Abstract
Purpose
To assess Serum Prealbumin in the severity of illness and monitor response to treatment in odontogenic space infection.
Patients and Methods
This was a prospective cohort study comprising patients being managed for odontogenic space infection at the Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria. The calculated sample size was 69. Clinical parameters (Swelling Size, Visual Analogue Scale for pain intensity, and Maximal Interincisal Distance) were measured on day 0, day 4, and day 8. Other clinical parameters were Number of Anatomic Spaces Involved, setting of treatment, and Length of Hospital Stay. Serum Prealbumin levels were also serially measured. The relationship between Serum Prealbumin level and the clinical parameters was established using Spearman’s correlation test, independent t test, Friedman’s test, and linear regression. Significance level was set at .05.
Results
The mean Serum Prealbumin level at presentation (day 0) was 19.19 ± 4.61 mg/dl, which was significantly lower among inpatients (p = 0.001). On days 0, 4, and 8, Serum Prealbumin negatively correlated with Number of Anatomic Spaces Involved (p < 0.001). Serum Prealbumin levels on days 0, 4, and 8 and response in Serum Prealbumin negatively correlated with Length of Hospital Stay. On each day, Serum Prealbumin negatively correlated with pain intensity and Swelling Size and positively correlated with mouth opening. The response in Serum Prealbumin also positively correlated with response in each of the three clinical parameters.
Conclusion
This study suggests that Serum Prealbumin is a reliable tool for grading severity of illness and monitoring response to treatment in odontogenic space infection.
Keywords: Odontogenic space infection, Serum Prealbumin, Severity, Response, Clinical parameters
Introduction
Odontogenic space infection (OSI) signifies the spread of infection from the dental pulp or the periodontium into a potential anatomic head and neck space. It is a polymicrobial infection involving bacterial species of the normal oral flora predominantly anaerobes and usually presents with swelling and pain over the anatomic space involved, trismus, dysphagia, fever, and prostration. Akinbami et al. [1] reported that OSI constituted 11.3% of all the cases presenting to the oral and maxillofacial surgeon, and thus, it was commonly encountered in practice.
The general principles of the management of OSI are removal of the underlying cause, incision and drainage, antibiotics, airway control, and medical support [2]. Management decisions like the need for hospital admission are based on the severity of illness at presentation with clinicians tending to manage the most severe cases on admission [2]. Once treatment begins, it is also important for clinicians to closely monitor clinical response to treatment so as to diagnose treatment failure early and reappraise the treatment protocol as necessary.
Traditionally, clinicians have used clinical parameters to grade severity of illness and monitor response to treatment. Sharma et al. [3] used clinical parameters like pain, swelling, and trismus to measure levels of severity and monitor response to treatment during OSI. Other clinical parameters like the number and the distribution of the anatomic spaces involved have been used to grade severity and monitor response to treatment [4]. In a clinical trial, Cachovan et al. [5] utilized clinical parameters to compare the efficacy of antibiotics of odontogenic infections.
However, the clinical presentation of odontogenic infections may vary, and therefore, it may not be adequate for monitoring therapeutic efficiency and patient’s response [6]. This awareness has led to the use of laboratory measures like microbiological samples [7], serum sodium levels and blood mean corpuscular volume (MCV) [8], and full blood count [4] in grading severity and monitoring response to treatment in OSI. However, white blood count has been found to be unreliable in grading severity and monitor response to treatment in OSI [9, 10]. Husain and Kim [11] similarly reported that clinical screening like clinical examination, erythrocyte sedimentation rate, and white blood cell count have been associated with high rates of false positives and false negatives and thus suggested the use of acute-phase protein assay which on the other hand has been found to be more reliable.
An acute-phase reactant is a protein whose serum concentration increases (positive acute-phase reactant) or decreases (negative acute-phase reactant) during systemic inflammatory response syndrome (SIRS) [12]. A positive acute-phase reactant like C-reactive protein (CRP) has been successfully used to measure levels of severity of odontogenic infections and determine which of the patients would need an admission into the intensive care unit [13], while the serum levels of prealbumin, a negative acute-phase reactant, have been used to grade severity of illness and response to treatment in OSI [3, 14]. Prealbumin is synthesized majorly in the liver and is a tetrameric protein whose molecular weight is 54 kDa [15] and half-life is about 2 days [16]. The purpose of this study was to assess the severity of illness and monitor response to treatment of OSI using Serum Prealbumin levels.
Patients and Methods
This prospective cohort study was approved by the Research Ethics Committee of the Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria, and all participants signed an informed consent agreement. The study involved patients who presented with OSI at the Oral and Maxillofacial Surgery Unit of the hospital from May 13, 2014, to April 18, 2015. Patients aged 11–90 years and with a demonstrable dental source of infection like carious lesion, periodontal lesion, pericoronitis, fractured tooth, or a retained tooth root were included in the study, while patients with a history suggestive of liver disease that might affect hepatic production of Serum Prealbumin, pregnant patients in whom medications may be modified with potential delay in clinical recovery, and patients on steroid medications in whom hepatic metabolism might be altered, were excluded. Sample size was calculated to be 69.
The patients were managed on an inpatient basis if there was the involvement of multiple or secondary anatomic spaces, fever higher than 38.3 °C, need for general anesthesia, need for in-hospital control of a medical comorbidity, need for airway control, need for use of intravenous medications, or dehydration. Otherwise, patients were managed on an outpatient basis.
At three time points (T1, T2, and T3), blood samples were taken for Serum Prealbumin assay and clinical parameters were measured. T1 was a time before the commencement of treatment (that is, at presentation, day 0), while T2 was a time on the fourth day, and T3 was a time on the eighth day after the commencement of treatment. In each patient, 1 ml of blood was drawn at the antecubital fossa at T1, T2, and T3 for the estimation of Serum Prealbumin levels (SP). Blood samples were analyzed using the NeoBioLab® Human ELISA Kit (NeoScientific, USA), and SP values were recorded in mg/dl.
At T1, Number of Anatomic Spaces Involved (NAS), pain intensity by Visual Analogue Scale (VAS), Swelling Size (SS), and Maximal Interincisal Distance (MID) were recorded for each patient. VAS was recorded by asking patients to place a mark on a 10-cm non-graduated line to rate the intensity of their pain [17]. SS was recorded in cm2 as the product of two measurements in two widest dimensions of a swelling using a tape measure. Patients were asked to open maximally, and the maximum distance between the incisal edges of the upper and lower central incisors was measured with a meter rule in the midline and recorded as MID in cm [18]. A male patient was taken to have trismus if his MID was < 44 mm, while a female patient was taken to have trismus if her MID was < 41 mm [18]. Patients who presented with trismus and had missing central incisors were excluded. VAS, SS, and MID were repeated at T2 and T3. At the end of the study, Length of Hospital Stay (LHS) was recorded in days for the inpatients.
VAS, SS, MID, and SP at T1 indicated the level of severity at presentation, while the differences between VAS, SS, MID, SP at T1 and T3 indicated response to treatment.
Descriptive statistics were carried out for clinical parameters, and SP and the differences in SP values were examined using independent-samples t test and Friedman’s test. The relationship between SP and each of NAS and LHS was investigated using linear regression. Spearman’s correlation was used to highlight the relationship between SP and clinical parameters at T1, T2, and T3. Spearman’s correlation was also used to examine the relationship between RSP and RVAS, RSS, and RMID. Results were reported in a table and graphs, and level of significance was set at .05 for all statistical tests.
Results
Eighty-one patients participated in the study. Seventeen patients (21.0%) were managed on an inpatient basis, and the mean Length of Hospital Stay was 18.65 ± 16.76 days. The mean Serum Prealbumin level at presentation was 19.19 ± 4.61 mg/dl: It was 16 ± 5.42 mg/dl among the inpatients and 20.04 ± 4.01 mg/dl among outpatients (Fig. 1). An independent t test found the difference in Serum Prealbumin level at presentation between both groups to be statistically significant (p = 0.001).
Fig. 1.
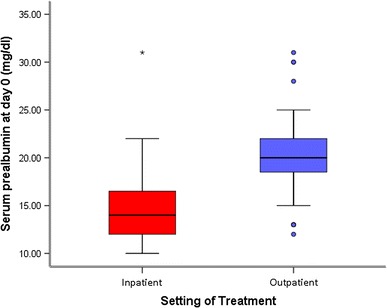
Box-and-whisker plot showing the distribution of Serum Prealbumin levels at presentation between inpatients and outpatients
Linear regression (y = β1x + β0) showed that there was a consistent inverse relationship between Serum Prealbumin levels and Number of Anatomic Spaces Involved (Fig. 2). The inverse relationship between each of Serum Prealbumin levels at T1 (SP1), T2 (SP2), and T3 (SP3) and Number of Anatomic Spaces Involved (NAS) was statistically significant (p < 0.001 in each of the three relationships.
Fig. 2.
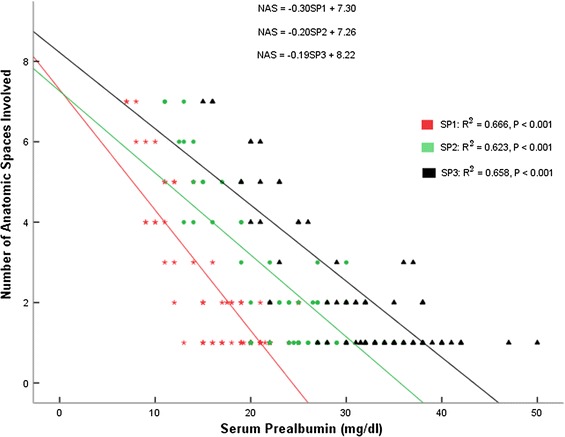
Relationship between Serum Prealbumin at presentation (SP1) and Number of Anatomic Space Involved (NAS), Serum Prealbumin on day 4 (SP2) and NAS, and Serum Prealbumin on day 8 (SP3) and NAS
Similarly, there was a consistent negative relationship between Serum Prealbumin levels and Length of Hospital Stay (LHS) (Fig. 3). Patients who ended up spending more days on admission (LHS) had lower Serum Prealbumin levels at presentation (SP1), on day 4 (SP2), and on day 8 (SP3). The relationship between response in Serum Prealbumin levels (RSP) and Length of Hospital Stay was also negative. Table 1 shows the significant negative correlation between Serum Prealbumin and severity of illness at every point of measurement and the significant positive correlation between response to treatment as measured by SP levels and response to treatment in clinical parameters.
Fig. 3.
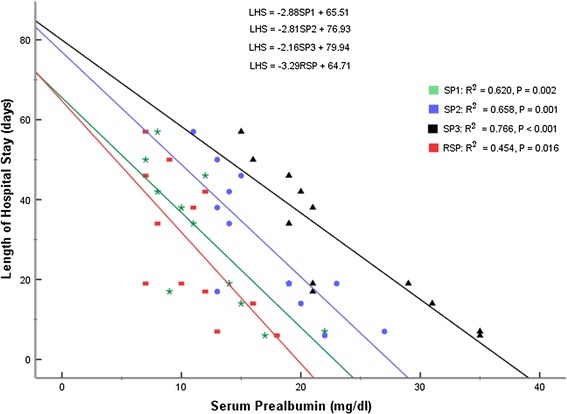
Relationship between Serum Prealbumin at presentation (SP1) and Length of Hospital Stay (LHS), Serum Prealbumin on day 4 (SP2) and LHS, Serum Prealbumin on day 8 (SP3) and LHS, and Response in Serum Prealbumin (RSP) and LHS
Table 1.
Spearman’s correlation between Serum Prealbumin and clinical parameters
| VAS1 | SS1 | MID1 | |
|---|---|---|---|
| SP1 | |||
| Correlation coefficient (ρ) | − 0.832 | − 0.733 | 0.663 |
| p (2-tailed) | 0.038 | 0.036 | 0.782 |
| VAS2 | SS2 | MID2 | |
|---|---|---|---|
| SP2 | |||
| Correlation coefficient (ρ) | − 0.571 | − 0.649 | 0.718 |
| p (2-tailed) | 0.001 | < 0.001 | 0.042 |
| VAS3 | SS3 | MID3 | |
|---|---|---|---|
| SP3 | |||
| Correlation coefficient (ρ) | − 0.723 | − 0.851 | 0.607 |
| p (2-tailed) | 0.027 | 0.016 | 0.035 |
| RVAS | RSS | RMID | |
|---|---|---|---|
| RSP | |||
| Correlation coefficient (ρ) | 0.615 | 0.816 | 0.610 |
| p (2-tailed) | 0.037 | 0.045 | 0.039 |
The mortality rate in this study was 2.5%. The two patients that died had stayed in the hospital for 14 and 57 days, respectively, and the cause of death was unknown as the family declined autopsy, but they both had systemic comorbidities—diabetes mellitus alone in one patient and both hypertension and diabetes mellitus in the other patient. Their SP1 values, 13 and 14 mg/dl, respectively, were in the lowest 18.5% of the entire study.
Discussion
Acute-phase reactants have been widely employed in the management of disease conditions in other parts of the body, while very little has been done in orofacial conditions. For instance, Serum Prealbumin has been used in prognosticating treatment outcomes and monitoring of patient’s response to treatment of malnutrition-inflammation syndrome in nephrology [19, 20], as an inflammatory and nutritional marker in prognosticating treatment outcome in lung cancer patients [21], in the early diagnosis of lower limb infections [22], and in the management of community-acquired pneumonia [23]. On the other hand, as at the time of the present study, an English PubMed search on the use of Serum Prealbumin in the management of odontogenic infections returned only two entries.
In the present study, prealbumin levels were significantly different between inpatients and outpatients (p = 0.001). The setting of treatment is a measure of severity of illness and thus prealbumin reliably predicted the setting of treatment for these patients. Another guide of severity of illness is Number of Anatomic Spaces Involved. Serum Prealbumin values on days 0, 4, and 8 negatively correlated with Number of Anatomic Spaces Involved which means that patients who recorded lower Serum Prealbumin values at the three points of measurement tended to present with more severe clinical disease as evidenced by higher numbers of anatomic spaces.
The traditional method of grading severity of illness and monitoring response to treatment in OSI is the use of clinical parameters like pain intensity, Swelling Size, and mouth opening. When compared with these clinical parameters, Serum Prealbumin proved to be a reliable method of grading severity of illness and monitoring response to treatment. At every point of measurement in this study, Serum Prealbumin negatively correlated with severity of pain, swelling, and trismus.
In the present study, Length of Hospital Stay correlated with Serum Prealbumin levels. This negative correlation also existed between response in Serum Prealbumin and Length of Hospital Stay which implies that patients whose Serum Prealbumin levels increased more stayed in the hospital for fewer days. This suggests that Serum Prealbumin can be used to predict patient’s response to treatment and thus Length of Hospital Stay.
Serum Prealbumin is a serum marker of both malnutrition and SIRS, and whether it is primarily a marker for malnutrition or SIRS is still controversial [24, 25]. Malnutrition and SIRS are two conditions that may characterize OSI. Malnutrition can predispose to OSI, while OSI through local features like pain, trismus, and dysphagia and systemic features like anorexia can predispose to malnutrition. It is not yet clear whether the derangements in Serum Prealbumin levels observed in OSI are due solely to malnutrition, SIRS, or both.
Prealbumin is a negative acute-phase reactant whose serum levels fluctuate in response to either or both of SIRS and malnutrition which are two conditions that are associated with OSI. Thus, its levels reflect the clinical severity at presentation and during treatment. In this study, Serum Prealbumin remarkably correlated with the severity of clinical parameters—Number of Anatomic Spaces Involved, pain, swelling, and trismus—while the derangements in Serum Prealbumin levels might be due to either malnutrition or SIRS or both.
Serum Prealbumin is an objective measure of severity of illness and response to treatment in OSI and should be included in the management of this condition. As Serum Prealbumin derangements might be due to either malnutrition or SIRS or both, nutritional support should be given adequate consideration in the management of OSI.
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
References
- 1.Akinbami BO, Akadiri O, Gbujie DC. Spread of odontogenic infections in Port Harcourt, Nigeria. J Oral Maxillofac Surg. 2010;68(10):2472–2477. doi: 10.1016/j.joms.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Vieira F, Allen SM, Stocks RM, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008;41(3):459–483. doi: 10.1016/j.otc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Sharma A, Giraadi G, Krishnan G, Shahi AK. Efficacy of serum prealbumin and CRP levels as monitoring tools for patients with fascial space infections of odontogenic origin: a clinicobiochemical study. J Maxillofac Oral Surg. 2012;13(1):1–4. doi: 10.1007/s12663-012-0376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng L, Yang C, Zhang W, Cai X, Kim E, Jiang B, et al. Is there association between severe multispace infections of the oral maxillofacial region and diabetes mellitus? J Oral Maxillofac Surg. 2012;70(7):1565–1572. doi: 10.1016/j.joms.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Cachovan G, Boger RH, Giersdorf I, Hallier O, Streichert T, Haddad M, et al. Comparative efficacy and safety of Moxifloxacin and clindamycin in the treatment of odontogenic abscesses and inflammatory infiltrates: a phase II, double-blind, randomized trial. Antimicrob Agents Chemother. 2011;55(3):1142–1147. doi: 10.1128/AAC.01267-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drazic R, Jurisic M, Markovic A, Colic S, Gacic B, Stojcev-Stajcic L. C-reactive protein as an inflammatory marker in monitoring therapy effectiveness of acute odontogenic infections. Srp Arh Celok Lek. 2011;139(7–8):446–451. doi: 10.2298/SARH1108446D. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Piriz R, Aguilar L, Gimenez MJ. Management of odontogenic infection of pulpal and periodontal origin. Med Oral Patol Oral Cir Bucal. 2007;12(2):E154–E159. [PubMed] [Google Scholar]
- 8.Zamiri B, Hashemi SB, Hashemi SH, Rafiee Z, Ehsani S. Prevalence of odontogenic deep head and neck spaces infection and its correlation with length of hospital stay. J Dent (Shiraz) 2012;13(1):29–35. [Google Scholar]
- 9.Callaham M. Inaccuracy and expense of the leukocyte count in making urgent clinical decisions. Ann Emerg Med. 1986;15(7):774–781. doi: 10.1016/S0196-0644(86)80371-7. [DOI] [PubMed] [Google Scholar]
- 10.Bakathir AA, Moos KF, Ayoub AF, Bagg J. Factors contributing to the spread of odontogenic infections: a prospective pilot study. Sultan Qaboos Univ Med J. 2009;9(3):296–304. [PMC free article] [PubMed] [Google Scholar]
- 11.Husain TM, Kim DH. C-reactive protein and erythrocyte sedimentation rate in orthopaedics. UPOJ. 2002;15:13–16. [Google Scholar]
- 12.Epstein FH, Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 13.Ylijoki S, Suuronen R, Jousimies-Somer H, Meurman JH, Lindqvist C. Differences between patients with or without the need for intensive care due to severe odontogenic infections. J Oral Maxillofac Surg. 2001;59(8):867–872. doi: 10.1053/joms.2001.25017. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham LL, Jr, Madsen MJ, Van Sickels JE. Using prealbumin as an inflammatory marker for patients with deep space infections of odontogenic origin. J Oral Maxillofac Surg. 2006;64(3):375–378. doi: 10.1016/j.joms.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Sann L, Bienvenu F, Bienvenu J, Bourgeois J, Bethenod M. Evolution of serum prealbumin, C-reactive protein, and orosomucoid in neonates with bacterial infection. J Pediatr. 1984;105(6):977–981. doi: 10.1016/S0022-3476(84)80094-3. [DOI] [PubMed] [Google Scholar]
- 16.Shenkin A. Serum prealbumin: is it a marker of nutritional status or of risk of malnutrition? Clin Chem. 2006;52(12):2177–2179. doi: 10.1373/clinchem.2006.077412. [DOI] [PubMed] [Google Scholar]
- 17.Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8(12):1153–1157. doi: 10.1111/j.1553-2712.2001.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 18.Chima O, Obiechina AE. Mouth opening among Nigerians. Odonto-Stomatologie Tropicale. 1995;18(70):22–24. [Google Scholar]
- 19.Rattanasompattikul M, Molnar MZ, Zaritsky JJ, Hatamizadeh P, Jing J, Norris KC, et al. Association of malnutrition-inflammation complex and responsiveness to erythropoiesis-stimulating agents in long-term hemodialysis patients. Nephrol Dial Transplant. 2013;28(7):1936–1945. doi: 10.1093/ndt/gfs368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He T, An X, Mao H-P, Wei X, Chen J-H, Guo N, et al. Malnutrition–inflammation score predicts long-term mortality in Chinese PD patients. Clin Nephrol. 2013;79(6):477–483. doi: 10.5414/CN107659. [DOI] [PubMed] [Google Scholar]
- 21.Bobbio A, Alifano M, Lupo A, Roche N, Regnard J-F, Cremer I, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome in resected non-small cell lung cancer. Eur Respir J. 2014;44(Suppl 58):505. doi: 10.1371/journal.pone.0106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourguignat A, Ferard G, Jenny JY, Gaudias J, Kempf I. Diagnostic value of C-reactive protein and transthyretin in bone infections of the lower limb. Clin Chim Acta. 1996;255(1):27–38. doi: 10.1016/0009-8981(96)06388-7. [DOI] [PubMed] [Google Scholar]
- 23.Hansson LO, Hedlund JU, Ortqvist AB. Sequential changes of inflammatory and nutritional markers in patients with community-acquired pneumonia. Scand J Clin Lab Invest. 1997;57(2):111–118. doi: 10.1080/00365519709056378. [DOI] [PubMed] [Google Scholar]
- 24.Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study. Kidney Int. 1999;55(5):1945–1951. doi: 10.1046/j.1523-1755.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 25.Myron Johnson A, Merlini G, Sheldon J, Ichihara K. Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition: International Federation of Clinical Chemistry and Laboratory Medicine (IFCC): IFCC Scientific Division Committee on Plasma Proteins (C-PP) Clin Chem Lab Med. 2007;45(3):419–426. doi: 10.1515/CCLM.2007.051. [DOI] [PubMed] [Google Scholar]


