Abstract
Background and Purpose
Sufficient evidence exists recommending the use of honey in the management of wounds. Studies revealed that the healing effect of honey could be classified by its antibacterial and anti-inflammatory properties of its components. Since surgical extraction of impacted molars is one of the most common operations in the oral cavity and the postoperative pain disturbing the patient may reduce the quality of health service, this study aimed to assess the analgesic potential of Manuka honey application into the extraction socket of impacted mandibular third molars.
Methods
This randomized split-mouth controlled study included 33 patients undergoing impacted bilateral lower third molars surgery under local anesthesia (n = 66). Randomization was carried out by coin flipping. One of the two impacted third molars was assigned to treatment group (Manuka honey applied just before suturing), other side to control group (nothing applied). Postsurgical pain was evaluated using visual analogue scale (VAS) of faces 7 days after extraction. The total analgesic dose used was also evaluated.
Results
In treatment group, postoperative VAS scores were significantly lower compared to that in control group regarding first and second days postoperatively (P < 0.05). Total analgesic intake in the control group was significantly higher (P = 0.0001).
Conclusion
This study demonstrated that intrasocket application of Manuka honey after surgical extraction of impacted lower third molar is an effective method for reducing acute postsurgical pain.
Keywords: Manuka honey, Impaction, Third molar surgery, Pain, Postoperative analgesia
Introduction
Honey has been one of the oldest and most commonly used agents in alternative and folk medicine since ancient times, besides being a sweet food. It has recently shifted from a food or folk-medicine product to a medicinal substance with high medical properties that attracted the attention of researchers in many medical fields [1, 2]. Sufficient evidence exists recommending the use of honey in the management of wounds and burns [1, 3–5]. Studies revealed that the healing effect of honey could be classified by its antibacterial, antiviral, anti-inflammatory, and antioxidant properties of its components [6]. Honey is reported to be soothing when applied to wounds and burns. Honey dressing is highly effective as compared to povidone-iodine dressing in reducing pain and increasing comfort in subjects with chronic wounds [7], and reducing acute postoperative pain and analgesic requirements in patients after tonsillectomy [8, 9]. Honey could be used as medicament for pain management of alveolar osteitis [10]. Manuka honey (Fig. 1) is derived from flowers of Manuka tree (Leptospermum scoparium) in New Zealand. This honey is unique because it is superior to other kinds of honey [11–15].
Fig. 1.

Active Manuka honey (activity level 25 +) used in this study showing applicable, viscous and semisolid structure
Since surgical extraction of impacted molars is one of the most common operations in the oral cavity and the postoperative pain disturbing the patient may reduce the quality of health service, this study aimed to assess the analgesic potential of Manuka honey application into the extraction socket of impacted mandibular third molars.
Materials and Methods
The study design was randomized split-mouth controlled clinical trial, conducted in 33 patients attending Clinics of Oral and Maxillofacial Department in Damascus University between June 2015 and May 2016. All patients involved in the study provided informed consent, and the study protocol was reviewed and approved by Research Ethics Committee of Damascus University (registration no. 1957; research ID: 18600936). The trial was registered in the ClinicalTrials.gov database (identifier NCT02483741). The inclusion criteria were asymptomatic, symmetrical, bilateral impacted mandibular third molars. Difficulty index for third molars is same for both groups. Patients with pericoronitis, infection, pathological condition in the region of surgery, alcoholism or diabetes were excluded.
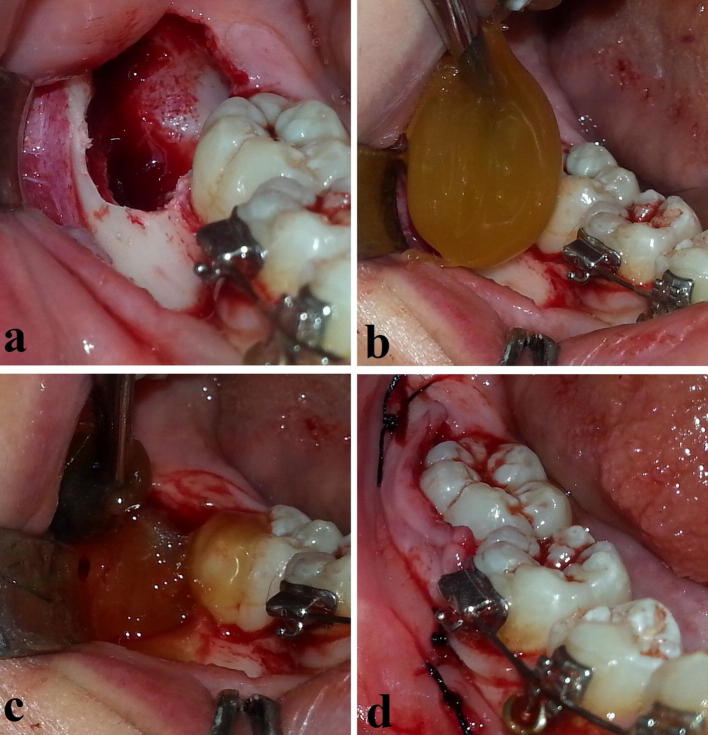
The two bilateral impacted molars were distributed by simple randomization (coin flipping) to test and control groups, so that one was surgically managed at the same day and that on the other side after 14 days (± 3 days) so as to ensure that the symptoms of the first surgery completely disappeared. Further randomization step decided which side to begin with. On both sides, surgical management was the same except for the last step (Fig. 2); local anesthesia was performed with 2% lidocaine + 1:80,000 epinephrine solution. Modified Ward’s flap was reflected. Necessary bone removal was performed by slow-speed straight surgical headpiece (rotation speed was fixed at 15,000 rpm) with continuous irrigation of saline solution. After the impacted molar was removed and the socket was well rinsed with saline (Fig. 2a), and before suturing was carried out, Manuka honey (Fig. 1; Activity Level 25 +; NZQueenBee Ltd: Auckland, New Zealand) was applied into the extraction socket in the test side only (Fig. 2b, c). Manuka honey was applied in a way that the socket was ensured to be completely filled with honey. Its physical properties had a role in making the honey more applicable and retainable (Fig. 1). Moreover, the surgical flap was sutured over the applied Manuka honey to keep it in place. The releasing incision was sutured to minimize the risk of losing Manuka. Time of surgery was recorded in minutes, from the time of initial incision to the time of final suture. Any patient who had more-than-7-minute difference between the first and second surgical times was excluded. All patients were operated on by the same surgeon and surgical assistants.
Fig. 2.
Test (Manuka) group. a–c Intrasocket application of Manuka honey into the extraction socket. d Suturing was done after
After every surgery, patients received fixed postoperative instructions regarding local homeostasis, cleaning, food and prescription. They were given instructions to take one tablet of diclofenac potassium 50 mg, as necessary, with a maximum daily dosage of 3 tablets for the 7 postsurgical days. Total analgesic dose used was assessed. In addition, patients were asked to indicate the intensity of pain on 5-level scale, each postsurgical day till the seventh day. The scale displayed five faces showing expressions which range from a minimum score of 1 (no pain) to score 5 (excruciating pain) [16]. Signs of inflammation and soft tissue healing were assessed at the seventh postoperative day through gingival healing index given by Landry et al. [17]. The index is consisted of 5 levels ranging from score 1 (very poor) to score 5 (excellent), and the scores were given on the basis of gingival color, bleeding on palpation, epithelialization of incision margins, and presence or absence of suppuration.
Data were analyzed using Statistical Package for the Social Sciences for Windows V19 (SPSS Inc, Chicago, IL, USA). Analysis included descriptive statistics of each of the variables. Student’s t test and Mann–Whitney U test were used to compare groups. The significance level was set at P < 0.05 with a confidence interval of 95%.
Results
Initially, a total of 33 patients were enrolled in the study. Figure 3 displays the flow diagram for patient recruitment and selection. Five patients did not participate in all postoperative stages, two patients underwent only one of the surgeries, two were lost to follow-up (left the country), and one were excluded because the difference between the first and second “surgery times” was more than 7 min. Thus, 28 patients included in all stages of the study. The mean patient age (8 males and 20 females) was 22.25 years (± 4.1).
Fig. 3.
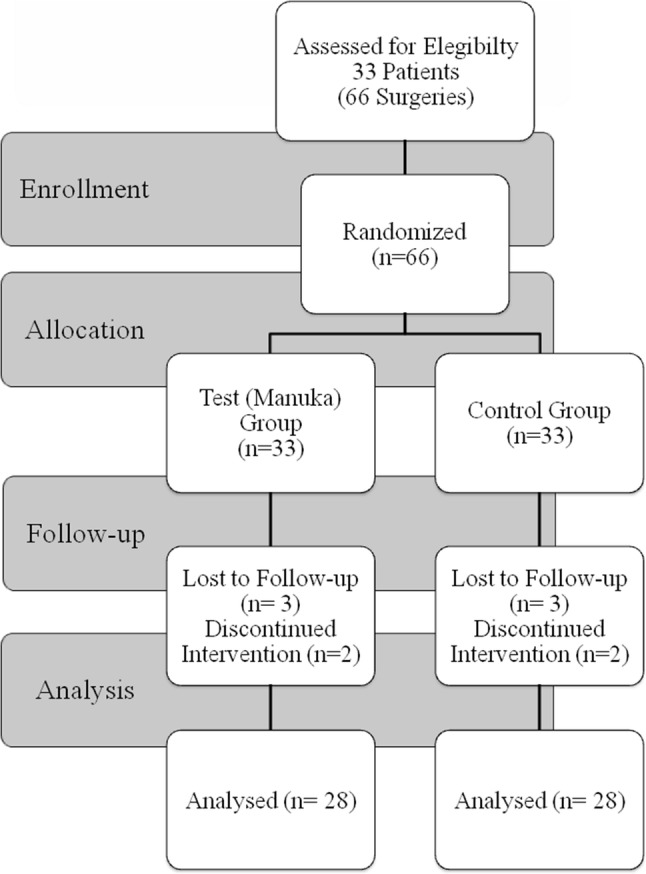
Flowchart of the study
The distribution of variables among the groups is shown in Table 1. There were no significant group differences in duration of surgical extraction (P = 0.827). No complications were associated with the surgical procedure. The VAS scores of the control group at the first and second postsurgical days were significantly higher compared to the Manuka group (P < 0.05; Table 1). There were no significant group differences in VAS scores at the third, fourth, fifth, sixth, and seventh postsurgical days (P > 0.05; Table 1). Soft tissue healing index scores were significantly higher in Manuka group at the seventh day in comparison with the control group (P = 0.0001; Table 1). Total analgesic intake in the control group was significantly higher (P = 0.0001; Table 1). Pain peak for the control group was in the first day and for the Manuka group in the third postsurgical day.
Table 1.
Distribution of variables and comparative tests results; patient age, surgery time, visual analogue scale (VAS), soft tissue healing index, analgesic dose
| Manuka group | Control group | Test value | P | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Age (years) | 22.25 ± 4.124 | 22.25 ± 4.124 | 0.000 | 1.000a |
| Surgery time (min) | 29.64 ± 8.156 | 26.60 ± 7.460 | 1.453 | 0.827a |
| VAS—first day | 2.39 ±0.956 | 3.25 ± 1.142 | − 2.767 | 0.006b |
| VAS—second day | 1.89 ±0 994 | 2.46 ± 1.035 | − 2.077 | 0.038b |
| VAS—third day | 2.42 ±1.103 | 2.64±0.869 | −0.688 | 0.491b |
| VAS—fourth day | 2.35 ±0.951 | 2.75 ± 1.109 | − 1.319 | 0.187b |
| VAS—fifth day | 1.78 ± 1.031 | 2.25 ± 1.205 | − 1.595 | 0.111b |
| VAS—sixth day | 1.46 ± 0.792 | 1.85 ± 1.112 | − 1.685 | 0.092b |
| VAS—seventh day | 1.42 ± 0.790 | 1.82 ± 1.218 | − 1.084 | 0.278b |
| Soft tissue healing—seventh day | 4.39 ±0.567 | 3.57 ± 0.634 | − 4.278 | 0.000b |
| Total analgesic dose | 10.57 ± 4.492 | 15.35 ± 4.983 | − 3.775 | 0.000a |
aStudent’s t test (test value “t”)
bMann–Whitney U test (test value “z”)
Discussion
The literature offers a number of studies on the analgesic and anti-inflammatory effects of Manuka honey. A meta-analysis showed that postsurgical administration of honey after tonsillectomy significantly reduces pain [8]. However, no study was found assessing these effects after surgical extractions. The inflammatory response and postsurgical symptoms associated with third molar surgical extraction are influenced by factors such as surgical difficulty and patient characteristics. The design of the study (split-mouth design) worked to minimize these influencing factors, as far as methodologically possible, by making the same person receives the study intervention and the control procedure. Not only that, but the setup of each individual case was made to ensure best symmetry possible in molars impaction level, angulation, covering bone, and so surgical difficulty. Moreover, the distribution of cases (right and left sides) into treatment and control was not always the same. Simple randomization (using a coin flip) was the best way to even out any differences that might occur due to right and left side-difficulty-relating issues or to the order of surgeries, which one to begin with. The researchers took every effort to restore the same baseline pain level by allowing a period of 14 days (± 3) for the symptoms to disappear, including pain. Of course, one might argue that the patient retained a memory of the pain. This is why a further step of randomization which decided which side to begin with eliminates this objection.
The choice was between balancing out differences between separate subjects from backgrounds that can vary widely (including race, age, gender, genetic makeup, and pain tolerance) and minimizing all those differences to one individual who has the exact same values for all these background variables between control and treatment. Given these parameters that could easily introduce bias, randomized split-mouth design became a clear winner.
On top of that, if one was to look at the results, the mean procedure time for the treatment group was longer due to the extra time needed to apply honey and the slightly longer time needed for suturing after honey application. And yet the treatment group still demonstrated a lower VAS at the first and second postoperative days and lower total need for painkillers.
The analgesic effect referred to in the present study after intrasocket application of Manuka honey could be on account of its direct and indirect anti-inflammatory potential. The fact that Manuka honey is an effective antimicrobial agent might help to explain its ability to reduce the inflammatory state indirectly. The compounds responsible for antimicrobial activity in honey are mainly: high sugar content, H2O2, methylglyoxal, bee defensin-1, low pH, and other antibacterial agents [13–15]. A low pH value contributes to wound debridement and reduction in bacterial sepsis and colonization [18]. High sugar content of honey is sufficient to prevent bacterial growth. This is believed to be the result of osmotic effect [19]. In fact, high sugar content is not the only reason for this activity. Honey, when diluted to reduce its sugar content and osmotic concentration, remains able to prevent bacterial growth [6]. The antibacterial activity of honey can be explained more clearly as a result of enzyme-produced peroxide activity, which continues to be produced even after dilution [11]. The peroxide activity of honey can be easily broken down by the application of heat or treatment with catalase. When this is done in vitro to Manuka honey, the effectiveness of this antimicrobial honey remains remarkably high [12]. This non-peroxide antibacterial (NPA) activity can be explained by the presence of high levels of the antimicrobial compound methylglyoxal and other phytochemical constituents [12, 14, 15]. The NPA activity is expressed by a factor, representing the concentration of a phenol solution yielding a similar zone of bacterial growth inhibition as Manuka honey. NPA rating of 10 is considered the minimum for therapeutic efficacy [20], and so this study used Manuka honey with NPA activity 25 + (Fig. 1).
Manuka honey is a major medical-grade honey which is approved for clinical application [11, 15]. Experiments showed that it is free of any type of microorganisms [13]. It has been recently used safely in many clinical studies [1–12, 21]. This study agreed, where no complication occurred regarding its use. The physical properties of the Manuka honey used (Fig. 1), namely its high viscosity and semi-solidity, make it more applicable and retainable.
Besides the fact that honey is able to inhibit the growth of bacteria that cause inflammation, honey has direct role in inflammatory response. Even when there is no infection, the anti-inflammatory effect is shown on experimental animals showing a decreased number of white blood cells in the inflammatory media [19]. Honey also was found to lower prostaglandin levels and elevate nitric oxide end products, which play a major role in inflammation [5, 12]. The anti-inflammatory effect of honey includes pain relieving [12]. Honey modulates adrenergic and muscarinic receptors to produce its anti-inflammatory and analgesic effects [22]. 5.8-kDa component isolated from Manuka honey is responsible for stimulating production of inflammatory cytokines in human monocytes. This component and its mechanism might be important in wound healing [23]. Pain and inflammation are so much related to each other. In the present study, signs of inflammation, assessed through the soft tissue healing index, were significantly lower in Manuka group in comparison with the control (P = 0.0001).
To the best of our knowledge, this study is the first study that indicates to the benefits of intrasocket application of Manuka honey after surgical extraction of mandibular third molars. However, this study coincides with the findings of Boroumand et al. [9], which showed that honey has analgesic effect after tonsillectomy. Malhotra et al. [21] indicated to the early postsurgical benefits of honey application on eyelid wounds, which somewhat agrees with the results of the current study; VAS scores of Manuka side was significantly lower only in the first and second postoperative days.
All patients were told to take the same painkiller as and when needed up to a maximum of 3 tablets a day, rather than taking them regularly. Thus, the measure that is derived here is the actual need to take analgesics, which is shown to be reduced in the treatment group. This is to complement the other indicator which is the subjects’ own evaluation of pain intensity via VAS. There is no denying that the analgesics controlled some of the pain, but the subject were instructed to write down their VAS value at the highest point of pain for every day post-surgery. The combination of instructing patients to take painkillers as necessary (as opposed to regularly) along with recording the pain value at the highest point of the day verifies the soundness of both indicators used in the study. Additionally, tablet counts were summed up as a single summery indicator for each group. The number tablets taken every day were recorded by the patients, but no comparison was made across individual days on this measure because a summery measure suffices. As the results showed, pain peak was shifted from the first day into the third day post-surgery by the intrasocket application of Manuka honey.
Conclusion
This study demonstrated that intrasocket application of Manuka honey after surgical extraction of impacted lower third molars is an effective method for reducing acute postsurgical pain.
Funding
This study was funded by Damascus University, Syria (Grant Number: 749).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and national research committee and with the 1964 Declaration of Helsinki and its amendments.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Contributor Information
Nuraldeen M. Al-Khanati, Phone: +963 956589272. Email: nuraldeen.alkhanati@gmail.com
Yasser Al-Moudallal, Email: yassermd11@hotmail.com.
References
- 1.Molan PC, Betts JA. Clinical usage of honey as a wound dressing: an update. J Wound Care. 2004;13(9):353–356. doi: 10.12968/jowc.2004.13.9.26708. [DOI] [PubMed] [Google Scholar]
- 2.Khan FR, Ul Abadin Z, Rauf N. Honey: nutritional and medicinal value. Int J Clin Pract. 2007;61(10):1705–1707. doi: 10.1111/j.1742-1241.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- 3.Moore OA, Smith LA, Campbell F, Seers K, McQuay HJ, Moore RA. Systematic review of the use of honey as a wound dressing. BMC Complement Altern Med. 2001;1:2. doi: 10.1186/1472-6882-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wijesinghe M, Weatherall M, Perrin K, Beasley R. Honey in the treatment of burns: a systematic review and meta-analysis of its efficacy. N Z Med J. 2009;122(1295):47–60. [PubMed] [Google Scholar]
- 5.Al-Waili N, Salom K, Al-Ghamdi AA. Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. Sci World J. 2011;11:766–787. doi: 10.1100/tsw.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaghoobi R, Kazerouni A, Kazerouni O (2013) Evidence for clinical use of honey in wound healing as an anti-bacterial, anti-inflammatory anti-oxidant and anti-viral agent: a review. Jundishapur J Nat Pharm Prod 8(3):100–104. PMCID: PMC3941901 [DOI] [PMC free article] [PubMed]
- 7.Gulati S, Qureshi A, Srivastava A, Kataria K, Kumar P, Ji AB. A prospective randomized study to compare the effectiveness of honey dressing versus povidone iodine dressing in chronic wound healing. Indian J Surg. 2014;76(3):193–198. doi: 10.1007/s12262-012-0682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang SH, Song JN, Jeong YM, Lee YJ, Kang JM. The efficacy of honey for ameliorating pain after tonsillectomy: a meta-analysis. Eur Arch Otorhinolaryngol. 2016;273(4):811–818. doi: 10.1007/s00405-014-3433-4. [DOI] [PubMed] [Google Scholar]
- 9.Boroumand P, Zamani MM, Saeedi M, Rouhbakhshfar O, Hosseini Motlagh SR, Aarabi Moghaddam F. Post tonsillectomy pain: Can honey reduce the analgesic requirements? Anesth Pain Med. 2013;3(1):198–202. doi: 10.5812/aapm.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh V, Pal US, Singh R, Soni N. Honey a sweet approach to alveolar osteitis: a study. Natl J Maxillofac Surg. 2014;5(1):31–34. doi: 10.4103/0975-5950.140166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011;1(2):154–160. doi: 10.1016/S2221-1691(11)60016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon A, Traynor K, Santos K, Blaser G, Bode U, Molan P. Medical honey for wound care—still the ‘latest resort’? Evid Based Complement Alternat Med. 2009;6(2):165–173. doi: 10.1093/ecam/nem175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider M, Coyle S, Warnock M, Gow I, Fyfe L. Anti-microbial activity and composition of manuka and portobello honey. Phytother Res. 2013;27(8):1162–1168. doi: 10.1002/ptr.4844. [DOI] [PubMed] [Google Scholar]
- 14.Kwakman PH, Zaat SA. Antibacterial components of honey. IUBMB Life. 2012;64(1):48–55. doi: 10.1002/iub.578. [DOI] [PubMed] [Google Scholar]
- 15.Mavric E, Wittmann S, Barth G, Henle T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol Nutr Food Res. 2008;52(4):483–489. doi: 10.1002/mnfr.200700282. [DOI] [PubMed] [Google Scholar]
- 16.Rathnam A, Madan N, Madan N. The language of pain: a short study. Contemp Clin Dent. 2010;1(3):142–145. doi: 10.4103/0976-237X.72778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pippi R. Post-surgical clinical monitoring of soft tissue wound healing in periodontal and implant surgery. Int J Med Sci. 2017;14(8):721–728. doi: 10.7150/ijms.19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res. 2007;298(9):413–420. doi: 10.1007/s00403-006-0713-x. [DOI] [PubMed] [Google Scholar]
- 19.Molan PC. The role of honey in the management of wounds. J Wound Care. 1999;8(8):415–418. doi: 10.12968/jowc.1999.8.8.25904. [DOI] [PubMed] [Google Scholar]
- 20.Schmidlin PR, English H, Duncan W, Belibasakis GN, Thurnheer T. Antibacterial potential of Manuka honey against three oral bacteria in vitro. Swiss Dent J. 2014;124(9):922–924. doi: 10.61872/sdj-2014-09-01. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra R, Ziahosseini K, Poitelea C, Litwin A, Sagili S. Effect of Manuka honey on eyelid wound healing: a randomized controlled trial. Ophthal Plast Reconstr Surg. 2017;33(4):268–272. doi: 10.1097/IOP.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 22.Owoyele BV, Oladejo RO, Ajomale K, Ahmed RO, Mustapha A. Analgesic and anti-inflammatory effects of honey: the involvement of autonomic receptors. Metab Brain Dis. 2014;29(1):167–173. doi: 10.1007/s11011-013-9458-3. [DOI] [PubMed] [Google Scholar]
- 23.Tonks AJ, Dudley E, Porter NG, Parton J, Brazier J, Smith EL, Tonks A. A 5.8-kDa component of manuka honey stimulates immune cells via TLR4. J Leukoc Biol. 2007;82(5):1147–1155. doi: 10.1189/jlb.1106683. [DOI] [PubMed] [Google Scholar]