Abstract
Case
We describe a rare case of antibiotic‐associated fulminant pseudomembranous enterocolitis caused by Klebsiella oxytoca. A 79‐year‐old man with a history of antibiotic therapy was admitted to our emergency department, complaining of consciousness disturbance. Initially, we suspected septic shock and diabetic ketoacidosis caused by intestinal infection. Although we administered sufficient extracellular fluid, his blood pressure was not elevated and his abdomen gradually swelled.
Outcome
The patient died of shock and abdominal compartment syndrome. Autopsy revealed widespread jejunal necrosis in conjunction with colitis, suggesting fulminant pseudomembranous enterocolitis caused by K. oxytoca infection.
Conclusion
As the clinical features of pseudomembranous enterocolitis caused by K. oxytoca resemble the features of colitis caused by Clostridium difficile, conservative therapy is applied first. However, fulminant pseudomembranous enterocolitis is a lethal disease, necessitating early operation for resection of the necrotic lesion. This report highlights the need for better surgical criteria at an early stage.
Keywords: Abdominal compartment syndrome, Clostridium difficile, inflammatory bowel disease, Klebsiella oxytoca, septic shock
Introduction
Drug‐associated diarrhea frequently occurs during antibiotic treatment. Clostridium difficile is a spore‐forming gram‐positive bacterium, and the clinical spectrum of C. difficile infection ranges from simple diarrhea without severe colitis to pseudomembranous colitis and fulminant colitis.1 Some types of pseudomembranous colitis infrequently occur in close association with ischemic colitis or infections by organisms such as cytomegalovirus, Shigella dysenteriae, Escherichia coli O157:H7, and Klebsiella oxytoca.2
Klebsiella oxytoca is a gram‐negative bacterium that provokes bacteremia, septic shock coupled with systemic organ dysfunction, and even death in serious cases.3 In 2006, K. oxytoca was first reported as a cause of antibiotic‐associated hemorrhagic colitis.4 However, to our knowledge, no case report has determined that K. oxytoca causes antibiotic‐associated fulminant pseudomembranous enterocolitis. Herein, we describe a rare case of antibiotic‐associated fulminant pseudomembranous enterocolitis caused by K. oxytoca.
Case
A 79‐year‐old man with diabetes mellitus was admitted to our emergency department with a complaint of consciousness disturbance. He received clarithromycin 400 mg/day to treat diarrhea before admission. On arrival, his vital signs were as follows: Glasgow Coma Scale score, E4V1M1; blood pressure, 144/102 mmHg; heart rate, 85 b.p.m.; respiratory rate, 25 cycles/min; peripheral oxygen saturation (SpO2) of 100%, with a 10 L/min oxygen reservoir mask. Blood gas analysis findings were as follows: pH, 7.219; PaCO2, 59.4 mmHg; PaO2, 529 mmHg; , 20.4 mEq/L; base excess, −3.3 mEq/L. Severe respiratory and mild metabolic acidosis with hyperglycemia (glucose, 491 mg/dL) were also noted. Because we initially suspected diabetic ketoacidosis and severe hypoventilation, we promptly intubated the patient and initiated the simultaneous i.v. injection of Ringer's lactate solution and regular insulin (8 IU). Serum examination strongly indicated renal dysfunction (blood urea nitrogen, 74 mg/dL; creatinine, 2.96 mg/dL), hyperkalemia (potassium, 5.3 mEq/L), and marked inflammatory reaction (C‐reactive protein, 15.6). Computed tomography revealed no abnormal lesions in the brain; however, marked intestinal dilation and swelling were observed between the duodenum and jejunum. These findings led to an initial diagnosis of septic shock and diabetic ketoacidosis caused by intestinal infection. Despite sufficient extracellular fluid, his blood pressure was not elevated and his abdomen gradually swelled (Fig. 1A). Contrast‐enhanced computed tomography revealed intestinal dilatation but no sign of ischemia or necrosis (Fig. 1B,C). Physiological examination (intra‐abdominal pressure >50 mmHg) also clearly showed abdominal compartment syndrome. We attempted an emergency operation, but the patient's condition deteriorated so rapidly that we could not ameliorate his hemodynamics. Finally, he died of shock and abdominal compartment syndrome 10 h after admission.
Figure 1.
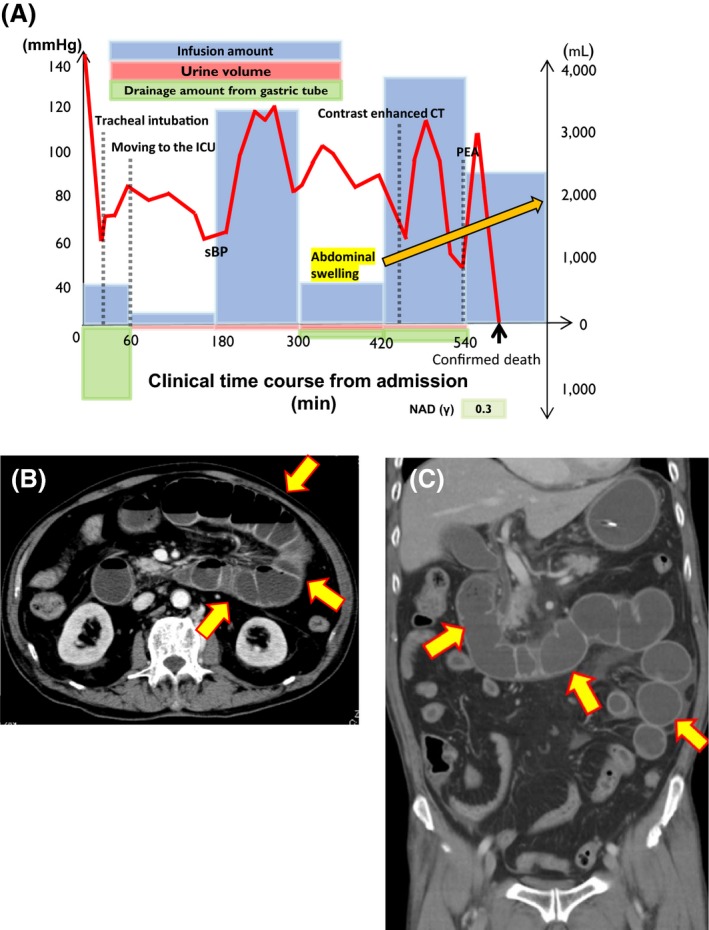
Time course and imaging findings of a 79‐year‐old man with fulminant pseudomembranous enterocolitis caused by Klebsiella oxytoca. A, Schema of the patient's clinical time course (min) from admission to death. ICU, intensive care unit; NAD (γ), nor‐adrenaline (mg/kg/min); PEA, pulseless electrical activity; sBP, systolic blood pressure. B,C, Contrast‐enhanced computed tomography scan (6 h after admission). Arrows indicate intestinal dilatation and swelling, with the fluid level between the duodenum and the jejunum. However, there were no signs of ischemia or necrosis because the intestinal wall was well‐enhanced with contrast media.
In autopsy, thinning, petechiae, and intestinal dilation were observed from the ligament of Treitz to 100 cm caudal to the jejunum. Pseudomembrane formation was observed on the intestinal mucosa (Fig. 2B). On histological analysis, severe inflammatory cell infiltration was observed in the lung, trachea, esophagus, liver, and spleen. In addition, hemophagocytosis was also observed in the bone marrow, indicating that systemic inflammation clearly occurred because of sepsis.
Figure 2.
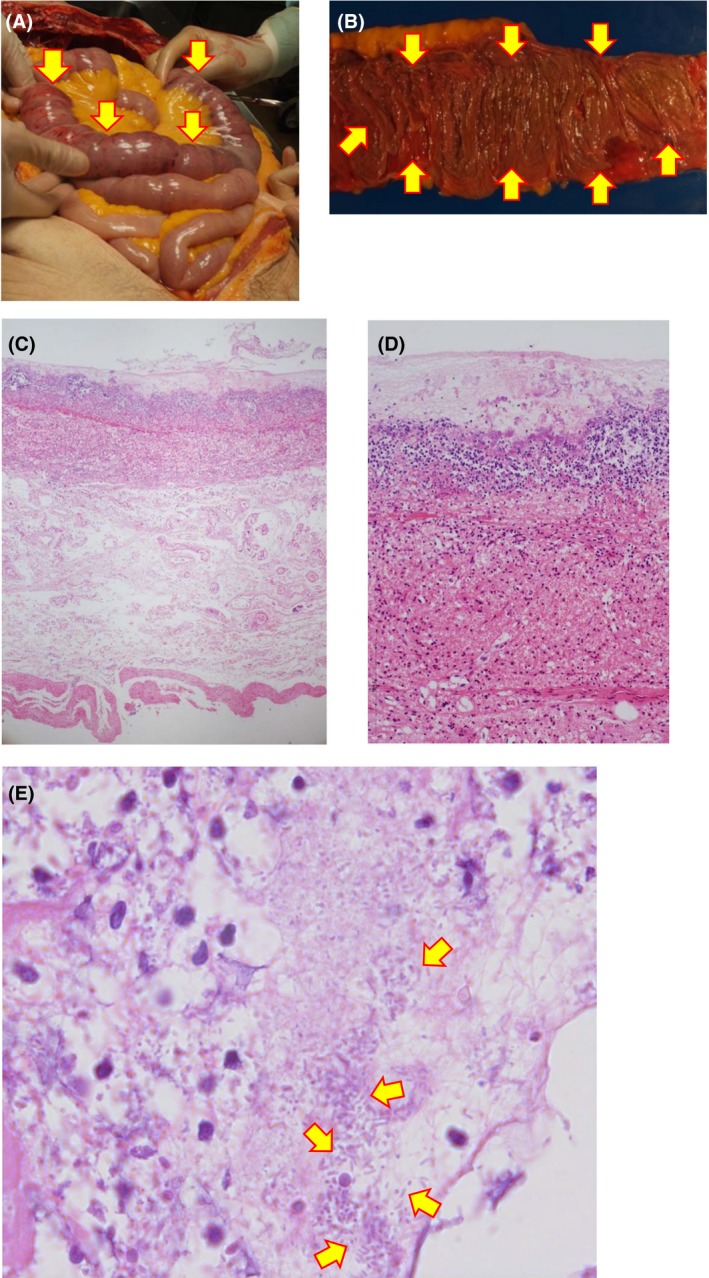
Autopsy findings in a 79‐year‐old man with fulminant pseudomembranous enterocolitis caused by Klebsiella oxytoca. A, Images of intestinal necrosis in the autopsy. Mucosal necrosis is observed between the duodenum and jejunum (arrows). B, Intestinal necrosis is observed between the duodenum and jejunum. Arrows indicate pseudomembrane formation. C, Microscopic view. Hematoxylin–eosin staining (×40). D, High‐power view (×100) of (C). Hematoxylin–eosin staining reveals mucosal hemorrhagic necrosis and submucosal edema in conjunction with pseudomembranous formation. E, Maximum‐power view (×1,000) of (C). Arrows indicate gram‐negative bacilli that correspond to K. oxytoca.
Gram‐negative bacilli were histologically detected in close association with the pseudomembrane. The mucous membrane was completely destroyed and replaced with inflammatory cell filtration, submucosal edema, and cellular debris (Fig. 2C,D). Klebsiella oxytoca was also identified by a post‐mortem bacterial culture from pseudomembranous contents of the jejunum. The patient had received clarithromycin before admission. Furthermore, the only bacterium cultured from the patient's first sputum was K. oxytoca, suggesting that K. oxytoca infection was already present on the day of admission. Therefore, our final diagnoses were as follows: (i) fulminant pseudomembranous enterocolitis caused by K. oxytoca, (ii) septic shock, (iii) diabetic ketoacidosis, (iv) abdominal compartment syndrome.
Discussion
To the best of our knowledge, this paper is the first autopsy report on fulminant pseudomembranous enterocolitis caused by K. oxytoca. The symptoms of K. oxytoca‐induced colitis resemble those of C. difficile‐induced colitis, as both bacteria cause opportunistic infections in immunocompromised hosts. In particular, our case clearly differed from previous cases because all damage was localized not in the colon but in the jejunum. To our knowledge, to date, only two case reports on pseudomembranous colitis caused by K. oxytoca 2, 5 have been published (Table 1). Conservative therapy following the cessation of prescribed medication was selected as the initial treatment in both cases.
Table 1.
Published case reports of pseudomembranous colitis caused by Klebsiella oxytoca
| Author (year) | Tomita et al. (1984) | Sweetser et al. (2002) | Our case (2018) |
|---|---|---|---|
| Diagnosis | Pseudomembranous colitis | Pseudomembranous colitis | Fulminant pseudomembranous enterocolitis |
| Age, years; gender | 15, male | 67, female | 79, male |
| Antibiotic | Cefatrizine | Unknown | Clarithromycin |
| Symptoms | Diarrhea, melena | Watery diarrhea | Diarrhea |
| Lesion | 20–45 cm from the anal ring | Sigmoid colon | 100 cm distal from ligament of Treitz |
| Treatment | Conservative | Metronidazole | Conservative |
| Result | Survival | Survival | Death |
The mechanisms of K. oxytoca‐induced antibiotic‐associated enterocolitis are still controversial because K. oxytoca has no enterotoxin. In C. difficile‐induced pseudomembranous colitis, toxin A plays a key role as an enterotoxin. In K. oxytoca, tilivalline and kleboxymycin (precursor of tilivalline) play roles as cytotoxins.6, 7
We could not find any scientific papers published to date that reference fulminant pseudomembranous enterocolitis caused by K. oxytoca. We believe that the pathogen causing pseudomembranous enterocolitis was K. oxytoca because of two factors. First, histology apparently revealed severe inflammation due to K. oxytoca infection (Fig. 2). Second, the bacteriological culture from the patient's sputum and post‐mortem contents from the jejunal pseudomembrane also showed K. oxytoca bacterial growth, suggesting that septic shock occurred because of fulminant enterocolitis caused by K. oxytoca infection. In addition, K. oxytoca often causes bacteremia when the patients are immunocompromised hosts, such as those with hepatopancreatic failure, malignant diseases, or diabetes mellitus.3 In our case, the patient's clinical condition might have become more serious because of uncontrolled diabetes mellitus.
Fulminant pseudomembranous colitis should be suspected when patients show systemic signs of toxicity including fever, hypotension, tachycardia, leukocytosis, and severe dehydration that requires massive transfusion.8 Surgical treatment leads to better results than conservative therapy. Postoperative mortality after urgent colectomy was reported to range from 34% to 57%.9 Indications for surgery could include toxic megacolon, perforation and/or peritonitis, fulminant disease in the presence of septic shock, multiple organ failure, and failure of conservative therapy.9
In our case, we rapidly administered sufficient fluid but it was very difficult to stabilize the patient's hemodynamics. The patient's clinical course was so rapid that it made surgery impossible. We should have carried out an urgent operation as soon as his abdominal swelling was noted. However, it is difficult to predict the failure of conservative therapy in the early phase. Better criteria are required to aid surgery selection in the early phase of fulminant pseudomembranous colitis.
Conclusion
We reported a rare case of fulminant pseudomembranous enterocolitis caused by K. oxytoca infection. Fulminant pseudomembranous enterocolitis is lethal and early surgery is necessary if it is suspected. The treatment is restricted to an “early diagnosis–early operation” policy, although the basic mechanisms and essential treatment of this condition remain unknown.
Disclosure
Approval of the research protocol: N/A.
Informed consent: Yes.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: N/A.
Acknowledgments
We thank Ms. Rika Ono for her technical assistance with submitting the manuscript. We also acknowledge Editage for their English editing.
Funding information
No funding information provided.
References
- 1. Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N. Engl. J. Med. 1994; 330: 257–62. [DOI] [PubMed] [Google Scholar]
- 2. Sweetser S, Schroeder KW, Pardi DS. Pseudomembranous colitis secondary to Klebsiella oxytoca . Am. J. Gastroenterol. 2009; 104: 2366–8. [DOI] [PubMed] [Google Scholar]
- 3. Al‐Anazi KA, Al‐Jasser AM, Al‐Zahrani HA, Chaudhri N, Al‐Mohareb FI. Klebsiella oxytoca bacteremia causing septic shock in recipients of hematopoietic stem cell transplant: two case reports. Cases J. 2008; 1: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Högenauer C, Langner C, Beubler E et al Klebsiella oxytoca as a causative organism of antibiotic‐associated hemorrhagic colitis. N. Engl. J. Med. 2006; 355: 2418–26. [DOI] [PubMed] [Google Scholar]
- 5. Tomita O et al A case of pseudomembranous colitis caused by Klebsiella oxytoca . Ann. Jap. Assoc. Endoscopic. Gastroenterol. 1984; 26: 308 (Japanese). [Google Scholar]
- 6. Tse H, Gu Q, Sze KH et al A tricyclic pyrrolobenzodiazepine produced by Klebsiella oxytoca is associated with cytotoxicity in antibiotic‐associated hemorrhagic colitis. J. Biol. Chem. 2017; 292: 19503–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schneditza G, Rentnerb J, Roiera S et al Enterotoxicity of a nonribosomal peptide causes antibiotic‐associated colitis. PNAS 2014; 111: 13181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sailhamer EA, Carson K, Chang Y et al Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch. Surg. 2009; 144: 433–9. [DOI] [PubMed] [Google Scholar]
- 9. Dallal RM, Harbrecht BG, Boujoukas AJ et al Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann. Surg. 2002; 235: 363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


