0.01 μmol min−1 mg−1 and 0.20 ± 0.02 μmol min−1 mg−1, respectively. These results indicate that the puc promoter from R. capsulatus is actually upregulated under anaerobic-light conditions in R. sphaeroides.
FIG 4.
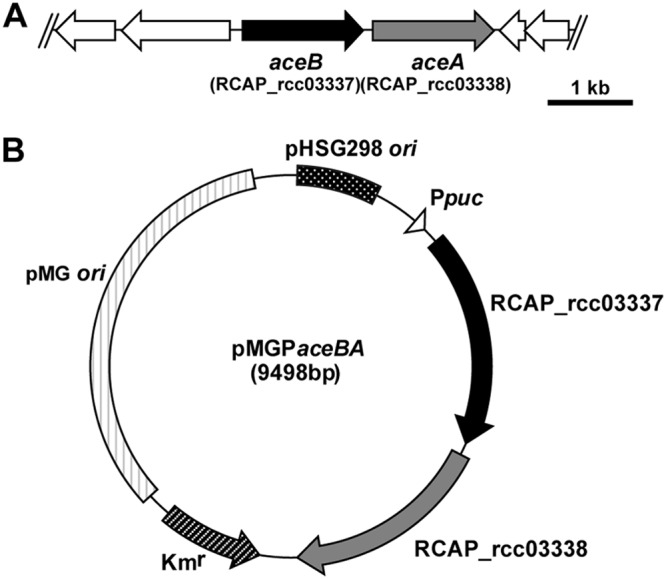
Construction of pMGPaceBA. (A) Orientation of aceA and aceB in the genome of R. capsulatus SB1003. Kyoto Encyclopedia of Genes and Genomes (KEGG) locus tags are shown in parentheses. (B) Schematic representation of pMGPaceBA. Ppuc denotes the puc promoter from R. capsulatus SB1003.
In vivo function of the heterologously expressed glyoxylate bypass in acetate assimilation.
Under photoheterotrophic growth conditions with acetate and NH4Cl, WT(pMGPaceBA) reached a higher optical density than WT(pMGP) for the first 48 h of culturing, although their maximal optical densities and acetate consumption were similar (Fig. 5A and B). To confirm the in vivo function of the heterologously expressed glyoxylate bypass genes in acetate assimilation, pMGPaceBA was introduced into the EMC pathway-disrupted Δccr mutant. The resulting strain [Δccr(pMGPaceBA) mutant] exhibited growth with a concomitant decrease in acetate in the culture medium, while the Δccr mutant harboring pMGP as a plasmid control [Δccr(pMGP) mutant] did not assimilate acetate (Fig. 5C and D). These results indicate that the heterologously expressed aceA and aceB genes derived from R. capsulatus function as glyoxylate bypass genes in R. sphaeroides for acetate assimilation.
FIG 5.
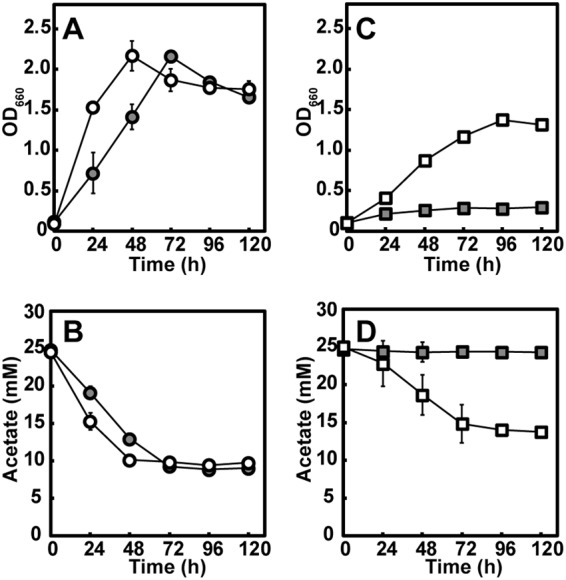
Photoheterotrophic growth of constructed R. sphaeroides strains expressing heterologous aceBA involved in the glyoxylate bypass with acetate and NH4Cl under an N2 atmosphere. (A and C) Growth. (B and D) Acetate consumption. Each value represents the mean ± standard deviation of the results from at least three independent cultures; error bars that are not visible are smaller than the symbol. Gray circle, wild-type strain carrying a vector plasmid [WT(pMGP)]; open circle, wild-type strain carrying a plasmid expressing aceBA [WT(pMGPaceBA)]; gray square, Δccr mutant carrying a vector plasmid [Δccr(pMGP) mutant]; open square, Δccr mutant carrying a plasmid expressing aceBA [Δccr(pMGPaceBA) mutant].
Effects of the glyoxylate bypass introduction on H2 production from acetate and l-glutamate.
H2 production of the R. sphaeroides strains expressing heterologous glyoxylate bypass genes was evaluated under photoheterotrophic growth conditions with acetate and l-glutamate in the absence of NH4Cl. The WT(pMGPaceBA) strain produced 1.54 ± 0.05 ml H2 per ml of culture, which corresponds to 175% of the amount of H2 produced by the WT(pMGP) strain (Fig. 6A). This result indicates that the addition of the glyoxylate bypass to R. sphaeroides, which also has an endogenous EMC pathway, enhances H2 production from acetate and l-glutamate. The WT(pMGPaceBA) strain produced 2.7-fold more H2 than the WT(pMGP) strain for the first 96 h of fermentation; the concomitant consumption of acetate as well as that of l-glutamate was also improved (Fig. 6C and D), and WT(pMGPaceBA) reached a higher optical density than did the WT(pMGP) strain (Fig. 6B).
FIG 6.
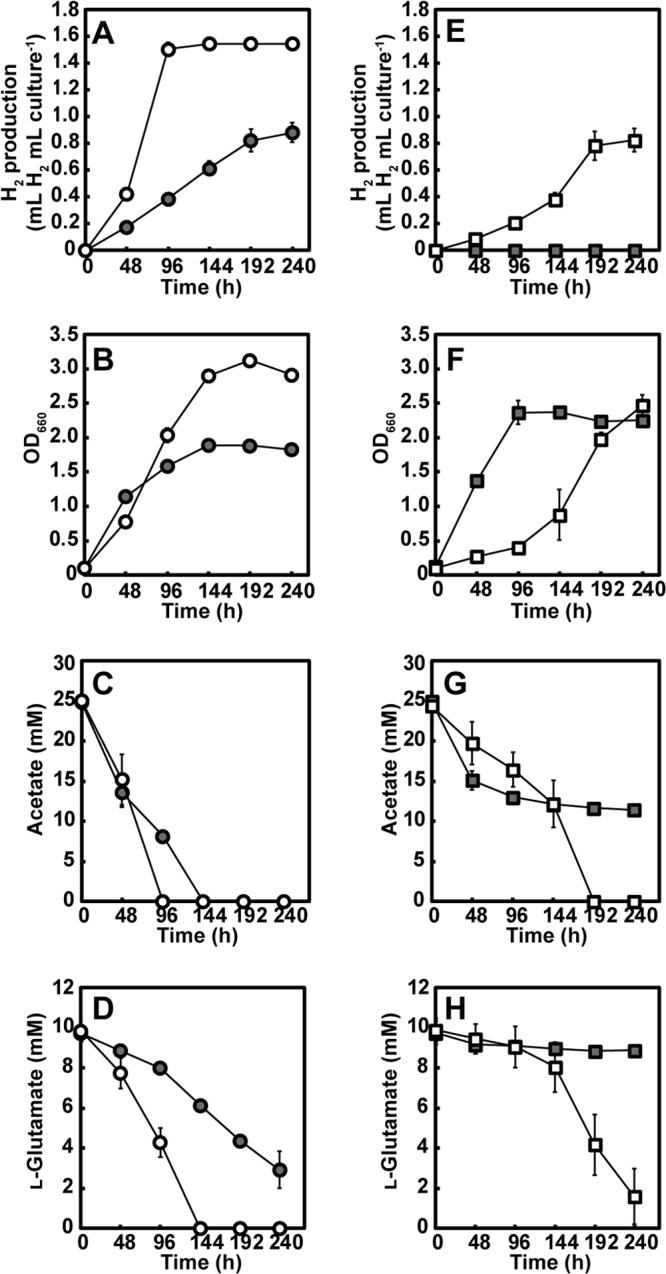
Photoheterotrophic growth of constructed R. sphaeroides strains expressing heterologous aceBA involved in the glyoxylate bypass with acetate and l-glutamate in the absence of NH4Cl under an Ar atmosphere. (A and E) H2 production. (B and F) Growth. (C and G) Acetate consumption. (D and H) l-Glutamate consumption. Each value represents the mean ± standard deviation of the results from at least three independent cultures; error bars that are not visible are smaller than the symbol. Gray circle, wild-type strain carrying a vector plasmid [WT(pMGP)]; open circle, wild-type strain carrying a vector plasmid [WT(pMGPaceBA)]; gray square, Δccr mutant carrying a vector plasmid [Δccr(pMGP) mutant]; open square, Δccr mutant carrying a plasmid expressing aceBA [Δccr(pMGPaceBA) mutant].
While the Δccr(pMGP) mutant did not produce H2, the Δccr(pMGPaceBA) mutant produced 0.82 ± 0.09 ml H2 per ml of culture (Fig. 6E), indicating that the introduced glyoxylate bypass genes function in H2 production from acetate and l-glutamate; however, the amount of H2 produced by the Δccr(pMGPaceBA) mutant was 53% of that produced by WT(pMGPaceBA). It was also noted that acetate consumption in the Δccr(pMGPaceBA) mutant was slower than that in WT(pMGPaceBA). These results indicate that the EMC pathway in WT(pMGPaceBA) also contributes to H2 production. Notably, the growth of the Δccr(pMGPaceBA) mutant was considerably delayed compared with that of the Δccr(pMGP) mutant (Fig. 6F), indicating that the introduction of the glyoxylate bypass impairs the acetate metabolic pathway mediating Δccr(pMGP) mutant growth with acetate and l-glutamate. The growth of the Δccr(pMGPaceBA) mutant was apparently exponential when a logarithmic scale was applied (data not shown), suggesting that the growth of the Δccr(pMGPaceBA) mutant is not dependent on suppressor mutations. During the late stage of fermentation, the optical density of the Δccr(pMGPaceBA) mutant markedly increased and reached a slightly higher level than that of the Δccr(pMGP) mutant. In accordance with these growth patterns and H2 production, acetate and l-glutamate consumption during Δccr(pMGPaceBA) mutant culturing was delayed, but it greatly accelerated at the later stages of fermentation, in contrast to much lower consumption of these substrates by the Δccr(pMGP) mutant (Fig. 6G and H).
Effects of the glyoxylate bypass introduction on PHB accumulation.
Since PHB biosynthesis has been proposed to compete with H2 production for reducing power (16), we analyzed PHB accumulation in the cells of the constructed strains during photoheterotrophic growth with acetate and l-glutamate in the absence of NH4Cl (Fig. 7). The WT strain accumulated PHB at 72 ± 8 and 95 ± 28 μg OD660−1 ml culture−1 (OD660, optical density at 660 nm) after 48-h and 96-h cultures, respectively, and the cellular content of PHB decreased to 38 ± 16 μg OD660−1 ml culture−1 after culturing for 240 h. The Δccr mutant accumulated amounts of PHB similar to those of the WT after 48 h and 96 h, but the content of PHB in Δccr mutant cells grown for 240 h was 1.6-fold higher than that in the WT cells, indicating that the disruption of ccr increases PHB accumulation. By the introduction of the glyoxylate bypass, the PHB accumulation in WT(pMGPaceBA) cells grown for 48 h, 96 h, and 240 h was 46%, 60%, and 92% lower, respectively, than that in the WT cells. Likewise, the amount of PHB accumulated in the Δccr(pMGPaceBA) mutant cells was lower than that in the Δccr mutant cells, indicating that introduction of the glyoxylate bypass represses PHB accumulation. However, a certain amount of PHB still accumulated in WT(pMGPaceBA) cells cultured for 48 h and 96 h.
FIG 7.
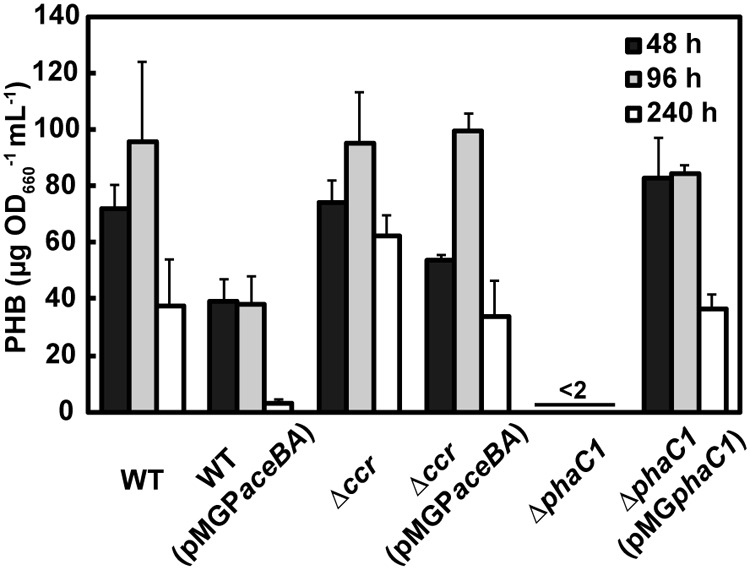
Polyhydroxybutyrate (PHB) accumulation in constructed R. sphaeroides strains during photoheterotrophic growth with acetate and l-glutamate in the absence of NH4Cl under an Ar atmosphere for 48 h (black bars), 96 h (gray bars), and 240 h (white bars). Each value represents the mean ± standard deviation of the results from at least three independent cultures.
Introduction of the glyoxylate bypass into a PHB-deficient mutant.
Since PHB accumulation was observed in WT(pMGPaceBA) during photoheterotrophic growth with acetate and l-glutamate, we attempted to examine whether a combination of the introduction of the glyoxylate bypass and disruption of the PHB biosynthesis improves H2 production. Although two PHB synthase genes, RSP_0382 and RSP_1257, are present in the genome of R. sphaeroides 2.4.1, the insertion of transposon Tn5 to RSP_0382 has been reported to eliminate PHB accumulation as well as PHB synthase activity during photoheterotrophic growth with acetate and l-glutamate (16). Thus, we constructed an in-frame deletion mutant of RSP_0382 (ΔphaC1) and analyzed its PHB accumulation during photoheterotrophic growth with acetate and l-glutamate in the absence of NH4Cl. No PHB accumulation was observed in the ΔphaC1 mutant, while its PHB accumulation was restored by the introduction of a plasmid carrying phaC1 (Fig. 7). These results show that phaC1 is actually essential for PHB accumulation in R. sphaeroides under the conditions used in this study. Subsequently, pMGP and pMGPaceBA were introduced into the ΔphaC1 mutant to generate the ΔphaC1(pMGP) and ΔphaC1(pMGPaceBA) mutants, respectively. Figure 8 details the H2 production, optical density, acetate consumption, and l-glutamate consumption during culturing of the ΔphaC1, ΔphaC1(pMGphaC1), ΔphaC1(pMGP), and ΔphaC(pMGPaceBA) mutant strains. In accordance with previous observations (16), phaC1 deletion apparently increased the growth yield and acetate consumption during photoheterotrophic growth with acetate and l-glutamate compared with that of the ΔphaC1(pMGphaC1) mutant (Fig. 8B and C compared with Fig. 3B and C). It should be noted that the results observed for the ΔphaC1(pMGphaC1) mutant were comparable to those for the WT (Fig. 3B and C). In addition, l-glutamate consumption significantly improved by phaC1 disruption (Fig. 8D compared with Fig. 3D). The ΔphaC1 and ΔphaC(pMGP) mutant strains produced 1.13 ± 0.15 ml H2 per ml culture and 1.15 ± 0.14 ml H2 per ml culture, respectively, corresponding to 120% and 130% of the WT and WT(pMGP) H2 production levels, respectively (Fig. 8A). While the ΔphaC1(pMGphaC1) mutant slowly produced H2 until the end of culturing, as observed for the WT, H2 production in the ΔphaC1 and ΔphaC1(pMGP) mutants plateaued early. Although the effects were moderate, these results are consistent with those from a previous report (16) that disruption of PHB biosynthesis enhances R. sphaeroides H2 production from acetate in the presence of l-glutamate. The ΔphaC1 mutant strain harboring a plasmid expressing the glyoxylate bypass genes, the ΔphaC1(pMGPaceBA) mutant, produced 1.53 ± 0.15 ml H2 per ml culture (Fig. 8A), which corresponds to 130% of the ΔphaC1(pMGP) H2 production. This result indicates that introduction of the glyoxylate bypass is also effective for H2 production in the genetic background of the ΔphaC1 mutant. However, no significant difference was observed in the amount of H2 production between the WT(pMGPaceBA) strain and the ΔphaC1(pMGPaceBA) mutant. It should be noted that H2 production in the ΔphaC1(pMGPaceBA) mutant was clearly slower than that in WT(pMGaceBA) in accordance with delayed growth and slowed substrate consumption. These results indicate that disruption of PHB biosynthesis was not effective for H2 production when the glyoxylate bypass was introduced.
FIG 8.
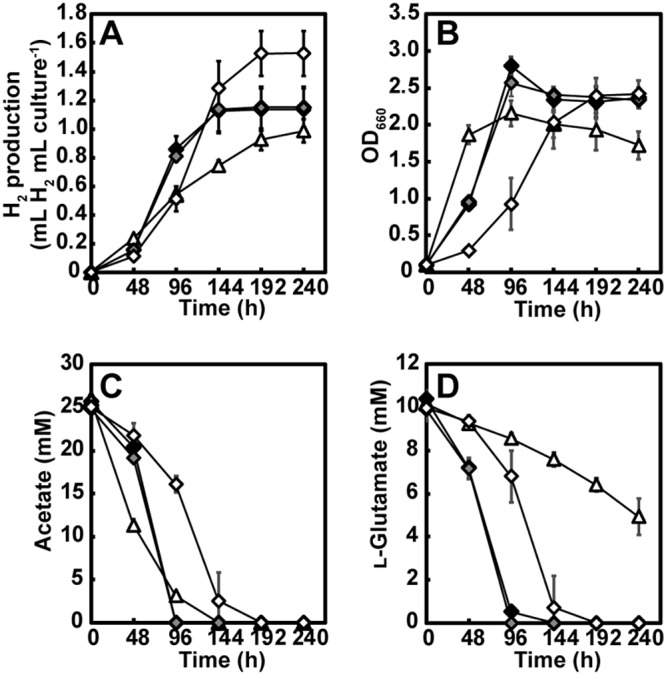
Photoheterotrophic growth of the PHB biosynthesis-deficient mutant, phaC1 complemented strain, and ΔphaC1 mutant expressing heterologous aceBA involved in the glyoxylate bypass with acetate and l-glutamate in the absence of NH4Cl under an Ar atmosphere. (A) H2 production. (B) Growth. (C) Acetate consumption. (D) l-Glutamate consumption. Each value represents the mean ± standard deviation of at least three independent cultures; error bars that are not visible are smaller than the symbol. Black diamond, ΔphaC1 mutant; open triangle, phaC1 mutant carrying a phaC1 complementation plasmid [ΔphaC1(pMGphaC1) mutant]; gray diamond, ΔphaC1 mutant carrying a vector plasmid [ΔphaC1(pMGP) mutant]; open diamond, ΔphaC1 mutant carrying a plasmid expressing aceBA [ΔphaC1(pMGPaceBA) mutant].
DISCUSSION
Figure 9 summarizes H2 yields from acetate and l-glutamate observed for the recombinant R. sphaeroides strains constructed in this study. Introduction of the glyoxylate bypass into the WT strain resulted in higher H2 yields than that into the genetic background of the mutant strain deficient in the EMC pathway (Δccr mutant), indicating that the introduced glyoxylate bypass and the endogenous EMC pathway additively enhanced the H2 yields in R. sphaeroides, at least under the conditions used in this study. Volumetric H2 productivity was significantly enhanced by the introduction of the glyoxylate bypass in the WT strain. It should be noted that the disruption of the EMC pathway abolished H2 production during photoheterotrophic growth with acetate and l-glutamate, and introduction of the glyoxylate bypass to the EMC pathway-disrupted mutant restored H2 production. Despite the differences in the enzymes and reducing equivalents required for acetate assimilation, both the EMC pathway and the glyoxylate bypass ultimately replenish the intermediates of the TCA cycle, which has been suggested as a major source of reducing power for H2 production (14, 25, 31). Thus, we suggest that the high H2 yields observed in WT(pMGPaceBA) are primarily due to an increase in the flux to the TCA cycle by a combination of the two anaplerotic pathways, even though the EMC pathway itself consumes reducing equivalents. This suggestion is supported by the reduced accumulation of PHB observed in WT(pMGPaceBA). In contrast, the glyoxylate bypass introduced in the Δccr mutant markedly delayed photoheterotrophic growth with acetate and l-glutamate. The endogenous EMC pathway-consuming reducing power may play a key role in adjusting the redox state for well-balanced photoheterotrophic growth with concomitant H2 evolution, as observed for WT(pMGPaceBA). However, the association with uncertain pathway(s) of acetate metabolism should be considered and are discussed later. On the other hand, H2 yields of WT(pMGPaceBA) were significantly higher than those of the ΔphaC1 mutant strain deficient in PHB biosynthesis, which has been suggested as one of the electron sinks competing with H2 production for the reducing power (16), and introduction of the glyoxylate bypass into the ΔphaC1 mutant strain resulted in an increase in the H2 yield to the same level as that in WT(pMGPaceBA) but slowed H2 production, as discussed later. Altogether, introduction of the glyoxylate bypass into the genetic background of the R. sphaeroides wild-type strain is an effective strategy for improving H2 yields from acetate in the presence of l-glutamate. Since another representative of PNSB, Rhodospirillum rubrum, has been reported to use the EMC pathway for acetate assimilation (32), it would be interesting to examine if introduction of the glyoxylate bypass to R. rubrum would also improve H2 yields from acetate and l-glutamate.
FIG 9.
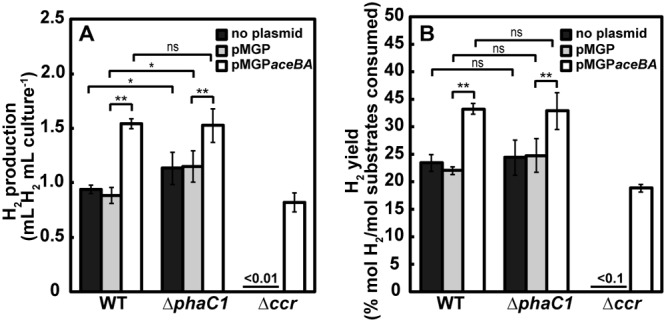
H2 production (A) and H2 yield (B) of constructed R. sphaeroides strains after 240 h of photoheterotrophic growth with acetate and l-glutamate in the absence of NH4Cl under an Ar atmosphere. Chromosomal genetic background of R. sphaeroides strains is indicated. Each bar is represented as follows: black bars, without plasmid; gray bars, pMGP as a vector control; white bars, pMGPaceBA for heterologous expression of aceBA, which is involved in the glyoxylate bypass. The molar amount of H2 produced was calculated from the volume of H2 produced based on the assumption that 1.00 ml of H2 is equal to 40.9 μmol. H2 yields from acetate and l-glutamate are represented as percentage of the theoretical maximal H2 yield from acetate (4.00 mol H2/mol) and l-glutamate (9.00 mol H2/mol) consumed. Results of Student’s t test are indicated as follows: ns, no significant difference; *, P < 0.05; **, P < 0.01.
Unexpectedly, disruption of the EMC pathway did not eliminate acetate consumption of R. sphaeroides during photoheterotrophic growth with acetate and l-glutamate. Moreover, the Δccr mutant accumulated PHB in amounts which were equivalent to 42% of the consumed acetate based on the assumption that the PHB monomer is derived from two molecules of acetate. Thus, acetate appears to be assimilated in the EMC pathway-disrupted mutant for cell growth during photoheterotrophic growth with acetate and l-glutamate. It is currently unclear how acetate is assimilated in the absence of the EMC pathway; however, l-glutamate is essential for acetate assimilation in the Δccr mutant, as acetate consumption was not observed in the absence of l-glutamate when ammonium salt was used as the sole nitrogen source. In addition to the EMC pathway, the citramalate cycle has been proposed as an anaplerotic pathway for acetate assimilation in R. rubrum (33), as well as R. sphaeroides (34). Since the EMC pathway has been shown to be essential for photoheterotrophic growth of R. sphaeroides with acetate (19), it is clear that the citramalate cycle alone is not sufficient for growth of R. sphaeroides with acetate as a sole carbon source; however, it may contribute to acetate assimilation when other carbon sources, such as l-glutamate, are available. Additionally, the involvement of glutaryl-CoA dehydrogenase (Rru_A2005) in acetate assimilation was recently proposed through the proteomic analysis of R. rubrum (32); a homolog (RSP_1295) showing 70% amino acid sequence identity with the R. rubrum glutaryl-CoA dehydrogenase is encoded in the R. sphaeroides genome. However, it is unlikely that acetate is assimilated via glutaryl-CoA dehydrogenase in the Δccr mutant because Ccr is required for the proposed acetate assimilation. Alternatively, it is possible that acetate is used solely for the anabolic reaction consuming acetyl-CoA, such as fatty acid biosynthesis and/or amino acid biosynthesis when l-glutamate is present, and any compounds converted from l-glutamate may be used for the condensation of acetyl-CoA. In any case, the acetate assimilation observed in the EMC pathway-disrupted mutant is clearly disadvantageous for H2 production despite the efficient growth observed. Since the same pathway(s) would also operate in the wild-type strain of R. sphaeroides during photoheterotrophic growth with acetate and l-glutamate, identification of the acetate-assimilating pathway(s) in the EMC pathway-disrupted mutant might lead to the discovery of novel target genes in R. sphaeroides that increase H2 yields from acetate and l-glutamate.
The glyoxylate bypass seems to be superior to the EMC pathway in terms of H2 production because of the differences in the reducing equivalents required for acetate assimilation; however, the H2 yields of the Δccr(pMGPaceBA) mutant were slightly lower than that of the WT strain. In the EMC pathway, the reduction of crotonyl-CoA catalyzed by Ccr is specific to NADPH (20). In addition, NADPH-dependent reduction of acetoacetyl-CoA is primarily responsible for the pathway in R. sphaeroides during photoheterotrophic growth with acetate and l-glutamate in the absence of ammonia (16), although NADH-dependent acetoacetyl-CoA reduction by (S)-3-hydroxybutyryl-CoA dehydrogenase is supposed to be functional under different conditions (35). Therefore, NADPH would be in excess by replacing the EMC pathway with the glyoxylate bypass. The RnfABCDGH complex, a membrane protein complex proposed to reduce ferredoxin in R. sphaeroides, mediates electron transfer to ferredoxin from NADH but not from NADPH (36). Thus, the lower H2 yields observed in the Δccr(pMGPaceBA) mutant presumably indicate inefficient electron transfer to nitrogenase from NADPH. Nevertheless, H2 yields in the WT strain and those in the Δccr(pMGPaceBA) mutant strain may not necessarily depend on the EMC pathway and the glyoxylate bypass, respectively, because there may be other acetate-assimilating pathway(s) in R. sphaeroides during photoheterotrophic growth with acetate and l-glutamate, as mentioned above. For accurate comparisons of the two acetate-assimilating pathways for H2 production, ammonium salt should be used as the sole nitrogen source instead of l-glutamate to omit any effects of the amino acid as a carbon source. To achieve this, construction of R. sphaeroides strains that express nitrogenase in the presence of ammonium salt would be required.
Since volumetric H2 production was apparently enhanced by the deletion of phaC1, PHB biosynthesis could be disadvantageous for H2 production by storing the carbon source as PHB (16), as well as by lowering the flux to the TCA cycle (15). However, no significant difference was observed in the H2 yields between the ΔphaC1 mutant and WT. In this context, PHB that accumulated in the WT cells at the end of culturing corresponds to only 6% of the total acetate consumed. Thus, at least under the conditions used in this study, the disruption of PHB biosynthesis increased the consumption of acetate and l-glutamate, whereas the efficiency of H2 production from the consumed substrates was not significantly improved. These observations also indicate that the reducing power stored as PHB may contribute to H2 production if the PHB stored is efficiently degraded; however, the reducing power consumed for cell growth is no longer available for H2 production. When the glyoxylate bypass was introduced, H2 production in the ΔphaC1(pMGPaceBA) mutant was clearly slower than that in WT(pMGPaceBA), owing to the growth delay. Thus, it is conceivable that temporal accumulation of PHB is rather beneficial in WT(pMGPaceBA) for H2 production by increasing fitness to the culture. It is noteworthy that a positive correlation between PHB accumulation and growth was also reported in R. rubrum (37) during photoheterotrophic growth with acetate. Nevertheless, it is currently unclear how PHB accumulation is regulated in R. sphaeroides. Moreover, the effects of a phaC1 deletion on intracellular metabolic flux during photoheterotrophic growth with acetate and l-glutamate are unknown. For a clear understanding of the role of PHB biosynthesis in H2 production, further analysis regarding the regulation of PHB accumulation and its role in intracellular redox states of R. sphaeroides is necessary.
Toward the establishment of photofermentative H2 production by PNSB, various approaches have been proposed to increase the H2 yields and/or production rates (25, 38), such as disruption of uptake hydrogenase (39), disruption of the Calvin cycle (40), and enhancement of the hydrogenase activity of nitrogenase (41). A combination of these approaches and the engineering of the acetate metabolic pathway presented in this study may lead to the development of high-yield photofermentative H2 production from acetate by R. sphaeroides. In parallel, further analysis regarding the regulation of nitrogen fixation, photosynthesis, PHB biosynthesis, the Calvin cycle, and the TCA cycle, as well as the cross talk among them, is also necessary to gain fundamental knowledge for guiding strain improvement.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture media.
The strains and plasmids used in this study are summarized in Table 1. R. sphaeroides 2.4.1 (ATCC 17023) and its mutant derivatives were photoheterotrophically precultured in 10 ml van Niel’s yeast medium (per liter; 10 g yeast extract, 0.5 g MgSO4⋅7H2O, and 1 g K2HPO4 [pH 7.0]) in a 33-ml rubber stopper-equipped tube purged with N2 at 30°C with continuous illumination (3,000 K fluorescent lamp, 7,000 lx). After 3 days of culture, cells were harvested by centrifugation at 20,400 × g for 5 min at 20°C and resuspended in 0.9% NaCl, followed by inoculation of defined mineral medium (pH 7.0) consisting of the following components per liter: 0.866 g KH2PO4, 0.733 g K2HPO4, 0.2 g MgSO4⋅7H2O, 0.075 g CaCl2⋅2H2O, 1.5 g NaHCO3, 1 ml metal solution (per 100 ml: 1 g ZnSO4·7H2O, 0.7 g FeSO4·7H2O, 0.15 g MnCl2·4H2O, 0.05 g CuSO4·5H2O, 0.02 g CoCl2·6H2O, 2.5 g EDTA·2Na, 0.075 g Na2MoO4·2H2O, and 0.01 g H3BO4), and 1 ml vitamin solution (per 100 ml: 1 mg biotin, 100 mg nicotinic acid, 20 mg thiamine-HCl, and 100 mg p-aminobenzoic acid), with carbon and/or nitrogen sources supplemented in the following combinations: (i) 25 mM sodium acetate and 10 mM NH4Cl; (ii) 10 mM sodium l-glutamate; and (iii) 25 mM sodium acetate and 10 mM sodium l-glutamate. For evaluation of photoheterotrophic growth with acetate and NH4Cl, headspace gas was replaced with N2. For evaluation of photoheterotrophic growth with l-glutamate or acetate and l-glutamate in the absence of NH4Cl, headspace gas was replaced with Ar. In all cases, the initial optical density at 660 nm was set to 0.1. For culturing of R. sphaeroides carrying plasmids, 20 μg ml−1 kanamycin was added. E. coli HST02 was used as a host for gene cloning and plasmid construction, and E. coli S17-1 was used as a donor for conjugation. E. coli BL21(DE3) was used for producing the recombinant enzyme. E. coli strains were grown in LB medium supplemented with 50 μg ml−1 kanamycin or 50 μg ml−1 ampicillin.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant feature(s)a | Reference or sourceb |
|---|---|---|
| Strains | ||
| Rhodobacter sphaeroides | ||
| 2.4.1 | WT strain | NBRC |
| Δccr mutant | In-frame deletion mutant of RSP_0960 | This study |
| ΔphaC1 mutant | In-frame deletion mutant of RSP_0382 | This study |
| Escherichia coli | ||
| HST02 | Cloning strain | TaKaRa |
| BL21(DE3) | Protein production strain | Novagen |
| S17-1 | Conjugation strain | NBRC |
| Plasmids | ||
| pUC18 | Cloning vector, Apr | TaKaRa |
| pET22b (+) | Expression vector in E. coli, Apr | Novagen |
| pETRcAceB | Production of C-terminal His6-tagged RcAceB, Apr | This study |
| pK19mobsacB | Mobilizable suicide vector for gene deletion, Kmr | 42 |
| pKDRsccr | pK19mobsacB backbone, deletion of RSP_0960, Kmr | This study |
| pKDRsphaC1 | pK19mobsacB backbone, deletion of RSP_0382, Kmr | This study |
| pMG170 | Shuttle vector stably maintained in R. sphaeroides, Kmr | 43 |
| pMG180 | lac promoter removed from pMG170, Kmr | This study |
| pMGccr | pMG180 backbone, RSP_0960 with 241-bp upstream region, Kmr | This study |
| pMGphaC1 | pMG180 backbone, RSP_0382 with 350-bp upstream region, Kmr | This study |
| pMGP | pMG180 backbone, puc promoter from R. capsulatus, Kmr | This study |
| pMGPaceBA | pMGP backbone, aceBA from R. capsulatus, Kmr | This study |
Apr, ampicillin resistance; Kmr, kanamycin resistance.
NBRC, National Institute of Technology and Evaluation (NITE) Biological Resource Center.
Construction of plasmids.
During the construction of plasmids, sequences of DNA fragments cloned into pUC18 or pMG derivatives were confirmed by sequencing with primers LSV-MCS-F (5ʹ-TTCCGGCTCGTATGTTGTG-3ʹ) and LSV-MCS-R (5ʹ-GATGTGCTGCAAGGCGATTAAG-3ʹ). For construction of the ccr (RSP_0960) deletion plasmid, a 3,506-bp fragment was amplified by PCR from the genomic DNA of R. sphaeroides (GenBank accession number GCA_000012905.2) using a pair of primers, RSP_ccr_u1120_Xba_Fw (5ʹ-GGGTCTAGACGGTATTTCTCTTCCTCGAT-3ʹ) and Rs_ccr_d1103_Eco_Rv (5ʹ-GGGGAATTCTCGTGTCGAACACCATCTTG-3ʹ), and then ligated into the XbaI-EcoRI restriction sites of pUC18 to generate pRs_ccr. Subsequently, inverse PCR was performed with a pair of 5ʹ-phosphorylated primers, Rs_ccr_1208_Fw (5ʹ-TACAGAAACCAGCACAAGCC-3ʹ) and Rs_ccr_30_Rv (5ʹ-GACGATATCGCTCTGCAC-3ʹ), with pRs_ccr as the template, and then the amplified fragment was self-ligated to generate pDRs_ccr, of which the 1,868-bp XbaI-EcoRI fragment was ligated into the corresponding sites of pK19mobsacB (42) to generate pKDRsccr. For construction of the phaC1 (RSP_0382) deletion plasmid, PCR was performed with genomic DNA of R. sphaeroides using Rs_phaC1_SphI_Fw (5ʹ-AATGCATGCAGTCGACGACGTAGAGCGTGT-3ʹ) and Rs_phaC1_EcoRI_Rv (5ʹ-ATTGAATTCTTGGCAGCCGTGGTCACGTCG-3ʹ). The amplified fragment was ligated into the SphI-EcoRI restriction sites of pUC18 to generate pRs_phaC1. Subsequently, inverse PCR was performed with a pair of 5ʹ-phosphorylated primers, Rs_phaC1_1803_Fw (5ʹ-TGAAGCCGAAGCCCAGACG-3ʹ) and Rs_phaC1_21_Rv (5ʹ-AGACTGCTCTTCGGTTGCCA-3ʹ), with pRs_phaC1 as the template, and then the amplified fragment was self-ligated to generate pDRs_phaC1, of which the 927-bp SphI-EcoRI-digested fragment was ligated into the corresponding sites of pK19mobsacB to generate pKDRsphaC1.
For gene complementation analysis, the lac promoter region was removed from pMG170 (43) by inverse PCR using a pair of 5ʹ-phosphorylated primers, MCS-7_Fw (5ʹ-GATTACGAATTCGAGCTCGG-3ʹ) and MCS-200_Rv (5ʹ-GCTGCATTAATGAATCGGCC-3ʹ), and the PCR product was self-ligated to generate pMG180. For complementation of the ccr deletion mutant, PCR was performed with genomic DNA of R. sphaeroides as the template using Rs_ccr_u640_Fw (5ʹ-GTCATCGAGGTGTTCATCTG-3ʹ) and Rs_ccr_3ʹ_Hind_Rv (5ʹ-GGGAAGCTTCTTGCGGATCGCTCCGATCA-3ʹ). The PCR product was digested with BamHI and HindIII and then ligated into corresponding sites of pMG180 to generate pMGccr. For complementation of the phaC1 deletion mutant, PCR was performed with genomic DNA of R. sphaeroides as the template using Rs_phaC1_u350_XbaI_Fw (5ʹ-GGGTCTAGACCGAAAGGCTTTTCCACCAC-3ʹ) and Rs_phaC1_3ʹ_XbaI_Rv (5ʹ-GGGTCTAGAGTCTGGGCTTCGGCTTCAAG-3ʹ). The PCR product was digested with XbaI and then ligated into corresponding sites of pMG180 to generate pMGphaC1.
To produce recombinant RcAceB, PCR was performed using a pair of primers, Rc_aceB_Nde_Fw (5ʹ-GGCCCCATATGTCGCTGGATTGCCCATC-3ʹ) and Rc_aceB_Xho_Rv (5ʹ-GGCCCCTCGAGGTCGAGCGCGTTCAAAACCG-3ʹ), with R. capsulatus genomic DNA (GenBank accession number GCA_000021865.1) as the template. The amplified fragment was digested with NdeI and XhoI and ligated into the corresponding restriction sites of pET22b to generate pETRcAceB.
To construct a plasmid for R. capsulatus aceBA expression in R. sphaeroides, PCR was performed using a pair of primers, MCS-7_Fw (5ʹ-GATTACGAATTCGAGCTCGG-3ʹ) and MCS-200_Rv (5ʹ-GCTGCATTAATGAATCGGCC-3ʹ), with pMG170 (43) as the template. Additionally, the light-inducible puc promoter (44) from R. capsulatus SB1003 was amplified by a pair of 5ʹ-phosphorylated primers, Ppuc_Fw (5ʹ-TTTGCAGCATGGCTCTTGC-3ʹ) and Ppuc_Rv (5ʹ-TGTGATCCGACCTCGTGAGA-3ʹ), from the genomic DNA of R. capsulatus SB1003. Amplified fragments were ligated to generate pMGP. Subsequently, the aceBA gene from R. capsulatus SB1003 was amplified by a pair of primers, Rc_aceB_u12_BamHI_Fw (5ʹ-GGGGGATCCGAAAGGAACCCCATGTCG-3ʹ) and Rc_aceA_XbaI_Rv (5ʹ-GGGTCTAGACCCTTGCTTCACTCTGCC-3ʹ), from the genomic DNA of R. capsulatus SB1003 and cloned into the BamHI-XbaI restriction sites of pMGP to generate pMGPaceBA.
Production and purification of recombinant C-terminal His6-tagged RcAceB.
E. coli BL21(DE3) cells harboring pETRcAceB were cultured in 100 ml of LB medium supplemented with 50 μg ml−1 ampicillin at 37°C until the OD610 reached 0.5, followed by addition of isopropyl-β-d-thiogalactopyranoside to the culture medium at a final concentration of 0.1 mM, and then the culture was continued at 25°C for an additional 12 h. Cells were harvested by centrifugation at 3,000 × g for 10 min at 4°C, washed with buffer A (20 mM Tris HCl [pH 8.0] and 150 mM NaCl), and then resuspended in the same buffer. Cells were disrupted by sonication (20 kHz, 2-s pulse with 10-s intervals, for a total of 10 min) on ice using the Astrason ultrasonic processor XL2020 (QSonica), and then cell debris was removed by centrifugation at 20,400 × g for 15 min at 4°C. The supernatant was loaded onto HisTrap crude FF columns (1 ml; GE Healthcare) and then washed with 10 ml buffer A containing 20 mM imidazole. RcAceB with a C-terminal hexahistidine tag was eluted with buffer A containing 500 mM imidazole and then dialyzed against buffer A containing 50% glycerol and 1 mM dithiothreitol (DTT) for storage at −20°C until further use.
Enzyme assays.
Extracts were prepared from R. sphaeroides cultured in van Niel’s yeast medium that was grown to stationary phase (72 h for anaerobic cultures, 48 h for aerobic culture). Cells collected from 2 ml of culture were washed once with 1 ml of 100 mM potassium phosphate (pH 7.0) containing 10% (vol/vol) glycerol and then resuspended in 0.7 ml of the same buffer. Cells were disrupted by sonication (20 kHz, 2-s pulse with 10-s intervals, for a total of 15 min) using Bioruptor UCD-250 (Cosmo Bio) at 4°C. After centrifugation (20,400 × g, 15 min, and 4°C), the supernatant was used for enzyme assays. Protein concentrations were determined by protein assay dye reagent (Bio-Rad) with bovine serum albumin as a standard, according to the manufacturer’s instructions.
Isocitrate lyase activity was measured at 30°C using a DU800 spectrophotometer (Beckman) in 1-ml reaction mixtures consisting of 100 mM sodium phosphate (pH 7.0), 5 mM dl-isocitrate, 5 mM MgSO4, and 3 mM phenylhydrazine hydrochloride. The reaction was started by the addition of cell extract containing 50 μg protein; the increase in absorbance at 324 nm by glyoxylate phenylhydrazone formation (45) (experimentally determined molar extinction coefficient at pH 7.0, 16,400 cm−1) was recorded for 2 min.
Malate synthase activity was measured at 30°C using the DU800 spectrophotometer in 1-ml reaction mixtures consisting of 100 mM potassium phosphate (pH 7.0), 5 mM MgSO4, 1 mM glyoxylate, 0.2 mM acetyl-CoA, and 0.15 mM 2,6-dichlorophenolindophenol. The reaction was started by adding cell extract containing 50 μg protein; the decrease in absorbance at 600 nm by the reduction of 2,6-dichlorophenolindophenol (experimentally determined molar extinction coefficient at pH 7.0, 16,800 cm−1) associated with formation of CoA (46) was recorded for 2 min. To subtract glyoxylate-independent formation of CoA, blank experiments were performed with the above-mentioned reaction mixture omitting glyoxylate.
The kinetic parameters of RcAceB using a fixed concentration of acetyl-CoA and various concentrations of glyoxylate were measured in 1-ml reaction mixture composed of 100 mM potassium phosphate (pH 7.0), 5 mM MgSO4, 0.2 mM acetyl-CoA, 0.15 mM 2,6-dichlorophenolindophenol, 1 μg RcAceB, and 10 to 1,000 μM glyoxylate. The kinetic parameters of RcAceB using a fixed concentration of glyoxylate and various concentrations of acetyl-CoA were measured in the same buffer as described above but with a fixed concentration of 1 mM glyoxylate and various concentrations of acetyl-CoA (5 to 400 μM). The data obtained were fit to the Michaelis-Menten equation using the nonlinear regression tool of KaleidaGraph 3.5 J (Hulinks, Inc.).
Gene manipulation of R. sphaeroides.
R. sphaeroides deletion mutants were constructed using a previously described method, with slight modifications (47). E. coli S17-1 harboring pKDRsccr or pKDRsphaC1 was grown at 33°C in LB medium containing 50 μg ml−1 kanamycin until the OD610 reached 0.5. Cells were harvested from 500 μl of culture by centrifugation at 20,400 × g for 1 min at 20°C and then washed twice with 1 ml van Niel’s yeast broth. E. coli cells were mixed with 500 μl of an overnight van Niel’s culture of R. sphaeroides, and then the mixture of cells was resuspended in 30 μl van Niel’s yeast broth. The cell suspension was dropped onto a nitrocellulose membrane (Merck Millipore) on van Niel’s yeast agar plate, and then conjugation was performed aerobically overnight at 30°C. Single-crossover candidates were selected for aerobically on a van Niel’s yeast agar plate containing 20 μg ml−1 kanamycin and 10 μg ml−1 potassium tellurite at 30°C, followed by the selection of double-crossover candidates performed on a van Niel’s yeast agar plate containing 10% (wt/vol) sucrose. In-frame deletion of ccr (RSP_0960), of 1,158 bp out of 1,293 bp, was confirmed by PCR and sequencing using Rs_ccr_u1480_Fw (5ʹ-ATGTAGTCGATGGCCTCGAT-3ʹ) and Rs_ccr_d1439_Rv (5ʹ-ATCCGCTTCTTGCGGTGCTT-3ʹ), corresponding to 420 bp upstream and 346 bp downstream, respectively, of the homologous region used for recombination. In-frame deletion of phaC1 (RSP_0382), of 1,782 bp out of 1,806 bp, was confirmed by PCR and sequencing using Rs Rs_phaC1_u890_Fw (5ʹ-CATGGAGATGAAGCTCTGAA-3ʹ) and Rs_phaC1_d914_Rv (5ʹ-ATGATCTGGAGGAGATATTG-3ʹ), corresponding to 299 bp upstream and 316 bp downstream, respectively, of the homologous region used for recombination.
Transformation of R. sphaeroides strains for the introduction of a replicating plasmid was carried out using electroporation, as described previously (43).
Evaluation of H2 production and substrate consumption.
Hydrogen was quantified using a GC-2014 gas chromatograph (Shimadzu) equipped with a thermal conductivity detector and molecular sieve-13X 60/80 (GL Sciences). Argon was used as the carrier gas at a flow rate of 35 ml min−1. The oven temperature was set to 50°C, the injection temperature was 100°C, and the detector temperature was 100°C. Concentrations of acetate and l-glutamate in culture supernatants were analyzed using a Nextera XR ultrahigh-performance liquid chromatograph (Shimadzu) equipped with a photodiode detector. Samples were separated by a Synergi Hydro-RP-HST column (Phenomenex) in 20 mM phosphate at a flow rate of 0.4 ml min−1 at 40°C, and acetate and l-glutamate were quantified by measuring the absorbance at 210 nm.
Quantification of PHB.
Cells in 0.5 ml of culture were withdrawn at 48, 96, and 240 h after inoculation, collected by centrifugation (20,400 × g for 5 min at 4°C), and washed with 1 ml deionized water, followed by digestion in 1 ml of 5% (vol/vol) sodium hypochlorite solution at 37°C for 2 h. After centrifugation (20,400 × g for 10 min at 4°C), the resulting pellet was washed once with 1 ml deionized water, followed by a wash with 1 ml of 1:1 alcohol-acetone, and then dried in vacuo. After the pellet was resuspended in 1 ml concentrated sulfuric acid, the solution was transferred to a glass tube and heated at 100°C for 15 min. PHB was spectrophotometrically quantified as crotonic acid, according to a previous report (48), using poly[(R)-3-hydroxybutyric acid] (Sigma-Aldrich) as a standard. For each sample, the amount of PHB was normalized by optical density at 660 nm recorded for each time point.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by grants from the Ministry of Economy, Trade and Industry (METI), Japan.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01873-18.
REFERENCES
- 1.Hillmer P, Gest H. 1977. H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: H2 production by growing cultures. J Bacteriol 129:724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gest H, Kamen MD. 1949. Photoproduction of molecular hydrogen by Rhodospirillum rubrum. Science 109:558–559. doi: 10.1126/science.109.2840.558. [DOI] [PubMed] [Google Scholar]
- 3.Prince RC, Kheshgi HS. 2005. The photobiological production of hydrogen: potential efficiency and effectiveness as a renewable fuel. Crit Rev Microbiol 31:19–31. doi: 10.1080/10408410590912961. [DOI] [PubMed] [Google Scholar]
- 4.Rivera-Ortiz JM, Burris RH. 1975. Interactions among substrates and inhibitors of nitrogenase. J Bacteriol 123:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida A, Nishimura T, Kawaguchi H, Inui M, Yukawa H. 2005. Enhanced hydrogen production from formic acid by formate hydrogen lyase-overexpressing Escherichia coli strains. Appl Environ Microbiol 71:6762–6768. doi: 10.1128/AEM.71.11.6762-6768.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren N, Guo W, Liu B, Cao G, Ding J. 2011. Biological hydrogen production by dark fermentation: challenges and prospects towards scaled-up production. Curr Opin Biotechnol 22:365–370. doi: 10.1016/j.copbio.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Ding J, Liu BF, Ren NQ, Xing DF, Guo WQ, Xu JF, Xie GJ. 2009. Hydrogen production from glucose by co-culture of Clostridium butyricum and immobilized Rhodopseudomonas faecalis RLD-53. Int J Hydrogen Energy 34:3647–3652. doi: 10.1016/j.ijhydene.2009.02.078. [DOI] [Google Scholar]
- 8.Fang HHP, Zhu H, Zhang T. 2006. Phototrophic hydrogen production from glucose by pure and co-culture of Clostridium butyricum and Rhodobacter sphaeroides. Int J Hydrogen Energy 31:2223–2230. doi: 10.1016/j.ijhydene.2006.03.005. [DOI] [Google Scholar]
- 9.Alalayah WM, Kalil MS, Kadhum AA, Jahim JM, Jaapar SZ, Alauj NM. 2009. Bio-hydrogen production using a two-stage fermentation process. Pak J Biol Sci 12:1462–1467. doi: 10.3923/pjbs.2009.1462.1467. [DOI] [PubMed] [Google Scholar]
- 10.Zagrodnik R, Laniecki M. 2015. The role of pH control on biohydrogen production by single stage hybrid dark- and photo-fermentation. Bioresour Technol 194:187–195. doi: 10.1016/j.biortech.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Barbosa MJ, Rocha JM, Tramper J, Wijffels RH. 2001. Acetate as a carbon source for hydrogen production by photosynthetic bacteria. J Biotechnol 85:25–33. doi: 10.1016/S0168-1656(00)00368-0. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson SJ, Jackson JB, McEwan AG. 1987. Anaerobic respiration in the Rhodospirillaceae: characterisation of pathways and evaluation of roles in redox balancing during photosynthesis. FEMS Microbiol Lett 46:117–143. doi: 10.1111/j.1574-6968.1987.tb02455.x. [DOI] [Google Scholar]
- 13.Koku H, Eroğlu İ, Gündüz U, Yücel M, Türker L. 2002. Aspects of the metabolism of hydrogen production by Rhodobacter sphaeroides. Int J Hydrogen Energy 27:1315–1329. doi: 10.1016/S0360-3199(02)00127-1. [DOI] [Google Scholar]
- 14.Kontur WS, Ziegelhoffer EC, Spero MA, Imam S, Noguera DR, Donohue TJ. 2011. Pathways involved in reductant distribution during photobiological H2 production by Rhodobacter sphaeroides. Appl Environ Microbiol 77:7425–7429. doi: 10.1128/AEM.05273-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao Y, Liu D, Yan X, Zhou Z, Lee JK, Yang C. 2012. Network identification and flux quantification of glucose metabolism in Rhodobacter sphaeroides under photoheterotrophic H2-producing conditions. J Bacteriol 194:274–283. doi: 10.1128/JB.05624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hustede E, Steinbüchel A, Schlegel HG. 1993. Relationship between the photoproduction of hydrogen and the accumulation of PHB in non-sulphur purple bacteria. Appl Microbiol Biotechnol 39:87–93. doi: 10.1007/BF00166854. [DOI] [Google Scholar]
- 17.Kobayashi J, Yoshimune K, Komoriya T, Kohno H. 2011. Efficient hydrogen production from acetate through isolated Rhodobacter sphaeroides. J Biosci Bioeng 112:602–605. doi: 10.1016/j.jbiosc.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Albers H, Gottschalk G. 1976. Acetate metabolism in Rhodopseudomonas gelatinosa and several other Rhodospirillaceae. Arch Microbiol 111:45–49. doi: 10.1007/BF00446548. [DOI] [PubMed] [Google Scholar]
- 19.Alber BE, Spanheimer R, Ebenau-Jehle C, Fuchs G. 2006. Study of an alternate glyoxylate cycle for acetate assimilation by Rhodobacter sphaeroides. Mol Microbiol 61:297–309. doi: 10.1111/j.1365-2958.2006.05238.x. [DOI] [PubMed] [Google Scholar]
- 20.Erb TJ, Berg IA, Brecht V, Muller M, Fuchs G, Alber BE. 2007. Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: the ethylmalonyl-CoA pathway. Proc Natl Acad Sci U S A 104:10631–10636. doi: 10.1073/pnas.0702791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erb TJ, Fuchs G, Alber BE. 2009. (2S)-Methylsuccinyl-CoA dehydrogenase closes the ethylmalonyl-CoA pathway for acetyl-CoA assimilation. Mol Microbiol 73:992–1008. doi: 10.1111/j.1365-2958.2009.06837.x. [DOI] [PubMed] [Google Scholar]
- 22.Erb TJ, Retey J, Fuchs G, Alber BE. 2008. Ethylmalonyl-CoA mutase from Rhodobacter sphaeroides defines a new subclade of coenzyme B12-dependent acyl-CoA mutases. J Biol Chem 283:32283–32293. doi: 10.1074/jbc.M805527200. [DOI] [PubMed] [Google Scholar]
- 23.Laguna R, Tabita FR, Alber BE. 2011. Acetate-dependent photoheterotrophic growth and the differential requirement for the Calvin-Benson-Bassham reductive pentose phosphate cycle in Rhodobacter sphaeroides and Rhodopseudomonas palustris. Arch Microbiol 193:151–154. doi: 10.1007/s00203-010-0652-y. [DOI] [PubMed] [Google Scholar]
- 24.Hädicke O, Grammel H, Klamt S. 2011. Metabolic network modeling of redox balancing and biohydrogen production in purple nonsulfur bacteria. BMC Syst Biol 5:150. doi: 10.1186/1752-0509-5-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinlay JB, Harwood CS. 2011. Calvin cycle flux, pathway constraints, and substrate oxidation state together determine the H2 biofuel yield in photoheterotrophic bacteria. mBio 2:e00323-10. doi: 10.1128/mBio.00323-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kars G, Gündüz U. 2010. Towards a super H2 producer: Improvements in photofermentative biohydrogen production by genetic manipulations. Int J Hydrogen Energy 35:6646–6656. doi: 10.1016/j.ijhydene.2010.04.037. [DOI] [Google Scholar]
- 27.Schneider K, Asao M, Carter MS, Alber BE. 2012. Rhodobacter sphaeroides uses a reductive route via propionyl coenzyme A to assimilate 3-hydroxypropionate. J Bacteriol 194:225–232. doi: 10.1128/JB.05959-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui J, Good NM, Hu B, Yang J, Wang Q, Sadilek M, Yang S. 2016. Metabolomics revealed an association of metabolite changes and defective growth in Methylobacterium extorquens AM1 overexpressing ecm during growth on methanol. PLoS One 11:e0154043. doi: 10.1371/journal.pone.0154043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puckett S, Trujillo C, Wang Z, Eoh H, Ioerger TR, Krieger I, Sacchettini J, Schnappinger D, Rhee KY, Ehrt S. 2017. Glyoxylate detoxification is an essential function of malate synthase required for carbon assimilation in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 114:E2225–E2232. doi: 10.1073/pnas.1617655114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meister M, Saum S, Alber BE, Fuchs G. 2005. l-Malyl-coenzyme A/beta-methylmalyl-coenzyme A lyase is involved in acetate assimilation of the isocitrate lyase-negative bacterium Rhodobacter capsulatus. J Bacteriol 187:1415–1425. doi: 10.1128/JB.187.4.1415-1425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinlay JB, Oda Y, Ruhl M, Posto AL, Sauer U, Harwood CS. 2014. Non-growing Rhodopseudomonas palustris increases the hydrogen gas yield from acetate by shifting from the glyoxylate shunt to the tricarboxylic acid cycle. J Biol Chem 289:1960–1970. doi: 10.1074/jbc.M113.527515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leroy B, De Meur Q, Moulin C, Wegria G, Wattiez R. 2015. New insight into the photoheterotrophic growth of the isocytrate lyase-lacking purple bacterium Rhodospirillum rubrum on acetate. Microbiology 161:1061–1072. doi: 10.1099/mic.0.000067. [DOI] [PubMed] [Google Scholar]
- 33.Ivanovsky RN, Krasilnikova EN, Berg IA. 1997. A proposed citramalate cycle for acetate assimilation in the purple non-sulfur bacterium Rhodospirillum rubrum. FEMS Microbiology Lett 153:399–404. doi: 10.1016/S0378-1097(97)00280-2. [DOI] [Google Scholar]
- 34.Filatova LV, Berg IA, Krasil’nikova EN, Ivanovsky RN. 2005. A study of the mechanism of acetate assimilation in purple nonsulfur bacteria lacking the glyoxylate shunt: enzymes of the citramalate cycle in Rhodobacter sphaeroides. Microbiology 74:270–278. doi: 10.1007/s11021-005-0062-3. [DOI] [PubMed] [Google Scholar]
- 35.Fales L, Kryszak L, Zeilstra-Ryalls J. 2001. Control of hemA expression in Rhodobacter sphaeroides 2.4.1: effect of a transposon insertion in the hbdA gene. J Bacteriol 183:1568–1576. doi: 10.1128/JB.183.5.1568-1576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biegel E, Schmidt S, González JM, Müller V. 2011. Biochemistry, evolution and physiological function of the Rnf complex, a novel ion-motive electron transport complex in prokaryotes. Cell Mol Life Sci 68:613–634. doi: 10.1007/s00018-010-0555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin H, Nikolau BJ. 2012. Role of genetic redundancy in polyhydroxyalkanoate (PHA) polymerases in PHA biosynthesis in Rhodospirillum rubrum. J Bacteriol 194:5522–5529. doi: 10.1128/JB.01111-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kontur WS, Noguera DR, Donohue TJ. 2012. Maximizing reductant flow into microbial H2 production. Curr Opin Biotechnol 23:382–389. doi: 10.1016/j.copbio.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Lee IH, Park JY, Kho DH, Kim MS, Lee JK. 2002. Reductive effect of H2 uptake and poly-beta-hydroxybutyrate formation on nitrogenase-mediated H2 accumulation of Rhodobacter sphaeroides according to light intensity. Appl Microbiol Biotechnol 60:147–153. doi: 10.1007/s00253-002-1097-2. [DOI] [PubMed] [Google Scholar]
- 40.McKinlay JB, Harwood CS. 2010. Carbon dioxide fixation as a central redox cofactor recycling mechanism in bacteria. Proc Natl Acad Sci U S A 107:11669–11675. doi: 10.1073/pnas.1006175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barahona E, Jimenez-Vicente E, Rubio LM. 2016. Hydrogen overproducing nitrogenases obtained by random mutagenesis and high-throughput screening. Sci Rep 6:38291. doi: 10.1038/srep38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schäfer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 43.Inui M, Nakata K, Roh JH, Vertes AA, Yukawa H. 2003. Isolation and molecular characterization of pMG160, a mobilizable cryptic plasmid from Rhodobacter blasticus. Appl Environ Microbiol 69:725–733. doi: 10.1128/AEM.69.2.725-733.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LeBlanc HN, Beatty JT. 1993. Rhodobacter capsulatus puc operon: promoter location, transcript sizes and effects of deletions on photosynthetic growth. J Gen Microbiol 139:101–109. doi: 10.1099/00221287-139-1-101. [DOI] [PubMed] [Google Scholar]
- 45.Dixon GH, Kornberg HL. 1959. Assay methods for key enzymes of the glyoxylate cycle. Biochem J 72:3. [Google Scholar]
- 46.Andi B, West AH, Cook PF. 2004. Stabilization and characterization of histidine-tagged homocitrate synthase from Saccharomyces cerevisiae. Arch Biochem Biophys 421:243–254. doi: 10.1016/j.abb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Chi SC, Mothersole DJ, Dilbeck P, Niedzwiedzki DM, Zhang H, Qian P, Vasilev C, Grayson KJ, Jackson PJ, Martin EC, Li Y, Holten D, Neil Hunter C. 2015. Assembly of functional photosystem complexes in Rhodobacter sphaeroides incorporating carotenoids from the spirilloxanthin pathway. Biochim Biophys Acta 1847:189–201. doi: 10.1016/j.bbabio.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Law JH, Slepecky RA. 1961. Assay of poly-beta-hydroxybutyric acid. J Bacteriol 82:33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.