Abstract
Purpose
Frontal bone deformities can be acquired due to trauma or ablative tumor resection surgeries and osteomyelitis. It may also occur due to congenital malformations. Repair of these defects have long been a challenge to oral and maxillofacial surgeons. We report our experience in the reconstruction of acquired frontal bone defects by titanium mesh implant.
Patients and Methods
Titanium mesh was used for reconstruction in 35 patients (18–55 years age-group) (34 males and 01 females) of acquired frontal bone defects secondary to trauma (RTA). All these patients have been referred to author by Department of Neurosurgery of the institute of Affiliation.
Results
All these cases acquired defects as a result of trauma. Follow-up ranged from 12 to 18 months after the reconstruction. Patients were followed up for the progress of healing, stability of implants, infection, wound dehiscence, discharging sinus, exposure of implants, collections, patient satisfaction regarding esthetics and reaction to thermal changes. No postoperative complications were found.
Conclusion
In reconstruction of frontal bone defects, titanium mesh gives satisfactory results.
Keywords: Frontal bone defects, Reconstructions, Titanium implants
Introduction
The primary function of the skull is to house and protect the brain along with important components of the central nervous system. It is, therefore, imperative to correct and reconstruct any loss in the continuity of this protective shield.
Any sizeable defect in this bony covering is far from ideal and leaves the patient vulnerable to external forces. Moreover, the loss of the bony framework in the frontal region influences the esthetics that has a direct bearing on the personality of the individual.
Frontal bone deformities as sequel to trauma may be as high as 70% [1]. The other causes are congenital malformations, ablative tumor resection surgeries and osteomyelitis. Repair of these defects have long been a challenge to surgeons dealing with reconstruction in which an effort is made to return the patient to some semblance of normality. The goal of reconstruction is to achieve a lifelong stable structural reconstruction of the cranium covered by a healthy skin and scalp flap.
Cranioplasty per se and repair of frontal bone defects in particular remains a difficult procedure for all facial surgeons, particularly when concerning the reconstruction of large lacunae in the skull. Considering the significant clinical and economic impact of the procedure, the search for materials and strategies to provide more comfortable and reliable surgical procedures is one of the most important challenges faced by modern craniofacial surgery [2].
While assessing the reconstructive options in a patient who has a frontal bone deformity, one must consider timing of the reconstruction, location of the defect, biomaterial, medical history and surgical technique.
The various materials and methods are available which includes autologous bone, biomaterial as bioceramics, hydroxyapatite, polyetherketone, polymethylmethacrylate (PMMA), porous polyethelyene and titanium (Ti).
A diverse range of techniques are available for reconstruction of full-thickness defects including intraoperative adaptation of implants and/or custom made implants either by preoperative CAD–CAM or stereolithography [3, 4].
The goal of this study was to demonstrate our use of titanium mesh regarding its clinical efficacy and safety for the reconstruction of the skull defects.
Material and Method
Study Design
Retrospective, observational and descriptive.
Duration
April 2013 to April 2017.
Inclusion Criteria
Thirty-five adult male patients (age range 18–55 years) peak age-group (25–35 years) were managed for acquired frontal bone defects either 1° due to trauma or 2° as a result of craniotomies done to evacuate subdural hematomas sustained in road traffic accidents. All these cases were referred from Department of Neurosurgery. The time gap between primary surgery for head injury and surgery for reconstruction varied from 2 to 12 months. It depended on patient’s perceived need for correction of the deformity. There was no correlation between time gap in referral by neurosurgeon and patient reporting for the correction of deformity. Diagnosis on reference was calvarial defect, as per neurosurgery department (Table 1).
Table 1.
Demography & etiology
| Chronological age range (years) | Number (N)/gender (M/F) | Cause | Remarks |
|---|---|---|---|
| 18–25 | 12 11 M 1 F |
Acquired posthead injury | 11 reported following frontal craniotomy after 2–6 months postdischarge from Neurosurgery Ward (NSW) 02 cases female N = 1 reported following frontal craniotomy after 2 week postdischarge from NSW |
| 25–35 | 18 18 M 0 F |
Acquired posthead injury | 01 case primary cause to frontal bone destruction by RTA 17 cases reported following frontal craniotomy after 2–12 months postdischarge from NSW |
| 35–45 | 04 04 M 0 F |
Acquired posthead injury | 4 cases reported following frontal craniotomy after 2–6 months postdischarge from NSW |
| 45–55 | 01 01 M 0 F |
Acquired posthead injury | Reported following frontal craniotomy after 8 months postdischarge from NSW |
Method
Diagnosis and treatment plan was based on patient’s history of present condition, clinical examination, plain radiographs (PA skull/lateral view skull) and CT scan. Thirty-four patients had defects of frontal bone and in one partial frontotemporoparietal bone was missing.
Three-dimensional CT reconstructions accurately measured defect size and location. Dimensions of defects size of varied from minimum of 06 cm by 06 cm and maximum defect size was 10 cm by 10 cm with margin of error kept at ± 0.5 cm. Titanium mesh (dimension: 0.9 mm thickness, length and width of mesh according to defect size) and Ti screws (dimension: diameter of 2.00 mm with thread core diameter of 1.6 mm, pitch of 10/10 one turn corresponding to 1 mm screw head diameter of 2.8 mm designed to allow insertion at 30°) self-tapping were used in all these patients. The drill bit used was of 1.6 mm diameter.
Under GA bicoronal flap was raised to access the defect. During elevation of flap in the region of the defect, supraperiosteal dissection was performed to avoid dural tear as during healing, periosteum fuses with dural layer. The margins of the defect were carefully exposed by subpericranial dissection. Dural repair was carried out wherever necessary (Figs. 1 picture 5, 6, 2 picture 7, 8).
Fig. 1.
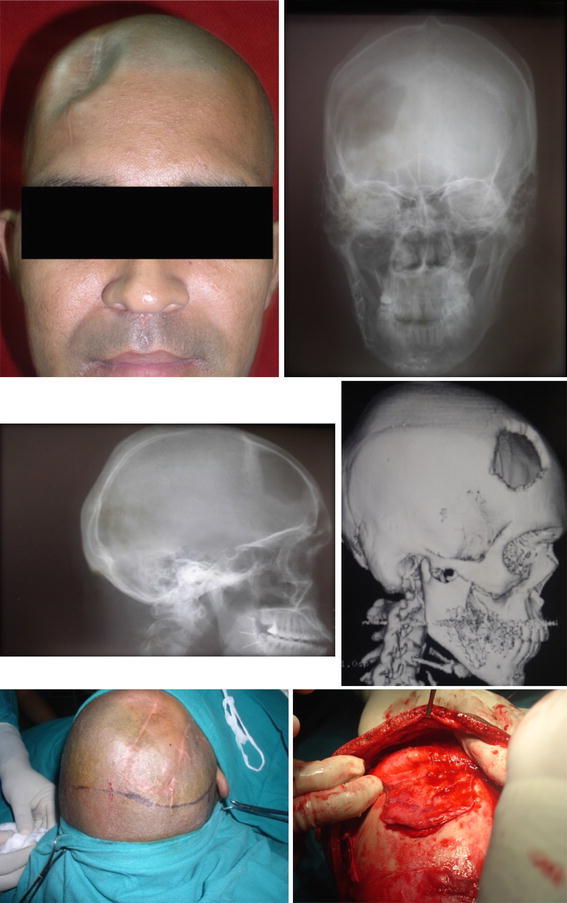
(Picture 1–6) A single random case
Fig. 2.
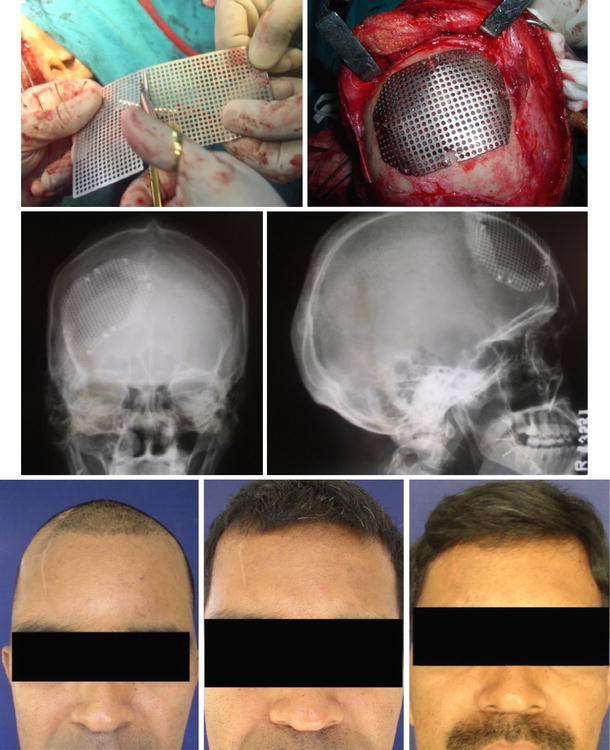
(Picture 7–13) Mesh adaptation and Post operative
Implant was finally contoured by directly placing it over the defect and adapted over the defect using mesh cutting scissors and fixed rigidly using Ti mini screws to the surrounding healthy bony margins. Hemostasis was achieved (Fig. 2 picture 7, 8).
Surgical wound was closed in layers using 3-0 vicryl and 2-0 silk sutures, after insertion of suction activated drain (no. 14 French gauge diameter). Pressure dressing was applied using bandage. Postoperative antibiotics and analgesics were administered for 5 days.
Follow-Up
Patients were followed up from 12 to 18 months. They were evaluated for the progress of healing, infections, wound dehiscence, discharging sinus, collection of fluids, stability of implants, implant exposure, satisfaction regarding esthetics and reaction to thermal change (Fig. 2 picture 9–13).
Results
All cases had good wound healing; implants were stable as evident from radiographs. There were no episodes of infection, no collection of fluids, no wound dehiscence or draining sinus and no cases of implant exposure. Patients were satisfied with postoperative esthetic correction. One patient complained of discomfort with temperature variation during winters but it did not interfere with his daily routine and did not require any intervention.
Discussion
Trauma is the cause of 70% cases of frontal bone deformities. The major accepted indications for cranial reconstructions are protective and cosmetic. Reconstruction of these defects also improves other vague symptoms namely headache, dizziness, discomfort at the defect edge and depression.
Age of patient, timing of the surgical intervention, extent of the defect along with the type of material used for reconstruction has direct influence on success of surgical repair. Reconstruction of the cranial defect can be undertaken as a primary or secondary procedure depending upon the duration, severity of injury, location of the defect and condition of the overlying soft tissues.
It remains a difficult procedure for all craniofacial surgeons, particularly when concerning the reconstruction of large defects. Considering the clinical and economic impact of the procedure, the search for materials and strategies to provide more comfortable and reliable surgical procedures is one of the most important challenges faced by modern craniofacial surgery [2].
Various grafts and alloplastic materials have been employed for the repair of cranial defects [2]. The grafts used for reconstruction are mainly autogenous. Use of autografts from iliac crest, rib and calvarium either as a free graft or transferred on a vascularized pedicle is preferred [5–8]. They have normal radiodensity, autogenous in nature, become a viable part of host and are rarely rejected.
However, sometimes it is difficult to shape the graft to conform to contours of the cranial vault because the harvested grafts are rigid. It also requires a separate donor site surgery and has unpredictable resorption [8].
Other graft materials which may be used are allograft and xenografts but success with these grafts has not been encouraging because of the antigenicity and limited supply. The alloplastic materials which have been used for cranial reconstruction include polymers and metals.
The most popular alloplastic materials for use in reconstruction of skull defects are polymethylmethacrylate (PMMA), porous polyethylene and titanium. PMMA—an acrylic polymer produced from esters of methacrylic acid—was first used for reconstruction of cranial defect by Zendar in 1940. It is used either as a heat cured preformed implant or self-curing form. A preformed polymethylmethacrylate implant is preferred as there is no heat on polymerization and no excess liquid monomer which might irritate and damage the underlying structures [9]. The implant is radiolucent and noncarcinogenic. It possesses low thermal conductivity. The implant is biocompatible and tissue response is minimal. It does not interfere with computed tomography or magnetic resonance imaging studies [10, 11]. However, acrylic implant has a tendency to shatter on impact, particularly in large defects. Some schools suggest incorporation of titanium mini plate [12] or stainless steel wire mesh [13] in the acrylic implant which offer better strength to the implant. To overcome the demerit of being radiolucent, some authors recommend impregnation of these plates with small amount of barium, to make it detectable on radiographs [12]. Porous polyethylene implant is an addition in alloplasts for reconstruction of the frontal bone defects. Its advantages and limitations are almost similar to acrylic plates. The main advantage of porous polyethylene implant is that it allows growth of fibrous tissues through it as pore diameter is 150–250 microns, can be shaped, contoured and being soft when immersed in hot water can be adapted to any shape intraoperatively [4].
Newer innovations include use of bioceramics, a calcium phosphate-based implant was developed and previously shown to potentially stimulate bone growth [14].
Gold and Silver were used first as implant metals followed by metal alloys such as vitallium, tantalum and stainless steel. Some components of these alloys specially nickel and cadmium are known to evoke an inflammatory response, and these materials are susceptible to corrosion after cold working and are rigid thereby causing difficulty in bending of the implant to anatomic contour of the bone. These metals also have a problem of high thermal conductivity and produces significant scattering in CT [15].
Simpson in 1965 used titanium for reconstruction and fixation of bony fragments of the cranial vault [16, 17]. The introductions of implants fabricated out of commercially pure titanium have overcome the problem of biocompatibility. Titanium mesh has been used in various other craniofacial deformities, treatment of facial trauma, mandibular osteotomies, Le Fort osteotomies and reconstruction after cancer surgery for patients with mandibular defects. The use of titanium mesh in craniofacial trauma has gained wide acceptance.
This material possesses higher strength, corrosion resistant, low weight-to-volume ratio, malleable to a reasonable extent which allows easy molding of plate to the anatomic contour of the bone and has low thermal conductivity. Modulus of elasticity of Ti (l5 psi × 106)2 is close to modulus of elasticity of bone (2.4 psi × 106)2. This property leads to even distribution of stress at bone implant interphase [15–17].
Recent studies have pointed out complications associated with titanium such as infection, exposure, collection and loosening, ultimately requiring implant removal. However, it is more related to the timing of cranioplasty related to late repair (> 18 months) [18, 19].
In all our cases of frontal bone defects reconstruction with use of titanium mesh, the results are very encouraging but a longer follow-up is required to draw any definite conclusion about superiority of one alloplast over the other.
Conclusion
Reconstruction of frontal bone defects requires knowledge of local anatomy, nature of defect, thorough evaluation and examination. Proper planning, selection of implant material, correct timing of surgery and standardized approach. Though use of autografts, conventional alloplasts such as PMMA, porous polyethylene and titanium have been giving satisfactory results, search for better and biodegradable material for frontal bone reconstruction is under evaluation. Bioresorbable implants and nonceramic hydroxyapatite cements which stimulate osteoid tissue formation are few alloplasts in this group.
In our study of 35 cases, all titanium implants were well tolerated without any case of rejection. Success rate for a period up to 18 months was 100%.
Acknowledgements
Prof. (Col) P Suresh Menon, Department of Neurosurgery and Anesthesia. Dr S.N. Medical College Jodhpur.
Conflict of interest
The author declares that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Haug RH, Adams JM, Conforti PJ, Likavek MJ. Cranial fractures associated with facial fractures. J Oral Maxillofac Surg. 1994;52:729–733. doi: 10.1016/0278-2391(94)90488-X. [DOI] [PubMed] [Google Scholar]
- 2.Zanotti B, Zingaretti N, Verlicchi A, Robiony M, Alfieri A, Parodi PC. Cranioplasty: review of materials. J Craniofac Surg. 2016;27(8):2061–2072. doi: 10.1097/SCS.0000000000003025. [DOI] [PubMed] [Google Scholar]
- 3.Williams LR, Fan KF, Bentley RP. Custom-made titanium cranioplasty: early and late complications of 151 cranioplasties and review of the literature. Int J Oral Maxillofac Surg. 2015;44(5):599–608. doi: 10.1016/j.ijom.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Lin AY, Kinsella CR, Jr, Rottgers SA, Smith DM, Grunwaldt LJ, Cooper GM, Losee JE. Custom porous polyethylene implants for large-scale pediatric skull reconstruction: early outcomes. J Craniofac Surg. 2012;23(1):67–70. doi: 10.1097/SCS.0b013e318240c876. [DOI] [PubMed] [Google Scholar]
- 5.Manson PN. Facial injuries. In: McCarthy JG, editor. Plastic surgery. Philadelphia: WB Saunders; 1990. pp. 867–1141. [Google Scholar]
- 6.McCarthy J, Zide BM. The spectrum of calvarial bone grafting: introduction of the vascularized calvarial bone flap. Plast Reconstr Surg. 1984;74:10. doi: 10.1097/00006534-198407000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Hunter PD, Pelofsky S. Classification of autogenous skull grafts in cranial reconstruction. J Craniomaxillofac Trauma. 1995;14:8–15. [PubMed] [Google Scholar]
- 8.Cohen SR, Kawamoto HK. Analysis and results of treatment of established pest traumatic facial deformities. Plast Reconstr Surg. 1992;90(4):574–584. doi: 10.1097/00006534-199210000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Van Gool AY. Preformed polymethyl methacrylate cranioplasties. J Oral Maxillofac Surg. 1985;13:2–8. doi: 10.1016/s0301-0503(85)80005-9. [DOI] [PubMed] [Google Scholar]
- 10.Brown KE. Fabrication of an alloplastic cranioimplant. J Prosthet Dent. 1970;24:213–224. doi: 10.1016/0022-3913(70)90148-4. [DOI] [PubMed] [Google Scholar]
- 11.Sabin H, Karvounis P. The neurosurgeon-dentist team in cranioplasty. JADA. 1969;79:1183–1188. doi: 10.14219/jada.archive.1969.0069. [DOI] [PubMed] [Google Scholar]
- 12.Replogle RE, Lanzino G, Francel P. Acrylic cranioplasty using miniplate struts. Neurosurgery. 1996;39:747–749. doi: 10.1097/00006123-199610000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Galicich JH, Hovind KH. Stainless steel mesh-acrylic cranioplasty technical note. J Neurosurg. 1967;27:376–378. doi: 10.3171/jns.1967.27.4.0376. [DOI] [PubMed] [Google Scholar]
- 14.Engstrand T, Kihlström L, Lundgren K, Trobos M, Engqvist H, Thomsen P. Bioceramic implant induces bone healing of cranial defects. Plast Reconstr Surg Glob Open. 2015;3(8):e491. doi: 10.1097/GOX.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malis LI. Titanium mesh and acrylic cranioplasty. Neurosurgery. 1989;25:351–355. doi: 10.1227/00006123-198909000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Patel MF, Langdon ID. Titanium mesh osteosynthesis: a fast and adaptable method of semirigid fixation. Br J Oral Maxillofac Surg. 1991;29:316–324. doi: 10.1016/0266-4356(91)90118-O. [DOI] [PubMed] [Google Scholar]
- 17.Simpson D. Titanium in cranioplasty. Br Med J. 1954;11(2):19–225. [Google Scholar]
- 18.Mukherjee S, Thakur B, Haq I, Hettige S, Martin AJ. Complications of titanium cranioplasty—a retrospective analysis of 174 patients. Acta Neurochir (Wien) 2014;156(5):989–998. doi: 10.1007/s00701-014-2024-x. [DOI] [PubMed] [Google Scholar]
- 19.Hill CS, Luoma AM, Wilson SR, Kitchen N. Titanium cranioplasty and the prediction of complications. Br J Neurosurg. 2012;26(6):832–837. doi: 10.3109/02688697.2012.692839. [DOI] [PubMed] [Google Scholar]


