Abstract
Background
Cerebrospinal fluid bacterial culture is the gold-standard for confirmation of acute bacterial meningitis, but many cases are not culture confirmed. Antibiotics reduce the chance of a microbiological diagnosis. Objective to evaluate efficacy of Heparin-binding protein in diagnosis of bacterial meningitis.
Patients
30 patients diagnosed with acute bacterial meningitis, 30 viral meningitis, and 30 subjects with normal CSF findings.
Design
Diagnosis was based on history, clinical criteria, CSF examination, latex agglutination & culture, and sensitivities and response to therapy. HBP was measured using enzyme-linked immunosorbent technique in both serum & CSF.
Results
Cerebrospinal fluid HBP levels averaged 0.82 ± 0.3 ng/mL in controls, 3.3 ± 1.7 ng/mL in viral and 174.8 ± 46.7 ng/mL in bacterial meningitis. Mean serum level was 0.84 ± 0.3 ng/mL in the controls, 3.7 ± 1.9 ng/mL in viral, and 192.2 ± 56.6 ng/mL in bacterial meningitis. Both HBP levels were significantly higher in patients with bacterial meningitis. Cut-offs of 56.7 ng/ml and 45.3 ng/ml in cerebrospinal fluid & serum showed 100% overall accuracy. Even in patients who received prior antibiotics, remained elevated.
Conclusion
Serum Heparin-binding protein serves as a non-invasive potential marker of acute bacterial meningitis even in partially treated cases.
Keywords: Community acquired meningitis, Acute bacterial meningitis, Heparin binding protein, CSF biomarker, Serum biomarker
Introduction
Meningitis is the most common infectious central nervous system syndrome. Acute bacterial meningitis (ABM) is a life-threatening neurological emergency. It is one of the top 10 causes of infection-related death worldwide. If left untreated, its mortality could approach 100%.1 Even with current antibiotics and advanced intensive care, the mortality rate of the disease is approximately 10%; and 30–50% of its survivors live with permanent neurological sequel following hospital discharge. The clinical distinction between viral and acute bacterial meningitis is difficult in the acute phase of illness because the symptoms are often similar. Community acquired meningitis refers to bacterial meningitis caused by Haemophilus influenzae (mostly type b), Streptococcus pneumonia or Neisseria meningitidis. These comprise 70–90% of ABM cases.1, 2
According to WHO guidelines, CSF (cerebrospinal fluid) analysis is essential to establish the diagnosis, distinguish bacterial from viral meningitis, and to identify the etiological agent.3 Bacterial culture is the gold-standard technique for ABM confirmation, due to its high specificity for identification of the etiologic agent (WHO, 2011). However, approximately 50% of suspected cases are not culture-confirmed. Thus, clinical criteria, culture, Gram staining, and bacterial antigen testing of CSF as well as the classical markers in CSF: CSF lactate, protein, glucose, white blood cell (WBC), and neutrophil count are used. Unfortunately, no isolated laboratory test has distinguished bacterial meningitis from aseptic meningitis with 100% sensitivity & specificity; and the most accurate combination of clinical features to raise or lower suspicion of meningitis is still unclear.4, 5
Potential applications of biomarkers in infectious diseases include distinguishing bacterial from nonbacterial infection, monitoring response to therapy, and predicting outcomes. Heparin-binding protein (HBP), also known as Azurocidin and Cationic Antimicrobial Protein (CAP37), is a positively charged 37 kD glycoprotein released from the secretory vesicles and azurophilic granules of activated neutrophils in severe sepsis. Structurally, it belongs to a hematopoietic serine protease superfamily with antimicrobial function. It forms a part of the innate defenses of human neutrophils. HBP is easily and rapidly mobilized from migrating neutrophils.6
The aim of this study is to evaluate the role of serum and CSF heparin binding protein in diagnosis of bacterial meningitis, and its efficacy in differentiating bacterial from non-bacterial meningitis; in order to verify the possibility of using the HBP in initial evaluation of ABM.
Methods
Study setting: The present study was conducted in the Ministry of Health Specialized hospital, Alexandria, Egypt. It is the largest hospital of its kind in Alexandria; a tertiary care facility and a center of expertise in care and management of fever patients. It serves the population of Alexandria city and nearby governorates with a considerable case load that warrants the accumulation of a sufficient number of cases during a reasonable period of time.
Study design: Cross-sectional study.
Study population: the study population encompassed 90 patients who were admitted to the Fever Hospital in Alexandria, Egypt, and were clinically suspected to have acute meningitis end of 2016 & early 2017. Diagnosis was based on history, clinical criteria, and CSF examination criteria.3, 7, 8, 9, 10 According to diagnosis and therapeutic outcome, the study population was classified into three groups: Group I included 30 patients diagnosed with ABM, Group II 30 patients were diagnosed with non-bacterial (mostly viral) meningitis, and, finally, Group III included 30 subjects with normal CSF examination findings (control group).
Inclusion criteria: Patients with meningitis admitted to the Ministry of Health Specialized Hospital.
Exclusion criteria: patients with tuberculous meningitis, brain space occupying lesions, HIV infection (or who were on immunosuppressive therapy), chronic bacterial meningitis, post-surgical meningitis, and those with a history of head trauma, were excluded from the study.
Sample size calculation: a minimum sample size of 29 for each group was calculated to achieve 80% power; and to detect difference of 0.219 between the area under the curve of the null hypothesis (0.78) and the alternative hypothesis of (0.999) with a significance level of 0.05
Tools of data collection: the study follows the principles of the Declaration of Helsinki. Consequently, after gaining approval from: the Ethical committee, Medical Research Institute, Alexandria University, Ministry of Health Specialized Hospital, and Ministry of Health; signed informed consents were obtained from all patients or their guardians, professing their acceptance to participate in the study and have the results published.
Three tools were used to obtain relevant information: Interviewing questionnaire, clinical examination, and investigations.
-
-
Interviewing questionnaire: an interview schedule was designed to collect relevant information from all eligible patients or their guardians enrolled in this study. The schedule included the following items: personal, past medical history and present complaint.
-
-
Clinical examination: complete clinical examination of the study subjects was performed.
-
-
Investigations: a laboratory workup was done; CSF and blood samples were drawn simultaneously under aseptic precautions. CSF was collected by the attending physician in sterile tubes for chemical, microbiological and cytological examination. Biochemical examination included glucose, protein and lactate, which were determined in CSF supernatant samples after centrifugation at 2000 × g for 5 min. A wet preparation of CSF was used for total WBCs count. Gram stain for detection of bacteria and differential WBC count on CSF sediment was done. For CSF bacterial culture, CSF was centrifuged at 1000 × g for 10–15 min to sediment bacteria, cultured on blood agar and chocolate agar plates, incubated for 18–24 h at 37 °C in a candle-jar, and examined after 18–24 h. Agglutination testing for H. influenzae, N. meningitidis, and S. pneumoniae using the CSF supernatant for the detection of soluble bacterial antigens (capsular polysaccharide) was also done.
For serum heparin binding protein, whole venous blood was drawn from each subject. Blood was allowed to clot for 20 min at room temperature, centrifuged and aliquoted at −20 °C for the determination of the concentrations of serum HBP, using Enzyme Linked Immunosorbant Technique (Glory science Co., Ltd, Hangzhou, China). Serum & CSF HBP were measured according to the manufacturer's instructions.11
Statistical analysis: was performed using IBM SPSS software package version 20.0.
-
-
The qualitative variables were summarized by frequency and percentage. Chi square test was used to test for association between the type of meningitis and the other qualitative variables. Monte Carlo test was used when more than 20% of total cells had expected cell counts < 5.
-
-
The distributions of quantitative variables were tested for normality using Kolmogorov–Smirnov test and revealed normal data distribution; and parametric tests were applied.
-
-
The quantitative variables were summarized by the mean and standard deviation. One-way ANOVA was used for Comparisons of group differences regarding the continuous variables. Correlations between quantitative variables were assessed using Pearson correlation technique.
The Receiver Operating Characteristic (ROC) curve was used to evaluate accuracy of HBP marker in prediction of bacterial meningitis. Area under the curve values is reported with the 95% confidence interval (CI). All accuracy markers were calculated: Sensitivities, specificities, positive and negative predictive values. Significant test results were quoted as two-tailed probabilities. Significance of the obtained results was judged at the 5% level (p ≤ 0.05).
Results
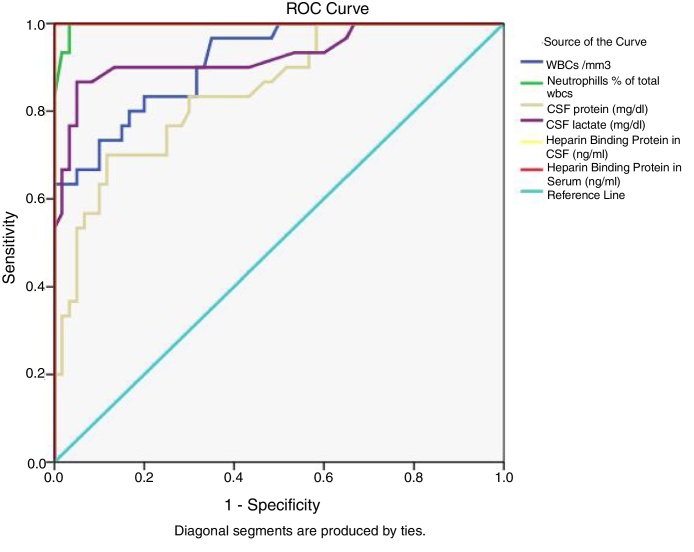
Characteristics of the study sample and HBP levels in CSF and serum are illustrated in Table 1. CSF HBP & serum HBP levels were both significantly higher in patients with acute bacterial meningitis than in patients with viral meningitis and controls. This suggests the potential role of both CSF & serum HBP in distinguishing between acute bacterial and viral meningitis (p < 0.01) (Fig. 1).
Table 1.
Characteristics of the study sample and HBP levels in CSF and serum.
| Characteristics | Bacterial group | Viral group | Control group | Total |
|---|---|---|---|---|
| n = 30 | n = 30 | n = 30 | n = 90 | |
| Gender | ||||
| Male | 19 (63.3%) | 18 (60)% | 12 (40%) | 49 (54.4%) |
| Female | 11 (30.6%) | 12 (40)% | 18 (60)% | 41 (45.6%) |
| Age in years | ||||
| Mean ± SD | 24.7 ± 14.7 | 24.7 ± 14.8 | 24.9 ± 14.3 | 24.9 ± 14.5 |
| (Minimum–maximum) | (1.5–50) | (1.0–49) | (1.5–50) | (1.0–50) |
| Pre-admission antibiotic | 25 (83%) | 27 (90%) | 24 (80%) | 76 (84.4%) |
| CSF HBP level in ng/ml | ||||
| Mean ± SD | 174.8 ± 46.7 | 3.3 ± 1.7 | 0.82 ± 0.3 | |
| (Minimum–maximum) | 56.7–295 | 1.30–7.5 | 0.31–1.8 | |
| Serum HBP levels in ng/ml | ||||
| Mean ± SD | 192.2 ± 56.6 | 3.7 ± 1.9 | 0.84 ± 0.3 | |
| (Minimum–maximum) | 45.3–289 | 1.1–7.6 | 0.4–1.51 | |
Fig. 1.
Receiver operator characteristics curve of cerebrospinal fluid total white blood cells, % neutrophils, protein, lactate and serum Heparin binding protein in prediction of bacterial meningitis.
Table 2 and Fig. 1 show the accuracy measures (at cutoff point with highest sensitivity and specificity) of the conventional CSF parameters and HBP as a new marker. For diagnosis of bacterial meningitis, the overall accuracy of CSF WBCs at a cutoff of 877.5 cells/cmm, CSF neutrophils at a cutoff of 59%, CSF protein at a cutoff of 45.5 mg/dl, CSF glucose at a cutoff of 39.5 mg/dl, and that of CSF lactate at a cutoff of 29.5 mg/dL were: 91.1%, 99.7%, 84.6%, 82.5%, and 92.7% respectively.
Table 2.
Accuracy measures with the cut off points of HBP and old markers in prediction of bacterial meningitis.
| Biomarker | Cutoff | Sensitivity | Specificity | Overall accuracy (area under the curve 95% C.I) | p value |
|---|---|---|---|---|---|
| CSF WBCs | 877.5 cells/mm3 | 63.3% | 100% | 91.1% (85.1–97.1%) | 0.000 |
| CSF neutrophils % | 59% | 100% | 97% | 99.7% (99.1–100%) | 0.000 |
| CSF protein | 45.5 mg/dl | 83.3% | 70% | 84.6% (76.2–93%) | 0.000 |
| CSF Glucose | 39.5 mg/dl | 50% | 100% | 82.5% (71.7–92.8) | 0.000 |
| CSF Lactate | 29.5 mg/dl | 90% | 87% | 92.7% (86.1–99.4%) | 0.000 |
| CSF HBP | 56.7 ng/ml | 100% | 100% | 100% | 0.000 |
| Serum HBP | 45.3 ng/ml | 100% | 100% | 100% | 0.000 |
A cutoff point of CSF HBP level at 56.7 ng/ml showed 100% sensitivity and specificity; positive and negative predictive values of 100%; and overall accuracy of 100%. At a cutoff point of 45.3 ng/ml serum HBP also showed 100% sensitivity and specificity; positive and negative predictive values of 100%; and overall accuracy of 100%. Areas under the curve were 1.0 for both CSF and serum HBP; which were higher than all other investigated parameters (Fig. 1).
Table 3 illustrates a highly significant positive correlation between CSF HBP, serum CSF and other CSF findings; WBCs, neutrophils percent, protein, lactate and decreased glucose levels.
Table 3.
Correlation between CSF and Serum HBP levels and CSF traditional markers.
| Traditional CSF markers | CSF HBP | Serum HBP |
|---|---|---|
| CSF WBCs count/mm3 | r = 0.349, p = 0.001 | r = 0.295, p = 0.005 |
| CSF neutrophils % of total CSF WBCs | r = 0.907, p = 0.000 | r = 0.916, p = 0.000 |
| Absolute CSF neutrophils count/mm3 | r = 0.347, p = 0.001 | r = 0.295, p = 0.005 |
| CSF glucose (mg/dl) | r = −0.583, p = 0.000 | r = −0.617, p = 0.000 |
| CSF Protein (mg/dl) | r = 0.574, p = 0.000 | r = 0.589, p = 0.000 |
| CSF Lactate (mg/dl) | r = 0.662, p = 0.000 | r = 0.687, p = 0.000 |
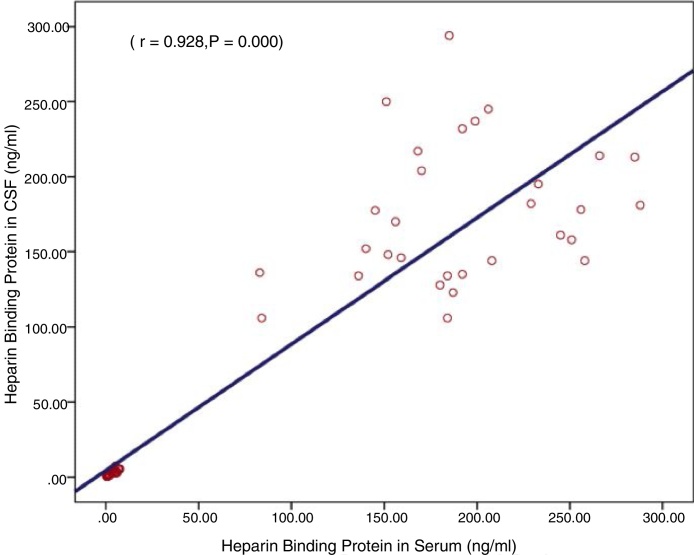
Lastly, Fig. 2 shows a highly significant positive correlation between CSF and serum HBP levels (r = 0.93, p = 0.000) in the three groups (Fig. 2).
Fig. 2.
Correlation between cerebrospinal fluid and serum HBP levels.
Discussion
Adverse effects of lumbar puncture include headache and backache. Complications may also occur in limited cases such as cerebral herniation, intracranial subdural hemorrhage, spinal epidural hemorrhage, and cranial and subdural hematomas from decreased CSF pressure. Most importantly, there is a number of situations where LP may be contraindicated in cases at the age of 60 or older, or cases suffering from: coagulopathies, immunocompromised state, history of central nervous system disease, seizure and local infection at the puncture site in mass lesions, and ventricular obstruction causing raised intracranial pressure and brain abscess.12 Hence, a less invasive sample (as blood serum), to aid or even replace LP for the diagnosis and follow up of meningitis, would be of a great benefit; not only to the patients but also to physicians and clinical laboratories.
In this study, all 30 patients with positive CSF findings in ABM group had elevated serum HBP levels (192.2 ± 56.6 ng/mL). Considering the significant morbidity associated with lumbar puncture and contraindication to perform a CSF tapping, the positive correlation between CSF and serum HBP levels may further support the potential use of serum HBP as a diagnostic marker in substitution of traditional CSF analysis. Therefore, serum HBP may be used in confirming or ruling out the diagnosis of clinically suspected bacterial meningitis, especially in cases where lumbar puncture is contraindicated. Also, the possibility of replacing follow up LP with serum HBP is worth further investigation.
In many clinical settings, involving patients with clinical suspicion of bacterial meningitis, antibiotics are instituted before lumbar puncture; reducing the chance of a microbiological diagnosis. Eighty three percent of our patients in the bacterial group received antibiotics 48–72 h before lumbar puncture. However, these patients still maintained significantly elevated CSF and serum HBP levels.
In this study, meningitis was confirmed by bacterial culture, Gram staining and/or agglutination testing, only in 16.7% of patients. However, the remaining 83.3% of patients in the bacterial group were diagnosed as bacterial according to: positive traditional CSF laboratory findings, clinical findings, and response to treatment. The low detection rates of culture, Gram staining and agglutination compared could be explained by low concentration of pathogens or empirical antibiotic treatment prior to sample collection which reduces the diagnostic sensitivity of these tests. It has also been shown for patients of bacterial group that CSF levels of protein (in 16.7% of patients), glucose (in 50% of patients), and lactate (in 10% of patients) could not differentiate between bacterial and viral meningitis; denoting the need of additional sensitive and specific markers.
As a granule-associated protein, HBP is rapidly mobilized from migrating polymorphonuclear leukocytes. It acts as a chemoattractant, an activator of monocytes and macrophages, and induces vascular leakage and edema formation. The release of HBP is triggered by ligation of neutrophilic β2-integrins; a process that may be initiated by bacteria. The overall outcome is powerful vascular leakage.13 When these effects are applied to the case of ABM, neutrophil-derived HBP production could result from the inflammatory response to bacterial infection and could have a major role in the resultant brain injury and brain edema in ABM.
HBP was proposed as a marker of bacterial infection for early detection of patients in the emergency department at risk of developing severe sepsis and septic shock. It demonstrated significant predictive values of elevated levels of HBP in blood of patients with severe sepsis and septic shock as compared to HBP levels in patients with noninfectious critical illness, and as compared to other markers. Elevated HBP levels (>30 ng/ml) were found in 80% of the patients while elevated procalcitonin levels (>0.5 ng/ml) were detected in 59%, ten and a half hours (median) before developing severe sepsis.14 Linder et al. (2012)15 found significantly higher plasma HBP in patients with severe sepsis or septic shock, as compared to patients with a non-septic illness in the intensive care unit. HBP was associated with severity of disease, and an elevated HBP at admission was associated with an increased risk of death. They recommended repeated HBP measurement in the ICU may help monitor treatment and predict outcome in patients with severe infections. Holub, Beran (2012),16 and Bentzer (2016)17 reported similar findings. Other studies present the role of HBP as a serum biomarker of bacterial infections.13, 18, 19, 20 Linder (2015)21 suggested HBP as the best predictor and an early indicator of infection-related organ dysfunction; and a strong predictor of disease progression to severe sepsis within 72 h in patients presenting at the emergency department. Finally, in 2017 Fisher & Linder acknowledge that HBP has a major role in the pathophysiology of severe bacterial infections and, thus, represents a potential diagnostic marker and a target for the treatment.22 Another study for them later that year states that elevated plasma HBP is associated with development of sepsis-induced acute kidney injury.23
Presumably, HBP was scarcely investigated in CSF of patients. Linder et al. (2011)11 analyzed CSF samples for the concentrations of HBP, lactate, protein, glucose, neutrophils, and mononuclear cells. HBP levels in CSF were significantly higher (p < 0.01) in patients with ABM (median 376 ng/mL, range 12–858 ng/mL) than in patients with viral meningitis (median 4.7 ng/mL, range 3.0–41 ng/mL), or control patients with normal CSF cell count (median 3.5 ng/mL, range 2.4–8.7 ng/mL). They suggested a CSF HBP cutoff concentration exceeding 20 ng/mL to diagnose ABM; with sensitivity 100%, specificity 99.2%, and positive and negative predictive values of 96.2% and 100%. The area under the ROC curve for heparin-binding protein was 0.994, which was higher than that for the other investigated parameters. Linder et al. concluded that elevated CSF levels of HBP could distinguish between patients with acute bacterial meningitis and patients with other central nervous system infections. We note the relatively small number of patients included in their study (41 bacterial meningitis patients and 10 viral meningitis patients). The results in this study concur with and confirm these results. Moreover, the present study took a step further as it is the first study to investigate serum HBP in meningitis and to correlate between CSF and serum HBP. The study also locates a weak positive correlation between CSF HBP and CSF polymorphonuclear cell counts. This was also noted by Linder et al.; and they suggested that HBP level probably reflects the number of activated neutrophils rather than the absolute neutrophil count.
PCR testing is more sensitive than CSF culture, particularly in patients who received previous antimicrobials. CSF bacterial culture requires that laboratories receive living bacteria which is quiet restricting taking into consideration the fragility of the three causative bacteria of community acquired meningitis. However, PCR cannot be routinely done in the diagnosis of every case of bacterial meningitis; it is more expensive and not available in resource limited settings, as is the case in developing countries. Therefore PCR is not usually available for routine case management; it can be retrospectively used in surveillance purposes for adapting the most appropriate preventive strategy.24 In patients with suspected bacterial meningitis, empirical therapy should not be delayed for more than one hour while awaiting diagnostic testing. In the meantime, HBP testing can offer a rapid, affordable tool to detect patients with early bacterial meningitis or patients who have been partially treated.
Given the lack of specificity of clinical findings, the key to the diagnosis of meningitis is the evaluation of CSF. Initiation of antimicrobials before lumbar puncture decreases the yield of CSF culture; Gram staining; CSF pleocytosis; the likelihood of a low CSF glucose level; and the degree of elevation of CSF protein and of CSF lactate. Moreover, patients with early bacterial meningitis might not have the classical positive CSF findings of bacterial meningitis. However, Heparin-binding protein is a promising new biomarker that might assist with early identification of bacterial meningitis as well as partially treated bacterial meningitis. It is recommend to have further studies on larger scales. CSF HBP level ≥56.7 ng/mL; and serum HBP level ≥45.3 ng/mL should prompt empirical therapy for bacterial meningitis while awaiting results of CSF culture or even molecular assays.
CSF and serum HBP were both significantly higher in patients with acute bacterial meningitis than in patients with viral meningitis and controls; despite the intake of antibiotics. CSF HBP level at 56.7 ng/ml, and serum HBP level at 45.3 ng/ml showed 100% sensitivity and specificity, and positive and negative predictive values of 100%, and overall accuracy of 100% for this parameter.
Our study sheds light on the excellent diagnostic performance of HBP in the diagnosis and possibly follow up of acute bacterial meningitis. Since CSF HBP was higher than serum HBP in ABM patients, it is safe to assume that HBP is produced locally in the CSF and is not coming from a systemic source. The correlation of serum and CSF levels & other CSF parameters suggests that serum levels of HBP can be used as a non-invasive test of ABM. However, due to the lack of specificity, serum HBP cannot confidently replace the first CSF tap. High levels of serum HBP when used alone could also indicate systemic bacterial infection & sepsis. Thus the use of serum HBP (alone without CSF HBP) in diagnosis of ABM has to be in context with clinical findings. We suggest that serial HBP measurements could be further studied as a replacement for the second CSF tap in the follow up of ABM.
Measuring HBP in CSF from patients with suspected meningitis could improve the diagnostic accuracy in differentiating between bacterial and viral meningitis, thereby allowing the clinician to start adequate treatment earlier; especially in situations or places when the time and resources are limited. Results of this study point out the possibility of considering serum HBP as a diagnostic marker in substitution of traditional CSF analysis in confirming or ruling out the diagnosis of community acquired ABM; especially with absolute and relative contraindicated lumbar puncture.
HBP could be a promising biomarker that might assist with early identification of bacterial meningitis as well as partially treated bacterial meningitis. HBP could be introduced as a routine test for patients with ABM; especially when kits for its determination are available on a commercial automated basis and not only for research studies. These results warrant evaluation in different populations and larger scale. Further research for serial measurements of HBP plasma levels for close monitoring of critically ill meningitis patients, instead of repeated lumbar puncture which is currently used in follow up of acute bacterial meningitis cases, is recommended.
Disclosures
-
•
This work was self-funded by the authors themselves and no funding sources made any contribution to it.
Dataset
-
•
El-Attar, Eman; Khalil, Gihane; Shehata, Gihan; Kandil, Mona; Hassan, Salwa (2017), “Heparin Binding Protein in Acute Bacterial Meningitis”, Mendeley Data, v2 https://doi.org/10.17632/g5c9w4pw3r.2
Conflicts of interest
The authors declare no conflicts of interest.
Associate Editor: Afonso Barth
References
- 1.Chaudhuri A., Martinez-Martin P., Kennedy P.G. EFNS guideline on the management of community-acquired bacterial meningitis: report of an EFNS Task Force on acute bacterial meningitis in older children and adults. Eur J Neurol. 2008;15:649–659. doi: 10.1111/j.1468-1331.2008.02193.x. [DOI] [PubMed] [Google Scholar]
- 2.Scheld W.M., Whitley R.J., Marra M.C. 4th ed. Wolters Kluwer Health, United States; Philadelphia: 2014. Infections of the Central Nervous System. [Google Scholar]
- 3.World Health Organization: Laboratory Methods for the Diagnosis of Meningitis Caused by Neisseria meningitides, Streptococcus pneumoniae, and Hemophilus Influenza. WHO Manual. 2nd ed. 2011. Available electronically at: http://www.who.int/csr/resources/publications/meningitis/WHO_CDS_CSR_EDC_99_7_EN/en/ Accessed 19.10.17.
- 4.Fitch M.T., Van de Beek D. Emergency diagnosis and treatment of adult meningitis. Lancet Infect Dis. 2007;7:191–200. doi: 10.1016/S1473-3099(07)70050-6. [DOI] [PubMed] [Google Scholar]
- 5.Águeda S., Campos T., Maia A. Prediction of bacterial meningitis based on cerebrospinal fluid pleocytosis in children. Braz J Infect Dis. 2013;17:401–404. doi: 10.1016/j.bjid.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gautam N., Olofsson A.M., Iversen H., Lundgren-Åkerlund E., Hedqvist P., Arfors K.E. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med. 2001;7:1123–1127. doi: 10.1038/nm1001-1123. [DOI] [PubMed] [Google Scholar]
- 7.Abro A.H., Ahmed Saleh Abdou A.S., Ali H., Ustadi A.M., Hasab A.A.H. Cerebrospinal fluid analysis acute bacterial versus viral meningitis. Pak J Med Sci. 2008;24(5):645–650. [Google Scholar]
- 8.Bamberger D.M. Diagnosis initial management, and prevention of meningitis. Am Fam Physician. 2010;82(12):1491–1498. 15. [PubMed] [Google Scholar]
- 9.Başpınar E., Dayan S., Bekçibaşı M. Comparison of culture and PCR methods in the diagnosis of bacterial meningitis. Braz J Microbiol. 2017;48(2):232–236. doi: 10.1016/j.bjm.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viallon A., Desseigne N., Marjollet O. Meningitis in adult patients with a negative direct cerebrospinal fluid examination: value of cytochemical markers for differential diagnosis. Crit Care. 2011;15(3):R136. doi: 10.1186/cc10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linder A., Åkesson P., Brink M. Heparin-binding prote: diagnostic marker of acute bacterial meningitis. Crit Care Med. 2011;39:812–817. doi: 10.1097/CCM.0b013e318206c396. [DOI] [PubMed] [Google Scholar]
- 12.Moghtaderi A, Naini RA, Sanatinia S. Lumbar Puncture. Techniques, Complications and CSF Analyses: Emergency Medicine – An. Int. Perspect. Dr. Michael Blaivas (Ed.), ISBN: 978-953-51-0333-2, InTech. 2012. Available electronically at: http://www.intechopen.com/books/emergency-medicine-an-international-perspective/lumbar-puncture-techniques-complications-and-csf-analyses Accessed 19.10.17.
- 13.Linder A., Soehnlein O., Åkesson P. Roles of heparin-binding protein in bacterial infections. J Innate Immunol. 2010;2:431–438. doi: 10.1159/000314853. [DOI] [PubMed] [Google Scholar]
- 14.Linder A., Arnold R., Zindovic M. Heparin-binding protein improves prediction of severe sepsis in the emergency department. Crit Care. 2013;17(suppl 4):P3. doi: 10.1097/CCM.0000000000001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linder A., Åkesson P., Inghamma M., Treutiger C.J., Linnér A., Sundén-Cullberg J. Elevated plasma levels of heparin-binding protein in intensive care unit patients with severe sepsis and septic shock. Crit Care. 2012;16:90–96. doi: 10.1186/cc11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holub M., Beran M. Should heparin-binding protein levels be routinely monitored in patients with severe sepsis and septic shock? Crit Care. 2012;16:133–138. doi: 10.1186/cc11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentzer P., Fisher J., Kong H.J. Heparin-binding protein is important for vascular leak in sepsis. ICMx. 2016;4:33. doi: 10.1186/s40635-016-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjölvmark C., Påhlman L.I., Åkesson P., Linder A. Heparin-binding protein (HBP) – a diagnostic biomarker of urinary tract infection in adults. Open Forum Infect Dis. 2014;23:4–10. doi: 10.1093/ofid/ofu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llewelyn M.J., Berger M., Gregory M., Ramaiah R., Taylor A.L., Curdt I. Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Crit Care. 2013;17:60–65. doi: 10.1186/cc12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariscalco M.M. Heparin-binding protein: another neutrophil granule protein.. another new biomarker? Crit Care Med. 2011;39(4):910–911. doi: 10.1097/CCM.0b013e31820e6a43. [DOI] [PubMed] [Google Scholar]
- 21.Linder A., Arnold R., Boyd J.H., Zindovic M., Zindovic I., Lange A. Heparin-binding protein measurement improves the prediction of severe infection with organ dysfunction in the emergency department. Crit Care Med. 2015;43(11):2378–2386. doi: 10.1097/CCM.0000000000001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher J., Linder A. Heparin-binding protein: a key player in the pathophysiology of organ dysfunction in sepsis. J Int Med. 2017;281(6):562–574. doi: 10.1111/joim.12604. [DOI] [PubMed] [Google Scholar]
- 23.Fisher J., Russell J.A., Bentzer P. Heparin-binding protein (HBP): a causative marker and potential target for heparin treatment of human sepsis-induced acute kidney injury. Shock. 2017;48(3):313–320. doi: 10.1097/SHK.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 24.Brouwer M.C., Tunkel A.R., de Beek D.V. Epidemiology, diagnosis and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467–492. doi: 10.1128/CMR.00070-09. [DOI] [PMC free article] [PubMed] [Google Scholar]