Abstract
Aim
The purpose of this study was to evaluate the use of buccal fat pad-derived stem cells (BFPSCs) as a source for full thickness bone defect repair secondary to pathology in maxilla or mandible.
Methods
Fat-derived stem cells were isolated from buccal fat pad, differentiated into osteocytes in osteogenic medium, and seeded onto human bone defects. Autologous buccal fat pad was harvested and BFPSCs cultured within 4–6 weeks. Bone defects secondary to enucleation of pathologic cyst or tumors were reconstructed with osteogenically differentiated fat-derived stem cells. Hematoxylin and eosin staining, immunohistochemical staining for osteocalcin, alkaline phosphatase and genotypic and phenotypic marker analysis, and histomorphometric measurements of new bone were performed.
Results
Maxillofacial bone defects were successfully reconstructed by BFPSCs, which after implantation at an in vivo site yielded faster osseous regeneration. BFPSCs were associated with superior bone density formation, better blending of margins with enhanced bone trabecular formation, well-organized and well-vascularized lamellar bone with Haversian channels and osteocytes resulting in superior functional and cosmetic results with better quality of life and with significant decrease in secondary complications.
Conclusion
Buccal fat pad is an ideal tool in the hands of an oral and maxillofacial surgeon for tissue engineering and clinical use requiring bone tissue growth and repair, secondary to large osseous defects. This study demonstrates the feasibility of reconstructing bony defects with fat-derived stem cells.
Keywords: Buccal fat pad, Buccal fat pad-derived stem cells, Enucleation, Tissue engineering
Introduction
Regenerative medicine has the potential to revolutionize the field of craniofacial skeletal repair through a cell-based approach to engineer bone [1, 2]. It incorporates the use of multi-potent building blocks combined with molecular and environmental cues for the repair of damaged or diseased tissue. Recent investigations have focused upon the adult stromal (progenitor/stem) cell population that has been demonstrated to possess the ability to differentiate into multiple lineages in appropriate environments [3].
Bone marrow-derived mesenchymal stem cells (MSCs) from ileum to reconstruct a critical size defect in maxillofacial region has been initiated not only in animals but also in humans [4–6]. Lendeckel [7] extracted adipose-derived MSCs from gluteal region to reconstruct a calvarial defect following head injury. However, these procedures have their shortcomings, like harvested cells with heterogeneous populations [8], inadequate volume of aspirates [9, 10], donor site morbidity, painful intervention [11, 12], postoperative ambulatory difficulties [11].
Although these modalities are still in infancy, another source of adult stem cells has now come into existence wherein buccal fat pad-derived stem cells (BFPSCs) have proven to be a valuable source of osteo-progenitor cells.
Buccal fat pad (BFP) is an easily accessible niche housing neural-crest-derived stem cells, and there is limited morbidity after retrieval [13]. It is being considered an interesting and potentially important source of autologous stem/progenitor cells, which can readily be used for therapeutic purposes, such as the repair/regeneration of craniofacial bones [7].
Thus, a study was designed to repair human bone defects in mandible and maxilla by grafting of autologous adipose stem/progenitor cells (ASCs) in the bone defect site secondary to enucleation of jaw pathologies.
Aims and Objectives
A pilot study on maxillofacial bone defect repair by grafting of BFPSCs at the site of an enucleated jaw pathology was conducted in the Department of Oral and Maxillofacial Surgery, Government Dental College and Hospital, Ahmedabad, in five patients with the following aims and objectives:
To demonstrate that BFP can be considered a feasible source of ASCs which can be used to repair bone defects in the human mandible and maxilla.
To demonstrate that BFP-derived stem cells can regenerate bone of optimal quality and quantity as compared to standard techniques commonly used. Thus, to prove that the need for various allogenic and alloplastic materials can be avoided.
To demonstrate faster osseous regeneration of a defect site with autologous BFPSCs, when compared with the normal healing process of bone.
Hence, to better define and analyze the merits and demerits of this new tissue engineering-based treatment modality, thereby considering its future clinical implications.
Materials and Methods
Sample Size of the Study
This study consisted of a sample of five patients who reported to the Department of Oral and Maxillofacial Surgery, Govt. Dental College and Hospital, Ahmedabad, with a pathologic lesion in the maxilla or mandible, which was amenable to conservative treatment and reconstruction with autologous BFPSCs. Out of these, there were three female and two male patients. All procedures described here comply with the institutional ethics committee guidelines.
Criteria for Case Selection
Patients were selected irrespective of sex, caste, creed, religion, and socioeconomic status.
Inclusion Criteria
Patients presenting with benign pathology either in the maxilla or mandible, which could be treated by conservative management and reconstruction with autologous BFPSCs.
Patients with no systemic diseases, autoimmune diseases, familial heredity, or chromosomal abnormalities.
Patients within the age group of 18–55 years.
Patients willing to participate for the entire duration of the study and readily available for periodic follow-up.
Patients willing to provide informed and written consent.
Patients with good oral hygiene.
Exclusion Criteria
Patients unable to undergo a minor oral surgical procedure.
Patients with uncontrolled metabolic diseases, compromised immune system, uncompensated systemic disease, pregnancy, and prior radiation therapy of the surgical site.
Patients with history of alcoholism, drug abuse, or excessive smoking.
Patients with psychotic illness or prisoner status.
Patients with poor oral hygiene.
Methods
Preoperative preparation of the patient:
Detailed history, clinical and radiographic examination, and baseline investigations were performed. Radiological examination was done by panoramic radiography and CT scans. Written and informed consent for stem cell transplantation and surgery was obtained from the patient. Patients were subjected to a minor surgical procedure under local anesthesia for obtaining BFP prior to main surgery (enucleation) to obtain autologous ASCs from the stromal vascular filtrate (SVF) of the same patients in the laboratory.
-
2)
Laboratory Procedure
-
i)
Harvesting BFP with minimal contamination:
After obtaining signed informed consent from the patient, BFP was planned to be taken from any one side under local anesthesia. The procedure was done in a facility (fumigated operation theater) with careful attention paid to close monitoring and absolute control of sterility. A single stab incision in the depth of the vestibule opposite to the upper second molar and gentle soft tissue dissection superiorly resulted in release of the buccal fat pad. Meticulous handling and manipulation of the fat pad was done with a fresh set of sterile surgical instruments, and around 5–10 ml of tissue was extricated under close aseptic conditions. Once the tissue was excised, it was quickly placed in a tube containing transport medium (Dulbecco’s modified Eagle’s medium, DMEM with 1% antibiotic solution) and swiftly transferred to the laboratory in aseptic conditions (Figs. 1, 2). A case of keratocystic odontogenic tumour (KCOT) in right body region of mandible of a 50-year old female treated by enucleation and reconstruction with BFPSC is presented here:
-
ii)
Obtaining BFPSCs:
Fig. 1.
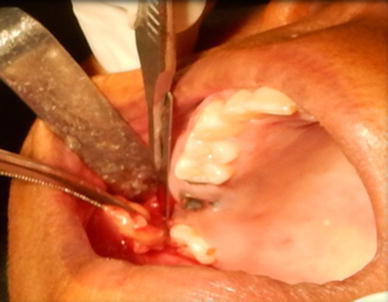
Adequate amount of fat tissue excised from right buccal fat pad
Fig. 2.
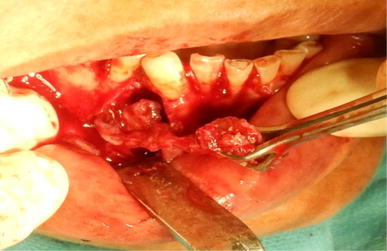
Total removal of pathology from defect
The BFP tissue was put for sterility check for 48 h. After confirming the sterility, the fat biopsy was then put for digestion in collagenase for 3–4 h. Thereafter, DMEM was again added in the tube. The tube was centrifuged at 2000 RPM for 10 min. The pellet was then resuspended in MSC media—DMEM, 20% human serum albumin (HSA), 2% l-glutamine, 1% antibiotic solution, fibroblast growth factor (FGF) and planted in T25 culture flask. Every alternate day the medium was changed and fresh media were added. The cells were differentiated in MSC media for 3 passages and then transdifferentiated into osteoblast lineage. The cells were then cultured in osteoblast media (DMEM, 20% HSA, 2% l-glutamine, 1% antibiotic solution, 4 mg/ml dexamethasone, 50 µg/ml ascorbic acid, and 10 mM beta glycerophosphate) till the cells reached the confluency stage.
After differentiating the cells into particular lineage, gene expression study was carried out for the confirmation of the cells. For MSC characterization, CD 29, CD 44, CD 54, CD 73, CD 90, and CD 105 markers were checked, and for osteoblast characterization, alkaline phosphatase (ALP) and osteocalcin marker study was performed (Fig. 5). When the cells were confirmed for the positive markers for osteoblast, they were lifted from the plates with the help of trypsin activity. The cells were collected in the tube and centrifuged to make a pellet. The pellet was then resuspended in minimum volume of osteoblast media and implanted into the patient.
Fig. 5.
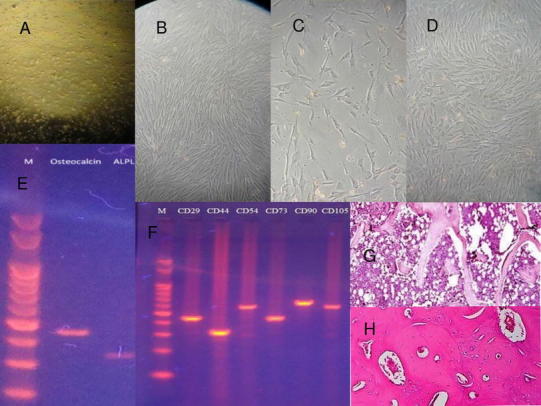
Histologic assessment: A mononuclear cells, initial cells retrieved from the buccal fat pad; B initial mesenchymal cell differentiation; C initial osteoblastic cell differentiation; D osteoblasts reached confluency; E isolation and characterization for osteoblastic cells with osteocalcin and alkaline phosphatase (ALPL) with RT-PCR for gene expression study; F isolation and characterization of mesenchymal stem cells from RT-PCR; G postoperative 3rd month biopsy from defect site indicating well-vascularized woven bone with cancellous structure; H postoperative 6th month biopsy indicating dense compact bone
Cell Count
The counting of cells was done with the help of trypan blue in the Neubar’s chamber. The dye will penetrate only the dead cells imparting the blue color. Hence, under the microscope the dead cells appeared blue and the live cells appeared transparent which were counted.
Stem Cell Transplantation: The BFPSCs were gently collected in a syringe, and this pellet of stem cells was used drop by drop to fill the intra-osseous defect left by the enucleation procedure in a dry surgical field under strict aseptic condition (Fig. 3).
Fig. 3.
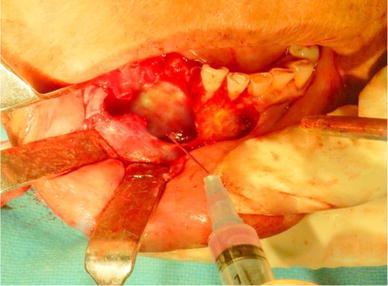
Implantation of buccal fat pad-derived stem cells at defect site
Results
Postsurgical Outcomes and Follow-Up
The study was designed for intra-individual comparison and the assessment of preoperative and postoperative bone level and soft tissue health at various stages.
Clinical and radiological controls were performed.
Patient was observed on third day postoperatively to evaluate clinical presence of pain, edema, bleeding, mouth opening, altered sensation, and functionality.
The next control was scheduled at day 7 after surgery, where clinical parameters were assessed, the first panoramic radiograph was taken, and suture removal was performed.
Patients were recalled thereafter, at 1, 3, 6, and 12 months postoperatively. During these recalls, clinical evaluation and radiographic analysis with orthopantomograms (OPG) was performed. Cone-beam computed tomograms (CBCTs) were taken during subsequent follow-ups.
During the third month postoperatively, bone sampling (biopsy) for histological evidence and marker flow cytometry was performed.
From Table 1, we can see that over a mean period of 43 days, 4.48 million ASCs (mean) were isolated from 2.88 gm of BFP.
Table 1.
Patient data
| Sr. no. | Patient | Age | Sex | Chief complaint | Final diagnosis | Site | Size (mm.) | Quantity of BFP extracted (gm) | Cell culture period (days) | Number of BFPSCs obtained (in millions/ml) | Mean bone density (% voxels) | Gain in mean bone density (% voxels) | Cell surface markers assessed | Postoperative complications | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative 6 months | ||||||||||||||
| 1. | A | 55 | F | Painful swelling | Type 1 unicystic ameloblastoma | Left maxilla | 35X45 | 2.58 | 63 | 3.5 | 14 | 88 | 74 | CD 9, 10, 13, 29, 34, 45, 49d, 90, 105, 106, 146, HLA-ABC, HLA-DR. ALP, OCN | Pain at 1 month, none later |
| 2. | B | 50 | F | Swelling | Keratocystic odontogenic tumor | Right mandible, body region | 25X45 | 3.17 | 35 | 3.8 | 18 | 86 | 68 | CD 9, 10, 13, 29, 34, 45, 49d, 90, 105, 106, 146, HLA-ABC, HLA-DR. ALP, OCN | None |
| 3. | C | 38 | M | Facial deformity | Keratocystic odontogenic tumor | Left mandible, angle-ramus region | 45X55 | 3.2 | 45 | 4.1 | 12 | 92 | 80 | CD 9, 10, 13, 29, 34, 45, 49d, 90, 105, 106, 146, HLA-ABC, HLA-DR. ALP, OCN | Paresthesia at 1 month, none later |
| 4. | D | 28 | F | Pain | Radicular cyst | Right mandible, body region | 25X35 | 2.32 | 33 | 5.8 | 15 | 88 | 73 | CD 10, 13, 29, 34, 45, 49d, 90, 105, 106, 146, HLA-ABC, HLA-DR. ALP, OCN | None |
| 5. | E | 44 | M | Pain | Type 1 unicystic ameloblastoma | Left mandible, body, angle region | 30X20 | 3.16 | 37 | 5.2 | 16 | 90 | 74 | CD 9, 10, 13, 29, 34, 45, 49d, 90, 105, 106, 146, HLA-ABC, HLA-DR. ALP, OCN | None |
Clinical Assessment
Postoperatively, patients were evaluated for pain, edema, infection, altered sensation, and complications. Each parameter was noted at the end of the 1st week, 1st, 3rd, and 6th month. Almost all the patients presented with mild pain, edema, and paresthesia at the end of the first week, which gradually reduced. One patient presented with pain at 1 month, and another patient presented with paresthesia at 1 month, which improved steadily (Table 1).
Radiologic Assessment
All the measurements for osseous regeneration like bone density, bone height were taken on OPGs by a single operator. OPGs were taken preoperatively and postoperatively—on the 3rd day, at the end of the 1st, 3rd and 6th month for all the five patients. Additionally, to detect bone formation, CBCTs were planned at 3, 6 months, and 1 year.
Postoperative radiological evaluation of patients was done with respect to peripheral blending of bone margins, presence or absence of trabecular bone, presence or absence of dense, compact cortical bone, osseous regeneration of defect site, and complications. Peripheral blending of bone margins was noted in OPGs of all the patients from the first month follow-up. In all the patients, coarse irregular trabecular bone was noted during the first month and replaced by dense compact bone in the subsequent third and 6th month OPGs and CBCTs (Fig. 4).
Fig. 4.
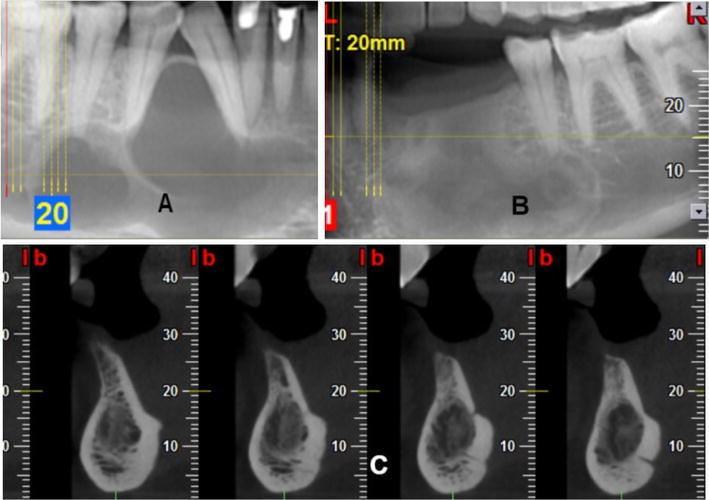
A Preoperative CBCT image showing a unilocular radiolucency in right body of mandible, B, C post-operative CBCT at 6 months showing dense bone at defect site
Bone Density Measurement
The densitometric analysis or bone density measurement was done for each patient on Radiant DICOM digital radiography software, and the measurements were taken in VOXELS from OPGs at stipulated time intervals.
Bone density in voxels was measured for each patient, preoperatively and postoperatively at the first, third and 6 month. Gain in bone density was noted as the difference between preoperative bone density level and postoperative bone density level at 6 months (refer Table 2).
Table 2.
Mean bone density measured in voxels at the defect sites preoperatively and on postoperative 1st, 3rd, and 6th month intervals from radiographs using DICOM software
| Patients name | Bone density (% voxels) | Gain in bone density (%) | |||
|---|---|---|---|---|---|
| Preoperative | Postoperative 1 month | Postoperative 3 months | Postoperative 6 months | ||
| Patient A | 14 | 22 | 48 | 88 | 74 |
| Patient B | 18 | 28 | 44 | 86 | 68 |
| Patient C | 12 | 37 | 49 | 92 | 80 |
| Patient D | 15 | 32 | 52 | 88 | 73 |
| Patient E | 16 | 36 | 58 | 90 | 74 |
| Mean | 15 | 31 | 50.2 | 88.8 | 73.8 |
The Z test was used to compare the mean bone density for the varied periods, where P < 0.05. This shows that significant difference in bone density exists at the interval of the 1st, 3rd, and 6th month
Thus, the densitometric analysis revealed that the mean preoperative bone densities were 15% voxels and the mean postoperative bone densities in % voxels at the 1st, 3rd, and 6th month are 31, 50.2, and 88.8. Also, the mean gain in bone density at 6 months is 73.8% voxels. The Z test was used to compare the mean bone density for the varied periods, wherein P < 0.05. This shows that significant difference in bone density exists at the interval of the 1st, 3rd and 6th month.
Histologic Assessment: (Fig. 5)
The reverse transcription polymerase chain reaction (RT-PCR) technique was employed in the laboratory for the gene expression study to isolate and characterize MSCs and osteoblastic differentiated cells with osteocalcin and alkaline phosphatase.
Postoperative histopathologic evaluation of all the patients was done by taking a bone biopsy from the operative site at 3 and 6 months. At the third month biopsy for all patients, a mixed histopathologic picture was present consisting of either osteoid with fibrous tissue, woven bone or dense compact bone. However, biopsy at 6 months for all patients revealed dense cortical compact bone. Also, no evidence of the primary pathologic lesion was noted in any of the biopsies (refer Fig. 5).
In addition to the above, cell surface marker expression study was performed by flow cytometry twice for all the patients. The first cell marker study was done on freshly cultured ASCs in the laboratory, while the second cell marker study was done in the third postoperative month on the tissue obtained from the bone biopsy of the surgical site.
As seen from Table 3, ASCs express human leukocyte antigen (HLA)-ABC, CD29, CD49e, CD51, and CD90 and show a variable expression of CD49d, CD9, CD34, CD105, and CD166. ASCs are negative to immunologically relevant surface antigens such as major histocompatibility complexes (MHC-II) (HLA-DR), CD40, CD40L, CD80, and CD86 and inhibit lymphocyte proliferation induced by allogenic cells. ASCs remain negative to these markers after osteogenic and chondrogenic differentiation. ASCs exclusively express CD54, CD 49d, and CD34 which are characteristic of their isolation.
Table 3.
Results of cell surface marker expression study of implanted buccal fat pad-derived stem cells and cells harvested from bone biopsies at 3 months by the technique of flow cytometry
| Marker | Protein | Patient A | Patient B | Patient C | Patient D | Patient E | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IBSC | RB | IBSC | RB | IBSC | RB | IBSC | RB | IBSC | RB | ||
| CD 9 | Tetraspanin 29 | NA | 99 | NA | 99 | NA | 100 | NA | – | NA | 98 |
| CD 10 | Common acute lymphocytic leukemia antigen |
NA | 96 | NA | 100 | NA | 99 | NA | 99 | NA | 100 |
| CD 13 | Aminopeptidase N | NA | 100 | NA | 99 | NA | 98 | NA | 100 | NA | 100 |
| CD 14 | 16 | NA | 14 | NA | 16 | NA | 15 | NA | 22 | NA | |
| CD 19 | Integrin-β1 | 14 | NA | 14 | NA | 16 | NA | – | NA | – | NA |
| CD 29 | 18 | 23 | 16 | 20 | 18 | 18 | 16 | 18 | 14 | 18 | |
| CD 34 | Sialomucin-like adhesion molecule | 1 | 13 | 1 | 12 | 2 | 15 | – | 13 | 1 | 12 |
| CD 45 | Leukocyte common antigen | 2 | 15 | 2 | 13 | 2 | 14 | 1 | 10 | 1 | 11 |
| CD 49d | Integrin-α2, VLA-4 | 98 | 100 | 99 | 99 | 98 | 98 | 99 | 100 | 97 | 99 |
| CD 73 | Ecto-5′-nucleotidase | 100 | NA | 99 | NA | 98 | NA | 102 | NA | – | – |
| CD 90 | Thymidylate synthase complementing protein | 96 | 100 | 98 | 99 | 95 | 100 | 96 | 99 | 98 | 100 |
| CD 105 | SH-2, endoglin | 100 | 100 | 99 | 100 | 98 | 100 | 98 | 100 | 99 | 99 |
| CD 106 | Vascular cell Adhesion molecule-1 |
32 | 90 | 35 | 78 | 31 | 85 | 36 | 84 | 33 | 99 |
| CD 146 | Melanoma cell Adhesion molecule |
NA | 99 | NA | 90 | NA | 100 | NA | 95 | NA | 98 |
| HLA-ABC | Major Histocompatibility Class I antigens |
91 | 98 | 92 | 100 | 91 | 100 | 90 | 99 | 93 | 100 |
| HLA-DR | Major Histocompatibility Class II antigens |
6 | NA | 5 | NA | 8 | NA | 6 | NA | 8 | NA |
| ALP | NA | + | NA | + | NA | + | NA | + | NA | + | |
| OCN | Osteocalcin | NA | + | NA | + | NA | + | NA | + | NA | + |
ASCs adipose stem cells; NA not applicable; + present; – absent; VLA very late antigen; SH-2 SRC homology 2; SRC sarcoma; ALP alkaline phosphatase; IBSC implanted BFPSCs; RB cells from regenerated bone
Discussion
This study was designed with the aim of utilizing BFPSCs by harnessing the excellent versatility of BFP, which is as such an almost vestigial entity [14]. A prospective study design was chosen for this study as it has fewer potential sources of bias.
It was found that the total number of cells in the oldest patient of the group aged 55 years was least, 3.5 million/ml, and also it took maximum time for culture, when compared to samples from younger patients. This suggests that with an increase in age the proliferative capacity of stem cells deteriorates [15].
Adipose tissue can be split into two different fractions, i.e., one fraction containing mature adipocytes and another fraction, SVF, containing a heterogeneous cell population. To determine the percentage of ASC present in the BFP, we characterized the SVF from 5 patients. The co-expression of different markers was determined by flow cytometry to quantify the cell population representing fresh ASC (Table 1). SVF from BFP contained a mixture of cells, as shown by percentages of marker co-expression [16].
The characterization data of the autogenous BFPSCs comply with previous results for ASCs [17], with a positive expression for markers substantiating the mesenchymal origin of cells and a negative expression of markers suggesting the hematopoietic and angiogenic origins of cells [17, 18].
The data from the flow cytometry in our study show that the BFPSCs expressed adhesion molecules CD9, CD29, CD49d, CD105, CD106, and CD166; receptor molecule CD44; surface enzymes CD10 and CD13; and extracellular matrix protein CD90. The cell source lacked the expression of CD31, CD34, CD45, and CD106, suggesting a lack of cells of hematopoietic and angiogenic lineages. However, the cells from the bone samples expressed markers CD9, CD10, CD13, CD34, CD49d, CD90, CD105, CD106, HLA-ABC, CD146, FGFR2, and FGFR3, confirming the osteogenic origin of the cells. All bone samples tested positive for cell-induced ALP and osteocalcin further confirming osteogenic characterization of the highly differentiated osteoblasts from BFPSCs [16].
ASC are negative to immunologically relevant surface antigens such as major histocompatibility complexes (MHC)-II (HLA-DR), CD40, CD40L, CD80, and CD86 and inhibit lymphocyte proliferation induced by allogenic cells. ASCs remain negative to these markers after osteogenic and chondrogenic differentiation. These immunoprivileged characteristics make these cells available for cell replacement therapies in HLA incompatible hosts. Besides this, their immunomodulatory effect could make them a suitable alternative to immunosuppressants, to prevent organ rejection after liver transplantation, avoiding the toxic side effects of these drugs [19].
In association with the characterization data, the in vitro functionality analyses proved that BFPSCs have osteogenic differentiation potential; moreover, the biomaterial (BFP) and medium used enhanced the osteogenic differentiation of the cells [20].
BFPSCs have an extraordinary innate capability of survival in low-oxygen environments, making them good candidates for cell-based therapies in which the oxygen supply may be limited, especially during the postimplantation period of the stem cells at the defect site when the blood supply is lacking, as also proven by Follmar et al. [21].
The ASCs secrete angiogenic cytokines such as vascular endothelial growth factor and hepatocyte growth factor, and these are considered to contribute to the angiogenic properties of ASCs [22, 23]. It has been suggested that the implanted cells enhance the development of vascular structures within the construct and increase the vitality and oxygen supply of the surgical defect site [24]. The presence of ASCs thus enhances the osteogenic and angiogenic conditions of the construct in vivo [25, 26].
The transplanted ASCs produce cytokines and chemokines that act as homing signals for endogenous stem cells and progenitor cells to the site of injury [27].
In contrast to the favorable aspects of autologous bone, the search for alternatives has been motivated by the drawbacks of the harvesting procedure [28]. Several investigators have reported that mesenchymal stem cells, when cultured under appropriate conditions, can maintain their viability and are able to differentiate into osteogenic cells [28, 29].
No control group was set for this study since to find lesions of similar size, age, gender, histopathology, etc., was a very difficult proposition. Therefore, the rate of bone formation in the study was compared with that established in the literature.
With regard to bone regeneration after cystectomy extensive ossification of defects up to 3 × 4 cm in diameter can be expected after 12 months, as proven by Ihan Hren and Miljavec [30]. They evaluated spontaneous bone healing of large mandible bone defects in 33 patients by computer-analyzed radiographs. Analysis revealed a mean gain of bone density of 7%, 27%, and 46% after 2, 6, and 12 months, respectively. In smaller defects measuring 2 × 3 cm in diameter a final bone density of 97% in relation to normal surrounding bone was observed after 12 months. In mandibular defects exceeding 3 cm in size they found a bone density of 84% of normal surrounding bone after 12 months.
Similar results were reported by Yim and Lee [31] on 74 patients after enucleation of jaw cysts. In this study panoramic radiograph analysis showed a recovery of radiopacity of more than 97% in defects smaller than 3 × 4 cm after 12 months. In another study by Chiapasco et al. [32] evaluating spontaneous bone regeneration after enucleation of 27 cysts larger than 4 cm, computed analysis of panoramic radiographs demonstrated almost complete bone healing of defects exceeding 4 cm in diameter after 24 months.
In sharp contrast to the above studies, we in our study have found that for lesions averaging 2 × 4 cm the mean bone density of 15%, 31%, 50.2%, and 88.8% has been recorded at preoperative stage and at 1, 3, and 6 months postoperative stages, respectively. Moreover, the mean gain in bone density is 73.8% at 6 months postoperative follow-up, which implies the rapid regeneration of bone at the defect site due to ASC implantation and highlights the efficiency and heightened tissue healing process of stem cells.
To recapitulate, the above-mentioned studies have scored that defects measuring 2 × 3 cm in diameter achieved a final bone density of 97% by spontaneous regeneration after 12 months [30, 31]. In mandibular defects exceeding 3 cm in size Ihan Hren and Miljavec [30] found a bone density of 84% of normal surrounding bone after 12 months and complete bone healing of defects exceeding 4 cm in diameter after 24 months [32]. However, we in our study obtained a final mean bone density of 88.8% of normal surrounding bone at 6 months post-ASC grafting for defects measuring mean 2 × 4 cm. This validates the clinical use of BFPSCs for faster osseous regeneration of maxillofacial bone defects owing to their excellent inherent properties.
The bone sampling done for histological evidence showed that a mature dense compact bone was formed at the defect site that was made up of well-organized and well-vascularized bone with a lamellar architecture surrounding the Haversian channels with osteocytes (Fig. 5). Laino et al. [33] proved that progenitor/stem cells in vitro produced compact lamellar osteoid bone (LAB), which is a fibrous bone tissue resembling adult bone during mineralization, with an external layer formed by osteoblasts markedly positive for osteocalcin. This newly formed tissue constitutes an ideal source of osteoblasts and mineralized tissue for bone regeneration. In fact, after in vivo transplantation, LAB formed lamellar bone containing osteocytes.
Over and above there was an added advantage of avoiding the use of a biomaterial scaffold in this study. Yang et al. [34] led a whole new dimension, where they avoided the use of any additional materials such as carrier substrates for stem cells or scaffolds and therefore the complications associated with traditional tissue engineering approaches such as host inflammatory responses to implanted polymer materials, were avoided. We too headed for a study where the cells were transplanted without the biodegradable scaffolds.
Limitations of this study include the short duration of follow-up, small sample size, and the high cost of BFPSC cultivation. The lesions treated within the study, although benign, were those with a high potential for recurrence. Whether the use of stem cells can increase the rate of recurrence has not been mentioned anywhere in the literature thus far. The literature although does mention the very favorable use of ASCs in bone regeneration [7, 14, 16, 20, 25], wherein complete bony continuity was seen in our study too on CT scans 3 months after the reconstruction.
Considering the nascent nature of the study, it is advised that the patients be thoroughly educated and motivated for long-term recalls involving multidisciplinary follow-up care with well-documented clinical, radiographic, and histopathologic reports to facilitate better long-term outcomes and aid in prompt and early management of any unforeseen complications.
Conclusion
All patients included in the study regularly presented for follow-up and appreciated early recovery of normal function, uneventful healing of wound and good quality of bone formation at the defect site without any complications.
Thus, the study validates BFP to be a remarkably rich source of stem cells, irrespective of age, sex, etc. Also, adjacent tissue traumatization is minimal when collecting BFP, hence low morbidity of the procedure. The cultured BFPSCs when transplanted led to the formation of vascularized bone of better quality as well as quantity. Faster osseous regeneration and repair of bony defects due to BFPSCs as compared to normal bone healing present a definite boon to patient’s overall health and validate the clinical translational application of tissue engineering.
Acknowledgements
We wish to acknowledge the fruitful contribution of our former H.O.D., Prof. Dr. Babu S. Parmar, to this study.
Conflict of interest
The authors hereby wish to state that this paper does not have any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Gosain AK. Distraction osteogenesis of the craniofacial skeleton. Plast Reconstr Surg. 2001;107(1):278–280. doi: 10.1097/00006534-200101000-00050. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SR, Burstein FD. The role of distraction osteogenesis in the management of craniofacial disorders. Ann Acad Med Singapore. 1999;28(5):728–738. [PubMed] [Google Scholar]
- 3.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Schantz JT, et al. Repair of calvarial defects with tissue engineered bone grafts I. Evaluation of osteogenesis in a 3D culture. Tissue Eng. 2003;9(Suppl 1):S113–S126. doi: 10.1089/10763270360697021. [DOI] [PubMed] [Google Scholar]
- 5.Schantz JT, et al. Repair of calvarial defects with tissue-engineered bone grafts II Evaluation of cellular efficiency in vivo. Tissue Eng. 2003;9(Suppl 1):S127–S139. doi: 10.1089/10763270360697030. [DOI] [PubMed] [Google Scholar]
- 6.Rohner D, et al. In vivo efficacy of bone-marrow-coated polycaprolactone scaffolds for the reconstruction of orbital defects in the pig. J Biomed Mater Res B Appl Biomater. 2003;66(2):574–580. doi: 10.1002/jbm.b.10037. [DOI] [PubMed] [Google Scholar]
- 7.Lendeckel S, et al. Autologous stem cells (adipose) and fibrin glue to treat widespread traumatic calvarial defects: case report. J Cranio Surg. 2004;32(6):370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Zuk PA, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 9.De Ugarte DA, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 10.Banfi A, et al. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol. 2000;28(6):707–715. doi: 10.1016/S0301-472X(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 11.Auquier P, et al. Comparison of anxiety, pain and discomfort in two procedures of hematopoietic stem cell collection: leukacytapheresis and bone marrow harvest. Bone Marrow Transpl. 1995;16(4):541–547. [PubMed] [Google Scholar]
- 12.Nishimori M, et al. Health-related quality of life of unrelated bone marrow donors in Japan. Blood. 2002;99(6):1995–2001. doi: 10.1182/blood.V99.6.1995. [DOI] [PubMed] [Google Scholar]
- 13.Graziano A, Papaccio G. Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev. 2008;4(1):21–26. doi: 10.1007/s12015-008-9015-3. [DOI] [PubMed] [Google Scholar]
- 14.Guasch EF (2011) Adipose stem cells from buccal fat pad and abdominal adipose tissue for bone tissue engineering. Ph.d. dissertation European Mention
- 15.Silva H, Conboy IM. Aging and stem cell renewal stem book. StemBook. Cambridge (MA): Harvard Stem Cell Institute; 2008. [PubMed] [Google Scholar]
- 16.Sandor GK, et al. Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: a case report and review of the clinical application of good manufacturing practice-level adipose stem cells for bone regeneration. J Oral Maxillofac Surg. 2013;71(5):938–950. doi: 10.1016/j.joms.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54(3):132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 18.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189(1):54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 19.Wan CD, Cheng R, Wang HB, Liu T. Immunomodulatory effects of mesenchymal stem cells derived from adipose tissues in a rat orthotopic liver transplantation model. Hepatobiliary Pancreat Dis Int. 2008;7(1):29–33. [PubMed] [Google Scholar]
- 20.Thesleff T, Lehtimäki K, Niskakangas T, et al. Cranioplasty with adipose-derived stem cells and biomaterial: a novel method for cranial reconstruction. Neurosurgery. 2011;68(6):1535–1540. doi: 10.1227/NEU.0b013e31820ee24e. [DOI] [PubMed] [Google Scholar]
- 21.Follmar KE, Decroos FC, Prichard HL, et al. Effects of glutamine, glucose, and oxygen concentration on the metabolism and proliferation of rabbit adipose derived stem cells. Tissue Eng. 2006;12:3525. doi: 10.1089/ten.2006.12.3525. [DOI] [PubMed] [Google Scholar]
- 22.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 23.Han J, Koh YJ, Moon HR, Ryoo HG, Cho CH, Kim I, et al. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood. 2010;115(5):957–964. doi: 10.1182/blood-2009-05-219923. [DOI] [PubMed] [Google Scholar]
- 24.Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5(7):1294–1311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conejero JA, Lee JA, Parrett BM, et al. Repair of palatal bone defect using osteogenically differentiated fat-derived stem cells. Plast Reconstr Surg. 2006;117:857. doi: 10.1097/01.prs.0000204566.13979.c1. [DOI] [PubMed] [Google Scholar]
- 26.Dragoo JL, Choi JY, Lieberman JR, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 27.Prockop DJ. ‘‘Stemness’’ does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin Pharmacol Ther. 2007;82:241. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 28.Meijer Gert J, et al. Cell based bone tissue engineering in jaw defects. Biomaterials. 2008;29:3053–3061. doi: 10.1016/j.biomaterials.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Cancedda R, Quarto R, et al. Bone marrow stromal cells and their use in regenerating bone. Novartis Found Symp. 2003;249:133–143. [PubMed] [Google Scholar]
- 30.Ihan Hren N, Miljavec M. Spontaneous bone healing of the large bone defects in the mandible. Int J Oral Maxillofac Surg. 2008;37:1111e–1116e. doi: 10.1016/j.ijom.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Yim JH, Lee JH (2009) Spontaneous bone regeneration after enucleation of bone cyst: a radiographic analysis. Free papers, e poster presentation
- 32.Chiapasco M, Rossi A, Motta JJ, Crescentini M. Spontaneous bone regeneration after enucleation of large mandibular cysts: a radiographic computed analysis of 27 consecutive cases. J Oral Maxillofac Surg. 2000;58:942–948. doi: 10.1053/joms.2000.8732. [DOI] [PubMed] [Google Scholar]
- 33.Graziano A, d’Aquino R, Laino G, Pirozzi G, De Rosa A, Papaccio G. Human CD34+ stem cells produce bone nodules in vivo. Cell Prolif. 2008;41:1. doi: 10.1111/j.1365-2184.2007.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Yamato M, Kohno C, et al. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26(33):6415–6422. doi: 10.1016/j.biomaterials.2005.04.061. [DOI] [PubMed] [Google Scholar]


