Abstract
Neutrophil extracellular traps (NETs) are DNA fibers associated with histones, enzymes from neutrophil granules and anti-microbial peptides. NETs are released in a process denominated NETosis, which involves sequential steps that culminate with the DNA extrusion. NETosis has been described as a new mechanism of innate immunity related to defense against different pathogens. The initial studies of NETs were carried out with bacteria and fungi, but currently a large variety of microorganisms capable of inducing NETs have been described including protozoan and helminth parasites. Nevertheless, we have little knowledge about how NETosis process is carried out in response to the parasites, and about its implication in the resolution of this kind of disease. In the best case, the NETs entrap and kill parasites in vitro, but in others, immobilize the parasites without affecting their viability. Moreover, insufficient studies on the NETs in animal models of infections that would help to define their role, and the association of NETs with chronic inflammatory pathologies such as those occurring in several parasitic infections have left open the possibility of NETs contributing to pathology instead of protection. In this review, we focus on the reported mechanisms that lead to NET release by protozoan and helminth parasites and the evidence that support the role of NETosis in the resolution or pathogenesis of parasitic diseases.
Keywords: helminths, neutrophils, NETosis, NETs, parasites, protozoa
Introduction
Parasitic diseases are a relevant public health problem of chronic diseases due to the high capacity of the parasites to evade immune responses [1]. Parasites greatly contribute to the global burden of infectious diseases in the developing world, mainly in tropical and subtropical communities. According to the World Health Organization (WHO) diarrheal diseases are the most frequent illness in humans, and several species of enteric parasites are partially responsible for them [2]. Drinking water contaminated with feces carrying parasite cysts or eggs can be the source of infection of parasitic diseases caused by protozoans such as amoebiasis, giardiasis, toxoplasmosis, cryptosporidiosis, and blastocystiosis or by helminths such as ascariasis and trichuriasis [3,4]. Despite the advances in the diagnosis and treatment of these diseases, the number of outbreak cases of water-borne parasitic diseases seems to have increased worldwide in recent years [5]. The reasons are unknown, but it may have to do in part with the climate changes [6] and the deterioration of drinking water sources in the communities [5]. The impact of the parasitic diseases is more frequent in developing countries but they also cause significant illness in the developed world [7]. In addition, the parasitic diseases represent great economic losses derived from the costs of diagnosis, treatment, and disability [8]. All the above indicates that parasitic infections remain one of the priorities within the scope of human and animal health and that the study of the strategies related to their control, including the host immune response, is of paramount importance in the field of parasitology.
Neutrophils are the most abundant leukocytes in the peripheral blood of mammals. These cells are rapidly recruited at the sites of infection where they form the first immunological barrier against pathogens using different combat strategies including phagocytosis [9–12], degranulation [13–16], and neutrophil extracellular traps (NETs) formation. NETs are nuclear DNA fibers associated with histones, enzymes of cytoplasmic granules and anti-microbial peptides, which are released into the extracellular space [17]. Different mechanisms of anti-pathogen action have been attributed to NETs including microorganism immobilization, degradation of virulence factors, and killing of bacteria, fungi, and parasites [17–20]. NETs are released in a process denominated NETosis that originally was described as a new mechanism of cell death [21]; nevertheless, cases of vital NETosis have been reported in which neutrophils do not lose their viability because they release mitochondrial DNA instead of nuclear DNA [22].
Initially, NETosis was extensively characterized using phorbol miristate acetate (PMA) as stimulus. It was shown that PMA activates protein kinase C (PKC) and Raf/MEK/ERK signaling pathway [23,24]. Posteriorly, NADPH oxidase complex is assembled, which generates reactive oxygen species (ROS) [22]. ROS induce an oxidative dissociation of neutrophil elastase (NE) from azurosomes present in azurofilic granules; this enzyme is translocated to the nuclei where it cleaves histones causing DNA decondensation [25] that is enhanced on the association of myeloperoxidase (MPO) to chromatin [26]. In the next step, internal membranes are dissociated, DNA associated with proteins derived from the granules, and, finally, cytoplasmic membrane disrupts releasing NETs [21]. NETosis process induced by calcium ionophores is independent of ROS generated by NADPH oxidase but it depends on peptidyl arginine deiminase 4 (PAD4) [27,28], a nuclear enzyme that catalyzes the conversion of positively charged arginine residues present in histones into non-charged citrulline residues [29]. PAD4 is activated by an increase in calcium influx and promotes chromatin decondensation [27]. Recently, it was described that ROS from mitochondria are required for NETosis induced by calcium ionophores [30]; nevertheless, the role of mitochondrial ROS remains unknown.
Both oxidative and non-oxidative responses have been detected during the interaction of neutrophils with parasites. It has been reported that oxidative burst is triggered in neutrophils by the lysates, excretory/secretory products and plasma membrane components from Trichomonas vaginalis [31], Leishmania amazonensis and Leishmania braziliensis amastigotes [32], and Entamoeba histolytica trophozoites [33], as well as by whole extracts, scolex, and membranes from Taenia solium metacestode [34], to mention some examples. Degranulation as a part of the non-oxidative mechanism used by the neutrophils in their struggle against parasites has been reported in cells stimulated with Leishmania [32]. In addition, phagocytosis has been effective in the control of small-size parasites as Trypanosoma cruzi and some species of Leishmania [35–37]; nevertheless, this mechanism was not effective against large parasites such as helminths since they cannot be engulfed. In this regard, it has been suggested that NETosis plays an active role in the defense against helminth parasites because they are entrapped by NETs preventing dissemination. Even though some small parasites are phagocytized by neutrophils, these immune cells can also release NETs that affect the viability of parasites and prevent their invasion.
The decision of neutrophils to generate NETs instead of phagocytosis is another area of intense study. The decision seems to be the result of the combination of multiple signals including the adhesive, metabolic, and activation conditions of the cells, the stimuli from the environment, and, very importantly, the size and signals coming from the stimulating particle (alive or inert; reviewed in [38]). Regarding the size of the stimulating particle, it has been suggested that large particles such as parasites would induce the formation of NETs, while small particles such as bacteria and viruses should be eliminated by phagocytosis [39,40]. It is well known, however, that both viruses and bacteria can induce NETs formation under certain conditions, so other aspects such as the signals derived from the particle (e.g., pathogenicity and/or virulence signals) are also important for the outcome under physiological conditions [41,42]. Noteworthy, whatever the decision made by the neutrophils, phagocytosis and NETosis seem mutually exclusive. The reasoning behind this has to do with the availability of the enzymes NE and MPO to perform functions in the nucleus or in the cytosol and phagolysosomes of neutrophils. During phagocytosis, both enzymes are recruited to the phagolysosomes that keep them away from the nucleus whereas during NETosis the enzymes are released from the azurosomes into the cytosol to degrade the actin cytoskeleton and then be translocated to the nucleus where they participate in chromatin decondensation. The degradation of the cytoskeleton and the sequestration of NE and MPO into the nucleus, keeping them away from the endocytic pathway, would impede the function of phagocytosis (reviewed in [38]). Despite all this, the physiological role of the NETs during parasitic infections remains unknown and is the subject of intense study.
Here, we provide an update of what is known regarding NETosis induced by parasites, the mechanisms involved in its triggering, and the outcome of the exposition of parasites to NETs in terms of their viability and infectivity. We also discuss the implications that manipulation of NETs may add to the control of parasitic diseases.
Protozoan parasites: an overview
Infections by protozoan parasites represent a high risk to public health, as they are responsible for many outbreaks worldwide [43,44]. Protozoan parasites are cited as one of the main causes of 1.7 billion annual cases of diarrhea contributing significantly to the 842000 deaths associated; these data place them as the second leading cause of death in children under 5 years old [45,46]. According to the Global Burden of Disease Study 2016, the neglected tropical diseases and malaria were responsible for 843600 deaths, malaria alone being responsible for 85% of them [47]. Other protozoan parasitic diseases such as leishmaniasis, Chagas, and African trypanosomiasis were responsible for 13700, 7100, and 2300 deaths, respectively, in 2016 [47]. On the other hand, amoebiasis that caused approximately 55500 deaths worldwide in 2010 is considered as the second leading cause of death by protozoan parasites [48].
During the infection with protozoan parasites, the innate immune cells such as macrophages and neutrophils contribute crucially to the host immune response. Together with dendritic cells, Natural killer cells, and natural killer T cells, macrophages and neutrophils promote the development of the pro-inflammatory Th1-profile adaptive response that controls the parasite and therefore the disease. Neutrophils are the first immune cells recruited to the infection sites, so constituting the first line of defense of the cellular innate immunity [49]. The early recruitment of neutrophils to the site of tissue invasion usually contributes to reduce the initial burden of protozoan parasites. As mentioned above, effector activities of neutrophils against protozoan parasites include degranulation, phagocytosis (when the parasite is enough small to be phagocyted), oxidative burst, and, ultimately, the formation of NETs.
Protozoan parasites and NETs
Leishmania spp.
Parasites of genera Leishmania actually have a relevant prevalence in endemic regions. According to WHO, more than 5000 cases of cutaneous leishmaniasis were reported in 2015 in endemic countries of South America (Peru, Colombia, and Brazil), Asia (Syria, Iraq, Iran, Afghanistan, and Pakistan), and Africa (Algeria and Tunisia), while more than 1000 cases of visceral leishmaniasis were reported during the same year in Brazil, Sudan, Uganda, Ethiopia, and Somalia [50]. This parasite is transmitted by female sandflies after a blood meal, injecting the promastigotes (infective stage) in the mammal host, which are phagocyted by resident host macrophages within which they transform into amastigotes [51].
Different groups have described in vivo and in vitro interaction of neutrophils with Leishmania species. Neutrophils are rapidly recruited at the infection sites after inoculation of BALB/c mice with L. amazonensis, reaching the highest infiltration levels after 18 h; when the neutrophils are depleted, a major progression of the lesions and an increase of parasite burden take place [52]. Neutrophils can phagocyte amastigote and promastigote forms of L. amazonensis, resulting in their activation denoted by an increase in CD11b expression and ROS generation by NADPH oxidase. This triggering of neutrophils leads to the parasite killing; nevertheless, amastigotes seem to be more resistant to the anti-parasitic action of neutrophils compared with promastigotes [37]. Neutrophils were also able to phagocyte L. major promastigotes through the CR3 complement receptor, so the phagocytosis increased when the parasites were opsonized. Interestingly, only under this situation promastigotes triggered neutrophils activation [53].
Guimaraes-Costa et al. [54] were the first to describe NETosis induced by Leishmania; the authors reported that L. amazonensis, L. chagasi, and L. major promastigotes induced NETs release in naïve neutrophils and detected the presence of histones and NE in these structures. It was observed using immunofluorescence and scanning electron microscopy that promastigotes were trapped in NETs and showed morphological alterations, suggesting loss of their viability. Interestingly, either amastigotes or promastigotes were able to induce NETosis in non-activated neutrophils, indicating that the parasite stage is not a determinant factor to induce NET release. This group considered that histones present in the NETs were the effector molecules because the use of anti-histone H2 antibodies increased the survival of parasites and purified histone H2 reduced the viability of promastigotes. Noteworthy, the use of lipopetidoglycan (LPG) from Leishmania also induced NETosis in a dose-dependent manner.
Posteriorly, it was reported that L. donovani promastigotes also induced NETosis in a dose-dependent manner and that no differences were observed when either opsonized or non-opsonized promastigotes were tested [55]. In this study, NETosis occurred rapidly 10 min after stimulation of neutrophils and required a close contact between neutrophils and promastigotes. It is important to mention that contrary to the previous observation, LPG present on the surface of L. donovani was not necessary to induce NETosis since mutant promastigotes lacking LPG did not show reduced capacity to induce NETs as well as LPG-coated zymozan failed to promote NET release. Nevertheless, LPG showed protective activity against the anti-parasitic effect of NETs since mutant promastigotes lacking LPG showed less survival percentage upon the action of NETs as compared with wild-type promastigotes. The authors also gave a first approximation to understand the mechanism involved in the NET release induced by L. donavani, probing that ROS generated by NADPH oxidase were not necessary for this NETosis process [55].
A work aimed to understand the mechanisms implicated in the NETosis by L. amazonensis was performed by Rochael et al. [56]. They demonstrated that inhibition of NE and PAD4 using MeOSuAAPV-CMK and chloroamidine, respectively, reduced the amount of NETs released after exposure of neutrophils to promastigotes; in contrast, MPO inhibition did not affect the NETosis. In this study, it was demonstrated that L. amazonensis promastigotes caused an increase of H2O2 levels in neutrophils and that ROS were generated by NADPH oxidase and mitochondrial respiratory chain. Nevertheless, only ROS derived from NADPH oxidase complex seemed to be necessary for the NETosis process since inhibition of other ROS sources such as xanthine oxidase did not affect the release of NETs induced by L. amazonensis, while nitric oxide synthase inhibition reduced the NET amounts. An early/rapid NETosis induced by promastigotes (only 10 min after stimulation) dependent on NE activity but independent of NADPH oxidase-derived ROS and PAD4 activity was also described in this work indicating that different pathways that converge in NET release could be triggered by the same stimulus [56].
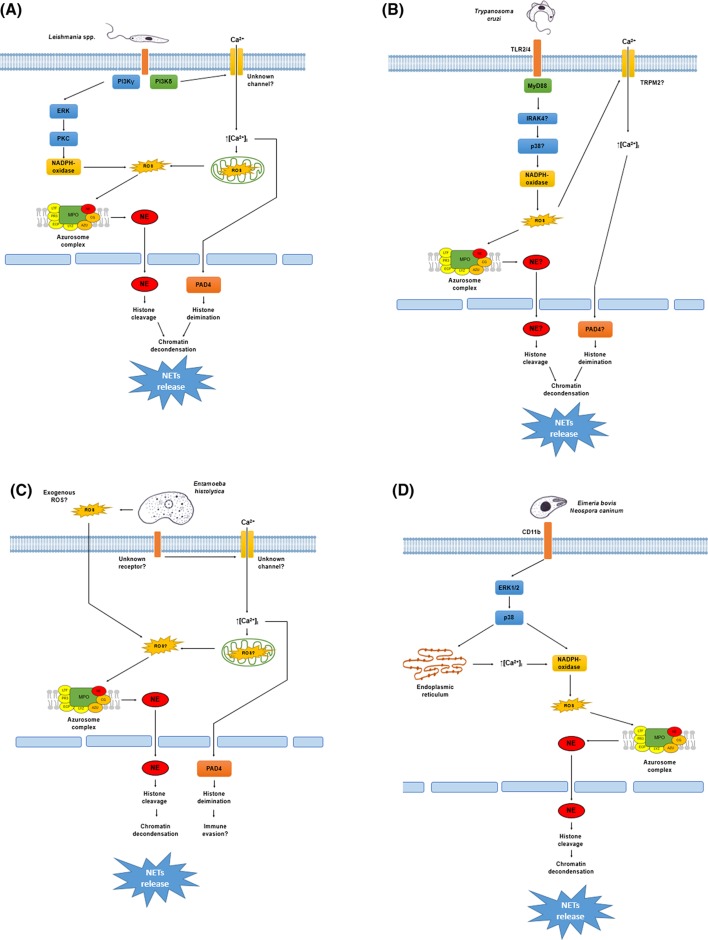
The molecular pathways involved in NETosis induced by L. amazonensis were studied by DeSouza-Vieira et al. [57]. They demonstrated that there are two pathways implicated in the NETosis by this parasite: one dependent, and another independent of ROS generated by NADPH oxidase. The ROS-dependent pathway implicates the activation of PI3K-γ, which activates ERK via MAPK; posteriorly, ERK activates PKC and ROS are generated by NADPH oxidase. ROS generation during ROS-dependent NETosis probably leads to the NE dissociation from the azurosomes and promotes histone cleavage that decondense DNA, as have been reported [25,26]. On the other hand, ROS-independent NETosis triggered by this parasite activates PI3K-δ that leads to an increase in calcium mobilization. No more inhibitors were tested to determine downstream components; nevertheless, NE activity was required to extrude DNA [57]. The ROS-independent NETosis that leads to an increase in cytoplasmic calcium levels could be able to activate PAD4 enzyme to decondense DNA [27]; PAD4 inhibition, however, did not affect this NETosis. Since reports exist indicating that mitochondria-derived ROS are necessary for NADPH oxidase-independent NETosis processes induced by calcium ionophores [30], it is likely that the mitochondria is the source of the ROS necessary for the induction of NETs by L. amazonensis. In addition, it is reported that the increase in calcium mobilization produced an increase of mitochondrial ROS in other cell types [58]. In this sense, ROS originated from mitochondria could dissociate NE to promote chromatin decondensation and release NETs (Figure 1A).
Figure 1. NETosis mechanisms triggered by protozoan parasites.
(A) Leishmania spp. promastigotes activate the PI3K signal pathway. PI3Kγ isoform leads to activation of the ERK pathway that phosphorylates PKC promoting the NADPH oxidase assemble. The NADPH oxidase generates ROS causing oxidative dissociation of the NE from the azurosome complex present in the membrane of azurophilic granules. NE is translocated to the nucleus where this enzyme cleaves the histones promoting chromatin decondensation and NET release. The PI3Kδ isoform probably causes calcium influx through an unknown membrane channel promoting ROS generation from the mitochondrial respiratory chain. ROS lead to the nuclear translocation of NE as mentioned above, or the influx of calcium activates the PAD4 causing histone citrullination and chromatin decondensation. (B) Trypanosoma cruzi trypomastigotes are sensed by the TLR2/4 receptor complex leading to NADPH oxidase assemble via the MyD88/IRAK4/p38 pathway. ROS promote NE translocation to the nucleus from the azurosome complex or activate the TRPM2 channels leading to calcium influx, which activates PAD4. Both pathways converge in the chromatin decondensation and NET release by histone cleavage or histone citrullination, respectively. (C) Entamoeba histolytica trophozoites trigger NETosis dependent on NE activity and extracellular calcium influx but independent of NADPH oxidase-derived ROS. It is likely that other ROS sources could be implicated in NETosis induced by amoebas since exogenous ROS from trophozoites or ROS from the mitochondrial respiratory chain can promote NE translocation, chromatin decondensation, and NET release as described elsewhere. In contrast, the trophozoites cause calcium influx that activates PAD4 promoting protein citrullination. Since PAD4 activity is not necessary for NETs release by amoebas, protein citrullination could be a mechanism of the parasite to evade the anti-microbial action of NETs. (D) The Apicomplexa parasites Eimeria bovis and Neospora caninum are sensed by neutrophils throughout the CD11b receptor, which in turn activates the ERK1/2 signaling pathway. The ERK pathway leads to calcium mobilization from the storages operated calcium entry (SOCE) that promotes NADPH oxidase activation or directly promotes NADPH oxidase assemble by phosphorylation. ROS are generated from this complex and the NE translocation takes place to decondense chromatin for release of NETs.
Trypanosoma cruzi
Chagas disease is a parasitosis with high prevalence in Mexico and Central and South America. According to the WHO, 8 million people are infected with T. cruzi worldwide causing more than 10,000 deaths per year [59]. T. cruzi is transmitted by different species of triatomine insects including Triatoma, Rhodnius, and Panstrongylus. After a blood meal, the insect vector releases in its feces the metacyclic trypomastigotes that enter the host through the lesions caused by the triatomine. Oral infection through ingestion of fruits and food contaminated with insect’s feces and via the transplacental route has also been reported. Inside the host, trypomastigotes invade adjacent cells and differentiate into amastigote forms. Amastigotes divide by binary fission and differentiate into trypomastigotes that are released into blood stream. Trypomastigotes can invade different organs as heart, colon, and esophagus, causing tissue damage with chronic manifestations [60,61].
Different groups have studied interaction between neutrophils and T. cruzi. It is described that neutrophils are able to phagocyte trypomastigote and amastigote forms of T. cruzi [36,62] and that neutrophils can kill the parasites in the phagolysosome vacuole through a mechanism dependent on the generation of hydrogen peroxide since neutrophils cultured with the parasite in the presence of catalase showed reduced killing efficiency [35,36]. In another study, neutrophil-depleted BALB/c mice showed increased parasitemia and less survival to acute infection, suggesting a protective role of neutrophils against T. cruzi [63]. In contrast, neutrophils apparently contribute to the pathology associated with trypanosomiasis because cardiac myoblasts are damaged when co-cultured with neutrophils and amastigotes, a trypanosomal state that cannot infect myoblast. The damage is likely mediated by secretory products from neutrophils since supernatants from neutrophil-parasite co-cultures also promote the detachment of myoblasts [64].
It has been described that T. cruzi trypomastigotes and their soluble extracts induce NETosis in a dose-dependent manner [65]. This mechanism appears to be triggered by sensing via TLR2/4 and involves the generation of ROS and histone citrullination. Actually, it is poorly understood how the TLR signaling pathway leads to NETosis; nevertheless, evidence exist that stimulation of neutrophils with LPS via TLR4 can activate NADPH oxidase by the phosphorylation of p47phox in an MyD88-IRAK4-p38MAPK-dependent manner with the subsequent ROS generation (reviewed in [66]). It can explain why NETosis triggered by T. cruzi is dependent on ROS from NADPH oxidase; it has been described, however, that phagocytosis inhibits NETosis since the phagolysosomes sequester the NE preventing its translocation to the nucleus and chromatin decondensation [39]. In this regard, it has been described that neutrophils possess TRPM2 channels, members of the transient receptor potential family, that can be activated by hydrogen peroxide produced via the respiratory burst under physiological conditions (reviewed in [67]). This calcium influx through the TRPM2 channels might trigger the activation of PAD4 that decondenses the chromatin. It is unknown if this channel is activated during T. cruzi–neutrophil interaction, however, it could explain why neutrophils enter NETosis even when they are able to phagocyte trypanosomes.
NETs can reduce the infectivity of T. cruzi trypomastigotes [65] but it remains unknown why it is possible. Different NET components, such as NE, cathepsin G, and Proteinase 3, possess proteolytic activity and these enzymes can likely act over the surface molecules of T. cruzi necessary for infection, degrade them in a similar manner as it occurs with other microorganisms [17], and block the entrance of the parasite into the cells (Figure 1B). Nevertheless, more studies are necessaries to confirm this theory.
Entamoeba histolytica
E. histolytica is an intestinal parasite with high prevalence in developing countries [68–70] that is responsible for intestinal amoebiasis and amoebic liver abscess. It is transmitted by the ingestion of mature cysts from food or water contaminated with stools. The excystation process takes place in the small intestine and the released trophozoites migrate to the large intestine where they divide by binary fission and penetrate the intestinal mucosa [71]. Hosts may suffer an asymptomatic infection or develop amoebic dysentery. In some cases, trophozoites can reach the blood stream and establish in other organs, mainly the liver, causing amoebic liver abscesses [72].
Neutrophils are rapidly recruited at E. histolytica infection sites and seem to participate in the protection from amoeba since CBA mice, which are partially resistant to intestinal amoebiasis, suffer a greater percentage of infection and an increase in the cecum thickness compared with the control group in the conditions when neutrophils are depleted [73]. In vitro experiments showed that trophozoites induce respiratory burst and apoptosis in human neutrophils [33,74]. Nevertheless, the exact role of neutrophils in this parasitic disease remains unknown.
Recently, it was observed that E. histolytica trophozoites induce NET release in human neutrophils, however, these NETs were incapable to kill trophozoites and the treatment of amoebas with PMA-induced NETs did not affect the capacity of trophozoites to form amoebic liver abscesses in a hamster model and neither reduced engulfment of neutrophil by trophozoites [75]. Different molecules with amoebicidal effect have been described to form part of the amoebas-induced NETs, including the anti-microbial peptide LL-37 and other defensins, lactoferrins, and enzymes such as MPO and NE [17,75,76]. Though anti-microbial peptides possess a characterized amoebicidal effect [77,78], recent studies indicate that association of these peptides with DNA significantly reduced their anti-microbial potential due to the negative charge of DNA that neutralizes the positive charges present in the peptides that are necessary for their microbicidal effect [79,80]. In addition, the particularly high levels of cholesterol in the cytoplasmic membrane of E. histolytica trophozoites confer them protection against cationic peptides in a similar manner as described for high membranal levels of cholesterol protecting amoebas against their own amoebopore [81]. This could also explain why histones, which effectively kill other protozoan parasites [82], do not reduce viability of amoebas.
Our working group has recently studied the molecular pathways implicated in the NETosis induced by E. histolytica. We found that NETosis triggered by trophozoites depends on serine proteases activity and extracellular calcium and requires translocation of NE to nucleus. On the other hand, it also depends on ROS generated by NADPH oxidase, as well as on PAD4 activity and Raf/MEK/ERK signaling pathway [83,84]. Interestingly, NE translocation took place independently of NADPH oxidase activity, despite previous reports indicate that the dissociation of NADPH oxidase from the azurosome depends on ROS [25]. Nevertheless, other ROS sources such as mitochondrial respiratory chain or ROS from the NETs-stimulating cells have not been discarded since NETosis induced by calcium ionophores is dependent on ROS from mitochondria [30] and NETosis induced by Candida albicans in neutrophils from chronic granulomatous disease patients (that cannot form ROS) is dependent on ROS from the fungi [85]. It is to notice that trophozoites induced protein citrullination but PAD4 activity was not necessary for NET release. The significance of the citrullination of neutrophil proteins induced by the amoeba during the NETosis process that does not require PAD4 is unknown, but it has been suggested that citrullination of NET components by E. histolytica trophozoites could act as a mechanism to evade their anti-microbial action [86], which could help to explain why NETs failed to kill E. histolytica trophozoites.
In our laboratory, we have observed that other cell types, such as eosinophils and monocytes, are also able to form extracellular traps (ETs) in contact with viable E. histolytica trophozoites and that trophozoites of the non-pathogenic E. dispar did not trigger NETosis, suggesting a relationship between pathogenicity and NETs formation (unpublished data); however, the role of NETs and the ETs from other immune cells in the protection against this parasite remains unknown (Figure 1C).
Eimeria spp.
Coccidiosis is a parasitic disease caused by apicomplexan protozoa of genera Eimeria that affects animals [87]. This disease causes diarrhea with or without blood and, in some cases, can result in the animal death. An animal is infected when it ingests sporulated oocysts that release sporozoites, which invade the intestinal epithelium. Sporozoites differentiate into meronts that divide by fission and develop into merozoites, which also invade the intestinal epithelium. Merozoites invade cells and develop into either macrogamonts or microgamonts. Macrogamonts develop into single macrogametes whereas microgamonts suffer multiple divisions resulting in numerous microgametes. Microgametes invade cells containing macrogametes and fertilization takes place resulting in oocysts formation [88].
The role of neutrophils in the protection against Eimeria is poorly studied; some evidence indicate that neutrophils may play an active role in the protection since neutrophil-depleted mice showed increased oocyst output after infection with E. papillate [89]. Nevertheless, the exact role of neutrophils in this infection is not yet clear.
The first description of NETosis induced by E. bovis was made by Behrendt et al. [90]. They found that E. bovis sporozoites trigger NETosis in bovine neutrophils much faster and stronger than PMA and that NETs were released in a time- and dose-dependent manner. Heat-inactivated sporozoytes and homogenates of active parasites failed to induce NETosis, suggesting that the viability of the parasite is indispensable for the induction NETs like it is observed with E. histolytica trophozoites [75,83]. This supports the notion that surface molecules present in pathogens are not the only stimuli necessary to induce NET release. The Eimeria-triggered process was dependent on ROS generated by NADPH oxidase, suggesting a classical NETosis pathway. In addition, sporozoytes that were co-cultured with neutrophils and underwent NETosis showed reduced infectivity in BUVEC cells. This effect was prevented using DNase, indicating the active role of NETs against this parasite. A more detailed work to study the mechanisms implicated in NETosis induced by E. bovis was performed by Muñoz-Caro et al. [91]. They described that CD11b receptor of neutrophils (an integrin component of complement receptor 3) was implicated in this NETosis since E. bovis sporozoytes induced its overexpression and the use of anti-CD11b receptor antibody reduced the NETs amount release by neutrophils co-cultured with the parasite. Like in other cases, NETosis required ROS from NADPH oxidase and the process was also dependent on calcium mobilization from store-operated calcium entry (SOCE), as well as on NE and MPO activities. E. bovis-induced NETosis was regulated by ERK1/2 and p38 MAPK pathways similar to what occurs with the cross-linking of FcγRIIIb receptor and PMA stimulation [24,92]. It is important to mention that merozoytes and oocyst stages of E. bovis also trigger NETosis, indicating that this process is not stage-specific. Moreover, not only bovine, but also neutrophils from other mammals release NETs when they are stimulated with E. bovis sporozoites, suggesting that NETosis is a conserved immune mechanism among animals.
It has been described that E. arloingi is also capable to induce in vitro NETosis in caprine neutrophils [93]. Like E. bovis, E. arloingi sporozoytes induce a rapid NETosis process that depends on ROS from NADPH oxidase; in the same manner, NETs entrapped and also reduced the infectivity of E. arloingi parasites over BUVEC cells, but the extruded DNA did not affect the viability of sporozoytes. NETosis was not a stage-specific mechanism since E. arloingi oocysts were also able to induce NET release.
The in vivo occurrence of Eimeria-induced NETosis has been recently documented [94]. In cross-sections from bovine and calf intestinal gut samples infected with E. bovis and E. arloingi, respectively, the presence of leukocytic infiltration mainly composed of neutrophils, and eosinophils and monocytes were detected. The immune cells were observed in close contact with oocytes, macrogamonts, and macromeronts. Interestingly, the presence of extracellular DNA co-localizing with histones and NE was also detected in this samples, indicating the occurrence of NETosis in infected tissues. It is to notice that the released DNA, which co-localized with anti-microbial proteins in the tissue samples, is principally attributed to neutrophils [17,54,95]; nevertheless, other leukocytes such as eosinophils, monocytes, and macrophages can also be able to drive ET formation [22,96,97]. For this reason, it is important to take care with data interpretation due to some of the principal NET hallmarks (e.g., histones, NE, or MPO) are not exclusive of neutrophils [98,99] (Figure 1D).
Toxoplasma gondii
Toxoplasmosis is caused by T. gondii, an obligate intracellular protozoan pathogen from phylum Apicomplexa. In various places throughout the world, it has been shown that in some locations, up to 95% of populations have been infected with Toxoplasma [100] and the prevalence varies widely from 10 to 80% between countries [101]. Oocysts are produced during the sexual reproduction of the parasite exclusively in felines, which are the definitive host. After oocyst ingestion by intermediate hosts such as humans, other mammals, and birds, sporozoites are released and penetrate the intestinal epithelium developing into tachizoytes. Tachyzoites divide by endodyogeny inside any type of nucleated cells and disseminate throughout the organism. Tachizoytes can differentiate into bradyzoites producing tissue cysts and may remain throughout life in most hosts, predominantly in the brain or musculature. Tissue cysts are infective when they are ingested by intermediate hosts. After ingestion of these tissue cysts, they are ruptured in the digestive tract releasing bradyzoites. The bradyzoites will infect the intestinal epithelium developing into the tachyzoite stage for dissemination throughout the body [101,102].
Neutrophils appear to play an important role in the protection during the acute stage of the infection by T. gondii. It has been reported that mice infected with T. gondii died rapidly when neutrophils were depleted [103] and CXCR2-/- mutant mice that showed a defective migration of neutrophils to the sites of infection had increased numbers of cysts [104]. In addition, neutrophils have an immunomodulatory role in this parasitosis [105]; evidence exist that different subsets of neutrophils are recruited at the infection sites to combat T. gondii pathogen [106].
It has been reported that in murine neutrophils NETosis was triggered by type I, II, and III strains of T. gondii and it did not require active parasitic invasion [95]. NETs caused damage in the plasma membrane of parasites and reduced their capacity to infect fibroblasts, indicating reduction in the parasite viability. Similar results were obtained in vivo using an intranasal infection mouse model. In lung sections, it was observed inflammatory infiltrate composed principally by neutrophils as seen from positive immunohistochemcal staining for MPO and from abundant dsDNA in bronchoalveolar lavage fluid (BLS) in control mice compared with neutrophil-depleted mice, suggesting that the detected DNA was from neutrophils. In addition, tachizoites recovered from BLS of neutrophil-depleted mice conserved their infectivity in fibroblast cultures; however, the infectivity of the tachizoites recovered from the control mice was reduced, indicating the impact of NETs on the control of toxoplasma infection.
Furthermore, T. gondii tachizoites induced NET release in a human promyelocytic leukemia cell line (HL-60) differentiated with dimethyl sulfoxide to neutrophil like-cells and in peripheral blood human neutrophils; in both cases, NETs entrapped tachizoite forms in presence of cytochalasin D, indicating that NETosis occurred independently of active parasitic invasion. It is important to mention that HL-60 cells present different responses depending on the stimuli used to their differentiation: all-trans retinoic acid-differentiated HL-60 cells released NETs only upon PMA stimulation, DMSO-differentiated HL-60 cells only upon A23187 calcium ionophore stimulation, while dimethylformamide-differentiated HL-60 cells formed NETs in response to either stimuli [107]. In this sense, T. gondii-induced NETosis can likely implicate a calcium influx taking into account the case of HL-60 cells differentiated with DMSO. The ERK1/2 MAPK signal pathway was involved in NETosis induced by T. gondii in human neutrophils since MEK inhibition decreased the extruded DNA levels; nevertheless, NETosis was not completely abolished, suggesting that other signaling pathways are involved in the process. It is worth to notice that recognition mediated by TLR was not necessary to trigger NET release by this parasite in contrast with T. cruzi-induced NETosis where TLR signaling was required [65]. These data confirm that different pathways drive NETosis depending upon the stimuli used as it has been previously observed [85].
Neospora caninum
N. caninum is an apicomplexan parasite that can infect different mammalian hosts including cattle, dogs, horses, goats, sheep, deer, and others. This parasite is the principal cause of abortion in cattle in many countries [108].
In vitro NETosis induced by N. caninum tachyzoites has been described in neutrophils from dogs, bovines, and dolphins [109–111]. In all cases, extracellular DNA fibers were detected entrapping tachyzoites and co-localizing with NE, MPO, and histone H3, which confirms that these structures corresponded to NETs. Nevertheless, the mechanism implicated in NET release by N. caninum varies depending on the neutrophils origin. NETosis induced in canine neutrophils showed a time- and dose-dependent kinetics and NADPH oxidase, NE and MPO activities were required for it, whereas time and dose dependency was not observed in bovine neutrophils and the inhibition of NE and MPO enzymatic activities reduced NETs but the differences were not statistically significant. In this regard, while ERK1/2 and p38 MAPK activation as well as the SOCE led to NETosis in canine neutrophils stimulated with N. caninum tachyzoites, the inhibition of these signaling pathways did not cause a significant decrease in NET release bovine neutrophils. Furthermore, NETosis induced by N. caninum in bovine neutrophils was also independent of PAD4 activity and CD11b (Figure 1D).
A summary of the characteristics of the NETosis process triggered by the protozoan parasites is shown in Table 1.
Table 1. Summary of the characteristics of NETosis mechanisms induced by protozoan parasites.
| Parasite | Receptor implicated | ROS dependency | Enzymatic activity required | Signal pathway involved | Anti-parasitic effect | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| NOX-2 | Mitochondria | NE | MPO | PAD4 | |||||
| Leishmania donovani | ND | (−) | ND | ND | ND | ND | ND | (+)1,3 | [55] |
| Leishmania amazonensis classical NETosis | ND | (+) | (−) | (+) | (−) | (+) | PI3Kγ/ERK/PKC | (+)3 | [56,57] |
| Leishmania amazonensis early/rapid NETosis | ND | (−) | ND | (+) | ND | (−) | PI3Kδ/Calcium | (+)3 | [56,57] |
| Trypanosoma cruzi | TLR2/4 | (+) | ND | ND | ND | (+)2 | ND | (+)3 | [65] |
| Entamoeba histolytica | ND | (−) | ND | (+) | ND | (−) | Raf/MERK/ERK | (−) | [75,83,84] |
| Eimeria bovis | CD11b | (+) | ND | (+) | (+) | ND | ERK1/2, p38 MAPK | (+)4 | [90,91] |
| Eimeria arloingi | ND | (+) | ND | (+) | (−) | ND | ND | (+)4 | [93] |
| Toxoplasma gondii | TLR independent | ND | ND | ND | ND | ND | ERK1/2 | (+)3 | [95] |
| Neospora caninum | ND | (+) | ND | (+) | (+) | (−) | ERK 1/2, p38 MAPK | (+)5 | [109–111] |
ND, Not determined.
LPG protects against anti-parasitic effect of NETs.
Determined by histone citrullination.
NETs caused death of parasites.
NETs reduced parasite infectivity.
NETs entrap parasites.
Helminths parasites: an overview
It is estimated that helminths infect more than 2 billion people in developing countries causing high rates of morbidity mainly associated with anemia, malnourishment, and weakness of the host [112]. Helminth infections are usually acquired by the ingestion of water or food contaminated with fecal matter that contains helminth eggs, the ingestion of encysted larvae with meat, or by the penetration of larva migrans through the skin into different target organs.
The helminth infections are generally chronic due to the ability of these parasites to manipulate the immune response of the host through the secretion of immunomodulatory products. As with most infections, helminths initially induce in the host an innate immune response that is essential for the development and establishment of protective adaptive immunity. This adaptive immune response has been suggested to be of Th2-type. The cross-talk between innate and CD4+TH2 cells results in finely adjusted effector responses that control the spreading of the parasite. Although there are larvae of parasite helminths small enough to be phagocytosed by polymorphonuclear cells and macrophages, most of them are too large to be eliminated by this route. The invasion of host tissues by helminths usually leads to the development of granulomas aimed to encapsulate the parasite preventing its dissemination and to destroy it, therefore reducing the possibility of dispersion of antigens that promote inflammation and tissue damage. Macrophages and neutrophils are the earliest cells to be recruited within the granuloma or adjacent tissues followed, depending on the helminth parasite, by the infiltration of eosinophils, basophils, and mast cells (reviewed in [113]).
The evidence suggest variable role of the neutrophils in mediating resistance to helminths, which seems to depend on the context of the immune response and the parasite in question. Thus, it has been described that human neutrophils were able to kill the infective stage of the nematode Strongyloides stercoralis in a complement-dependent way when these cells were cultured together with macrophages [114]. Nevertheless, when neutrophils or macrophages were cultured separately with the parasite, they failed to kill S. stercoralis larvae. Noteworthy, in this study it was also demonstrated that soluble factors released by neutrophils and macrophages cross-activate them to kill the parasite, showing that they cooperate to establish a protective defense. Similar cooperation for optimal killing had also been reported for eosinophils [115]. A major role of neutrophils against the early stages of skin infection by the filaria Litomosoides sigmodontis has also been reported in a neutropenic murine model [116]. In this study, the anti-filarial activity of infiltrating neutrophils was associated with degranulation, oxidative burst, and NETs release effector mechanisms. In contrast, depletion of neutrophils in a murine model had no effect on the severity of tissue damage caused by the trematode Schistosoma mansoni, suggesting that these neutrophils have no protective role in this infection [117]. More recently, it was reported that the recruitment of neutrophils to the liver of mice by gamma-delta T cell subset Vγ2 T, which secrete IL-17A, aggravates the fibrosis caused by an infection with S. japonicum, and contributes to the pathology of the disease [118].
Helminth parasites and NETs
Our understanding of the role of the NETs action against helminth parasites remains incipient. Few studies have been carried out compared with the work done with protozoa; however, some working groups have contributed to a better understanding of the role played by neutrophils in these diseases. Below, we provide the information available regarding the production of NETs induced by metazoan parasites.
Strongyloides stercoralis
S. stercoralis is the etiological agent of strongyloidiasis, a chronic parasitic infection of dogs, cats, and primates including humans principally in the tropical and subtropical regions and, in less extent, in countries with temperate climates. According to WHO, an estimate of 30–100 million people are infected worldwide with S. stercolaris; precise data on prevalence are unknown in endemic countries [119,120].
Infection is acquired when filariform larvae from contaminated soil penetrate the human skin and migrate to the small intestine where they molt twice and become adult female worms. The females live in the epithelium of the small intestine and produce eggs by parthenogenesis. The rhabditiform larvae pass either to the stool or become infective filariform larvae, which can penetrate either the intestinal mucosa or the skin of the perianal area causing autoinfection [119,121].
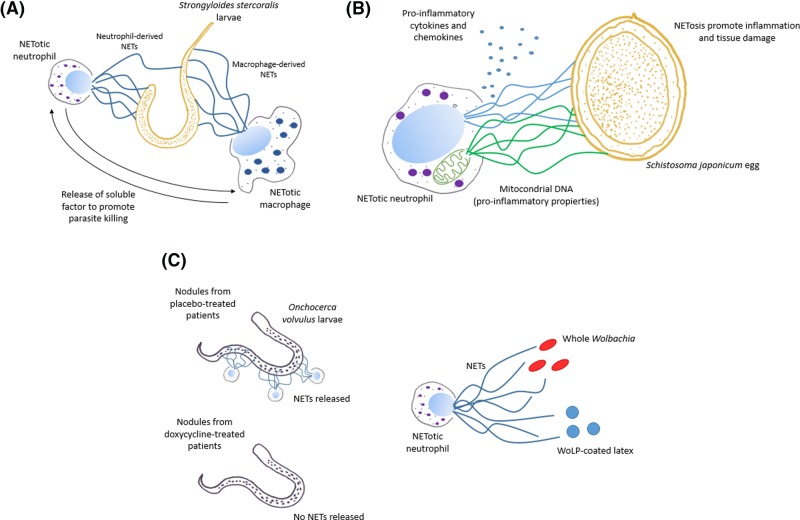
Human neutrophils co-cultured with viable and non-viable S. stercoralis larvae formed NETs in vitro and independently of complement [114]. As previously mentioned, neutrophils or macrophages separately cultured with S. stercoralis larvae did not kill worms; nevertheless, when both cells were cultured together with larvae, they killed 90% of parasites. Interestingly, this effect was reduced by the addition of DNase I, suggesting that the parasite killing was mediated by NETs. Furthermore, human macrophages were able to release ETs in response to S. stercoralis, suggesting that ETs released by macrophages could contribute to the killing activity. NETosis by S. stercoralis larvae, however, was also induced in murine neutrophils but not in murine macrophages. In this case, the anti-parasitic action of these cells was not mediated by NETs since the addition of DNase to the cultures did not reduce the killing efficiency of leukocytes. S. stercoralis-induced NETosis was detected in vivo inoculating L3 larvae in the peritoneal cavity of mice where it was observed increase of free DNA in peritoneal lavages in respect to the controls, and NETs were able to kill the larvae (Figure 2A).
Figure 2. NETosis triggered by helminth parasites and its implications.
(A) Strongyloides stercoralis larvae trigger NETosis in human neutrophils and macrophages. Both cell types are unable to kill the larvae separately; however, together they kill efficiently this parasite due to the release of soluble factors that enhance the anti-parasitic effect of NETs. (B) Schistosoma japonicum eggs induce NET release in human neutrophils. During this NETosis process, mitochondrial DNA and pro-inflammatory cytokines and chemokines are released. These molecules promote an inflammatory response that causes tissue damage observed in the hepatic granuloma caused by this parasite. (C) In nodules excised from placebo-treated patients infected with Onchocerca volvulus, the presence of NETs in close contact with the parasite cuticle was detected; in contrast, NETs were not detected in doxycycline-treated patients, suggesting that the symbiotic bacteria Wolbachia was responsible for triggering NETosis. Confirming this theory, whole Wolbachia and latex beads coated with its lipopeptide (WoLP) triggered in vitro NET release in human neutrophils.
Schistosoma japonicum
Schistosomiasis is caused by different species of trematodes from genus Schistosoma. It is prevalent in tropical and subtropical areas, especially in poor communities. It is estimated that at least 91.4% of the persons requiring treatment for schistosomiasis live in Africa. Intestinal schistosomiasis is caused by S. mansoni, S. japonicum, S. mekongi, and S. guineensis species and the infection is acquired when infective cercariae penetrate the skin of the human host and shed their forked tail, becoming schistosomulae. The schistosomulae migrate through tissues and stages to their residence in the veins. In humans, the adult worms reside in different locations in the mesenteric venules. The females deposit eggs in the small venules of the portal and perivesical systems. The eggs are moved progressively toward the lumen of the intestine and are eliminated with feces. Some of the eggs, however, may also become lodged in the host’s intestine, liver, or other sites causing granulomas [122–124]. During a transcriptomic study of hepatic granulomas caused by S. japonicum and S. mansoni in a mouse model [125], the presence of neutrophil infiltrations in the core and periphery of S. japonicum-induced granulomas was detected. Noteworthy, NET-like structures were visualized surrounding the parasite eggs in contrast with the S. mansoni granuloma where less abundant neutrophils were observed only in the periphery and no NETs were visualized. In vitro, only S. japonicum eggs were able to trigger NET release. Furthermore, it has been described that whole soluble protein or excretory/secretory protein from S. japonicum eggs is able to induce NETs accompanied by the release of mitochondrial DNA and pro-inflammatory cytokines and chemokines [126]. In this regard, it is probable that NET contributes to the damage associated with granuloma by propitiating inflammation since mitochondrial DNA released during NETosis possess pro-inflammatory properties and it has been linked to pathology associated with severe diseases [127,128]. Furthermore, NETs did not affect the viability of S. japonicum eggs entrapped, suggesting the non-protective role of NETosis in this parasitic disease (Figure 2B).
Haemonchus contortus
H. contortus is a highly pathogenic helminth, primarily of small ruminants, with a global distribution. The prevalence of H. contortus is particularly high in the tropical climatic zones of both hemispheres; nevertheless, H. contortus presents adaptability over a wide range of environments. Ruminants are infected when they ingest the L3 infective larvae stage, which pass through the first three stomachs establishing finally in abomasum. L3 larvae shed their cuticles and burrow into the internal layer, where they develop into L4 larvae stage. The L4 larvae then molt and develop into adult form. The adult male and female parasites feed on blood and may remove up to 30 μl of blood per day, rapidly causing anemia and subsequent death [129,130].
H. contortus L3 larvae induced NETosis in bovine neutrophils [131]. Three morphological types of NETs were observed by scanning electron microscopy and immunofluorescence during the interaction of the neutrophils with the larvae according to: diffuse NETs characterized by their globular and compact form, spread NETs observed as smooth elongated web-like structures composed by thin fibers, and at last, aggregated NETs denoted by large clusters of NET-like structures with a ‘ball of yarn’-like clumpy massive appearance involving a high number of neutrophils. Only the aggregated NETs showed the ability to immobilize the larvae, but like with other parasites, NETs did not kill larvae. NETosis was triggered independently of parasite viability as viable and heat-inactivated larvae indistinctly induced NET release. Neutrophils were able to extrude DNA in acidic conditions, indicating that NETosis can well take place in vivo in the abomasum that presents low pH values. Like in other cases, the process depended on NADPH oxidase, MPO, and NE activities.
Nyppostrongylus brasiliensis
N. brasiliensis is a nematode parasite that infects rodents including Rattus norvegicus, R. rattus, and Mus musculus. After percutaneous infection, the L3 larvae stage migrates to the lungs. Here they develop into L4 larvae stage and migrate via the trachea and esophagus to the small intestine. The larvae grow and transform into adult worms. The adults mature and female worms produce eggs. Egg production continues for about a week and then most of the worms are expelled from the rodent [132]. During inoculation of non-viable N. brasiliensis L3 larvae in ear skin of mice, inflammatory neutrophils and monocytes were rapidly recruited followed by the appearance of NET-like structures characterized by the presence of extracellular DNA co-localizable with NE, MPO, and citrullinated histone H3 [133]. These structures were also produced in both TLR2 KO and TLR4 KO mice, suggesting that NETosis triggered by N. brasiliensis did not require TLR signaling.
Filarial nematodes
Filarial diseases are a prevalent public health problem. According to WHO, an estimate of 120 million people in 73 countries from tropical and subtropical areas are infected with lymphatic filariasis [134,135]. Approximately, 90% of filarial infections are caused by Wuchereria bancrofti and the remainder by Brugia spp. Humans are the exclusive host of infection with W. bancrofti whereas certain strains of Brugia malayi can also infect some animal species. River blindness caused by Onchocerca volvulus is found in some foci in Latin America but more than 99% of infected people live in 31 countries in sub-Saharan Africa [135]. The life cycle of filarial nematodes involves uptake of the first-stage larvae (microfilariae, Mf) by a hematophagous arthropod followed, after two moults in the vector, by transmission of the L3 larvae stage to a new vertebrate host, where two further moults later, the nematodes mature as dioecious adults [136]. Noteworthy, filarial nematode species Brugia spp., W. bancrofti, and O. volvulus usually are infected by Wolbachia, a genus of Gram-negative intracellular bacteria belonging to the order Rickettsiales of the family Anaplasmataceae. These bacteria only infect invertebrate organisms and are naturally found in several nematodes [137,138]. The nature of the symbiosis between Wolbachia and nematodes has been considered mutualistic since antibiotic chemotherapy in various Onchocercid species has demonstrated that depletion of Wolbachia is associated with stunting, sterilization, and death of adult worms [139]. In addition, Wolbachia is associated with inflammatory process present in filarial diseases.
The presence of NETs containing NE, MPO, and citrullinated histones was detected in nodules excised from placebo-treated patients infected with O. volvulus nematodes, which were positive for Wolbachia. NETs were visualized by immunofluorescence in close contact with the parasite cuticle [140]. In contrast, NETs were not detected in doxycycline-treated patients and nematodes were negatives for Wolbachia, suggesting that the symbiotic bacteria was responsible for triggering NETosis. In this sense, whole Wolbachia and its lipopeptide (WoLP) bound to latex beads were able to induce NET release in human neutrophils while soluble WoLP failed to do it. This mechanism was triggered via TLR6 since neutrophils from mutant mice lacking TLR6 were unable to form NETs in the presence of whole Wolbachia and WoLP-coated latex beads (Figure 2C).
B. malayi microfilaria induced NETosis in human neutrophils, and in these structures the presence of MPO, NE, and citrullinated histone H4 was detected [141]. In the case of B. malayi microfilaria, NET release was dependent on ROS since NADPH oxidase inhibition led to a decrease in the extruded DNA. Interestingly, addition of autologous serum to the cultures blocked NETosis but this action cannot be attributed to the complement as the heat-treated serum also inhibited NETosis. NET release promoted the attachment of neutrophils to B. malayi microfilaria; nevertheless, NETs do not apparently participate in parasite killing because the addition of DNase I to the cultures did not reduce the killing efficiency of neutrophils.
Litomosoides sigmodontis is a filarial nematode that infects rodents and is commonly used as a model to study filarial diseases. NETosis was detected in murine neutrophils stimulated with L. sigmodontis accompanied by an increase in ROS generation and the release of MPO from cells. L. sigmodentis-induced NETosis was observed in vivo in skin from mice inoculated with the parasite as extracellular DNA co-localized with NE and MPO by immunofluorescence.
Concluding remarks
The study of NETs has advanced by leaps and bounds since they were first described only 14 years ago. Many of the studies have focused on the identification of the stimuli that induce NETs, and on the neutrophil signaling pathways involved. This is particularly true in the case of parasites, some of which trigger NETs by the classical routes, i.e. ROS and PAD4-dependent routes, while others, such as the amoeba, do it in a non-classical way. The panorama seems to be more complicated by the results obtained in the studies of NETs formation by helminth parasites and the identification of the Wolbachia symbiont of the filarial O. volvulus as one of the stimuli of NETs associated with this parasite. This suggests the possibility that the microbiota of helminths, as well as viruses and bacteria carried by protozoan parasites, would be able to influence the pathways of the formation of traps in the neutrophils and, perhaps, their outcome. This is of particular importance if we consider that it has been suggested that large particles such as parasites would induce the formation of NETs whereas small particles, such as bacteria and viruses, would induce phagocytosis [39,40]. Since both viruses and bacteria can induce NETs formation under certain conditions, it is likely that other signals derived from the particle (e.g., pathogenicity and/or virulence signals) and the condition of the neutrophil (primed or not, stressed or not, in circulation or in tissue) are also equal or more important for the outcome under physiological conditions [41,42].
The exact role that NETs play during parasitic infections remains unknown, being an area of intense study at present. Most in vitro evidence indicate that NETs can trap protozoa, and in some cases, such as promastigotes of Leishmania or tachyzoites of toxoplasma, to kill them. In contrast, in cases such as E. histolytica trophozoites, the NETs do not seem to affect the viability and infectivity of the parasite. Something similar has been observed in the case of helminth parasites, where NETs trap and kill the larvae of some of these parasites, but they do not seem to affect the viability of adult worms.
Even less known is the role of NETs during the invasion of host tissues by parasites. Advances in this regard have been hampered by the difficulty in the sure identification of NETs in infected tissues and by the absence of specific inhibitors of in vivo NETs formation. The possibility that NETs participate in the pathology rather than in the defense of the host against parasites also remains latent. In host tissues infected with parasites, the NETs may not spread enough to trap them and, instead, the traps may exacerbate the local inflammatory response by activating the complement and coagulation systems, contributing thus to tissue damage [142]. This could help explain the large tissue damage associated with some parasitic infections, such as amoebiasis and schistosomiasis.
Finally, the elucidation of the role of NETs and the understanding of the cellular mechanisms behind their regulation could contribute to the development of more efficient strategies of the control of parasitic infections and the amelioration of the damage caused by them in humans and animals of veterinary importance.
Acknowledgments
C.D.-G. is a PhD student of the Programa de Maestría y Doctorado en Ciencias Bioquímicas, UNAM, and received a scholarship from Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico (432205/596731). We thank Israel Rodríguez for the support in the search of the information and Pavel Petrossyan for the critical revision of the manuscript and corrections of the English.
Abbreviations
- BLS
bronchoalveolar lavage fluid
- ET
extracellular trap
- LPG
lipopetidoglycan
- MPO
myeloperoxidase
- NE
neutrophil elastase
- NET
neutrophil extracellular trap
- PAD4
peptidyl arginine deiminase 4
- PKC
protein kinase C
- PMA
phorbol miristate acetate
- ROS
reactive oxygen species
- WHO
World Health Organization
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
J.C.C. and C.D.-G. wrote the paper and made drawings.
Funding
This work was supported in part by [Grant 284830] from Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico (http://conacyt.mx), by [Grant IN206316] from Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México (http://dgapa.unam.mx), and by a special Grant for encouragement of medical research from the ‘Miguel Alemán Valdés’ Foundation, Mexico (https://www.miguelaleman.org). Funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zambrano-Villa S., Rosales-Borjas D., Carrero J.C. and Ortiz-Ortiz L. (2002) How protozoan parasites evade the immune response. Trends Parasitol. 18, 272–278 10.1016/S1471-4922(02)02289-4 [DOI] [PubMed] [Google Scholar]
- 2.WHO (2008) The Global Burden of Disease: 2004 Update, WHO Press, Geneva, Switzerland [Google Scholar]
- 3.Hunter P.R. (2003) Climate change and waterborne and vector-borne disease. J. Appl. Microbiol. 94, 37–46 10.1046/j.1365-2672.94.s1.5.x [DOI] [PubMed] [Google Scholar]
- 4.Omarova A., Tussupova K., Berndtsson R., Kalishev M. and Sharapatova K. (2018) Protozoan parasites in drinking water: A system approach for improved water, sanitation and hygiene in developing countries. Int. J. Environ. Res. Public Health 15, 1–18 10.3390/ijerph15030495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang K., LeJeune J., Alsdorf D., Lu B., Shum C.K. and Liang S. (2012) Global distribution of outbreaks of water-associated infectious diseases. PLoS Neglected Trop. Dis. 6, e1483 10.1371/journal.pntd.0001483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Short E.E., Caminade C. and Thomas B.N. (2017) Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect. Dis.: Res. Treat. 10, 1–7 10.1177/1178633617732296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harper S.L., Edge V.L., Schuster-Wallace C.J., Berke O. and McEwen S.A. (2011) Weather, water quality and infectious gastrointestinal illness in two inuit communities in Nunatsiavut, Canada: potential implications for climate change. EcoHealth 8, 93–108 10.1007/s10393-011-0690-1 [DOI] [PubMed] [Google Scholar]
- 8.Torgerson P.R. and Macpherson C.N.L. (2011) The socioeconomic burden of parasitic zoonoses: global trends. Vet. Parasitol. 182, 79–95 10.1016/j.vetpar.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 9.Hartmann P., Becker R., Franzen C., Schell-Frederick E., Romer J., Jacobs M.. et al. (2001) Phagocytosis and killing of Mycobacterium avium complex by human neutrophils. J. Leukocyte Biol. 69, 397–404, Retrieved from http://www.jleukbio.org/content/69/3/397.long%5Cnhttp://www.jleukbio.org/content/69/3/397.short [PubMed] [Google Scholar]
- 10.Lee W.L., Harrison R.E. and Grinstein S. (2003) Phagocytosis by neutrophils. Microbes Infect. 5, 1299–1306 10.1016/j.micinf.2003.09.014 [DOI] [PubMed] [Google Scholar]
- 11.Vonk A.G., Wieland C.W., Netea M.G. and Kullberg B.J. (2002) Phagocytosis and intracellular killing of Candida albicans blastoconidia by neutrophils and macrophages: a comparison of different microbiological test systems. J. Microbiol. Methods 49, 55–62 10.1016/S0167-7012(01)00348-7 [DOI] [PubMed] [Google Scholar]
- 12.van Kesse K.P.M., Bestebroer J. and van Strijp J.A.G. (2014) Neutrophil-mediated phagocytosis of Staphylococcus aureus. Front. Immunol. 5, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wazny T.K., Mummaw N. and Styrt B. (1990) Degranulation of human neutrophils after exposure to bacterial phospholipase C. Eur. J. Clin. Microbiol. Inf. Dis.: Off. Publ. Eur. Soc. Clin. Microbiol. 9, 830–832, Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2128277 10.1007/BF01967385 [DOI] [PubMed] [Google Scholar]
- 14.Nøorgaard A., Andersen L.P. and Nielsen H. (1995) Neutrophil degranulation by Helicobacter pylori proteins. Gut 36, 354–357, Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1382444&tool=pmcentrez&rendertype=abstract 10.1136/gut.36.3.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacy P. (2006) Mechanisms of degranulation in neutrophils. Allergy, Asthma, Clin. Immunol. 2, 98–108 10.1186/1710-1492-2-3-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swamydas M., Gao J.L., Break T.J., Johnson M.D., Jaeger M., Rodriguez C.A.. et al. (2016) CXCR1-mediated neutrophil degranulation and fungal killing promote Candida clearance and host survival. Sci. Transl. Med. 8, 322ra10 10.1126/scitranslmed.aac7718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S.. et al. (2004) Neutrophil extracellular traps kill bacteria. Science (New York, N.Y.) 303, 1532–1535 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 18.Bruns S., Kniemeyer O., Hasenberg M., Aimanianda V., Nietzsche S., Thywißen A.. et al. (2010) Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and Influenced by hydrophobin RodA. PLoS Pathog. 6, e1000873 10.1371/journal.ppat.1000873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zawrotniak M. and Rapala-Kozik M. (2013) Neutrophil extracellular traps (NETs) - Formation and implications. Acta Biochim. Pol. 60, 277–284, [pii] [PubMed] [Google Scholar]
- 20.Arazna M., Pruchniak M.P. and Demkow U. (2013) Neutrophil extracellular traps in bacterial infections: strategies for escaping from killing. Respir. Physiol. Neurobiol. 187, 74–77 10.1016/j.resp.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 21.Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V.. et al. (2007) Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yousefi S., Mihalache C., Kozlowski E., Schmid I. and Simon H.U. (2009) Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 16, 1438–1444 10.1038/cdd.2009.96 [DOI] [PubMed] [Google Scholar]
- 23.Takei H., Araki A., Watanabe H., Ichinose A. and Sendo F. (1996) Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukocyte Biol. 59, 229–240 10.1002/jlb.59.2.229 [DOI] [PubMed] [Google Scholar]
- 24.Hakkim A., Fuchs T.A., Martinez N.E., Hess S., Prinz H., Zychlinsky A.. et al. (2011) Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 7, 75–77 10.1038/nchembio.496 [DOI] [PubMed] [Google Scholar]
- 25.Metzler K.D., Goosmann C., Lubojemska A., Zychlinsky A. and Papayannopoulos V. (2014) Myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 8, 883–896 10.1016/j.celrep.2014.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papayannopoulos V., Metzler K.D., Hakkim A. and Zychlinsky A. (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191, 677–691 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Li M., Stadler S., Correll S., Li P., Wang D.. et al. (2009) Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 184, 205–213 10.1083/jcb.200806072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leshner M., Wang S., Lewis C., Zheng H., Chen X.A., Santy L.. et al. (2012) PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 3, 1–11 10.3389/fimmu.2012.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bicker K.L. and Thompson P.R. (2013) The protein arginine deiminases: Structure, function, inhibition, and disease. Biopolymers 99, 155–163 10.1002/bip.22127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douda D.N., Khan M.A., Grasemann H. and Palaniyar N. (2015) SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. U.S.A. 112, 2817–2822 10.1073/pnas.1414055112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song H.-O. and Ryu J.-S. (2013) Superoxide anion production by human neutrophils activated by Trichomonas vaginalis. Korean J. Parasitol. 51, 479–84 10.3347/kjp.2013.51.4.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlsen E.D., Jie Z., Liang Y., Henard C.A., Hay C., Sun J.. et al. (2015) Interactions between neutrophils and Leishmania braziliensis amastigotes facilitate cell activation and parasite clearance. J. Innate Immun. 7, 354–363 10.1159/000373923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sim S., Yong T.-S., Park S.-J., Im K.-i., Kong Y., Ryu J.-S.. et al. (2005) NADPH Oxidase-derived reactive oxygen species-mediated activation of ERK1/2 is required for apoptosis of human neutrophils induced by Entamoeba histolytica. J. Immunol. 174, 4279–4288 10.4049/jimmunol.174.7.4279 [DOI] [PubMed] [Google Scholar]
- 34.Pugine S.M.P., De Faria M.F., Maia A.A.M., Do Valle C.R., Boschini C., Poleti M.D.. et al. (2005) Effect of Cysticercus cellulosae fractions on the respiratory burst of pig neutrophils. Rev. Inst. Med. Trop. Sao Paulo 47, 91–94, [pii]/r/S0036-46652005000200006 10.1590/S0036-46652005000200006 [DOI] [PubMed] [Google Scholar]
- 35.Villalta F. and Kierszenbaum F. (1983) Role of polymorphonuclear cells in Chagas’ disease. I. Uptake and mechanisms of destruction of intracellular (amastigote) forms of Trypanosoma cruzi by human neutrophils. J. Immunol. 131, 1504–1510, [PubMed] [Google Scholar]
- 36.Villalta F. and Kierszenbaum F. (1986) Effects of human colony-stimulating factor on the uptake and destruction of a pathogenic parasite (Trypanosoma cruzi) by human neutrophils. J. Immunol. 137, 1703–1707 [PubMed] [Google Scholar]
- 37.Carlsen E.D., Hay C., Henard C.A., Popov V., Garg N.J. and Soong L. (2013) Leishmania amazonensis amastigotes trigger neutrophil activation but resist neutrophil microbicidal mechanisms. Infect. Immun. 81, 3966–3974 10.1128/IAI.00770-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manfredi A.A., Ramirez G.A., Rovere-Querini P. and Maugeri N. (2018) The neutrophil’s choice: Phagocytose vs make neutrophil extracellular traps. Front. Immunol. 9, 9–13 10.3389/fimmu.2018.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Branzk N., Lubojemska A., Hardison S.E., Wang Q., Gutierrez M.G., Brown G.D.. et al. (2014) Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 15, 1017–1025 10.1038/ni.2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warnatsch A., Tsourouktsoglou T.D., Branzk N., Wang Q., Reincke S., Herbst S.. et al. (2017) Reactive oxygen species localization programs inflammation to clear microbes of different size. Immunity 46, 421–432 10.1016/j.immuni.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonald B., Urrutia R., Yipp B.G., Jenne C.N. and Kubes P. (2012) Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 12, 324–333 10.1016/j.chom.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 42.Kim S.J. and Jenne C.N. (2016) Role of platelets in neutrophil extracellular trap (NET) production and tissue injury. Semin. Immunol. 28, 546–554 10.1016/j.smim.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 43.Baldursson S., Karanis P., Barardi C.R.M., Aquino V.R., Constante C.C., Bakos L.. et al. (2011) Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2004–2010. Water Res. 45, 6603–6614 10.1016/j.watres.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 44.Efstratiou A., Ongerth J.E. and Karanis P. (2017) Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2011–2016. Water Res. 114, 14–22 10.1016/j.watres.2017.01.036 [DOI] [PubMed] [Google Scholar]
- 45.Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S.. et al. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet North Am. Ed. 382, 209–222 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 46.Platts-Mills J.A., Babji S., Bodhidatta L., Gratz J., Haque R., Havt A.. et al. (2015) Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). The Lancet Global Health 3, 564–575 10.1016/S2214-109X(15)00151-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abajobir A.A., Abbafati C., Abbas K.M., Abd-Allah F., Abera S.F., Aboyans V.. et al. (2017) Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet North Am. Ed. 390, 1151–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V.. et al. (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet North Am. Ed. 380, 2095–2128 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amulic B., Cazalet C., Hayes G.L., Metzler K.D. and Zychlinsky A. (2012) Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30, 459–489 10.1146/annurev-immunol-020711-074942 [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization (2018) Leishmaniasis. Retrieved from https://www.who.int/leishmaniasis/en/ Accessed 2 May, 2018 [Google Scholar]
- 51.Torres-Guerrero E., Quintanilla-Cedillo M.R., Ruiz-Esmenjaud J. and Arenas R. (2017) Leishmaniasis: a review. F1000 Res. 6, 750 10.12688/f1000research.11120.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sousa L.M.A., Carneiro M.B.H., Resende M.E., Martins L.S., dos Santos L.M., Vaz L.G.. et al. (2014) Neutrophils have a protective role during early stages of Leishmania amazonensis infection in BALB/c mice. Parasite Immunol. 36, 13–31 10.1111/pim.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laufs H., Müller K., Fleischer J., Jahnke N., Jensenius J.C., Solbach W.. et al. (2002) Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infect. Immun. 70, 826–835 10.1128/IAI.70.2.826-835.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guimaraes-Costa A.B., Nascimento M.T.C., Froment G.S., Soares R.P.P., Morgado F.N., Conceicao-Silva F.. et al. (2009) Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. U.S.A. 106, 6748–6753 10.1073/pnas.0900226106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gabriel C., McMaster W.R., Girard D. and Descoteaux A. (2010) Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J. Immunol. 185, 4319–4327 10.4049/jimmunol.1000893 [DOI] [PubMed] [Google Scholar]
- 56.Rochael N.C., Guimarães-Costa A.B., Nascimento M.T.C., DeSouza-Vieira T.S., Oliveira M.P., Garcia e Souza L.F.. et al. (2015) Classical ROS-dependent and early/rapid ROS-independent release of neutrophil extracellular traps triggered by leishmania parasites. Sci. Rep. 5, 18302 10.1038/srep18302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeSouza-Vieira T., Guimaraes-Costa A., Rochael N.C., Lira M.N., Nascimento M.T., Lima-Gomez P.. et al. (2016) Neutrophil extracellular traps release induced by Leishmania: role of PI3K, ERK, PI3K, PKC, and [Ca2+]. J. Leukocyte Biol. 100, 801–810 10.1189/jlb.4A0615-261RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adam-Vizi V. and Starkov A.A. (2010) Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J. Alzheimer’s Dis. 20, S413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organization (2018) Chagas disease (American trypanosomiasis). Retrieved from https://www.who.int/chagas/en/ Accessed 5 May, 2018 [PMC free article] [PubMed] [Google Scholar]
- 60.Tyler K.M. and Engman D.M. (2001) The life cycle of Trypanosoma cruzi. Int. J. Parasitol. 31, 472–481 10.1016/S0020-7519(01)00153-9 [DOI] [PubMed] [Google Scholar]
- 61.Carrea A. and Diambra L. (2016) Systems biology approach to model the life cycle of Trypanosoma cruzi. PLoS One 11, 1–20 10.1371/journal.pone.0146947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanderson C.J. and de Souza W. (1979) A morphological study of the interaction between Trypanosoma cruzi and rat eosinophils, neutrophils and macrophages in vitro. J. Cell Sci. 37, 275–286 [DOI] [PubMed] [Google Scholar]
- 63.Grage-Griebenow E., Zawatzky R., Kahlert H., Brade L., Flad H.D. and Ernst M. (2001) Neutrophil depletion exacerbates experimental Chagas’ disease in BALB/c, but protects C57BL/6 mice through modulating the Th1/Th2 dichotomy in different directions. Eur. J. Immunol. 31, 265–275 10.1002/1521-4141(200101)31:1%3c265::AID-IMMU265%3e3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- 64.Molina H.a and Kierszenbaum F. (1989) Interaction of human eosinophils or neutrophils with Trypanosoma cruzi in vitro causes bystander cardiac cell damage. Immunology 66, 289–295 [PMC free article] [PubMed] [Google Scholar]
- 65.Sousa-Rocha D., Thomaz-Tobias M., Diniz L.F.A., Souza P.S.S., Pinge-Filho P. and Toledo K.A. (2015) Trypanosoma cruzi and its soluble antigens induce NET release by stimulating toll-like receptors. PLoS One 10, 1–16 10.1371/journal.pone.0139569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogier-Denis E., Mkaddem S.Ben and Vandewalle A. (2008) NOX enzymes and toll-like receptor signaling. Semin. Immunopathol. 30, 291–300 10.1007/s00281-008-0120-9 [DOI] [PubMed] [Google Scholar]
- 67.Heiner I., Eisfeld J. and Lückhoff A. (2003) Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium 33, 533–540 10.1016/S0143-4160(03)00058-7 [DOI] [PubMed] [Google Scholar]
- 68.Silva M.T.N., Santana J.V., Bragagnoli G., Marinho A.M., da N. and Malagueño E. (2014) Prevalence of Entamoeba histolytica/Entamoeba dispar in the city of Campina Grande, in Northeastern Brazil. Rev. Inst. Med. Trop. São Paulo 56, 451–454 10.1590/S0036-46652014000500015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leung P.O., Chen K.H., Chen K.L., Tsai Y.T., Liu S.Y. and Chen K.T. (2014) Epidemiological features of intestinal infection with Entamoeba histolytica in Taiwan, 2002–2010. Travel Med. Infect. Dis. 12, 673–679 10.1016/j.tmaid.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 70.Ghenghesh K.S., Ghanghish K., BenDarif E.T., Shembesh K. and Franka E. (2016) Prevalence of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium spp. in Libya: 2000–2015. Libyan J. Med. 11, 1–6 10.3402/ljm.v11.32088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chalmers R.M. (2013) Entamoeba histolytica. Microbiology of Waterborne Diseases: Microbiological Aspects and Risks, 2nd edn, Elsevier [Google Scholar]
- 72.Wuerz T., Kane J.B., Boggild A.K., Krajden S., Keystone J.S., Fuksa M.. et al. (2012) A review of amoebic liver abscess for clinicians in a nonendemic setting. Can. J. Gastroenterol. 26, 729–733 10.1155/2012/852835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Asgharpour A., Gilchrist C., Baba D., Hamano S. and Houpt E. (2005) Resistance to intestinal Entamoeba histolytica infection is conferred by innate immunity and Gr-1+ cells. Infect. Immun. 73, 4522–4529 10.1128/IAI.73.8.4522-4529.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sim S., Kim K.A., Yong T.S., Park S.J., Im K.I. and Shin M.H. (2004) Ultrastructural observation of human neutrophils during apoptotic cell death triggered by Entamoeba histolytica. Korean J. Parasitol. 42, 205–208, [pii] 10.3347/kjp.2004.42.4.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ávila E.E., Salaiza N., Pulido J., Rodríguez M.C., Díaz-Godínez C., Laclette J.P.. et al. (2016) Entamoeba histolytica trophozoites and lipopeptidophosphoglycan trigger human neutrophil extracellular traps. PLoS One 11, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brinkmann V. and Zychlinsky A. (2012) Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell Biol. 198, 773–783 10.1083/jcb.201203170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rico-Mata R., De Leon-Rodriguez L.M. and Avila E.E. (2013) Effect of antimicrobial peptides derived from human cathelicidin LL-37 on Entamoeba histolytica trophozoites. Exp. Parasitol. 133, 300–306 10.1016/j.exppara.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 78.Ayala-Sumuano J.-T., Téllez-López V.M., Domínguez-Robles M.D.C., Shibayama-Salas M. and Meza I. (2013) Toll-like receptor signaling activation by Entamoeba histolytica induces beta defensin 2 in human colonic epithelial cells: its possible role as an element of the innate immune response. PLoS Neglected Trop. Dis. 7, e2083 10.1371/journal.pntd.0002083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weiner D.J., Bucki R. and Janmey P.A. (2003) The antimicrobial activity of the cathelicidin LL37 is inhibited by F-actin bundles and restored by gelsolin. Am. J. Respir. Cell Mol. Biol. 28, 738–745 10.1165/rcmb.2002-0191OC [DOI] [PubMed] [Google Scholar]
- 80.Neumann A., Völlger L., Berends E.T.M., Molhoek E.M., Stapels D.A.C., Midon M.. et al. (2014) Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular Traps against degradation by bacterial nucleases. J. Innate Immun. 6, 860–868 10.1159/000363699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andrä J., Berninghausen O. and Leippe M. (2004) Membrane lipid composition protects Entamoeba histolytica from self-destruction by its pore-forming toxins. FEBS Lett. 564, 109–115 10.1016/S0014-5793(04)00324-2 [DOI] [PubMed] [Google Scholar]
- 82.Wang Y., Chen Y., Xin L., Beverley S.M., Carlsen E.D., Popov V.. et al. (2011) Differential microbicidal effects of human histone proteins H2A and H2B on Leishmania promastigotes and amastigotes. Infect. Immun. 79, 1124–1133 10.1128/IAI.00658-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Díaz-Godínez C., Fonseca Z., Néquiz M., Laclette J.P. and Rosales C. (2018) Entamoeba histolytica Trophozoites Induce a Rapid Non-classical NETosis Mechanism Independent of NOX2-Derived Reactive Oxygen Species and PAD4 Activity. Front. Cell Infect Microbiol. 8, 81–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fonseca Z., Díaz-Godínez C., Mora N., Alemán O.R., Uribe-Querol E., Carrero J.C.. et al. (2018) Entamoeba histolytica induce signaling via Raf/MEK/ERK for neutrophil extracellular trap (NET) formation. Front. Cell. Infect. Microbiol. 8, 1–19 10.3389/fcimb.2018.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muth A., Mondal S., Kenny E.F., Herzig A., Kru R., Thompson P.R.. et al. (2017) Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 6, 1–21 10.7554/eLife.24437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Konig M.F. and Andrade F. (2016) A critical reappraisal of neutrophil extracellular traps and NETosis mimics based on differential requirements for protein citrullination. Front. Immunol. 7, 461 10.3389/fimmu.2016.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chapman H.D., Barta J.R., Blake D., Gruber A., Jenkins M., Smith N.C.. et al. (2013) A selective review of advances in coccidiosis research. Adv. Parasitol. 83, 93–171 10.1016/B978-0-12-407705-8.00002-1 [DOI] [PubMed] [Google Scholar]
- 88.Lillehoj H.S. and Trout J.M. (1993) Coccidia: a review of recent advances on immunity and vaccine development. Avian Pathol. 22, 3–31 10.1080/03079459308418897 [DOI] [PubMed] [Google Scholar]
- 89.Schito M.L. and Barta J.R. (1997) Nonspecific immune responses and mechanisms of resistance to Eimeria papillata infections in mice. Infect. Immun. 65, 3165–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Behrendt J.H., Ruiz A., Zahner H., Taubert A. and Hermosilla C. (2010) Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria bovis. Vet. Immunol. Immunopathol. 133, 1–8 10.1016/j.vetimm.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 91.Muñoz-Caro T., Huertas S.J.M., Conejeros I., AlarcÃ3n P., Hidalgo M.A., Burgos R.A.. et al. (2015) Eimeria bovis-triggered neutrophil extracellular trap formation is cd11b-, ERK 1/2-, p38 MAP kinase- and soce-dependent. Vet. Res. 46, 23 10.1186/s13567-015-0155-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alemán O.R., Mora N., Cortes-Vieyra R., Uribe-Querol E., Rosales C.. et al. (2016) Differential use of human neutrophil Fc γ receptors for inducing neutrophil extracellular trap formation. J. Immunol. Res. 2016, 1–17 10.1155/2016/2908034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Silva L.M.R., Muñoz Caro T., Gerstberger R., Vila-Viçosa M.J.M., Cortes H.C.E., Hermosilla C.. et al. (2014) The apicomplexan parasite Eimeria arloingi induces caprine neutrophil extracellular traps. Parasitol. Res. 113, 2797–2807 10.1007/s00436-014-3939-0 [DOI] [PubMed] [Google Scholar]
- 94.Muñoz-Caro T., Machado Ribeiro da Silva L., Rentería-Solis Z., Taubert A. and Hermosilla C. (2016) Neutrophil extracellular traps in the intestinal mucosa of Eimeria-infected animals. Asian Pac. J. Trop. Biomed. 6, 301–307 10.1016/j.apjtb.2016.01.001 [DOI] [Google Scholar]
- 95.Abdallah Abi, S. D., Lin C., Ball C.J., King M.R., Duhamel G.E.. et al. (2012) Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect. Immun. 80, 768–777 10.1128/IAI.05730-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chow O.A., Von Köckritz-Blickwede M., Bright A.T., Hensler M.E., Zinkernagel A.S., Cogen A.L.. et al. (2010) Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 8, 445–454 10.1016/j.chom.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Granger V., Faille D., Marani V., Noël B., Gallais Y., Szely N.. et al. (2017) Human blood monocytes are able to form extracellular traps. J. Leukocyte Biol. 102, 775–781 10.1189/jlb.3MA0916-411R [DOI] [PubMed] [Google Scholar]
- 98.Kargi H.A., Campbell E.J. and Kuhn C. III (1990) Elastase and cathepsin G of human monocytes: heterogeneity and subcellular localization to peroxidase-positive granules. J. Histochem. Cytochem.: Off. J. Histochem. Soc. 38, 1179–1186 10.1177/38.8.2164060 [DOI] [PubMed] [Google Scholar]
- 99.Ueki S., Konno Y., Takeda M., Hirokawa M., Matsuwaki Y., Ohta N.. et al. (2017) HHS Public Access. 137, 258–267 [Google Scholar]
- 100.Center for Disease Control and Prevention (2017) Toxoplasmosis (Toxoplasma infection). Retrieved from https://www.cdc.gov/parasites/toxoplasmosis/epi.html Accessed 7 May, 2018 [Google Scholar]
- 101.Robert-Gangneux F. and Dardé M.L. (2012) Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25, 264–296 10.1128/CMR.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Black M.W. and Boothroyd J.C. (2000) Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64, 607–623 10.1128/MMBR.64.3.607-623.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sayles P.,C. and Johnson L.,L. (1996) Exacerbation of toxoplasmosis in neutrophil-depleted mice. Nat. Immun. 15, 249–258, 1996-1997 [PubMed] [Google Scholar]
- 104.Del Rio L., Bennouna S., Salinas J. and Denkers E.Y. (2001) CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J. Immunol. 167, 6503–6509 10.4049/jimmunol.167.11.6503 [DOI] [PubMed] [Google Scholar]
- 105.Bliss S.K., Gavrilescu L.C., Alcaraz A., Denkers Y. and Alcaraz A.N.A. (2001) Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect. Immun. 69, 4898–4905 10.1128/IAI.69.8.4898-4905.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Biswas A., French T., Düsedau H.P., Mueller N., Riek-Burchardt M., Dudeck A.. et al. (2017) Behavior of neutrophil granulocytes during Toxoplasma gondii infection in the central nervous system. Front. Cell. Infect. Microbiol. 7, 1–13 10.3389/fcimb.2017.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manda-Handzlik A., Bystrzycka W., Wachowska M., Sieczkowska S., Stelmaszczyk-Emmel A., Demkow U.. et al. (2018) The influence of agents differentiating HL-60 cells toward granulocyte-like cells on their ability to release neutrophil extracellular traps. Immunol. Cell Biol. 96, 413–425 10.1111/imcb.12015 [DOI] [PubMed] [Google Scholar]
- 108.Dubey J.P. (2003) Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 41, 1–16 10.3347/kjp.2003.41.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei Z., Hermosilla C., Taubert A., He X., Wang X., Gong P.. et al. (2016) Canine neutrophil extracellular traps release induced by the apicomplexan parasite Neospora caninum in vitro. Front. Immunol. 7, 436 10.3389/fimmu.2016.00436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Villagra-Blanco R., Silva L.M.R., Aguilella-Segura A., Arcenillas-Hernández I., Martínez-Carrasco C., Seipp A.. et al. (2017) Bottlenose dolphins (Tursiops truncatus) do also cast neutrophil extracellular traps against the apicomplexan parasite Neospora caninum. Int. J. Parasitol.: Parasites Wildl. 6, 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Villagra-Blanco R., Silva L.M.R., Muñoz-Caro T., Yang Z., Li J., Gärtner U.. et al. (2017) Bovine polymorphonuclear neutrophils cast neutrophil extracellular traps against the abortive parasite Neospora caninum. Front. Immunol. 8, 1–11 10.3389/fimmu.2017.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.WHO (1999) World Health Report, World Health Organization, Geneva, Switzerland [Google Scholar]
- 113.Anthony R.M., Rutitzky L.I., Urban J.F., Stadecker M.J. and Gause W.C. (2007) Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 7, 975–987 10.1038/nri2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bonne-Année S., Kerepesi L.A., Hess J.A., Wesolowski J., Paumet F., Lok J.B.. et al. (2014) Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microbes Infect. 16, 502–511 10.1016/j.micinf.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Galioto A.M., Hess J.A., Nolan T.J., Schad G.A., Lee J.J. and Abraham D. (2006) Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval Strongyloides stercoralis in mice. Infect. Immun. 74, 5730–5738 10.1128/IAI.01958-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pionnier N., Brotin E., Karadjian G., Hemon P., Gaudin-Nomé F., Vallarino-Lhermitte N.. et al. (2016) Neutropenic Mice Provide Insight into the Role of Skin-Infiltrating Neutrophils in the Host Protective Immunity against Filarial Infective Larvae. PLoS Neglected Trop. Dis. 10, 1–24 10.1371/journal.pntd.0004605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Herbert D.B.R., Ho C., Mohrs M., Arendse B., Schwegmann A., Radwanska M.. et al. (2004) Herbert et al. - 2004 - Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology.pdf. 21, 23845. [DOI] [PubMed] [Google Scholar]
- 118.Zheng L, Hu Y, Wang Y, Huang X, Xu Y, Shen Y. et al. (2017) Recruitment of neutrophils mediated by Vγ2 γδ T cells deteriorates liver fibrosis induced by Schistosoma japonicum Infection in C57BL/6 Mice. Infect. Immun. 85, e01020 10.1128/IAI.01020-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.World Health Organization (2018) Intestinal worms. Retrieved from http://www.who.int/intestinal_worms/epidemiology/strongyloidiasis/en/ Accessed 15 May, 2018 [Google Scholar]
- 120.Cimino R.O. and Krolewiecki A. (2014) The epidemiology of human strongyloidiasis. Appl. Health Econ. Health Policy 1, 216–222 [Google Scholar]
- 121.Center for Disease Control and Prevention (2017) Strongyloidiasis. Retrieved from https://www.cdc.gov/dpdx/strongyloidiasis/index.html Accessed 18 May, 2018 [Google Scholar]
- 122.World Health Organization (2018) Schistosomiasis. Retrieved from http://www.who.int/schistosomiasis/epidemiology/en/ Accessed 18 May, 2018 [Google Scholar]
- 123.Center for Disease Control and Prevention (2017) Schistosomiasis infection. Retrieved from https://www.cdc.gov/dpdx/schistosomiasis/index.html Accessed 18 May, 2018 [Google Scholar]
- 124.Hams E., Aviello G. and Fallon P.G. (2013) The schistosoma granuloma: friend or foe? Front. Immunol. 4, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chuah C., Jones M.K., Burke M.L., Owen H.C., Anthony B.J., McManus D.P.. et al. (2013) Spatial and temporal transcriptomics of Schistosoma japonicum-induced hepatic granuloma formation reveals novel roles for neutrophils. J. Leukocyte Biol. 94, 353–365 10.1189/jlb.1212653 [DOI] [PubMed] [Google Scholar]
- 126.Chuah C., Jones M.K., Burke M.L., Mcmanus D.P., Owen H.C. and Gobert G.N. (2014) Defining a pro-inflammatory neutrophil phenotype in response to schistosome eggs. Cell. Microbiol. 16, 1666–1677 10.1111/cmi.12316 [DOI] [PubMed] [Google Scholar]
- 127.Keshari R.S., Jyoti A., Kumar S., Dubey M., Verma A., Srinag B.S.. et al. (2012) Neutrophil extracellular traps contain mitochondrial as well as nuclear DNA and exhibit inflammatory potential. Cytometry A 81, 238–247 10.1002/cyto.a.21178 [DOI] [PubMed] [Google Scholar]
- 128.Lood C., Blanco L.P., Purmalek M.M., Carmona-Rivera C., De Ravin S.S., Smith C.K.. et al. (2016) Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 10.1038/nm.4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Besier R.B., Kahn L.P., Sargison N.D. and Van Wyk J.A. (2016) The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv. Parasitol. 93, 95–143 10.1016/bs.apar.2016.02.022 [DOI] [PubMed] [Google Scholar]
- 130.Emery D.L., Hunt P.W. and Le Jambre L.F. (2016) Haemonchus contortus: the then and now, and where to from here? Int. J. Parasitol. 46, 755–769 10.1016/j.ijpara.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 131.Muñoz-Caro T., Rubio R, M.C., Silva L.M.R., Magdowski G., Gärtner U., McNeilly T.N.. et al. (2015) Leucocyte-derived extracellular trap formation significantly contributes to Haemonchus contortus larval entrapment. Parasites Vectors 8, 1–12 10.1186/s13071-015-1219-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bridget M.,O. and Valerie E.,J. (1971) Nypostrongylus brasiliensis: a review of immunity and the host parasite relationship in the rat. Exp. Parasitol. 29, 138–177 10.1016/0014-4894(71)90021-X [DOI] [PubMed] [Google Scholar]
- 133.Pellefigues C., Tang S.C., Schmidt A., White R.F., Lamiable O., Connor L.M.. et al. (2017) Toll-like receptor 4, but not neutrophil extracellular traps, promote IFN type I expression to enhance Th2 responses to Nippostrongylus brasiliensis. Front. Immunol. 8, 1–14 10.3389/fimmu.2017.01575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Center for Disease Control and Prevention (2013) Lymphatic filariasis. Retrieved from https://www.cdc.gov/parasites/lymphaticfilariasis/epi.html Accessed 22 May, 2018 [Google Scholar]
- 135.World Health Organization (2018) Lymphatic filariasis. Retrieved from http://www.who.int/lymphatic_filariasis/epidemiology/en/ Accessed 22 May, 2018 [Google Scholar]
- 136.Armstrong S.D., Babayan S.A., Lhermitte-Vallarino N., Gray N., Xia D., Martin C.. et al. (2014) Comparative analysis of the secretome from a model filarial nematode (Litomosoides sigmodontis) reveals maximal diversity in gravid female parasites. Mol. Cell Proteomics 13, 2527–2544 10.1074/mcp.M114.038539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Slatko B.E., Taylor M.J. and Foster J.M. (2010) The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis 51, 55–65 10.1007/s13199-010-0067-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kamtchum-Tatuene J., Makepeace B.L., Benjamin L., Baylis M. and Solomon T. (2017) The potential role of Wolbachia in controlling the transmission of emerging human arboviral infections. Curr. Opin. Infect. Dis. 30, 108–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bouchery T., Lefoulon E., Karadjian G., Nieguitsila A. and Martin C. (2013) The symbiotic role of Wolbachia in Onchocercidae and its impact on filariasis. Clin. Microbiol. Infect. 19, 131–140 10.1111/1469-0691.12069 [DOI] [PubMed] [Google Scholar]
- 140.Tamarozzi F., Turner J.D., Pionnier N., Midgley A., Guimaraes A.F., Johnston K.L.. et al. (2016) Wolbachia endosymbionts induce neutrophil extracellular trap formation in human onchocerciasis. Sci. Rep. 6, 1–13 10.1038/srep35559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McCoy C.J., Reaves B.J., Giguère S., Coates R., Rada B. and Wolstenholme A.J. (2017) Human leukocytes kill Brugia malayi microfilariae independently of DNA-based extracellular trap release. PLoS Neglected Trop. Dis. 11, 1–20 10.1371/journal.pntd.0005279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Engelmann B. and Massberg S. (2013) Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 13, 34–45 10.1038/nri3345 [DOI] [PubMed] [Google Scholar]