Abstract
Aim
To examine lymphocyte counts as a predictive prognostic marker in patients with coma after cardiac arrest.
Methods
We retrospectively evaluated patients with coma after cardiac arrest admitted to the intensive care unit of Shiga University of Medical Science (Otsu, Japan). Lymphocyte counts were measured for 6 days from admission. Neurological outcome was assessed as favorable or unfavorable using cerebral performance categories. Associations between lymphocyte count and prognosis were investigated using multivariate logistic regression analysis and receiver operating characteristic curves.
Results
Forty‐six patients were assessed from February 2012 to December 2016. Survivors had significantly higher lymphocyte counts than non‐survivors on days 2 and 5. Multivariate analysis showed that lymphocyte count was not associated with 90‐day mortality. Patients with favorable neurological outcome at discharge had significantly higher lymphocyte counts on days 2–6 than patients with unfavorable outcomes. Multivariate logistic regression analysis, including possible confounders, showed that lymphocyte counts on days 2–4 and 6 were associated with neurological outcome (day 2: odds ratio [OR] = 0.75, 95% confidence interval [CI] = 0.58–0.97, P = 0.029; day 3: OR = 0.68, 95% CI = 0.47–0.98, P = 0.04; day 4: OR = 0.4, 95% CI = 0.16–1.00, P = 0.05; day 6: OR = 0.69, 95% CI = 0.48–0.99, P = 0.046). Receiver operating characteristic curve analysis indicated high accuracy for predicting neurological outcome for each lymphocyte count on days 2–6 using the area under the curve, day 4 values being most accurate (day 2: 0.776, day 3: 0.787, day 4: 0.909, day 5: 0.774, day 6: 0.839).
Conclusion
Lymphocyte counts on days 2–4 and 6 after cardiac arrest are associated with neurological outcome; counts on day 4 most accurately predict neurological outcome.
Keywords: Cardiopulmonary arrest, lymphocyte, lymphopenia, prognosis, therapeutic hypothermia
Introduction
Globally, out‐of‐hospital cardiac arrest occurs in approximately 20–140 people per 100,000 population per year.1 Despite advances in the resuscitation and management of patients in the post‐cardiac arrest period, many patients, including those who are initially resuscitated, die before discharge from the hospital, or are discharged with poor neurological status due to development of post‐cardiac arrest syndrome (PCAS).2 However, predicting patient prognosis, including neurological outcome, both of which are required to determine mild hypothermia (MHT) strategies following PCAS, is difficult.
Although neuron‐specific enolase is known to be a predictive marker of neurological outcome in PCAS patients and shows high accuracy in predicting neurological outcome on day 4,3 the number of facilities that can immediately measure neuron‐specific enolase are limited.
Recently, the relationship between lymphopenia in the acute phase and prognosis of PCAS patients was reported. One study showed that the neutrophil–lymphocyte ratio (NLR) is associated with mortality in cardiac arrest patients at admission.4, 5 Another study showed that lymphopenia occurs in PCAS patients at admission and is associated with neurological recovery in out‐of‐hospital cardiac arrest patients.6 However, the association between lymphocyte count and prognosis in the subacute phase (after day 2) is not well known.
In PCAS, whole‐body ischemia and reperfusion is associated with the development of systemic inflammatory response syndrome and elevation of plasma cytokine levels.7
This phenomenon is considered to share features with sepsis, which causes elevation of plasma cytokine levels, and with stroke, which causes ischemia and reperfusion of the brain. Clinically, in patients with sepsis, lymphopenia on day 4, but not day 1, due to apoptosis of lymphocytes can be used as a predictor of mortality;8, 9 in stroke patients, lymphopenia at admission and day 4 is reportedly associated with stroke area.10, 11 Given the similarities of PCAS with stroke and sepsis in terms of their association with lymphopenia, lymphocyte count in both the acute and subacute phase might be associated with mortality and neurological outcome in PCAS patients.
Here, we retrospectively examined the association between lymphocyte count in the first 6 days after cardiac arrest and mortality or neurologic outcomes in PCAS patients treated with MHT.
Methods
Study design and setting
We retrospectively investigated patients (>18 years old) at Shiga University of Medical Science (Otsu, Japan) who were successfully resuscitated following cardiac arrest and in whom MHT was induced between February 2011 and December 2016.
All patients developed unconsciousness (Glasgow Coma Scale [GCS] score < 8) after return of spontaneous circulation (ROSC). Exclusion criteria for MHT were aortic dissection, hemorrhagic disease and pregnancy, and exclusion criteria for study participation were death within 72 h, stroke, and hematological and autoimmune diseases, which would prevent evaluation of neurological outcome and can cause lymphopenia, respectively.
All patients received standardized medical treatment along with MHT to 35°C for 48 h and were rewarmed over 24 h, according to our local treatment guidelines.
Repeated neurological examinations were undertaken at least 48 h after cardiac arrest, after rewarming the patient to a core temperature of >36°C and when the patient was off sedation. This study was approved by the institutional review board of Shiga University of Medical Science (29‐042) and carried out in accordance with the principles of the Declaration of Helsinki (amended in 2013). We publicized the study by posting a summary of the protocol on the website of the Shiga University of Medical Science and the notice clearly informed patients of their right to refuse participation.
Measurements and data collection
We collected patient demographic data and information on other baseline characteristics, including age, sex, body mass index, cardiopulmonary resuscitation (initial cardiac rhythm, time to ROSC, witness report, provision of bystander cardiopulmonary resuscitation, etiology of cardiac arrest, provision of defibrillation, total adrenaline dose, and prehospital ROSC), and Sequential Organ Failure Assessment (SOFA) score for the first 6 days (excluding the GCS score). Based on previous studies, we estimated catecholamine doses, as the catecholamine index (CAI), hourly for each of the first 6 days using the formula: CAI = hourly doses (μg/kg/min) of dopamine + dobutamine + (adrenaline + noradrenaline) × 100 (μg/kg/min). The CAI of each day was calculated at the highest point of the day. Ninety‐day mortality, laboratory results, and neurological outcome were assessed on the basis of the cerebral performance categories (CPC) score.12, 13 The CPC score was retrospectively determined from the patients’ discharge charts. We defined CPC 1‐2 as favorable outcomes and CPC 3–5 as unfavorable outcomes.
Blood samples were collected daily for the first 6 days in all patients. Blood sampling on day 1 was defined as the value measured for the first 24 h after admission to the intensive care unit.
Outcomes
The primary outcomes evaluated were 90‐day mortality and neurological status at 90 days.
Data analysis
Statistical analyses were carried out with the IBM spss Statistics 22 software package (IBM Japan, Tokyo, Japan). Variables are expressed as the median and interquartile range for continuous variables and proportion for categorical variables. Demographic and clinical differences between groups were assessed using Student's t‐test or the Mann–Whitney U‐test, as appropriate. Lymphocyte counts, neutrophil counts, white blood cell (WBC) counts, C‐reactive protein (CRP) levels and NLR, which are related to inflammation, SOFA score (excluding GCS) and CAI were compared between favorable and unfavorable neurological outcome groups, and between survivors and non‐survivors, using repeated‐measures anova. Logistic regression analysis was carried out to assess the correlations between neurological outcomes and survival with lymphocyte count on days 1–6 and other candidate risk factors, such as age, time to ROSC, SOFA score without GCS on day 1, and shockable rhythm. All variables and risk factors initially considered in the univariate logistic analysis to be significantly associated with mortality or neurological outcome (P < 0.10) were included in multivariate logistic regression analysis. For the final model, we calculated the adjusted odds ratio and 95% confidence intervals for each variable. P < 0.05 was considered significant. The predictive accuracy of lymphocyte counts and NLR on each day for neurological outcome was calculated using analysis of the area under the receiver operating characteristic curve.
Univariate linear analysis was carried out to evaluate the relationship between the lymphocyte count on each day and candidate variables, such as age, time to ROSC, SOFA score on day 1 and catecholamine index on each day using univariate linear regression. We also undertook multivariate linear regression analysis to evaluate the relationship between lymphocytes and combinations of risk factors. We included all risk factors that were significantly associated with lymphocyte counts (P < 0.10) in the univariate analysis.
Results
A total of 46 cardiac arrest patients, all of whom received MHT and survived for more than 72 h, were enrolled in this study. There were no mortalities from days 3 to 6 in this study. The main characteristics of survivors and non‐survivors are shown in Table 1. Overall survival was seen in 27 patients. In survivors, the time to ROSC was shorter, dosage of adrenaline at resuscitation was lower, proportion of prehospital ROSC was higher, SOFA score without GCS on day 1 was lower, and CAI on days 5 and 6 were lower than in non‐survivors. There were no significant differences in the proportion of shockable rhythms between survivors and non‐survivors (Table 1).
Table 1.
Comparison of baseline characteristics between survivors and non‐survivors among Japanese patients with coma after cardiac arrest (n = 46)
| Total (n = 46) | Survivors (n = 33) | Non‐survivors (n = 13) | P‐value | |
|---|---|---|---|---|
| Age, years | 62.0 (48.5–71.3) | 59.0 (39.0–71.0) | 64.0 (60.5–73.0) | 0.113 |
| Sex, male | 38 (83) | 26 (79) | 11 (85) | 0.182 |
| BMI | 22.2 (18.4–24.2) | 21.7 (17.9–23.5) | 23.2 (20.2–28.1) | 0.054 |
| Initial rhythm of VT/VF | 26 (57) | 19 (58) | 7 (54) | 0.818 |
| Time from collapse to ROSC, min | 30.5 (18.0–50.0) | 24.0 (14.0–45.0) | 47.5 (28.3–57.0) | 0.018 |
| Witnessed cardiac arrest | 43 (94) | 30 (91) | 13 (100) | 0.261 |
| Adrenaline dose at resuscitation, mg | 3 (0.0–4.0) | 1 (0.0–3.5) | 4 (3.0–5.0) | 0.004 |
| Provision of defibrillation | 25 (54) | 19 (58) | 6 (46) | 0.484 |
| Prehospital ROSC | 14 (30) | 13 (39) | 1 (8) | 0.035 |
| In‐hospital cardiac arrest | 11 (24) | 6 (18) | 5 (38) | 0.147 |
| Bystander CPR | 19 (41) | 23 (70) | 5 (38) | 0.051 |
| Cardiac origin of arrest | 32 (70) | 23 (70) | 9 (69) | 0.975 |
| Coronary disease | 15 (33) | 9 (27) | 6 (46) | 0.206 |
| CAI | ||||
| Day 1 | 5.00 (2.00–12.48) | 5.00 (1.00–12.00) | 8.00 (2.00–21.25) | 0.087 |
| Day 2 | 4.50 (1.23–9.25) | 3.70 (5.00–8.00) | 6.00 (2.50–12.50) | 0.053 |
| Day 3 | 3.00 (0.25–8.00) | 2.50 (0.00–7.50) | 8.00 (1.50–8.00) | 0.053 |
| Day 4 | 2.00 (0.00–5.63) | 2.00 (0.00–5.00) | 4.00 (0.00–8.00) | 0.269 |
| Day 5 | 1.10 (0.00–4.13) | 0.00 (0.00–3.00) | 4.00 (0.00–11.50) | 0.025 |
| Day 6 | 0.00 (0.00–3.00) | 0.00 (0.00–1.65) | 3.00 (0.00–9.50) | 0.022 |
Data are shown as median (range) or n (%).
Catecholamine index (CAI) = hourly doses (μg/kg/min) of dopamine + dobutamine + (adrenaline + noradrenaline) × 100 (μg/kg/min).
CAI of each day was calculated at the highest point of the day.
BMI, body mass index; CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation; VF, ventricular fibrillation; VT, ventricular tachycardia.
Survivors had a significantly higher WBC count on day 2 and lymphocyte counts on days 2 and 5, and lower CRP on days 4–6, NLR on day 6, neutrophil counts on day 1 and SOFA scores on days 1–6, compared with non‐survivors (Fig. 1). Univariate analysis of mortality found the following additional variables to be statistically significantly related to survival: lymphocyte count on days 2, 5 and 6, age, SOFA score without GCS on day 1, and time to ROSC. Multivariate logistic analysis of each lymphocyte count revealed that none of these variables was associated with mortality (Table 2).
Figure 1.
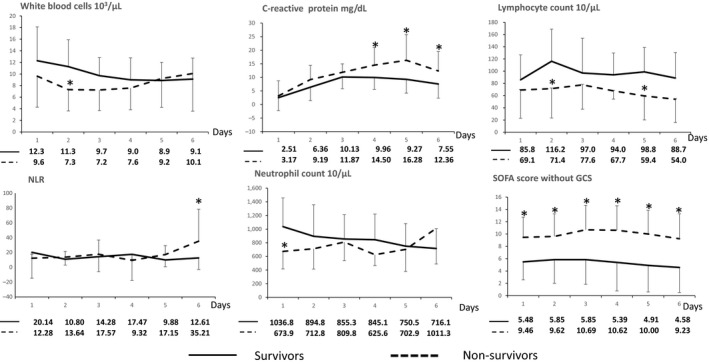
Comparison of white blood cell count, C‐reactive protein levels, lymphocyte count, neutrophil count, neutrophil–lymphocyte ratio (NLR) and Sequential Organ Failure Assessment (SOFA) scores between survivor (solid line) and non‐survivor (broken line) outcome groups of Japanese patients with coma after cardiac arrest (n = 46). The values of each point are shown below the graphs. The average of survivors is shown in the upper line of values; the average of non‐survivors is shown in the lower line of values. Whiskers in the graphs indicate standard deviation. SOFA score excluded Glasgow Coma Scale (GCS) score. *P < 0.05 compared with survivors.
Table 2.
Univariate and multivariate logistic regression analyses of mortality among Japanese patients with coma after cardiac arrest (n = 46)
| Lymphocyte count, 100/μL | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P‐value | Odds ratio | 95% CI | P‐value | ||
| Model 1 | |||||||
| Day 1 | 0.910 | 0.746–1.111 | 0.355 | Lymphocyte count on day 2 | 0.874 | 0.677–1.128 | 0.301 |
| Day 2 | 0.840 | 0.689–1.025 | 0.087 | SOFA score without GCS on day 1 | 1.064 | 0.719–1.575 | 0.756 |
| Day 3 | 0.923 | 0.750–1.137 | 0.453 | Age | 1.071 | 0.973–1.179 | 0.161 |
| Day 4 | 0.789 | 0.530–1.176 | 0.245 | Time from collapse to ROSC (min) | 1.032 | 0.966–1.101 | 0.349 |
| Day 5 | 0.768 | 0.592–0.995 | 0.046 | Model 2 | |||
| Day 6 | 0.798 | 0.612–1.040 | 0.095 | Lymphocyte count on day 5 | 0.957 | 0.699–1.309 | 0.782 |
| SOFA score without GCS on day 1 | 1.437 | 1.121–1.842 | 0.004 | SOFA score without GCS on day 1 | 1.275 | 0.879–1.849 | 0.201 |
| Age | 1.047 | 0.998–1.100 | 0.063 | Age | 1.033 | 0.945–1.130 | 0.476 |
| Time from collapse to ROSC (min) | 1.035 | 0.999–1.072 | 0.059 | Time from collapse to ROSC (min) | 1.054 | 0.985–1.128 | 0.129 |
| Initial rhythm of VT/VF | 0.860 | 0.236–3.125 | 0.818 | Model 3 | |||
| Lymphocyte count on day 6 | 0.908 | 0.659–1.253 | 0.557 | ||||
| SOFA score without GCS on day 1 | 1.172 | 0.843–1.629 | 0.345 | ||||
| Age | 1.022 | 0.944–1.107 | 0.591 | ||||
| Time from collapse to ROSC (min) | 1.025 | 0.986–1.065 | 0.218 | ||||
Sequential organ failure assessment (SOFA) score without Glasgow Coma Scale (GCS) on day 1 was measured as the worst data within the first 24 h, excluding GCS score.
Model 1 included lymphocyte count on day 2, SOFA score on day 1, age and time from collapse to return of spontaneous circulation (ROSC), for which the P‐values were < 0.1. Model 2 included lymphocyte count on day 5, SOFA score on day 1, age and time from collapse to ROSC, for which the P‐values were < 0.1. Model 3 included lymphocyte count on day 6, SOFA score on day 1, age and time from collapse to ROSC, for which the P‐values were < 0.1.
CI, confidence interval.
The main characteristics of patients with favorable and unfavorable neurological outcomes are shown in Table 3. Overall favorable neurological outcome was seen in 19 patients. In patients with favorable neurological outcomes, the time to ROSC was shorter, and CAI on day 1 was lower than those in patients with unfavorable neurological outcomes. There were no significant differences in the proportion of shockable rhythms between patients with favorable and unfavorable neurological outcomes.
Table 3.
Comparison of baseline characteristics among Japanese patients with coma after cardiac arrest (n = 46), according to favorable and unfavorable outcomes
| Total (n = 46) | Favorable outcome (n = 19) | Unfavorable outcome (n = 27) | P‐value | |
|---|---|---|---|---|
| Age, years | 62.0 (48.5–71.3) | 51.0 (34.0–68.0) | 67.0 (59.0–74.0) | 0.016 |
| Sex, male | 38 (83) | 16 (84) | 22 (82) | 0.810 |
| BMI | 22.2 (18.4–24.2) | 22.8 (19.4–24.5) | 21.7 (18.4–23.3) | 0.292 |
| Initial rhythm of VT/VF | 26 (57) | 12 (63) | 14 (52) | 0.446 |
| Time from collapse to ROSC, min | 30.5 (18.0–50.0) | 24.0 (15.0–41.0) | 45.0 (27.0–54.0) | 0.018 |
| Witnessed cardiac arrest | 43 (94) | 19 (100) | 24 (89) | 0.133 |
| Adrenaline dose at resuscitation, mg | 3.0 (0.0–4.0) | 1.0 (0.0–4.0) | 3.0 (1.0–5.0) | 0.072 |
| Provision of defibrillation | 25 (54) | 11 (58) | 14 (52) | 0.685 |
| Prehospital ROSC | 14 (30) | 8 (42) | 6 (22) | 0.149 |
| In‐hospital cardiac arrest | 11 (24) | 4 (21) | 7 (26) | 0.703 |
| Bystander CPR | 19 (41) | 11 (58) | 8 (30) | 0.055 |
| Cardiac origin of arrest | 32 (70) | 15 (79) | 17 (63) | 0.246 |
| Coronary disease | 15 (33) | 7 (37) | 8 (30) | 0.607 |
| CAI | ||||
| Day 1 | 5.00 (2.00–12.48) | 4.00 (0.00–12.00) | 8.00 (2.00–16.00) | 0.042 |
| Day 2 | 4.50 (1.23–9.25) | 2.50 (0.10–8.00) | 5.00 (1.00–11.00) | 0.533 |
| Day 3 | 3.00 (0.25–8.00) | 4.00 (0.90–8.00) | 3.00 (0.30–9.00) | 0.796 |
| Day 4 | 2.00 (0.00–5.63) | 2.00 (0.90–5.00) | 2.00 (0.00–6.00) | 0.697 |
| Day 5 | 1.10 (0.00–4.13) | 0.00 (0.00–4.00) | 2.00 (0.00–5.00) | 0.173 |
| Day 6 | 0.00 (0.00–3.00) | 0.00 (0.00–2.00) | 0.00 (0.00–4.00) | 0.163 |
| Survival | 33 (72) | 18 (95) | 15 (56) | 0.004 |
Data are shown as median (range) or n (%).
Favorable outcome = cerebral performance category score 1–2; unfavorable outcome = score 3–5.
Catecholamine index (CAI) = hourly doses (μg/kg/min) of dopamine + dobutamine + (adrenaline + noradrenaline) × 100 (μg/kg/min).
CAI of each day was calculated at the highest point of the day.
BMI, body mass index; CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation; VF, ventricular fibrillation; VT, ventricular tachycardia.
There were no significant differences in WBC count or CRP between patients in the favorable and unfavorable neurological outcome groups. Patients who had a favorable neurological outcome had significantly higher lymphocyte count on days 2–6, lower SOFA score on days 4–6, higher neutrophil count on day 1, lower neutrophil count on day 6 and lower NLR on day 6 than patients with unfavorable neurological outcomes (Fig. 2). Univariate analysis of neurological outcomes found the following factors to be statistically significant: lymphocyte count on days 2–6, age, SOFA score without GCS on day 1, and time to ROSC. Multivariate analysis of individual lymphocyte counts on days 2–6, age, SOFA score without GCS at admission and time to ROSC showed that each of the lymphocyte counts on days 2–4 and 6 were associated with neurological outcome, whereas lymphocyte counts on day 5 were not associated with neurological outcome (Table 4).
Figure 2.
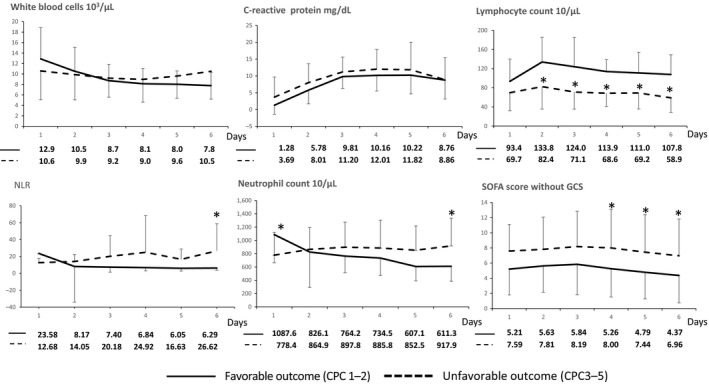
Comparison of white blood cell count, C‐reactive protein levels, lymphocyte count, neutrophil count, neutrophil–lymphocyte ratio (NLR) and Sequential Organ Failure Assessment (SOFA) scores between favorable (solid line) and unfavorable (broken line) neurological outcome groups of Japanese patients with coma after cardiac arrest (n = 46). The values of each point are shown below the graph. The average of patients with favorable neurological outcome is shown in the upper line of values; the average of patients with unfavorable neurological outcome is shown in the lower line of values. The whiskers indicate standard deviation. SOFA score excluded Glasgow Coma Scale (GCS) score. *P < 0.05 compared with the favorable outcome group. CPC, cerebral performance category.
Table 4.
Univariate and multivariate logistic regression analyses of neurological outcome among Japanese patients with coma after cardiac arrest (n = 46)
| Lymphocyte count, 100/μL | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P‐value | Odds ratio | 95% CI | P‐value | ||
| Model 1 | |||||||
| Day 1 | 0.877 | 0.727–1.059 | 0.172 | Lymphocyte count on day 2 | 0.753 | 0.583–0.972 | 0.029 |
| Day 2 | 0.824 | 0.693–0.980 | 0.029 | SOFA score without GCS on day 1 | 0.861 | 0.586–1.265 | 0.447 |
| Day 3 | 0.770 | 0.593–1.000 | 0.050 | Age | 1.075 | 0.986–1.177 | 0.099 |
| Day 4 | 0.432 | 0.200–0.935 | 0.033 | Time from collapse to ROSC (min) | 1.086 | 1.003–1.177 | 0.043 |
| Day 5 | 0.769 | 0.618–0.957 | 0.019 | Model 2 | |||
| Day 6 | 0.683 | 0.510–0.914 | 0.010 | Lymphocyte count on day 3 | 0.679 | 0.470–0.983 | 0.040 |
| SOFA score without GCS on day 1 | 1.224 | 1.011–1.482 | 0.039 | SOFA score without GCS on day 1 | 0.889 | 0.488–1.619 | 0.701 |
| Age | 1.047 | 1.008–1.087 | 0.019 | Age | 1.120 | 0.986–1.271 | 0.081 |
| Time from collapse to ROSC (min) | 1.044 | 1.005–1.084 | 0.025 | Time from collapse to ROSC (min) | 1.084 | 0.982–1.196 | 0.110 |
| Initial rhythm of VT/VF | 0.628 | 0.189–2.085 | 0.447 | Model 3 | |||
| Lymphocyte count on day 4 | 0.400 | 0.160–1.000 | 0.050 | ||||
| SOFA score without GCS on day 1 | 1.383 | 0.647–2.956 | 0.403 | ||||
| Age | 0.987 | 0.898–1.085 | 0.789 | ||||
| Time from collapse to ROSC (min) | 1.053 | 0.955–1.162 | 0.296 | ||||
| Model 4 | |||||||
| Lymphocyte count on day 5 | 0.791 | 0.593–1.055 | 0.111 | ||||
| SOFA score without GCS on day 1 | 0.976 | 0.711–1.340 | 0.881 | ||||
| Age | 1.053 | 0.980–1.119 | 0.169 | ||||
| Time from collapse to ROSC (min) | 1.075 | 0.996–1.159 | 0.062 | ||||
| Model 5 | |||||||
| Lymphocyte count on day 6 | 0.687 | 0.475–0.993 | 0.046 | ||||
| SOFA score without GCS on day 1 | 0.865 | 0.618–1.209 | 0.395 | ||||
| Age | 1.063 | 0.996–1.135 | 0.066 | ||||
| Time from collapse to ROSC (min) | 1.026 | 0.972–1.084 | 0.349 | ||||
Sequential organ failure assessment (SOFA) score without Glasgow Coma Scale (GCS) on day 1 was measured as the worst data within the first 24 h, excluding GCS score.
Model 1 included lymphocyte count on day 2, SOFA score on day 1, age and time from collapse to return of spontaneous circulation (ROSC), for which the P‐values were < 0.1. Model 2 included lymphocyte count on day 3, SOFA score on day 1, age and time from collapse to ROSC, for which the P‐values were < 0.1. Model 3 included lymphocyte count on day 4, SOFA score on day 1, age and time from collapse to ROSC, for which the P‐values were < 0.1. Model 4 included lymphocyte count on day 5, SOFA score on day 1, age and time from collapse to ROSC, for which the P‐values were < 0.1. Model 5 included lymphocyte count on day 6, SOFA score on day 1, age and time from collapse to ROSC, for which the P‐values were < 0.1.
CI, confidence interval.
Receiver operating characteristic curves showed a high accuracy for each lymphocyte count and neutrophil–lymphocyte ratio on days 2–6 to predict neurological outcome (Table 5).
Table 5.
Receiver operating characteristic curve analysis of neurological outcome among Japanese patients with coma after cardiac arrest (n = 46)
| AUC | P‐value | 95% CI | |
|---|---|---|---|
| Lymphocyte counts, /μL | |||
| Day 1 | 0.661 | 0.157 | 0.433–0.889 |
| Day 2 | 0.776 | 0.018 | 0.592–0.960 |
| Day 3 | 0.787 | 0.017 | 0.605–0.969 |
| Day 4 | 0.909 | 0.002 | 0.773–1.000 |
| Day 5 | 0.774 | 0.012 | 0.591–0.957 |
| Day 6 | 0.839 | 0.002 | 0.689–0.989 |
| NLR | |||
| Day 1 | 0.572 | 0.526 | 0.348–0.796 |
| Day 2 | 0.739 | 0.040 | 0.541–0.938 |
| Day 3 | 0.753 | 0.035 | 0.552–0.955 |
| Day 4 | 0.800 | 0.020 | 0.607–0.993 |
| Day 5 | 0.889 | >0.001 | 0.769–1.000 |
| Day 6 | 0.911 | >0.001 | 0.810–1.000 |
| SOFA score without GCS on day 1 | 0.701 | 0.022 | 0.542–0.860 |
| Age | 0.710 | 0.018 | 0.549–0.870 |
| Time to ROSC (min) | 0.707 | 0.018 | 0.557–0.856 |
Sequential Organ Failure Assessment (SOFA) score without Glasgow Coma Scale (GCS) on day 1 was measured as the worst data within the first 24 h, excluding GCS score.
AUC, area under the curve; CI, confidence interval; NLR, neutrophil–lymphocyte ratio; ROSC, return of spontaneous circulation.
On days 2–4, lymphocyte count showed higher accuracy for prediction of neurological outcome than NLR. On days 5 and 6, NLR showed higher accuracy for prediction of neurological outcome than lymphocyte count. In univariate and multivariate linear regression analyses, SOFA score on day 1 was independently associated with lymphocyte count on days 1, 2, 5 and 6. In univariate linear regression analysis, CAI on day 4 was associated with lymphocyte count on day 4. The CAI on day 5 and time to ROSC were associated with lymphocyte count on day 5, although neither variable was associated with lymphocyte count in multivariate linear regression analysis (Table 6).
Table 6.
Univariate and multivariate logistic regression analyses of lymphocyte count among Japanese patients with coma after cardiac arrest (n = 46)
| Univariate linear regression | Multivariate linear regression | |||||
|---|---|---|---|---|---|---|
| B | P‐value | 95% CI | B | P‐value | 95% CI | |
| Analysis of lymphocyte count on day 1 | ||||||
| CAI on day 1 | −0.097 | 0.434 | −0.154 to 0.348 | |||
| Age | −0.013 | 0.822 | −0.135 to 0.108 | |||
| Time from collapse to ROSC (min) | −0.035 | 0.306 | −0.104 to 0.034 | |||
| SOFA score without GCS on day 1 | −0.521 | 0.016 | −0.934 to −0.107 | |||
| Analysis of lymphocyte count on day 2 | ||||||
| CAI on day 2 | 0.126 | 0.565 | −0.319 to 0.571 | |||
| Age | −0.021 | 0.724 | −0.139 to 0.098 | |||
| Time from collapse to ROSC (min) | −0.036 | 0.566 | −0.165 to 0.093 | |||
| SOFA score without GCS on day 1 | −0.629 | 0.024 | −1.168 to −0.090 | |||
| Analysis of lymphocyte count on day 3 | ||||||
| CAI on day 3 | 0.214 | 0.386 | −0.289 to 0.714 | |||
| Time from collapse to ROSC (min) | −0.022 | 0.739 | −0.158 to 0.113 | |||
| Age | −0.012 | 0.831 | −0.132 to 0.107 | |||
| SOFA score without GCS on day 1 | −0.403 | 0.355 | −1.291 to 0.481 | |||
| Analysis of lymphocyte count on day 4 | ||||||
| CAI on day 4 | −0.293 | 0.081 | −0.612 to 0.039 | −0.236 | 0.132 | −0.551 to 0.079 |
| Time from collapse to ROSC (min) | −0.044 | 0.133 | −0.103 to 0.015 | |||
| Age | −0.075 | 0.052 | −0.150 to 0.001 | −0.064 | 0.086 | −0.138 to 0.010 |
| SOFA score without GCS on day 1 | −0.087 | 0.764 | −0.684 to 0.510 | |||
| Analysis of lymphocyte count on day 5 | ||||||
| CAI on day 5 | −0.293 | 0.066 | −0.607 to 0.021 | −0.161 | 0.379 | −0.530 to 0.209 |
| Time from collapse to ROSC (min) | −0.057 | 0.100 | −0.126 to 0.012 | −0.015 | 0.714 | −0.096 to 0.066 |
| Age | −0.076 | 0.103 | −0.169 to 0.016 | |||
| SOFA score without GCS on day 1 | −0.528 | 0.010 | −0.921 to −0.134 | −0.439 | 0.041 | −0.860 to −0.019 |
| Analysis of lymphocyte count on day 6 | ||||||
| CAI on day 6 | −0.253 | 0.166 | −0.618 to 0.111 | |||
| Time from collapse to ROSC (min) | −0.039 | 0.262 | −0.108 to 0.030 | |||
| Age | −0.076 | 0.078 | −0.162 to 0.009 | −0.042 | 0.339 | −0.130 to 0.046 |
| SOFA score without GCS on day 1 | −0.528 | 0.013 | −0.935 to −0.120 | −0.446 | 0.049 | −0.889 to −0.002 |
Sequential Organ Failure Assessment (SOFA) score without Glasgow Coma Scale (GCS) on day 1 was measured as the worst data within the first 24 h, excluding GCS score.
Catecholamine index (CAI) = hourly doses (μg/kg/min) of dopamine + dobutamine + (adrenaline + noradrenaline) × 100 (μg/kg/min).
CAI of each day was calculated at the highest point of the day.
B, partial regression coefficient; CI, confidence interval.
Discussion
In this retrospective study, we showed that lymphocyte count on days 2‐4 and 6 were associated with neurological outcome, but not with mortality, in PCAS patients. Receiver operating characteristic curves of lymphocyte count on days 2–6 were able to predict neurological outcome, with lymphocyte count on day 4 showing the highest accuracy to predict neurological outcome.
Lymphopenia might be caused by lymphocytic apoptosis. Lymphopenia sometimes occurs in patients with sepsis and stroke, which have similar features to PCAS.9, 10 Lymphocyte apoptosis is a possible mechanism to explain the lymphopenia in both sepsis and stroke.10, 14 Lymphocytic apoptosis could also occur pathophysiologically in PCAS. In fact, lymphocyte apoptosis in the spleen has been identified in an animal PCAS model.15 Hence, apoptosis of lymphocytes might be the reason for the lymphopenia observed in the PCAS patients in our study.
Catecholamines might influence lymphocyte count in PCAS patients. One study reported that catecholamines are involved in proliferation of lymphocytes,16 whereas another study reported that catecholamines are involved in apoptosis of lymphocytes.17 In this study, extrinsic catecholamines were not independently associated with neurological outcome or lymphocyte count. However, it has been previously reported that intrinsic catecholamines are elevated in PCAS patients.18 Evaluation of the association between plasma catecholamine concentration and lymphocyte counts might reveal the mechanism of lymphopenia in PCAS patients.
An association between lymphopenia and mortality in sepsis patients was also observed in a previous study.9 However, in the current study, lymphopenia was not associated with mortality in PCAS patients, despite the PCAS‐induced cytokinemia, as is seen in sepsis. This suggests that immunosuppression accompanied by lymphopenia might be fatal in sepsis, which is related to infection, but is not fatal in PCAS, which is not related to infection at admission.
In our study, lymphocyte count on days 2–6 showed higher accuracy in predicting neurological outcome than that on day 1.There were five patients who had unfavorable neurological outcomes and a higher lymphocyte count than 675/μL (the median value for all patients) on day 1. Lymphocyte count in the subacute phase tended to decrease in four of these patients. This phenomenon might be one of the reasons lymphocyte count on day 4 showed a higher accuracy to predict neurological outcome than that on day 1.
In our study, NLR showed the highest accuracy in predicting neurological outcome on day 6. Only on day 6, neutrophil counts were significantly lower in patients who had favorable neurological outcomes than patients who had unfavorable neurological outcomes. This could be the reason why NLR on day 6 showed the highest accuracy in the prediction of neurological outcome.
Hypothermia is reported to cause lymphopenia.19 However, although lymphocyte counts on day 3 could have been influenced by MHT, those on days 4 and 5 were not. Therefore, the association between lymphocyte count and neurological outcome in PCAS patients in this study was most likely not an effect of the MHT.
There are several limitations to this study. First, it was retrospective in nature. Second, this was a single‐center study, so the sample size was relatively small. However, the statistical power for logistic analysis of lymphocyte counts on days 2–6 and neurological outcome, which was calculated assuming a type 1 error = 0.05, showed sample size and prognostic proportion to be as follows: day 1, 0.396; day 2, 0.868; day 3, 0.961; day 4, >0.999; day 5, 0.885; and day 6, 0.986. The high statistical power might be the reason for the association between lymphocyte count on days 2–6 and neurological outcome, despite the small sample size. Third, our hospital is a tertiary care hospital. Hence, it is possible that only patients who are expected to have a favorable outcome with more intensive treatment are transported to our hospital for intensive care. This could be a reason for the more favorable neurological outcome in non‐ventricular fibrillation patients in our study compared with previous studies,20 and for the absence of association between neurological outcomes and initial cardiac rhythm. Finally, as our facility can measure neutrophil and lymphocyte counts only during the daytime, we missed several data points, and day 1 data were not always measured immediately after admission. This could be a reason why we could not find an association between lymphocyte and neutrophil counts on day 1 and mortality, as was shown in a previous study.4 Prospective multicenter studies are required to resolve these issues.
Conclusion
Lymphopenia on days 2–4 and 6 after cardiac arrest is associated with poor neurological outcomes, but not mortality. Lymphocyte count on day 4 showed the highest accuracy of days 1–6 to predict neurological outcome in patients with coma after cardiac arrest.
Disclosure
Approval of the research protocol: The present study was approved by the institutional ethics committee of Shiga University of Medical Science.
Informed consent: N/A.
Registry and registration no. of the study/trial: N/A.
Animal study: N/A.
Conflict of interest: None declared.
Acknowledgements
We are grateful to all the physicians who participated in this study for their contribution to its successful completion.
Funding Information
No funding information provided.
References
- 1. Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out‐of‐hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation 2010; 81: 1479–87. [DOI] [PubMed] [Google Scholar]
- 2. Nolan JP, Neumar RW, Adrie C et al Post‐cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008; 118: 2452–83. [DOI] [PubMed] [Google Scholar]
- 3. Vondrakova D, Kruger A, Janotka M. Association of neuron‐specific enolase values with outcomes in cardiac arrest survivors is dependent on the time of sample collection. Crit. Care 2017; 21: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiser C, Schwameis M, Sterz F et al Mortality in patients resuscitated from out‐of‐hospital cardiac arrest based on automated blood cell count and neutrophil lymphocyte ratio at admission. Resuscitation 2017; 116: 49–55. [DOI] [PubMed] [Google Scholar]
- 5. Baser K, Bas HD, Attaluri P, Rodrigues T, Nichols J, Nugen K. Changes in neutrophil‐to‐lymphocyte ratios in postcardiac arrest patients treated with targeted temperature management. Anatol. J. Cardiol. 2017; 18: 215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Villois P, Grimaldi D, Spadaro S et al Lymphopaenia in cardiac arrest patients. Ann. Intensive Care 2017; 7: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adrie C, Adib‐Conquy M, Laurent I et al Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis‐like” syndrome. Circulation 2002; 106: 562–8. [DOI] [PubMed] [Google Scholar]
- 8. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003; 348: 138–50. [DOI] [PubMed] [Google Scholar]
- 9. Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014; 42: 383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Urra X, Cervera A, Villamor N, Planas AM, Chamorro A. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience 2009; 158: 1174–83. [DOI] [PubMed] [Google Scholar]
- 11. Hug A, Dalpke A, Wieczorek N et al Infarct volume is a major determiner of post‐stroke immune cell function and susceptibility to infection. Stroke 2009; 40: 3226–32. [DOI] [PubMed] [Google Scholar]
- 12. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975; 1: 480–4. [DOI] [PubMed] [Google Scholar]
- 13. Yamashita C, Hara Y, Kuriyama N, Nakamura T, Nishida O. Clinical effects of a longer duration of polymyxin B‐immobilized fiber column direct hemoperfusion therapy for severe sepsis and septic shock. Ther. Apher. Dial. 2015; 19: 316–23. [DOI] [PubMed] [Google Scholar]
- 14. Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J. Immunol. 2005; 174: 5110–8. [DOI] [PubMed] [Google Scholar]
- 15. Gu W, Zhang Q, Yin W, Li C. Caspase‐3‐mediated splenic lymphocyte apoptosis in a porcine model of cardiac arrest. Am. J. Emerg. Med. 2014; 32: 1027–32. [DOI] [PubMed] [Google Scholar]
- 16. Burns AM, Keogan M, Donaldson M, Brown DL, Park GR. Effects of inotropes on human leucocyte numbers, neutrophil function and lymphocyte subtypes. Br. J. Anaesth. 1997; 78: 530–5. [DOI] [PubMed] [Google Scholar]
- 17. Cioca DP, Watanabe N, Isobe M. Apoptosis of peripheral blood lymphocytes is induced by catecholamines. Jpn. Heart J. 2000; 41: 385–98. [DOI] [PubMed] [Google Scholar]
- 18. Prengel AW, Lindner KH, Ensinger H, Grunert A. Plasma catecholamine concentrations after successful resuscitation in patients. Crit. Care Med. 1992; 20: 609–14. [DOI] [PubMed] [Google Scholar]
- 19. Bouma HR, Kroese FG, Kok JW et al Low body temperature governs the decline of circulating lymphocytes during hibernation through sphingosine‐1‐phosphate. Proc. Natl Acad. Sci. USA 2011; 108: 2052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim YM, Yim HW, Jeong SH, Klem ML, Callaway CW. Does therapeutic hypothermia benefit adult cardiac arrest patients presenting with non‐shockable initial rhythms? A systematic review and meta‐analysis of randomized and non‐randomized studies. Resuscitation 2012; 83: 188–96. [DOI] [PubMed] [Google Scholar]


